-
镉(Cd)[1]和砷(As)[2]是全球农田污染的高毒性(类)金属,对人体健康构成巨大威胁. 而工矿企业及冶炼厂附近的重金属复合污染问题突出,常常给土壤修复和治理带来挑战[3 − 4]. 例如Cd和As的复合污染问题是我国目前土壤污染治理的难点之一,因为Cd和As在地球化学行为和生物有效性方面存在显著差异[5 − 6],使得制定共同策略以减少它们在作物中积累非常困难. 研究表明,土壤氧化还原电位(Eh)、pH和溶解性有机碳(DOC)是Cd和As生物利用性的主要影响因素[7]. 在好氧条件下,大部分As以AsV形态存在,主要被土壤铁氧化物表面所持留[8],而Cd主要以CdⅡ形式存在,可被多种土壤活性胶体表面所吸附. 相比而言,碱性土壤有利于Cd的吸附,但As则相反[9]. 在厌氧条件下,As易随土壤铁氧化物的还原溶解而溶出,而Cd则由于吸附或沉淀反应而减少了溶出活性. 当前人们开发了多种技术用于控制土壤Cd+As复合污染,如水分管理[8, 10]、施用有机肥[11]、基因调控和新型材料等[12]. 其中,纳米颗粒(nanoparticles, NPs)因其高表面能、高反应性、流动性和强吸附能力成为抵抗非生物胁迫的重要工具[13]. 例如,磁性纳米颗粒(FeO·Fe2O3 NP)可以显著降低水培条件下日本萝卜中As和Cd吸收量[14]. ZnO NPs也可以显著抑制Cd+As复合污染水稻中As和Cd含量[15]. SiO2和TiO2 NPs可以通过调节抗氧化能力和Si/As转运体表达抑制As对水稻的毒性. CeO2 NPs对Cd胁迫下大豆的生长具有促进作用[16].
目前NPs的研究多采用水培的方式,但在实际土壤环境中,NPs由于其巨大的表面能极易被土壤胶体吸附,其生物化学行为可能与水培实验结果产生差异,因此有必要研究土培条件下NPs对重金属的阻抗效应. 小白菜是中国本土食用最广泛的蔬菜之一,本研究以小白菜为供试植物,研究了4种代表性NPs(ZnO、CeO2、SiO2、S )对As、Cd单一和复合污染下的综合影响,以期为未来的农业重金属治理提供科学依据.
-
实验土壤采自南京大学仙林校区附近花园的表层土壤(0—20 cm),采集后的土壤在室温下自然风干,去除石子等杂质后过2 mm筛,土壤pH的测定参考玻璃电极法(HJ962—2018),土壤有机质(SOM)采用重铬酸钾氧化法测定(HJ 615—2011);土壤铁氧化物含量(DCB-Fe)采用连二亚硫酸钠-柠檬酸钠-碳酸氢钠提取法测定[17];无定形铁氧化物含量(ox-Fe)采用草酸提取法测定[17];土壤中As和Cd等元素参考HNO3−H2O2消解法测定(USEPA 3050B). 土壤基本性质如表1所示.
在土壤中分别添加Na2HAsO4和CdCl2进行两个月的老化,使土壤As和Cd含量分别达到106.3 mg·kg−1 和2.8 mg·kg−1,污染物浓度均超过了我国农用地土壤污染风险筛选值(GB
15618 —2018),且在前期预实验中使植物表现出一定的受损症状. -
实验采用4种不同的NPs(CeO2、ZnO、SiO2、S)分别从江苏先丰纳米材料科技有限公司和上海攀田粉体材料有限公司购买,粒径分别为32、53、16、16 nm[17]. 在种植植物前,将一定量的NPs与经2 mm筛的土壤摇匀混合并稳定48 h. 实验处理包括未污染土壤(CK)、Cd染毒土壤(Cd)、As染毒土壤(As)、Cd/As复合污染土壤4种(Cd+As),此外,在前期预实验结果基础上,在这4种背景下分别施用100 mg·kg−1 CeO2、100 mg·kg−1ZnO、200 mg·kg−1 SiO2、200 mg·kg−1 S NPs,每个处理包括4个重复. 小白菜种子购自北京市农林科学院,经消毒后种植于不同处理的土壤中,每盆300 g土壤,施入0.15/0.05/0.1 g·kg−1的N/P/K底肥,植物生长1周后,对植物进行间苗,每盆培养4棵小白菜. 实验在校园温室中进行,温度范围约为18—28 ℃,小白菜生长至30 d后收获. 在收获前24 h,使用Rhizon土壤孔隙水采集器采集土壤孔隙水样品,冷藏保存后在一周内尽快完成相关参数的测定. 植物收获时,轻轻拨掉小白菜根系的土壤,在自来水下冲洗干净后再以去离子水清洗3次. 利用无尘纸吸去植物表面水分,测定株高、根长、地上和地下鲜重. 部分植物组织置于−80 ℃冰箱保存.
-
冻干的植物样品被研磨成粉末后,取
0.1000 g样品置于聚丙烯塑料消解管中,采用HNO3-H2O2消解法测定(USEPA 3050B). 待冷却至室温后,定容至25.00 mL,过滤后使用电感耦合等离子体发射光谱(ICP-OES,PQ9000,analytikjena)或电感耦合等离子体质谱(ICP-MS,NexION 300, PerkinElmer)测定元素含量. 孔隙水经0.22 μm滤膜过滤,用2%HNO3稀释后测定元素含量. 为保证测量精度,每30个样品插入一个已知浓度溶液以测定回收率. 消解采用国家标准物质GBW07602(GSV-1)灌木枝叶进行质量控制,元素回收率为96%—108%. 孔隙水在采集后的12 h内测定其pH, 将采集后的孔隙水过0.45 μm滤膜,加入过量2 mol·L−1HCl酸化后使用总有机碳测定仪(Elementar Vario TOC Cube analyzer,德国)测定孔隙水中的溶解性有机碳(DOC)含量. -
将新鲜的植物组织在液氮中迅速研磨,并以1:9(W:V)的比例加入0.1 mol·L−1 PBS缓冲液(pH7.2)进行匀浆,将匀浆液在4 ℃下以
10000 r·min−1的转速离心10 min,得到的上清液采用南京建成生物工程研究所的试剂盒测定超氧化物歧化酶(SOD,A001-1-1)、过氧化物酶(POD,A084-1-1)活性以及丙二醛(MDA,A003-3-1)的含量. -
所有数据以平均值±标准差SD表示,采用IBM SPSS 25进行处理间差异分析,使用单因素方差分析和Duncan法进行多重比较,P<0.05视为显著差异. 所有图表均使用Origin 2023制作. 每种植物营养元素含量进行Min-Max归一化处理(Min-Max Normalization),使得原始数据的结果值映射到0—1之间,最终以热图(Heatmap)的形式展示,转换函数如下:
-
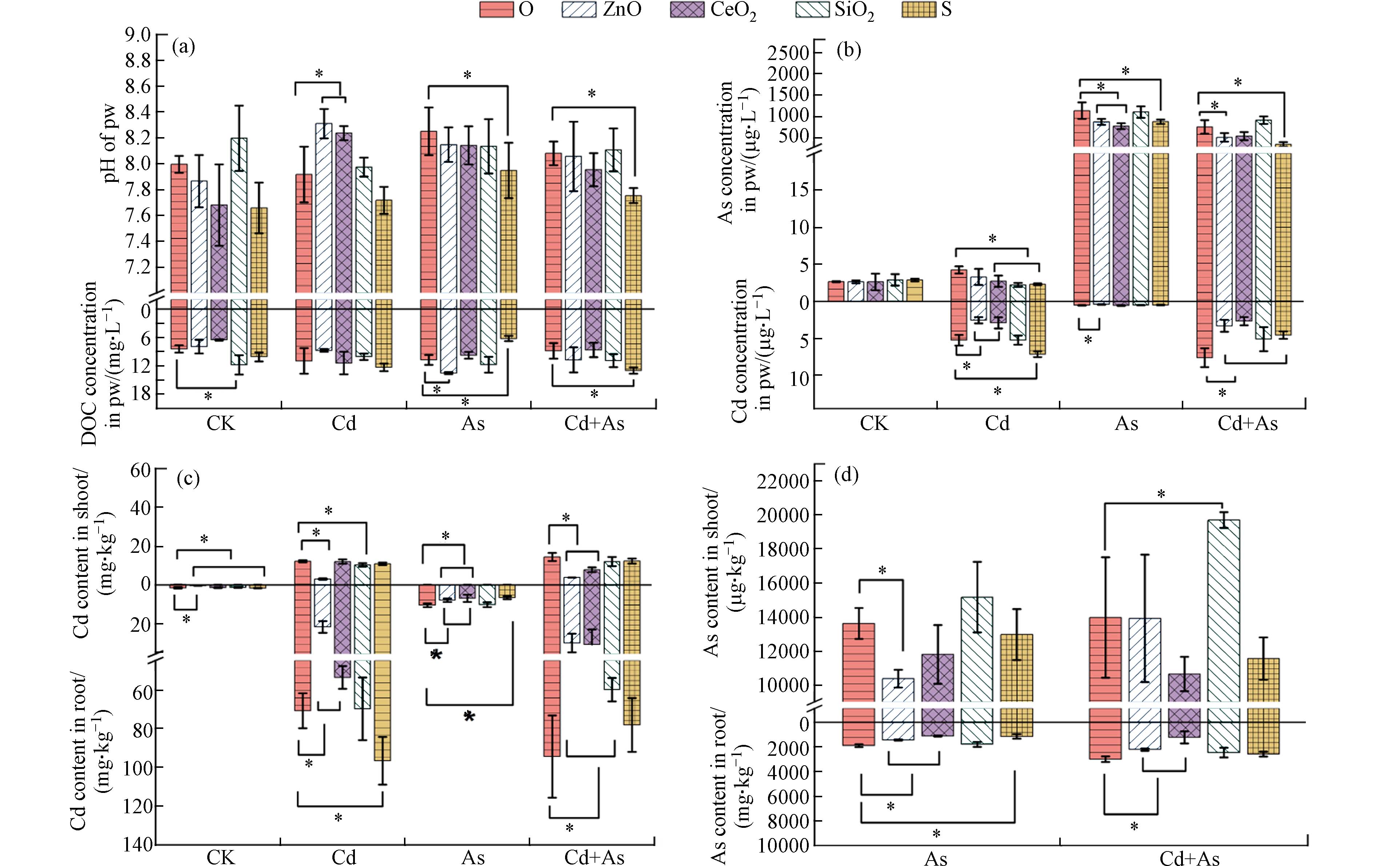
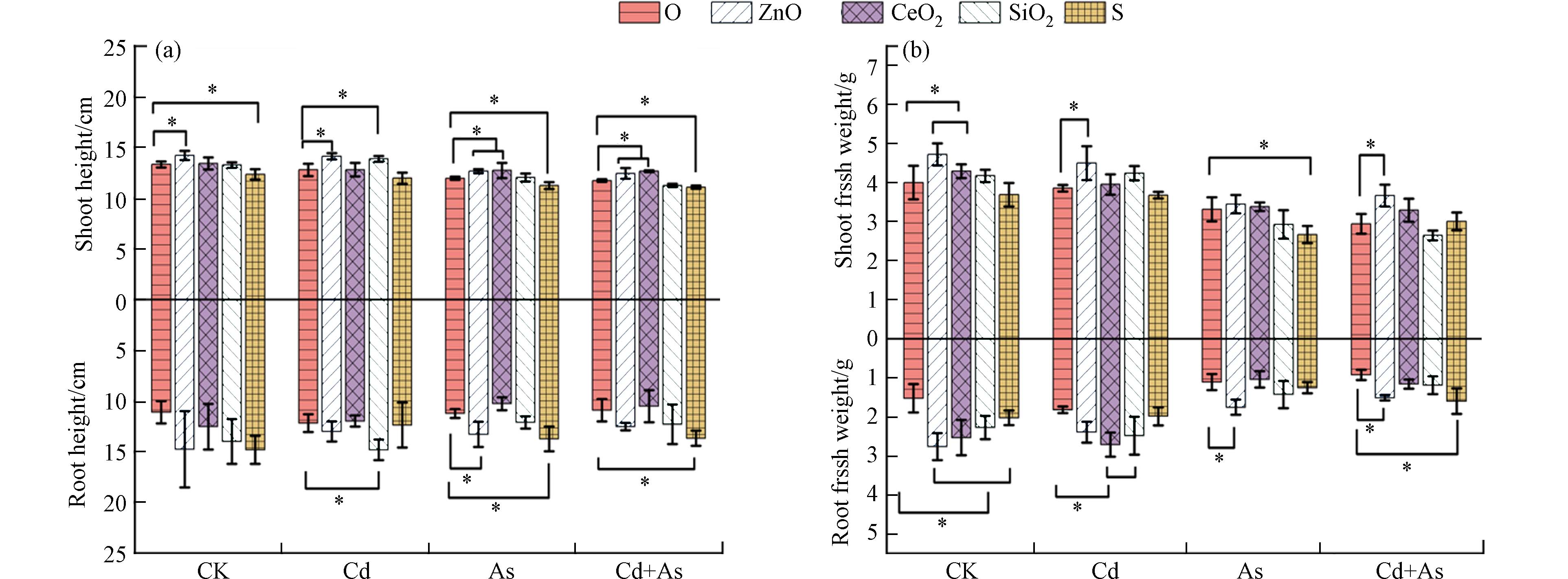
Cd和As单一或混合处理下对植物的生长和生物量均显示出抑制作用,表现为植物叶片黄化严重,而NPs的添加在一定程度上缓解或加剧了这种毒害效应. 在不同Cd和As处理下,ZnO NPs均可以显著促进植物地上部的生长,株高和地上鲜重分别增加了5.9%—10.5%和16.5%—24.6%(图1a、b). CeO2 NPs也促进了As和Cd+As胁迫下植物地上部株高(6.1%—7.3%). SiO2 NPs可促进Cd胁迫下小白菜8.5%的株高、21.5%的根长、36.4%的根部鲜重. 而S NPs虽然一定程度上促进了As和Cd+As胁迫下植物的根长(22.2%—25.3%),却显著抑制了5.5%—6%的株高(图1a、b).
已有研究证明在As[18]、Cd[19]或Cd+As[5]重金属胁迫下,植物生长会受到不同程度抑制. 由于碱性土壤更有利于Cd的吸附且易于As的溶出[7]. ZnO NPs在不同环境背景下对植物都是一种高效的生长促进剂,ZnO NPs可以通过提供Zn元素“微肥”来促进植物的生长发育[20]. 比如,ZnO NPs进入土壤后,可以水解释放出Zn2+. 例如在2种不同的土壤中掺杂4 g·kg−1的ZnO NPs后,在1 d内几乎全部溶解成Zn2+形式,从而促进了植物对营养元素Zn的吸收[21]. 有研究表明,在Cd胁迫,ZnO NPs可以通过促进叶绿素含量以及通过提高淀粉酶活性促进水稻幼苗多糖的积累,改善Cd胁迫下的水稻生长[22]. ZnO NPs还可以通过促进水稻在As胁迫下的光合作用,促进植物生物量的积累[23]. 重金属会造成植物细胞超微结构的损伤,如质壁分离、叶绿体、线粒体、过氧化物酶体的破裂紊乱、细胞壁产生裂缝等[24],使得叶片黄化,抑制植物生长. 此外,ZnO NPs可以抑制As或钴(Co)胁迫下植物的超微结构损伤,促进植物对抗植物的抗性,正常的细胞结构会促使植物更好的生长发育[25]. 因此,可以从图1a中看出,ZnO NPs抑制了植物的黄化. 以往结果表明叶面喷施CeO2 NPs可以显著提高小白菜在As胁迫下的生长[26],主要归因于CeO2 NPs是一种光敏感性材料,会通过促进植物光合速率以及水分利用效率来促进植物生长发育. 此外,CeO2 NPs可以通过提高气孔导度促进玉米在Cd胁迫下的光合作用[27]. 本研究发现根部暴露CeO2 NPs也可以一定程度上促进小白菜的生长. 对SiO2NPs而言,其对植物根部的促进作用大于植物地上部,可能原因是SiO2 NPs可以促进植物根部细胞壁的增厚以抵御Cd的吸收,最终使得植物根部生物量积累[10]. S NPs对As污染场景中植物根部生长的促进及对茎叶的显著抑制可能归因于S NPs对根际环境(如pH)的调节,这在2.2部分会进行进一步的讨论.
-
Cd环境下,ZnO NPs和CeO2 NPs可以分别提高土壤孔隙水中的pH,而S NPs显著降低As和Cd+As环境下的pH(图2a). CK和As环境中,SiO2 NPs和ZnO NPs分别显著提高39.8%和26.1%的孔隙水DOC浓度. S NPs比单独的As环境背景显著下降了41.7%的DOC含量,但在Cd+As环境中显著升高了46.9%(图2b). 对于孔隙水中的重金属元素含量来说,单独Cd处理中,ZnO NPs和CeO2 NPs显著降低了44.7%—51.3%的孔隙水中Cd浓度,但是在S NPs下提高了35.8%. 在单独的As胁迫处理中,ZnO NPs、CeO2 NPs、S NPs分别显著抑制了孔隙水中22.8%、31.5%、22.3%的As浓度(图2b). 在Cd+As环境中,ZnO NPs和S NPs不仅显著降低了孔隙水中As的含量,同时抑制了Cd的积累. 然而,和Cd+As环境中变化相反的是,S NPs在单独的Cd环境中显著提高了Cd的积累. 孔隙水中的As或Cd浓度通常代表植物可吸收的有效态重金属含量[28]. 图2显示孔隙水和植物体内(尤其是根部)的重金属元素变化具有一致性. 比如,ZnO NPs和CeO2 NPs可以显著抑制Cd和Cd+As环境中植物根部Cd的积累,然而S NPs却加剧了植物根部Cd的积累. 同时,ZnO NPs和CeO2 NPs对植物根部As的含量也表现出抑制作用. 除了在Cd+As环境中施用SiO2显著提高了植物地上41%的As含量外,地上部重金属含量的变化和根部基本保持一致. 相对而言,NPs对根部重金属元素含量的扰动作用强于植物地上部.
已有研究表明,ZnO NPs进入土壤中后会迅速水解释放出Zn2+,水解反应导致质子(H+)的消耗,土壤pH因此升高[21, 29]. 同时,ZnO NPs水解程度也受到土壤pH的影响,CK、Cd、As、Cd+As这4种土壤的本底pH分别为7.99、7.91、8.25、8.08,由于pH较低的土壤中ZnO NPs更容易水解[21],因此在Cd环境中添加了ZnO NPs后Zn2+的释放程度最多,孔隙水中的Zn2+浓度提高了51.4%,水解导致的pH增幅最高,达到显著水平. 和本研究一致的是,Wang等[30]发现了SiO2 NPs可以通过水解成SiO32−提高水稻土壤的pH,尽管这种水解程度有限,但却有效地改善了水稻根际土壤的酸碱微环境. 而土壤中S NPs会被迅速氧化为SO42−,进而降低土壤的pH[31],且土壤的碱性程度越高,则S对pH的降低程度越显著[32]. 因此,在pH更高的As和Cd+As环境中,土壤孔隙水pH的降幅达到显著水平. 对于孔隙水DOC浓度来讲,总体与pH的变化趋势一致,但Cd环境中施加ZnO NPs虽然提高了pH,但对DOC没有显著影响,以及As环境中S NPs反而增加了DOC浓度,推测这些反常可能与微生物活动差异有关. 如Zn促进了根际土壤有机碳的分解[33],而S NPs抑制了DOC的降解[34],具体原因还有待分析.
几种纳米材料中,ZnO NPs对植物对Cd和As的积累均有较好的抑制效果,而CeO2 NPs主要表现在对植物根部金属的具有一定抑制效果,SiO2 NPs和S NPs的表现并不一致,一定程度上分别出现“抑Cd促As”和“抑As促Cd”的效应(图1c、d). 本研究中,ZnO NPs通过增加土壤pH促进了Cd在土壤颗粒表面的吸附,因此孔隙水中的Cd浓度降低. 此外,Zn和Cd均是2价阳离子,共享转运酶和结合点位,存在竞争作用[35],因此当土壤中有效态Zn含量增多时,可以有效降低Cd的吸收. 释放的Zn2+不仅可以通过竞争Cd2+在根细胞质膜上进入通道,他们还共用一个共同的从根到地上部的运输和转运系统,因此,添加ZnO NPs的植物处理中地上和地下部位中Cd的含量都得到显著下降,这在受Cd胁迫的甜高粱中也得到了证实[36]. SNPs可以通过降低pH促进Cd在土壤颗粒表面的解吸,最终表现在孔隙水和根部吸收的Cd含量均升高,损害植物生长发育. SiO2 NPs之所以降低Cd胁迫下地上部Cd的积累可能是由于SiO2促进了Cd胁迫下小白菜的生物积累(图1a),对重金属有“生物稀释”作用,此外,有研究表明SiO2 NPs会通过增加细胞壁的组成成分—硅体,来促进植物细胞壁的韧性,进而将Cd固存在根系细胞壁,抑制Cd向地上的转运[37]. 虽然前人研究[10]发现SiO2 NPs在pH=7.11—7.63的土中可以同时抑制水稻对As和Cd的吸收,但在本研究中SiO2 NPs未表现出对As较好的抑制效果,反而一定程度上促进了As在白菜中的积累,说明土壤性质和水分条件差异对纳米材料的效果影响较大. DOC可以与As或Cd发挥络合反应抑制自由态重金属离子(如AsV、AsIII、Cd2+)的活度[38 − 39]. 例如,经绿肥处理的土壤中,土壤DOC与植物地下部Cd含量呈显著负相关(R2=0.80)[38]. 在As胁迫下,ZnO NPs可以通过提高与DOC结合的络合态重金属抑制孔隙水中自由态重金属离子的浓度,进而抑制植物对As的吸收. S NPs可以通过降低pH促进As和Cd+As胁迫下As在土壤表面的吸附,抑制孔隙水和植物体内As的吸收,同时Cd+As环境中DOC含量的升高促进了络合态Cd的比例,因此抑制了孔隙水中自由态Cd的浓度. 相比于其它3种NPs,CeO2的溶解度最低,孔隙水中的Ce检出浓度(<0.5 μg·L−1)远低于其它3种NPs,因此CeO2 NPs施加后未显著改变土壤孔隙水pH和DOC含量,推测CeO2 NPs可能以颗粒状对重金属发挥吸附作用进而抑制对As和Cd的吸收. 之前的研究也表明CeO2 NPs在植物生长基质中(水培、营养液)被认为是稳定和不溶性的,甚至在植物体内仍然相对稳定[40]. 如Wang等利用单颗粒-电感耦合等离子体质谱(sp-ICP MS)在植物体内观察到的粒径(30—39 nm)CeO2 NPs与原始的材料粒径(50 nm)基本一致[41]. CeO2 NPs已被证明对Cd、AsⅢ、AsV等具有较强的吸附作用[42].
-
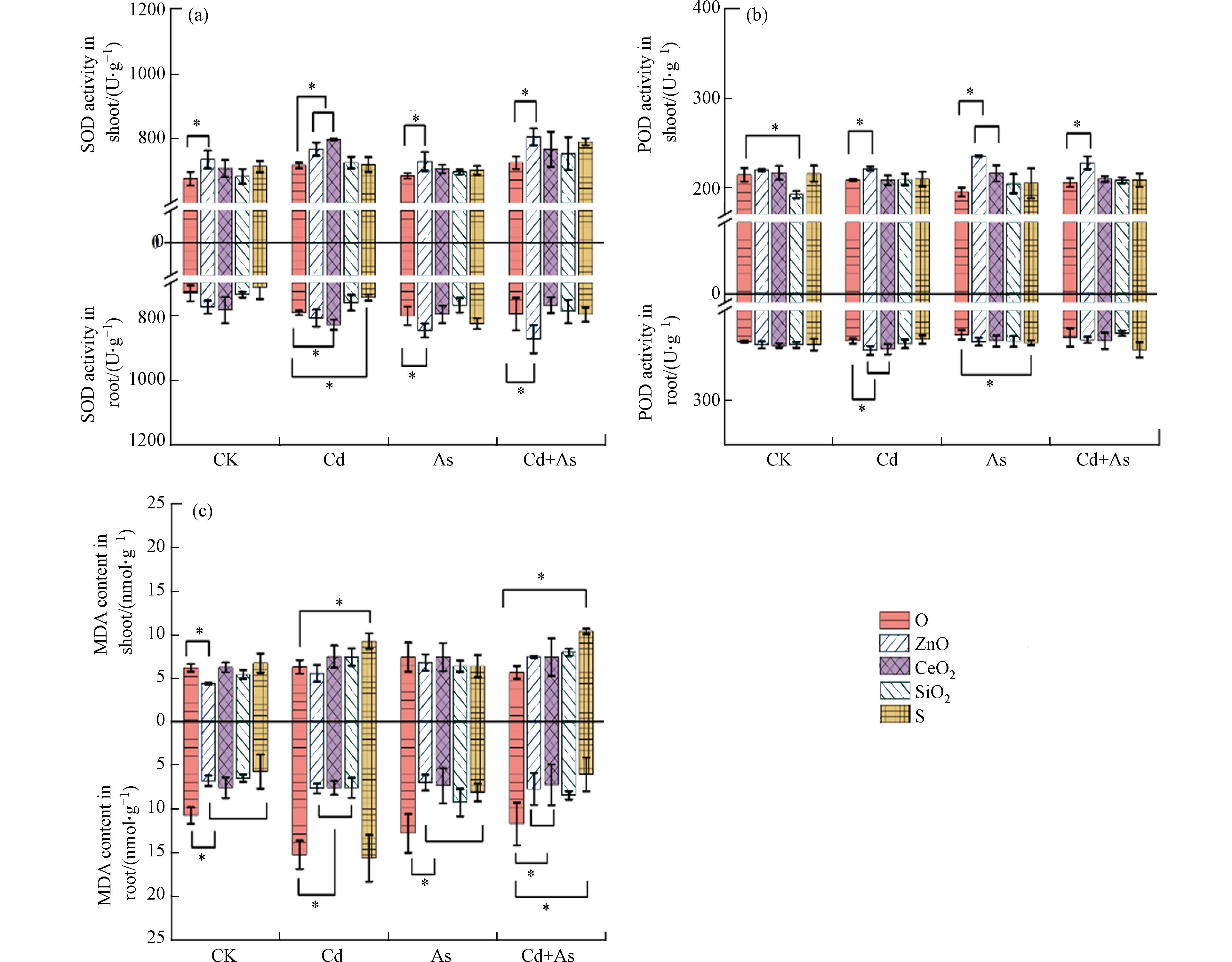
当植物受到重金属胁迫时,植物体内常产生过量的活性氧(ROS)引起植物的氧化应激,会导致植物的氧化损伤[43],最直接的影响结果就是导致植物细胞膜脂质过氧化[44],细胞膜中的不饱和脂肪酸发生氧化,生成MDA. 因此,MDA是植物细胞膜脂质过氧化的生物标志物[45]. 植物的抗氧化酶如SOD、POD等一直被认为是抵御ROS的第一道防线[45 − 47]. 在CK和几种处理中,ZnO NPs的添加显著增强了地上部SOD、POD的酶活水平(6.6%—11%)(图3a、b). ZnO NPs还对As和Cd+As环境中根部SOD活性、Cd环境中根部POD活性具有显著提升效果. CeO2 NPs显著提高了Cd胁迫下植物体内4.6%—11.2%的SOD活性,同时对Cd胁迫下植物根部以及As胁迫下植物地上部的POD活性具有显著增强效果. 对于MDA含量水平来说(图3c),ZnO NPs和CeO2 NPs降低了CK和3种土壤污染水平下植物根部29.6%—50.3%的MDA含量. SiO2 NPs分别降低了Cd或As胁迫下50.3%和27.6%的植物根部MDA含量. S NPs显著降低了As和Cd+As胁迫下根部36.7%—48.6%的MDA含量,但却提高了Cd胁迫以及Cd+As胁迫下地上47.1%—83.3%的MDA含量.
本研究中,ZnO NPs可以通过提高重金属胁迫下SOD和POD活性增强植物抵御ROS的能力,从而抑制MDA的积累,这与之前的研究保持一致[48]. 这是因为ZnO NPs可以稳定生物膜和提高蛋白质的稳定性,进而清除过量的ROS来平衡细胞稳态[49]. 此外,SOD主要在叶绿体内发挥清除·O2−的能力,ZnO NPs可以通过释放出Zn2+来提高Cu/Zn SOD基因的表达提高植物清除·O2−的能力,保护植物叶片的叶绿体免受损伤,进而提高植物光合作用并提高抗性[50]. 由于CeO2 NPs特殊的氧空穴结构,已被证明具有清除·O2−、H2O2等ROS的能力,可以发挥类SOD[51]、POD[52]的类酶活性,因此已经被发现对Cd[53]、As[26]引起的ROS积累具有强效的清除能力. SiO2 NPs已被证明可以增强细胞壁成分(如木质素和纤维素)的积累,进一步增强和稳定细胞膜,通过改善膜的完整性,来抑制MDA的积累[54]. 因此,尽管本研究中SiO2未显著改善Cd或As胁迫下的SOD和POD抗氧化酶活性,但却显著缓解了植物根部MDA积累. S NPs显著增加了Cd胁迫下植物的MDA积累,可能是由于S NPs对土壤酸化导致的Cd吸收和毒性效应增加. S NPs的负面效应表明纳米材料在实际应用前应充分评估其可能的潜在风险. 综上,ZnO NPs和CeO2 NPs可以通过提高SOD、POD活性和降低MDA含量来抑制Cd和As胁迫下对植物造成的氧化损伤.
-
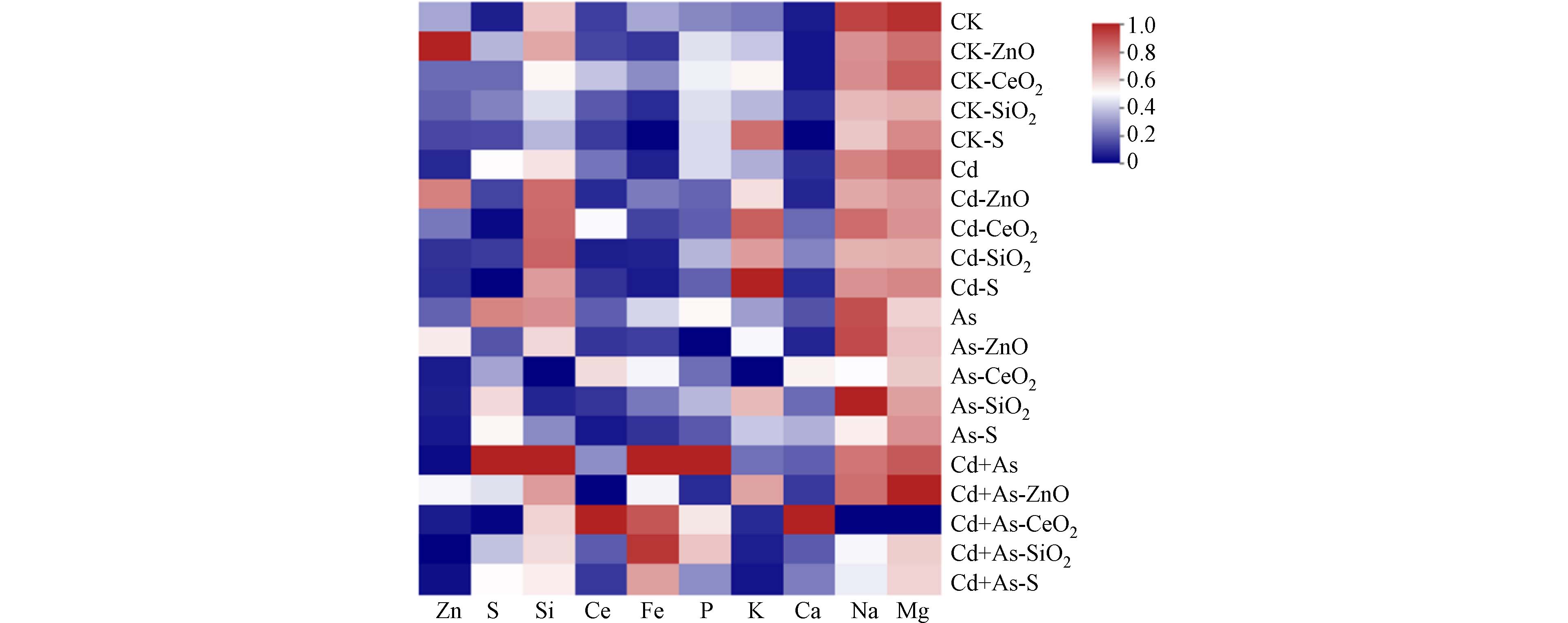
为探究几种NPs对不同污染条件下营养元素吸收的影响,本实验进一步测定了植物地上部营养元素(Zn、S、Si、Ce、Fe、P 、K、Ca、Na、Mg)的含量(图4). 结果显示,施用纳米材料后,植物中相应纳米材料元素显著提高. 例如ZnO NPs显著促进了植物中Zn元素积累. 由于Zn是植物必需营养元素,一方面印证了前面ZnO NPs对植物生长存在促进作用(图1),同时也印证了Zn对Cd吸收的竞争性抑制(图2c). 此外,纳米材料也提高了植物中部分元素含量,如ZnO显著提高了As或Cd+As环境下植物中7.8%—22.4%的K含量,以及1.7%—7.9%的Na和Mg的含量;CeO2 NPs显著提高了As或Cd+As环境中植物42.6%—92.6%的Ca含量;S NPs显著提高了CK或Cd环境下植物中26.7%—28.4%的K含量. Ca和Mg是叶绿体光合作用的重要组成部分[55],同时,Ca2+还在逆境胁迫下植物体内信号传导中起重要作用[56]. 说明CeO2 NPs或ZnO NPs可能通过增强Ca和Mg含量来增强植物生物量积累的潜力(图1a). ZnO NPs在Cd+As胁迫下植物体内K含量的增加可能是通过平衡阴离子[57]来缓解As胁迫. 但是,结果同时也发现ZnO NPs和CeO2 NPs一定程度上抑制了植物对Fe和P的吸收(图4),推测可能原因是由于两者增加了Fe和P在土壤中的吸附,由于P与As的环境行为具有相似性,因此ZnO NPs对As的抑制效应可能也反映在植物对P的吸收上. 以上结果表明,纳米材料在阻控植物对污染元素吸收的过程中,也会一定程度上影响对营养元素的吸收.
-
(1) 土壤中施用ZnO NPs能以“微肥”形式补充Zn2+,有效促进植物的生长,同时可以通过调节土壤pH和竞争吸收来抑制植物对Cd的吸收,通过增加孔隙水DOC含量减少植物对As的吸收. 此外,ZnO NPs通过调节抗氧化酶活性系统,调控元素含量来缓解Cd+As复合胁迫造成的胁迫效应.
(2)CeO2NPs可以通过促进As胁迫下植物的生长,以及降低孔隙水中重金属浓度来抑制As、Cd在植物根中的吸收. 同时,CeO2 NPs可以提高POD活性,减少根部MDA含量,降低膜损伤,增强植物抗性.
(3) SiO2 NPs促进了植物的根部生长,同时表现出一定的“抑Cd促As”效应,即抑制了Cd向植物地上的转运,但显著促进了Cd+As环境中小白菜地上As的积累.
(4) S NPs的施用导致了土壤的显著酸化,造成了一定的“抑As促Cd”效应,即显著促进了植物对Cd的吸收,而对As的吸收有轻微抑制效应,酸化同时抑制了土壤抗氧化酶活性和加剧了植物体内MDA的积累,从而对植物地上部生长表现出明显抑制.
几种典型纳米材料对土壤-植物体系中砷镉积累的阻控效应
Alleviation of arsenic and cadmium accumulation in soil-plant system by typical nanomaterials
-
摘要: 纳米材料(NPs)已成为近年来农业领域对污染控制具有较好前景的新兴技术. 本研究针对农田蔬菜中砷(As)和镉(Cd)复合污染问题,比较了4种代表性NPs(ZnO、CeO2、SiO2、S)在As/Cd单一和复合污染场景下对小白菜中重金属的富集和毒性效应的抑制效应和机制. 结果表明,在Cd环境中,ZnO NPs通过提高土壤pH和Zn2+抑制了植物对Cd的吸收;在As环境中,溶解性有机碳增加导致孔隙水和植物体中As含量下降. 此外,ZnO NPs还可以显著促进植物生长,增强地上部SOD、POD抗氧化酶活性,降低MDA水平,提高了植物对重金属的抗性. CeO2 NPs同样可以促进植物生长,降低了根部MDA水平,但对植物对As和Cd吸收的抑制能力程度不如ZnO NPs,主要降低了植物根中金属的积累量. SiO2 NPs虽然一定程度上抑制了植物根对Cd的吸收,但显著促进了复合污染中地上部分As累积. 而S NPs导致土壤明显酸化,抑制了植物地上部的生长,并导致小白菜根中Cd含量显著升高. 综合来看,ZnO和CeO2具有较好的阻控效果,SiO2和S纳米材料则需要谨慎使用,结果可以为As-Cd复合污染提供“因土配方”的纳米技术参考.Abstract: Nanoparticles (NPs) have emerged as a promising technology for pollution control in agriculture in recent years. This study investigates the mitigation effects and mechanisms of four typical NPs (ZnO, CeO2, SiO2, and S) on arsenic (As) and cadmium (Cd) accumulation and toxicity in Chinese cabbage under single and combined As/Cd pollution scenarios. The results showed that in Cd-contaminated soil, ZnO NPs effectively reduced plant Cd uptake by elevating soil pH and sequestering Zn2+. Conversely, in As contaminated soil, the increase of dissolved organic carbon led to a decline in both pore water and plant As concentrations. Additionally, ZnO NPs promoted significant plant growth and enhanced the antioxidative enzyme activities of superoxide dismutase (SOD) and peroxidase (POD) in shoot tissues. This is accompanied by a reduction in malondialdehyde (MDA) levels, ultimately enhancing the plants’ resistance to heavy metal stressors. Similarly, CeO2 NPs promoted plant growth and decreased root MDA levels; however, their ability on inhibition metal uptake is not as pronounced as that of ZnO NPs, primarily reducing the accumulation of metals in plant roots. While SiO2 NPs partially inhibited plant root-Cd, they notably promoted the shoot-As in co-contaminated scenarios. Conversely, S NPs induced significant soil acidification, suppressed plant growth, and significantly increased Cd concentration within Chinese cabbage roots. Overall, ZnO and CeO2 demonstrate superior mitigation efficacy, while caution is needed in the application of SiO2 and S nanomaterials. These findings offer valuable insights into nano-engineering strategies tailored to “soil-adapted” solutions for As-Cd co-contamination.
-
Key words:
- soil remediation /
- nanomaterial /
- As /
- Cd /
- vegetable
-

-
表 1 土壤基本理化性质
Table 1. Physical and chemical properties of soil
pH SOM/(g·kg−1) DCB-Fe/(g·kg−1) ox-Fe/(g·kg−1) As/(mg·kg−1) Cd/(mg·kg−1) Ca/(g·kg−1) Fe/(g·kg−1) K/(g·kg−1) Mg/(g·kg−1) Na/(g·kg−1) 8.31 6.3 10.4 6.8 10.8 0.1 7.2 25.5 5.2 4.7 2.6 -
[1] XIAO Y T, GUO W, QI X B, et al. Differences in cadmium uptake and accumulation in seedlings of wheat varieties with low- and high-grain cadmium accumulation under different drought stresses[J]. Plants, 2023, 12(19): 3499. doi: 10.3390/plants12193499 [2] SEVAK P, PUSHKAR B. Arsenic pollution cycle, toxicity and sustainable remediation technologies: A comprehensive review and bibliometric analysis[J]. Journal of Environmental Management, 2024, 349: 119504. doi: 10.1016/j.jenvman.2023.119504 [3] JIANG Z J, YANG S Z, LUO S. Source analysis and health risk assessment of heavy metals in agricultural land of multi-mineral mining and smelting area in the Karst region - a case study of Jichangpo Town, Southwest China[J]. Heliyon, 2023, 9(7): e17246. doi: 10.1016/j.heliyon.2023.e17246 [4] 邓海, 王锐, 严明书, 等. 矿区周边农田土壤重金属污染风险评价[J]. 环境化学, 2021, 40(4): 1127-1137. doi: 10.7524/j.issn.0254-6108.2020071601 DENG H, WANG R, YAN M S, et al. Risk assessment of heavy metal pollution in farmland soil around mining area[J]. Environmental Chemistry, 2021, 40(4): 1127-1137 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020071601
[5] ZHAO F J, WANG P. Arsenic and cadmium accumulation in rice and mitigation strategies[J]. Plant and Soil, 2020, 446(1): 1-21. [6] PAN D D, LIU C P, YU H Y, et al. A paddy field study of arsenic and cadmium pollution control by using iron-modified biochar and silica Sol together[J]. Environmental Science and Pollution Research International, 2019, 26(24): 24979-24987. doi: 10.1007/s11356-019-05381-x [7] HONMA T, OHBA H, KANEKO-KADOKURA A, et al. Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains[J]. Environmental Science & Technology, 2016, 50(8): 4178-4185. [8] ARAO T, KAWASAKI A, BABA K, et al. Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice[J]. Environmental Science & Technology, 2009, 43(24): 9361-9367. [9] YAO B M, WANG S Q, XIE S T, et al. Optimal soil Eh, pH for simultaneous decrease of bioavailable Cd, As in co-contaminated paddy soil under water management strategies[J]. Science of The Total Environment, 2022, 806: 151342. doi: 10.1016/j.scitotenv.2021.151342 [10] WANG X X, JIANG J C, DOU F G, et al. Simultaneous mitigation of arsenic and cadmium accumulation in rice (Oryza sativa L. ) seedlings by silicon oxide nanoparticles under different water management schemes[J]. Paddy and Water Environment, 2021, 19(4): 569-584. doi: 10.1007/s10333-021-00855-6 [11] SHEN B B, WANG X M, ZHANG Y, et al. The optimum pH and Eh for simultaneously minimizing bioavailable cadmium and arsenic contents in soils under the organic fertilizer application[J]. Science of the Total Environment, 2020, 711: 135229. doi: 10.1016/j.scitotenv.2019.135229 [12] HAN R X, WANG Z, WANG S Q, et al. A combined strategy to mitigate the accumulation of arsenic and cadmium in rice (Oryza sativa L. )[J]. Science of The Total Environment, 2023, 896: 165226. doi: 10.1016/j.scitotenv.2023.165226 [13] 魏晓贺, 苗欣宇, 欧阳少虎, 等. 根系分泌物介导的土壤金属氧化物纳米材料对植物毒性作用的研究进展[J]. 环境化学, 2024, 43(1): 199-209. doi: 10.7524/j.issn.0254-6108.2022071307 WEI X H, MIAO X Y, OUYANG S H, et al. Advances in phytotoxic effects of metal oxide nanomaterials mediated by root exudates in soils[J]. Environmental Chemistry, 2024, 43(1): 199-209 (in Chinese). doi: 10.7524/j.issn.0254-6108.2022071307
[14] SUN X T, MO H J, HATANO K I, et al. Simultaneous suppression of magnetic nanoscale powder and fermented bark amendment for arsenic and cadmium uptake by radish sprouts grown in agar medium[J]. Environmental Science and Pollution Research, 2019, 26(14): 14483-14493. doi: 10.1007/s11356-019-04756-4 [15] MA X M, SHARIFAN H, DOU F G, et al. Simultaneous reduction of arsenic (As) and cadmium (Cd) accumulation in rice by zinc oxide nanoparticles[J]. Chemical Engineering Journal, 2020, 384: 123802. doi: 10.1016/j.cej.2019.123802 [16] ROSSI L, ZHANG W L, SCHWAB A P, et al. Uptake, accumulation, and in planta distribution of coexisting cerium oxide nanoparticles and cadmium in Glycine max (L. ) Merr[J]. Environmental Science & Technology, 2017, 51(21): 12815-12824. [17] WANG Y Y, CHEN W L, GU X Y, et al. Comparison of the arsenic protective effects of four nanomaterials on pakchoi in an alkaline soil[J]. Science of The Total Environment, 2024, 912: 168918. doi: 10.1016/j.scitotenv.2023.168918 [18] MAWIA A M, HUI S Z, ZHOU L, et al. Inorganic arsenic toxicity and alleviation strategies in rice[J]. Journal of Hazardous Materials, 2021, 408: 124751. doi: 10.1016/j.jhazmat.2020.124751 [19] NAFEES M, SEHRISH A K, ALOMRANI S O, et al. Mechanism and synergistic effect of sulfadiazine (SDZ) and cadmium toxicity in spinach (Spinacia oleracea L. ) and its alleviation through zinc fortification[J]. Journal of Hazardous Materials, 2024, 464: 132903. doi: 10.1016/j.jhazmat.2023.132903 [20] SONG U, KIM J. Zinc oxide nanoparticles: a potential micronutrient fertilizer for horticultural crops with little toxicity[J]. Horticulture, Environment, and Biotechnology, 2020, 61(3): 625-631. doi: 10.1007/s13580-020-00244-8 [21] WU P, CUI P X, DU H, et al. Long-term dissolution and transformation of ZnO in soils: The roles of soil pH and ZnO particle size[J]. Journal of Hazardous Materials, 2021, 415: 125604. doi: 10.1016/j.jhazmat.2021.125604 [22] LI Y, LIANG L, LI W, et al. ZnO nanoparticle-based seed priming modulates early growth and enhances physio-biochemical and metabolic profiles of fragrant rice against cadmium toxicity[J]. Journal of Nanobiotechnology, 2021, 19(1): 75. doi: 10.1186/s12951-021-00820-9 [23] YAN S W, WU F, ZHOU S, et al. Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease the accumulation of arsenic in rice (Oryza sativa L. )[J]. BMC Plant Biology, 2021, 21(1): 150. doi: 10.1186/s12870-021-02929-3 [24] SALAM A, KHAN A R, LIU L, et al. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress[J]. Journal of Hazardous Materials, 2022, 423: 127021. doi: 10.1016/j.jhazmat.2021.127021 [25] SHEN M M, LIU W T, ZEB A, et al. Bioaccumulation and phytotoxicity of ZnO nanoparticles in soil-grown Brassica chinensis L. and potential risks[J]. Journal of Environmental Management, 2022, 306: 114454. doi: 10.1016/j.jenvman.2022.114454 [26] WANG Y Y, MA C X, DANG F, et al. Mixed effects and co-transfer of CeO2 NPs and arsenic in the pakchoi-snail food chain[J]. Journal of Hazardous Materials, 2024, 462: 132770. doi: 10.1016/j.jhazmat.2023.132770 [27] FOX J P, CAPEN J D, ZHANG W L, et al. Effects of cerium oxide nanoparticles and cadmium on corn (Zea mays L. ) seedlings physiology and root anatomy[J]. NanoImpact, 2020, 20: 100264. doi: 10.1016/j.impact.2020.100264 [28] NORTON G J, ADOMAKO E E, DEACON C M, et al. Effect of organic matter amendment, arsenic amendment and water management regime on rice grain arsenic species[J]. Environmental Pollution, 2013, 177: 38-47. doi: 10.1016/j.envpol.2013.01.049 [29] TOURINHO P S, VAN GESTEL C A M, LOFTS S, et al. Influence of soil pH on the toxicity of zinc oxide nanoparticles to the terrestrial isopod Porcellionides pruinosus[J]. Environmental Toxicology and Chemistry, 2013, 32(12): 2808-2815. doi: 10.1002/etc.2369 [30] WANG X X, LI X F, DOU F, et al. Elucidating the impact of three metallic nanoagrichemicals and their bulk and ionic counterparts on the chemical properties of bulk and rhizosphere soils in rice paddies[J]. Environmental Pollution, 2021, 290: 118005. doi: 10.1016/j.envpol.2021.118005 [31] SHAKOOR A, JILANI G, IQBAL T, et al. Synthesis of elemental- and nano-sulfur-enriched bio-organic phosphate composites, and their impact on nutrients bioavailability and maize growth[J]. Journal of Soil Science and Plant Nutrition, 2023, 23(3): 3281-3289. doi: 10.1007/s42729-023-01244-0 [32] TABAK M, LISOWSKA A, FILIPEK-MAZUR B. Bioavailability of sulfur from waste obtained during biogas desulfurization and the effect of sulfur on soil acidity and biological activity[J]. Processes, 2020, 8(7): 863. doi: 10.3390/pr8070863 [33] LIU L, TSYUSKO O V, UNRINE J M, et al. Pristine and sulfidized zinc oxide nanoparticles promote the release and decomposition of organic carbon in the legume rhizosphere[J]. Environmental Science & Technology, 2023, 57(24): 8943-8953. [34] HAMMERSCHMIEDT T, HOLATKO J, ZELINKA R, et al. The combined effect of graphene oxide and elemental nano-sulfur on soil biological properties and lettuce plant biomass[J]. Frontiers in Plant Science, 2023, 14: 1057133. doi: 10.3389/fpls.2023.1057133 [35] TIMILSINA A, ADHIKARI K, CHEN H. Foliar application of green synthesized ZnO nanoparticles reduced Cd content in shoot of lettuce[J]. Chemosphere, 2023, 338: 139589. doi: 10.1016/j.chemosphere.2023.139589 [36] WANG F Y, ADAMS C A, SHI Z Y, et al. Combined effects of ZnO NPs and Cd on sweet sorghum as influenced by an arbuscular mycorrhizal fungus[J]. Chemosphere, 2018, 209: 421-429. doi: 10.1016/j.chemosphere.2018.06.099 [37] CUI J H, JIN Q, LI F B, et al. Silicon reduces the uptake of cadmium in hydroponically grown rice seedlings: why nanoscale silica is more effective than silicate[J]. Environmental Science: Nano, 2022, 9(6): 1961-1973. doi: 10.1039/D1EN00973G [38] 王赟, 付利波, 梁海, 等. 绿肥作物对云南旱地土壤镉有效性的影响[J]. 农业环境科学学报, 2021, 40(10): 2124-2133. doi: 10.11654/jaes.2021-0457 WANG Y, FU L B, LIANG H, et al. Effects of green manure crops on cadmium availability in dryland soils in Yunnan, China[J]. Journal of Agro-Environment Science, 2021, 40(10): 2124-2133 (in Chinese). doi: 10.11654/jaes.2021-0457
[39] ANAWAR H M, TAREQ S M, AHMED G. Is organic matter a source or redox driver or both for arsenic release in groundwater?[J]. Physics and Chemistry of the Earth, 2013, 58: 49-56. [40] ZHANG Z Y, HE X, ZHANG H F, et al. Uptake and distribution of ceria nanoparticles in cucumber plants[J]. Metallomics, 2011, 3(8): 816-822. doi: 10.1039/c1mt00049g [41] WANG J, YUE L, ZHAO J, et al. Uptake and bioaccumulation of nanoparticles by five higher plants using single-particle-inductively coupled plasma-mass spectrometry[J]. Environmental Science: Nano, 2022, 9(8): 3066-3080. doi: 10.1039/D1EN01195B [42] SHARIFAN H, WANG X X, GUO B L, et al. Investigation on the modification of physicochemical properties of cerium oxide nanoparticles through adsorption of Cd and As(III)/As(V)[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(10): 13454-13461. [43] BERNI R, LUYCKX M, XU X, et al. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism[J]. Environmental and Experimental Botany, 2019, 161: 98-106. doi: 10.1016/j.envexpbot.2018.10.017 [44] GHORI N H, GHORI T, HAYAT M Q, et al. Heavy metal stress and responses in plants[J]. International Journal of Environmental Science and Technology, 2019, 16(3): 1807-1828. doi: 10.1007/s13762-019-02215-8 [45] DASTOGEER K M G, ZAHAN M I, TAHJIB-UL-ARIF M, et al. Plant salinity tolerance conferred by arbuscular mycorrhizal fungi and associated mechanisms: A Meta-Analysis[J]. Frontiers in Plant Science, 2020, 11: 588550. doi: 10.3389/fpls.2020.588550 [46] SILVA-GIGANTE M, HINOJOSA-REYES L, ROSAS-CASTOR J M, et al. Heavy metals and metalloids accumulation in common beans (Phaseolus vulgaris L. ): A review[J]. Chemosphere, 2023, 335: 139010. doi: 10.1016/j.chemosphere.2023.139010 [47] VINOGRADOVA N, VINOGRADOVA E, CHAPLYGIN V, et al. Phenolic compounds of the medicinal plants in an anthropogenically transformed environment[J]. Molecules, 2023, 28(17): 6322. doi: 10.3390/molecules28176322 [48] HUSSAIN A, ALI S, RIZWAN M, et al. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants[J]. Environmental Pollution, 2018, 242: 1518-1526. doi: 10.1016/j.envpol.2018.08.036 [49] FAIZAN M, FARAZ A, YUSUF M, et al. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants[J]. Photosynthetica, 2018, 56(2): 678-686. doi: 10.1007/s11099-017-0717-0 [50] LI M, AHAMMED G J, LI C, et al. Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling[J]. Frontiers in Plant Science, 2016, 7: 615. [51] HECKERT E G, KARAKOTI A S, SEAL S, et al. The role of cerium redox state in the SOD mimetic activity of nanoceria[J]. Biomaterials, 2008, 29(18): 2705-2709. doi: 10.1016/j.biomaterials.2008.03.014 [52] HUANG L J, SUN D W, PU H B. Photosensitized peroxidase mimicry at the hierarchical 0D/2D heterojunction - like quasi metal - organic framework interface for boosting biocatalytic disinfection[J]. Small, 2022, 18(20): 2200178. doi: 10.1002/smll.202200178 [53] WANG Y Y, WANG L Q, MA C X, et al. Effects of cerium oxide on rice seedlings as affected by co-exposure of cadmium and salt[J]. Environmental Pollution, 2019, 252: 1087-1096. doi: 10.1016/j.envpol.2019.06.007 [54] AL-MOKADEM A Z, SHETA M H, MANCY A G, et al. Synergistic effects of kaolin and silicon nanoparticles for ameliorating deficit irrigation stress in maize plants by upregulating antioxidant defense systems[J]. Plants, 2023, 12(11): 2221. doi: 10.3390/plants12112221 [55] YAN W, YANG L, WANG Q. Distribution of lanthanum among the chloroplast subcomponents of spinach and its biological effects on photosynthesis: location of the lanthanum binding sites in photosystem II[J]. Chinese Science Bulletin, 2005, 50(16): 1714-1720. doi: 10.1360/982004-876 [56] CHMIELOWSKA-BĄK J, DECKERT J. A common response to common danger?Comparison of animal and plant signaling pathways involved in cadmium sensing[J]. Journal of Cell Communication and Signaling, 2012, 6(4): 191-204. doi: 10.1007/s12079-012-0173-3 [57] KUMAR D, SINGH V P, TRIPATHI D K, et al. Effect of arsenic on growth, arsenic uptake, distribution of nutrient elements and thiols in seedlings of Wrightia arborea (Dennst. ) Mabb[J]. International Journal of Phytoremediation, 2015, 17(2): 128-134. doi: 10.1080/15226514.2013.862205 -



 下载:
下载: