-
根据2018年《中国生态环境状况公报》,我国111个重要湖泊(水库)中,富营养化湖泊占23.4%,富营养化造成的有害水藻水华(HABs),严重影响人民的饮用水安全、工业用水效率[1-3]。近年来,HABs的治理方案层出不穷,物理法、化学法在短时间内能够有效去藻,但不利于长足治理,微生物(溶藻细菌、噬藻体)作为控藻的有效手段具有标本兼治、容易从湖泊水体中分离、生态安全性好的优点,引起国内外研究者的关注[4-7]。生态学控藻是被国家列入生态环保领域的技术需求,如《太湖流域管理条例》(国务院令第604号)第37条“国家鼓励运用技术成熟、安全可靠的方法对蓝藻等有害藻类进行生态防治”。
随着微生物控藻研究的深入,国内外研究者在溶藻细菌筛选鉴定的基础上,将光谱分析技术应用于藻类有机物AOM(包括EOM和IOM)分析[8-9]。Wang 等[10]发现三维荧光光谱可用来区分自然水体中不同类型和来源的可溶性有机物DOM。Henderson等[11]研究了四种藻类分泌藻类有机物的光谱特征,发现生长期的藻类AOM中含有较少亲水性多糖物质和疏水性蛋白物质。但溶藻细菌溶藻过程中DOM的组成和特性不明确,溶藻机制尚不清晰。
本课题组发现太湖水系HABs爆发时期,当地农民用含HABs的藻水灌溉水稻田,而水稻田中并未出现大量蓝藻。由此引起本课题组关注,进而从稻田水稻根部土壤中筛选分离出一株高效溶藻菌Paenibacillus sp. XXG,为探究XXG菌溶藻机制,本研究通过对溶藻前后的铜绿微囊藻液进行紫外-可见光光谱、三维荧光光谱、红外光谱和倒置显微镜回放,系统分析溶藻产物并据此推测溶藻机制,以期为生态学控藻研究提供参考。
HTML
-
恒温振荡培养箱HZQ-X100A(上海一恒科学仪器有限公司)、净化工作台SW-CJ-1F(上海贺德实验设备厂)、数显光照培养箱GZP-250(上海精宏实验设备有限公司)、立式压力蒸汽灭菌器LDZX-50KBS(上海申安医疗器械厂)、冷冻干燥机SCIENTZ-12N(宁波新芝生物科技股份有限公司)、倒置显微镜TL4(日本奥林巴斯)、紫外可见光分光光度计UV-1800(日本岛津)、荧光分光光度计(美国Agilent Cary Eclipse)、红外光谱分析仪iS50(美国尼高力)等。
-
铜绿微囊藻来源:FACHB905,购自中国科学院武汉水生生物研究所。在50 mL三角瓶(高压灭菌且干燥)中加入购买的15 ml铜绿微囊藻液,在温度为25 ℃、光照强度2500 lux、光暗比为12 h:12 h的条件下培养2—3 d后,加入BG-11培养基15 mL,同等条件继续培养20—30 d,待藻细胞处于对数生长期,再进行转接,转接比例为1:5,藻种本身不带细菌且在无菌环境下转接。
菌种来源:水稻田(太湖水系灌溉)的水稻根部土壤中筛选出1株高效溶藻菌株Paenibacillus sp. XXG,已在“中国微生物菌种保藏管理委员会普通微生物中心”保藏,保藏编号为CGMCC No.17503。
-
藻类培养基:BG-11培养基,配方由中国科学院水生生物研究所提供。
细菌培养基:牛肉膏蛋白胨培养基(牛肉膏 5 g、鱼粉蛋白胨 10 g、NaCl 5 g,蒸馏水1000 mL,调pH值为7.2,高温灭菌冷却;固体培养基再加入20 g·L−1琼脂粉,加热溶解,调pH值为7.2,高温灭菌冷却)。
-
牛肉膏、鱼粉蛋白胨、琼脂粉、KH2PO4、MgSO4、NH4NO3、柠檬酸、柠檬酸铁铵、EDTA-Na2、FeSO4、KNO3、H3BO3、MnSO4·7H2O、NaMoO4·H2O、CuSO4·5H2O、Co(NO3)2·6H20、NaNO3、K2HPO4、MgSO4·7H2O、CaCl2·2H2O, 除牛肉膏、鱼粉蛋白胨、琼脂粉外,其余试剂均是分析纯。
-
加入灭菌牛肉膏蛋白胨培养基的铜绿微囊藻液为对照组,按菌藻体积比为5.6%投加XXG菌液的铜绿微囊藻液为实验组,采用UV-1800紫外可见光分光光度计,对溶藻前后的铜绿微囊藻液分别进行波长范围为300—800 nm的紫外-可见光光谱扫描分析并用origin9.1等间距作出紫外-可见光光谱图,间隔为0.2,确定溶藻菌XXG溶藻过程中铜绿微囊藻液中组分及含量变化情况。采用origin9.1中Data Analysis对区间积分算峰面积。
前期研究表明,溶藻时间取前6 d可排除培养基对溶藻效果的影响,XXG菌溶藻在菌藻体积比5.6%、XXG菌处于对数生长期、铜绿微囊藻液处于对数生长期前期的条件下,溶藻率最高,则本文实验均在上述条件进行[12]。
-
加入灭菌牛肉膏蛋白胨培养基的铜绿微囊藻液为对照组,按菌藻体积比为5.6%投加XXG菌液的铜绿微囊藻液为实验组,经0.22 um滤膜过滤,稀释5倍后,采用Cary Eclipse荧光光度计(λex: 220—400 nm,增量5 nm;λem: 280—550 nm,增量2 nm;PMT电压800 V,扫描速度1200 nm·min−1)测定溶藻进程的三维荧光谱图,确定溶藻菌XXG溶藻过程中产生溶藻产物。
-
加入灭菌牛肉膏蛋白胨培养基的铜绿微囊藻液为对照组,按菌藻体积比为5.6%投加XXG菌液的铜绿微囊藻液为实验组,放入冷冻干燥机中过夜处理至干粉,分别取1 mg冻干的样品与400 mg干燥的KBr混合后磨细,在10 t·cm−2下压成薄片并维持2 min,用Nicolet iS50 United States型红外光谱分析仪进行红外光谱扫描并用origin9.1等间距作出红外光谱图,间隔为3%,鉴别溶藻产物的结构组成,确定化学基团。采用origin9.1中Data Analysis对区间积分算峰面积。
-
以5.6%的菌藻比取初始Chl-a浓度为1124.08 µg·L−1的铜绿微囊藻液与OD600=1.261的XXG菌发酵液于无菌三角瓶中,在30 ℃、150 r·min−1的摇床中振荡培养,每天取0.5 mL于载玻片上,置于倒置显微镜上观察溶藻进程中藻细胞的动态形貌变化。
1.1. 仪器与试剂
1.2. 供试材料
1.2.1. 试验材料
1.2.2. 培养基
1.2.3. 试剂
1.3. 试验方法
1.3.1. 溶藻进程紫外-可见光光谱测定
1.3.2. 溶藻进程三维荧光光谱测定
1.3.3. 溶藻进程红外光谱测定
1.3.4. 溶藻进程显微镜记录
-
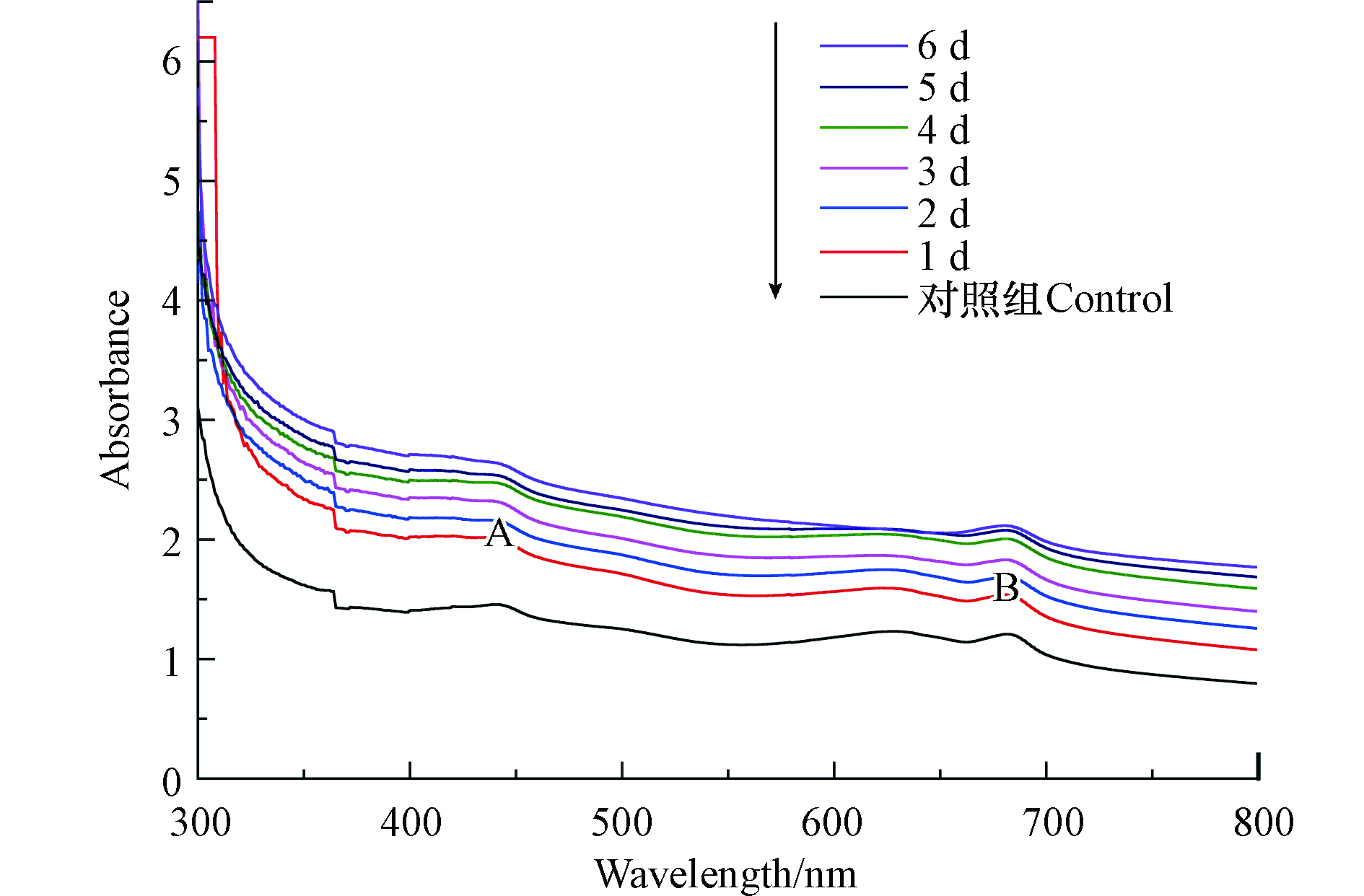
溶藻前后的藻液紫外-可见光光谱如图1所示,光谱主要出现2个吸收带(吸收带A、吸收带B),吸收带A的吸收峰位置为442 nm,位于可见光的蓝紫光区;吸收带B出现在630—750 nm,位于可见光的红光区,为叶绿素A(Chl-a)的吸收带[13]。紫外光区300—350 nm,所有样品均呈下降趋势,绿光区492—577 nm出现峰谷,因为铜绿微囊藻液是绿色的,不吸收绿色光。
如表1所示,吸收带A的吸收峰位置为442 nm,吸收带B的吸收峰位置为681 nm或682 nm,溶藻的1 —6 d,吸收带B峰面积逐渐减少,是铜绿微囊藻液在XXG菌的作用下藻细胞死亡,Chl-a浓度变小,面积变化最大主要发生在溶藻过程的第5、6 天,这与显微观察的结果一致,据此,溶藻产物的释放应该主要发生在此阶段;未加菌的铜绿微囊藻在吸收带B吸收峰处的吸光度和峰面积<溶藻第1 天的菌藻处理液在吸收带B吸收峰处的吸光度和峰面积,可能是XXG菌溶藻速率<铜绿微囊藻的生长速率。
-
紫外分光光度法只能用来初步断定溶藻进程中Chl-a浓度的变化,荧光光谱对铜绿微囊藻液分析要比紫外-可见光光度法分析高2—3个数量级[14]。图2表示溶藻前后的菌藻处理液,随着时间的推移,溶藻产物增多,三维荧光检测出3组荧光峰,A组峰出现在Ex/Em=(270—290) nm/(320—370) nm之间,此区域为可溶性微生物产物类(SMP,soluble microbial by-product-like),有机物组成主要是类蛋白及氨基酸类,是藻类有机物产生的荧光峰;B组峰出现在Ex/Em=(320—340) nm/(400—440) nm之间,此区域内有机物组成主要是胡敏酸(HA);C组峰出现在Ex/Em=(230—270) nm/(410—470) nm之间,此区域内有机物组成主要是富里酸(FA);D组峰出现在Ex/Em=(390—400) nm/(470—500) nm之间,根据对同浓度无菌液体培养基的三维荧光光谱图确定D峰为牛肉膏蛋白胨培养基中的主要物质。溶藻后溶液中包含氨基酸、类蛋白、HA及FA,其中HA是腐殖质中的主要有机成分,由有机生物死亡后经生物降解产生,且溶藻后的荧光图与二级处理的污水荧光光谱相似[15]。
如表2所示,溶藻前荧光峰A峰位置为激发波长280 nm,发射波长338 nm,根据水环境中主要荧光基团位置确定为类蛋白[16]。溶藻后荧光峰A峰强逐渐增强且位置右移,说明XXG菌在藻液中分泌新的物质。由于芳香族蛋白质类的荧光峰位置激发波长为205 nm,发射波长为270 nm,此类物质较藻液中类蛋白含量少,导致荧光峰A峰位置轻微右移,推测XXG分泌新物质[17]。荧光峰A的最大增幅发生在溶藻过程的第5、6 天,此时紫外-可见光光谱吸收带B峰面积减少最大,与显微观察中藻细胞膜破裂,Chl-a离体后被破坏结果相一致。荧光峰B峰值的增加说明藻细胞死亡后经XXG降解产生HA,溶藻时间越长,HA含量越多;荧光峰C从无到有,说明FA为溶藻产物,荧光峰C逐渐增加,是因FA化学性质稳定,不能被XXG菌利用[18],说明HA、FA为溶藻产物。
-
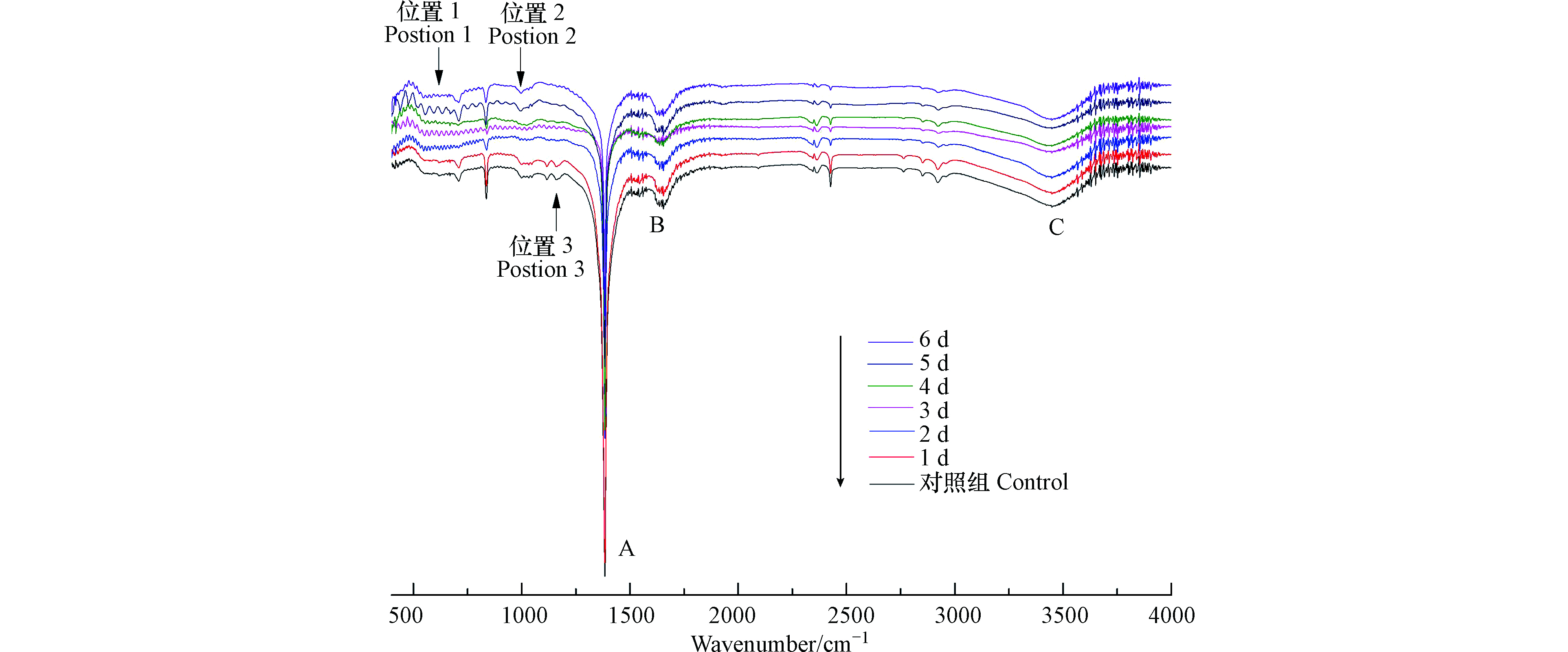
如图3所示,溶藻前后的样品光谱图均主要产生3个峰,峰的位置是由于吸收峰强度与化学键之间的极性呈正相关关系而出现不同强度的峰,说明溶藻前后的铜绿微囊藻液中存在相同的官能团,但是峰的强度发生变化。A峰位置在1600—1300 cm−1,1545.22 cm−1处吸收峰的是N—H的弯曲振动和N—H的弯曲振动,位于酰胺II带,表明蛋白质酰胺键遭到破坏[19];B峰位置在1690—1640 cm−1,酰胺类的C=O键伸缩振动,藻液和菌藻处理液中均含有酰胺类,与Gabriel Kenne等[20]对蓝藻进行主成分分析,发现高度标准化的酰胺I带结果高度一致;C峰位置在3500—3100 cm−1/3550—2500 cm−1,峰在波数为3452.56 cm−1处是藻细胞细胞壁壳聚糖和蛋白质中O—H键所在位置,溶藻过程中该处的O—H键伸缩振动,细胞壁与蛋白质结构被破坏,藻细胞破裂[21]。结合3个峰的透光度来看,在溶藻进程中蛋白质的酰胺键逐渐被破坏、藻细胞壁破裂。
波数在2930附近有来自核酸、蛋白质和脂类的—CH3、—CH2对称运动和反对称运动产生的峰[22],蛋白质被破坏,释放氨基酸。随着溶藻的进行,图中还存在突然出现的峰或突然消失的峰,如图3位置1所示,溶藻第1 d波数在550—600的伸缩区几乎没有振动峰,第2—5 天,振动峰振动强度逐渐加大,到第6天,伸缩吸收,此区为烯烃、端块烃、芳烃、胺、羧酸等氢单键面外弯曲振动频率区[23]。位置1特征表现与三维荧光A峰位置及峰面积变化规律一致,推测波数在550—600的伸缩区为芳烃,溶藻产物可能包括芳香族氨基酸。如图3位置2所示,第1—2 天波数在1100—1500处窄而短的振动峰,第3—6 天峰逐渐频率减小且弯曲振动。如图3位置3所示,波数在1100—1250处窄而尖的峰逐渐伸缩吸收。
结合峰的位置,溶藻前的铜绿微囊藻细胞中存在酰胺类物质,随着溶藻的进行,藻液中酰胺类物质浓度发生变化,藻细胞壁、蛋白质被破坏,产生类腐殖质类物质。
如表3所示,溶藻进程的1—3 d,3个峰面积逐渐增大,结合不同峰位置来看,溶藻的初期伴随着硝基化合物、酰胺类的增加,可能有芳环结构的氨基酸、类腐殖酸等物质的增加。随着XXG菌繁殖生长,藻细胞数量的减少,在溶藻第4—6 天,3个峰面积均先减少后增加,猜测由于XXG菌生长和藻细胞死亡导致溶藻产物量的变化,并影响细胞新陈代谢。
-
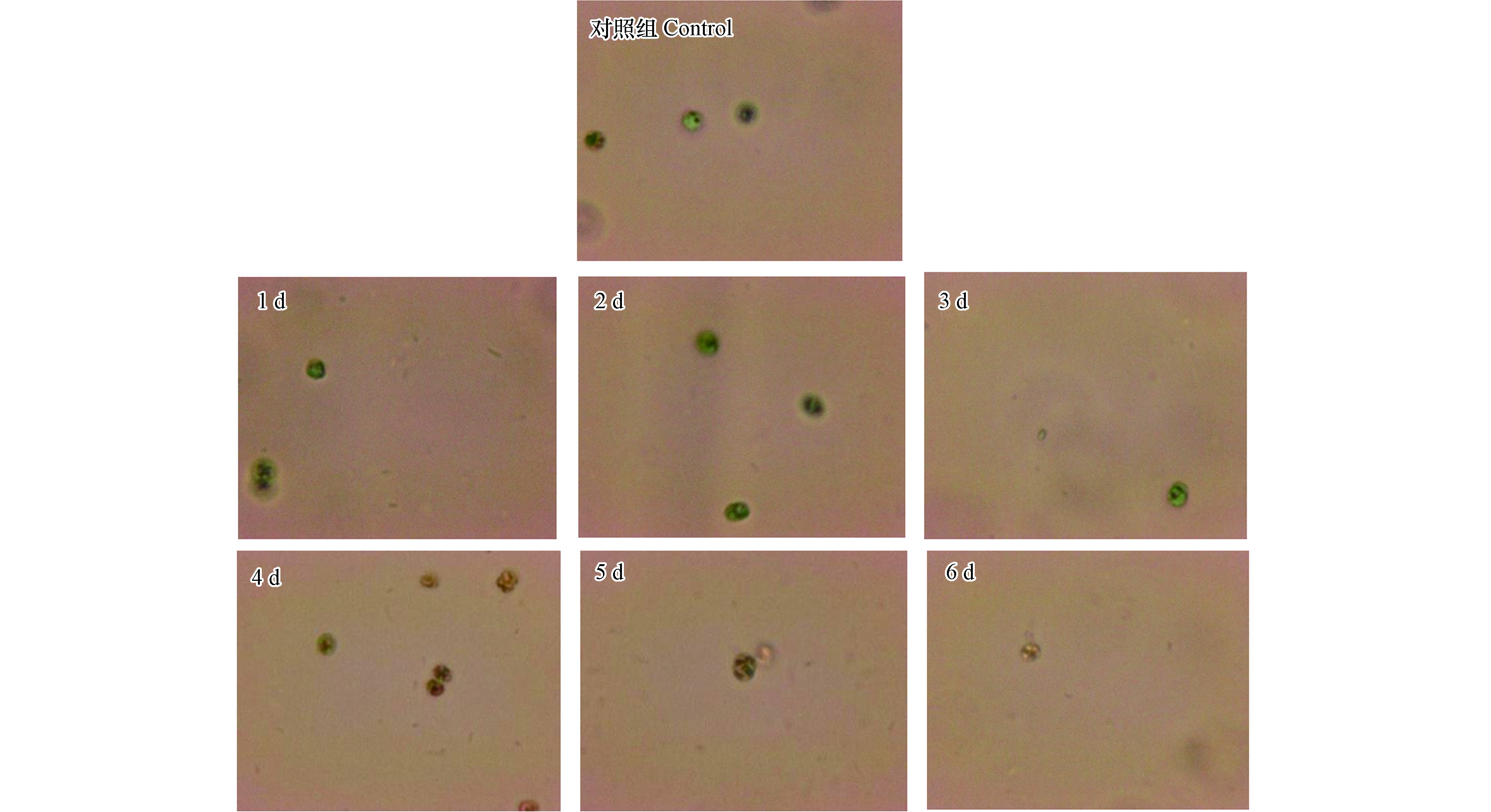
采用倒置显微镜对溶藻前及溶藻进程6 d进行观察,藻细胞形态变化见图4。
如图4所示,未加菌处理的藻细胞形态正常,边缘清晰,具有荧光特性,溶藻的1—2 d,藻细胞绿色加重,变为深绿色,第3 天,藻细胞颜色变浅,细胞边缘逐渐模糊,从第4 天起,藻细胞逐渐失去荧光特性,藻细胞破碎,胞内物质流出,细胞变小。通过显微镜的观察结果,推测溶藻进程为:XXG菌感染藻细胞→藻细胞膜收缩、凹陷→藻细胞壁破损,胞内物质流出→藻细胞死亡。根据溶藻产物初步解析结果,推测溶藻进程是:菌体感染铜绿微囊藻细胞,使藻细胞膜收缩、凹陷直至藻细胞壁破损,藻细泡内外渗透压失衡,致使藻细胞膜破裂,磷脂双分子层被分解,铜绿微囊藻细胞胞内物质流出,Chl-a离体后被破坏,蛋白质等有机物被分解,溶藻产物FA、HA含量大幅增加,藻细胞死亡、溶解。
2.1. 紫外-可见光光谱特征
2.2. 溶藻产物三维荧光光谱特征
2.3. 红外光谱特征
2.4. XXG菌溶藻进程显微观察与溶藻机制推测
-
(1)综合紫外-可见光光谱、三维荧光光谱、红外光谱特征分析,Paenibacillus sp. XXG菌溶藻进程中,铜绿微囊藻中Chl-a含量逐渐降低,藻细胞壁破裂,蛋白质的酰胺键逐渐被破坏,溶藻产物主要是类蛋白及氨基酸类,还包括Chl-a、类腐殖质类物质。在溶藻的第5 天、第6 天,铜绿微囊藻液中Chl-a含量大幅减少,类蛋白及氨基酸类含量大幅增加。
(2)依据溶藻产物光谱特征解析和连续显微录像回放观察分析,推测溶藻机制为:菌体感染铜绿微囊藻细胞,使藻细胞膜收缩、凹陷直至藻细胞壁破损,藻细泡内外渗透压失衡,在溶藻的第5、6 天藻细胞膜破裂,磷脂双分子层被分解,铜绿微囊藻细胞胞内物质流出,Chl-a离体后被破坏,蛋白质等有机物被分解,溶藻产物FA、HA含量大幅增加,藻细胞死亡、溶解。





 DownLoad:
DownLoad:
