-
多环芳烃(PAHs)广泛分布于大气、土壤、水、生活垃圾等各种环境中,由于具有遗传毒性和致癌活性,它们在食品和环境中的污染水平受到严格监管[1 − 4]. 含氧多环芳烃(oxy-PAHs)、硝基多环芳烃、烷基多环芳烃等衍生物通常与PAHs共同存在,其排放源与PAHs相似,主要包括化石燃料的不完全燃烧、汽车尾气排放以及多环芳烃的光化学与生物转化作用等[5]. 由于具有高极性、高水溶性、低蒸汽压等特点,oxy-PAHs在水、土壤、大气颗粒物等环境中的分布比母体PAHs更为广泛[6 − 7]. 且在各种食物以及电子垃圾等环境中也检测到较高浓度的oxy-PAHs[8 − 9]. 最近研究表明,与PAH相比,许多oxy-PAHs具有更高的遗传毒性、促肿瘤活性、发育毒性、心血管毒性和细胞毒性[10 − 13]. 然而,目前有关oxy-PAHs的毒性作用机制研究主要集中于遗传毒性、发育毒性等,对其促肿瘤活性作用机制的研究甚少.
9-芴酮(9-Fluorenone)是大气和水环境中含量最为丰富的oxy-PAHs之一,在多种环境介质中均有检出. 例如,法国南部地区大气环境中9-Fluorenone含量约占oxy-PAHs总浓度的60%[14]. 汽车尾气颗粒物中9-Fluorenone含量明显高于其他oxy-PAHs[10]. 我国多个地下水样本中均检出大量9-Fluorenone[15]. 对宜昌市化工园区的土壤样品有机污染物进行非靶标筛查中发现了9-Fluorenone的存在[16]. 此外,研究发现9-Fluorenone是矿质土壤和燃烧排放中最丰富的oxy-PAHs之一[17]. 目前,有关9-Fluorenone的毒性研究主要集中于发育毒性、遗传毒性等[18],有关9-Fluorenone的促癌作用研究较少. 本课题组前期研究发现,9-Fluorenone暴露可诱导肺癌细胞EMT的发生进而促进侵袭转移[19],但关于9-Fluorenone的促癌作用机制仍不明确.
Hippo-YAP通路被发现是调节癌细胞存活、增殖、转移、免疫、凋亡等的关键因子[20 − 21]. 与大量动物研究结果一致,美国TCGA(The Cancer Genome Atlas,癌症基因组图谱)项目对9125个肿瘤样本进行了多组学分析,结果表明Hippo通路失调发生在多种人类癌症中[22 − 23]. Hippo信号通路是一个激酶级联通路,主要作用是调节Yes相关蛋白(YAP和TAZ)、转录共激活因子和效应靶标分子. Hippo信号通路开始于肿瘤抑制性丝氨酸/苏氨酸激酶(MST1/2)的激活,然后磷酸化并激活大肿瘤抑制因子(LATS1/2),进而磷酸化并抑制YAP(Yes相关蛋白)向细胞核的转移[24]. 当Hippo信号通路受刺激失活时,MST1/2与LATS1/2的磷酸化激活被抑制,使得未磷酸化的YAP转移至细胞核以调节癌细胞增殖转移等相关靶基因的表达[25]. Hippo通路异常与肿瘤细胞的迁移和侵袭密切相关,Hippo通路的关键效应分子YAP和TAZ的异常积累和核转移在转移性肿瘤中经常发生[26]. YAP作为细胞核内TEAD等转录因子的共激活因子,可以调控肿瘤转移相关基因的表达[27]或者通过激活或抑制MAPK通路、WNT通路等促进肿瘤转移和侵袭[28 − 29]. 此外,Hippo通路失调可以通过诱导EMT和减少细胞之间或细胞与细胞外基质之间的黏附诱导肿瘤侵袭和转移发生[30 − 31]. 本课题组前期研究发现1-硝基芘暴露可以诱导Hippo-YAP通路失活,促进肺癌细胞EMT,诱导转移的发生[32]. 然而,目前仍不清楚oxy-PAHs的促肿瘤作用是否与Hippo通路有关.
为了解9-Fluorenone的促肿瘤作用是否与Hippo-YAP通路有关,本文采用肝癌细胞暴露模型,探究了9-Fluorenone对细胞侵袭、转移等的影响,检测了MST1/2、LATS1/2、YAP等蛋白磷酸化的发生,结合免疫荧光检测了YAP-TEAD的结合,并采用YAP-TEAD阻断剂处理进一步确证了Hippo-YAP通路在9-Fluorenone促肿瘤发生中的关键作用.
-
HepG2肝癌细胞购买自中国科学院上海细胞库,9-Fluorenone和YAP-TEAD抑制剂verteporfin购买自Sigma公司,DMEM培养基、链霉素、青霉素均购买自Gibco公司,胎牛血清购自于Biological Industries公司,Transwell小室、Matrigel基质胶购买自Corning公司,蛋白一抗(β-actin,bs-0061R;p-MST1/2,bs-3294R;MST1/2,bs-8477R;p-LATS1/2,bs-7913R;YAP,bs-3605R;p-YAP,bs-3475R)购自Bioss公司,免疫荧光一抗(YAP,ab52771;TEAD,ab58310)购自Abcam公司,荧光标记二抗(IRDye800 CW,926-32211)购自LICOR公司,反转录试剂与SYBR扩增酶购自TaKaRa公司.
-
将HepG2肝癌细胞于含10%血清的DMEM培养基(加100 U·mL−1链霉素和青霉素)中进行单层培养. 待HepG2细胞生长至80%时,用胰酶消化,铺细胞,将其随机分为对照组和暴露组,暴露组细胞用不同浓度(1、3、10 μmol·L−1)的9-Fluorenone处理不同时间并进行后续实验,对于YAP-TEAD阻断组,采用1 μmol·L−1 的verteporfin预处理细胞1 h后再进行9-Fluorenone暴露.
-
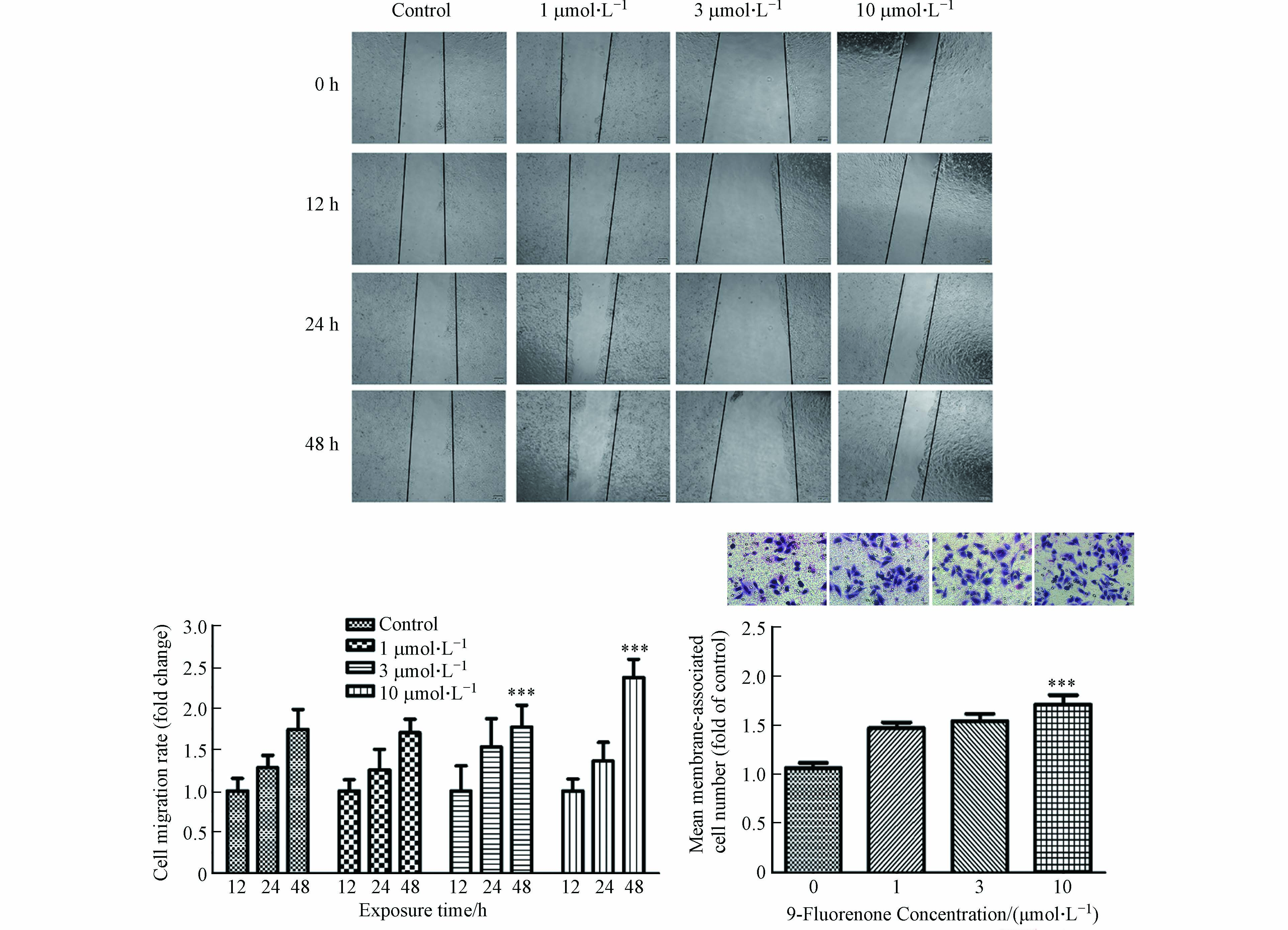
将HepG2细胞接种于12孔板中,待细胞生长至90%时,采用无菌移液枪头划出一个宽度区域,PBS洗涤后用不同浓度(1、3、10 μmol·L−1)的9-Fluorenone暴露处理,12 h、24 h和48 h后于光学显微镜下拍照观察细胞迁移情况,计算迁移速率.
-
将HepG2细胞接种于35 mm细胞培养皿中,待细胞生长至80%时,用不同浓度(1、3、10 μmol·L−1)的9-Fluorenone对细胞暴露48 h,胰酶消化处理后用无血清培养基重悬至5×105个·mL−1. 在Transwell小室内加入50 μL的Matrigel基质胶(1:8稀释),取104个细胞均匀接种到Transwell小室上方,下室中加入诱导剂即完全培养基,孵育24 h. 去掉上层的培养基,用棉签擦去上方未迁移的细胞,迁移到小室下表面的细胞用多聚甲醛固定,结晶紫染色,光学显微镜拍照计数.
-
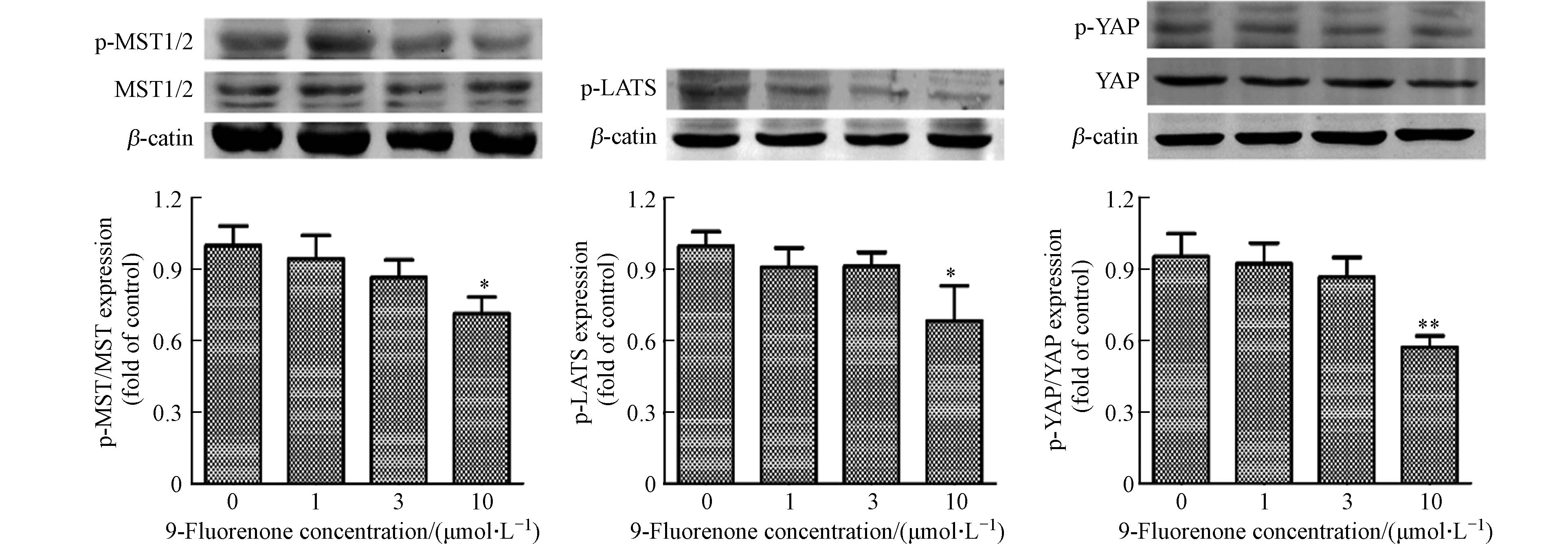
收集不同浓度(1、3、10 μmol·L−1)9-Fluorenone处理24 h后的细胞,用RIPA裂解液提取蛋白,用BCA试剂盒测定蛋白浓度. 采用8%的浓缩胶和分离胶进行SDS-PAGE电泳分离实验,之后90 V恒压转移至PVDF膜上,蛋白冻干粉封闭后选用不同的一抗(β-actin、p-MST1/2、MST1/2、p-LATS1/2、YAP、p-YAP)于4 ℃孵育过夜. 第二天用PBST清洗,荧光标记二抗(1:10000稀释,IRDye800 CW)避光孵育1 h,PBST清洗后使用Odyssey红外荧光成像系统对条带进行扫描.
-
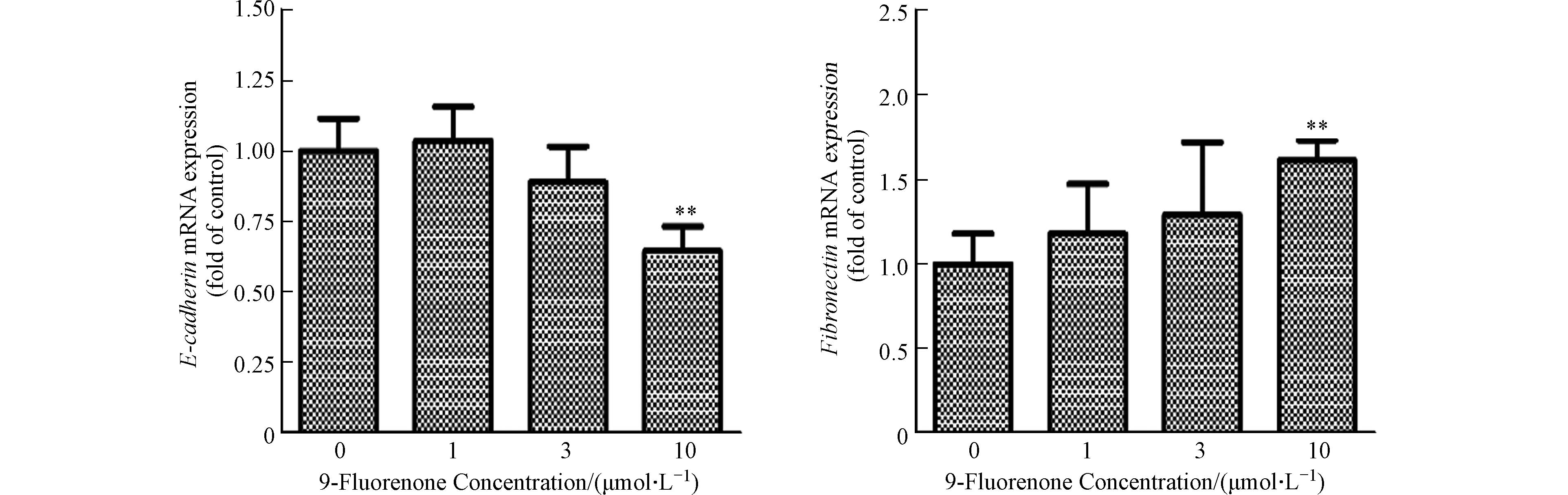
收集不同浓度(1、3、10 μmol·L−1)9-Fluorenone处理24 h后的细胞,用Trizol提取细胞RNA,用Nanodrop测定RNA浓度. 采用反转录试剂将1 μg的RNA反转为cDNA,6倍稀释后,选用SYBR Premix Ex TaqⅡ进行实时荧光定量PCR. 扩增结果依据ΔΔCt法计算. 引物序列信息见表1.
-
将2 cm×2 cm的细胞玻片进行高压灭菌处理,用10×多聚赖氨酸(Poly-L)预处理后置于60 mm细胞培养皿中. 将HepG2细胞接种于玻片上,分别用10 μmol·L−1的9-Fluorenone与1 μmol·L−1的verteporfin进行暴露处理. 之后用4%多聚甲醛进行固定,0.5%Triton X-100通透处理. 用山羊血清封闭后滴加稀释好的一抗混合液(YAP和TEAD),置于湿盒中过夜. 第二天用荧光二抗混合液(羊抗兔lgG(IRDye800 CW,926-32211)和羊抗鼠lgG(bs-40296G-IRDye8))避光孵育1 h,用含DAPI抗荧光猝灭封片液进行封片,荧光显微镜下观察拍照.
-
所有数据为3次独立重复实验的平均值±标准差. 数据采用SPSS单因素方差分析比较组间均值差异,采用GraphPad Prism 5作图.
-
许多研究发现,土壤样本、河流、柴油机尾气和城市灰尘中检测到的oxy-PAHs含量高于其母体PAHs[33 − 34]. 含氧多环芳烃会引发与苯并[a]芘相似或更高水平的遗传毒性作用,表明含氧多环芳烃可能对环境多环芳烃混合物的总致癌效力有显著贡献[13]. 暴露于含氧多环芳烃后HBEC和HepG2细胞的磷酸化CHK1水平升高,此结果与肿瘤细胞增殖相关[13]. 前期研究发现,9-Fluorenone暴露可诱导肺癌细胞EMT的发生进而促进侵袭转移[32]. 然而oxy-PAHs暴露是否可影响肝癌细胞的侵袭转移,目前仍无明确数据. 本研究选用人肝癌细胞HepG2为研究对象,对其进行9-Fluorenone暴露,检测了细胞迁移、细胞侵袭能力的变化. 前期的CCK-8实验结果表明[35],大于30 μmol·L−1的9-Fluorenone暴露会引起肝癌细胞HepG2活性的显著下降,而10 μmol·L−1的9-Fluorenone暴露对细胞活性无显著影响. 因此,本研究选择对细胞活性无显著影响的不同浓度(1、3、10 μmol·L−1)对HepG2进行暴露,以探究9-Fluorenone对细胞侵袭转移功能的影响. 划痕实验结果显示,与对照组相比,10 μmol·L−1的9-Fluorenone暴露处理48 h后细胞迁移明显增加,迁移速率变为12 h的2.36倍(图1). Transwell小室实验发现,10 μmol·L−1的9-Fluorenone暴露处理后细胞侵袭能力为对照组的1.7倍,其他浓度下侵袭能力的变化不显著(图1). 此结果证实,较高浓度的9-Fluorenone暴露具有一定的促肿瘤转移作用.
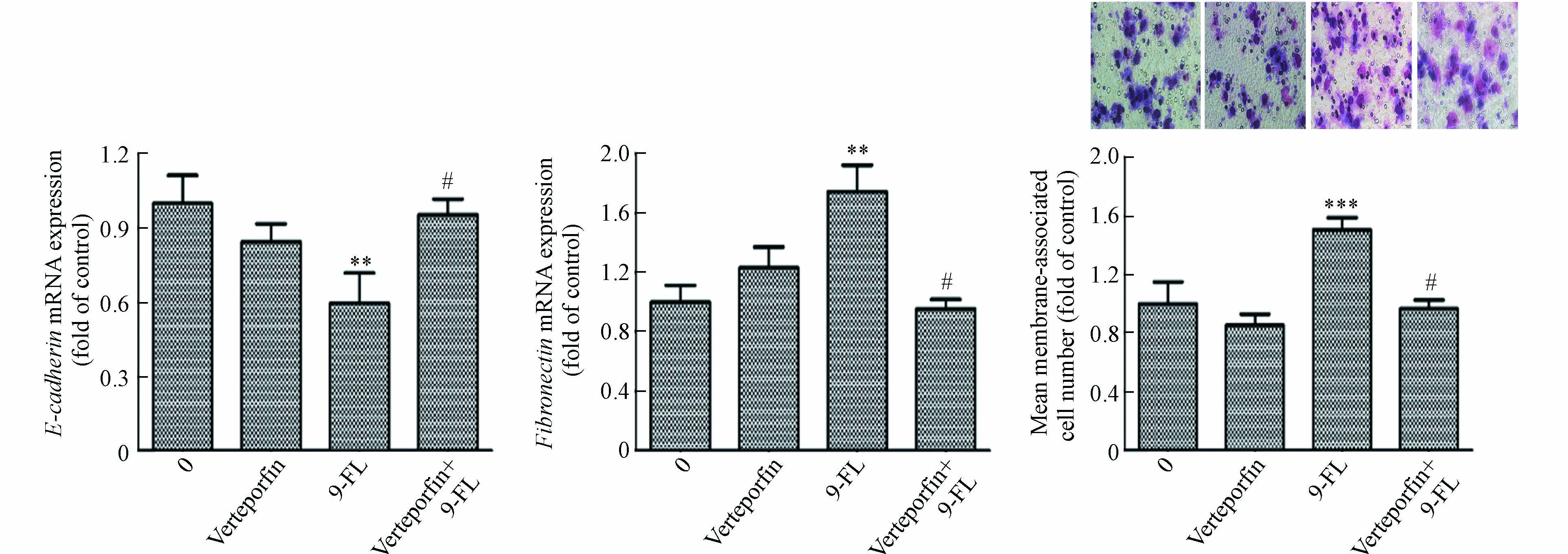
肿瘤细胞的上皮间质转化(EMT)是肿瘤细胞发生侵袭和转移的重要过程[36]. EMT是上皮细胞失去细胞-细胞黏附和顶端-基底极性等特征,并获得间充质特性(如增加的运动性和侵袭性)的过程,在此过程中细胞会失去上皮标志物E-钙黏蛋白(E-cadherin)并获得间质标志物纤连蛋白(Fibronectin)等[37]. 先前研究表明,9-Fluorenone暴露可诱导肺癌细胞EMT过程的发生[19],为了解高浓度9-Fluorenone暴露诱导肝癌细胞转移的发生是否与EMT有关,本实验检测了E-cadherin和Fibronectin基因的转录表达. 图2结果发现,10 μmol·L−1的9-Fluorenone暴露后E-cadherin基因的mRNA表达下降为对照组的0.65倍,Fibronectin基因的表达增加至1.6倍,表明高浓度的9-Fluorenone暴露促进了EMT的发生.
-
Hippo通路的失调对癌症的发展有重要的影响,MST和LATS是Hippo通路激酶的核心组成部分. Hippo通路激活状态下,MST发生磷酸化后可以激活LATS的磷酸化,进而顺位激活YAP的磷酸化[38]. 研究发现MST和LATS激酶在调节重金属诱导的细胞稳态中有重要作用[39]. 为了解9-Fluorenone暴露是否影响Hippo通路上游激酶的表达,通过Western Blot检测了细胞内p-MST1/2和p-LATS1/2的表达. 结果显示,高浓度9-Fluorenone暴露后Hippo上游激酶MST和LATS的磷酸化分别降低至对照组的0.76倍和0.68倍,低浓度暴露下的变化不明显(图3). 与此结果相同,LATS磷酸化的降低影响了YAP的磷酸化. Western Blot结果显示,9-Fluorenone暴露后p-YAP的蛋白表达降低至对照组的0.57倍(图3). 此结果表明,9-Fluorenone暴露诱导了Hippo通路的失活,导致Hippo通路级联效应MST-LATS-YAP的磷酸化降低,该过程可能与9-Fluorenone的促肿瘤转移作用有关.
-
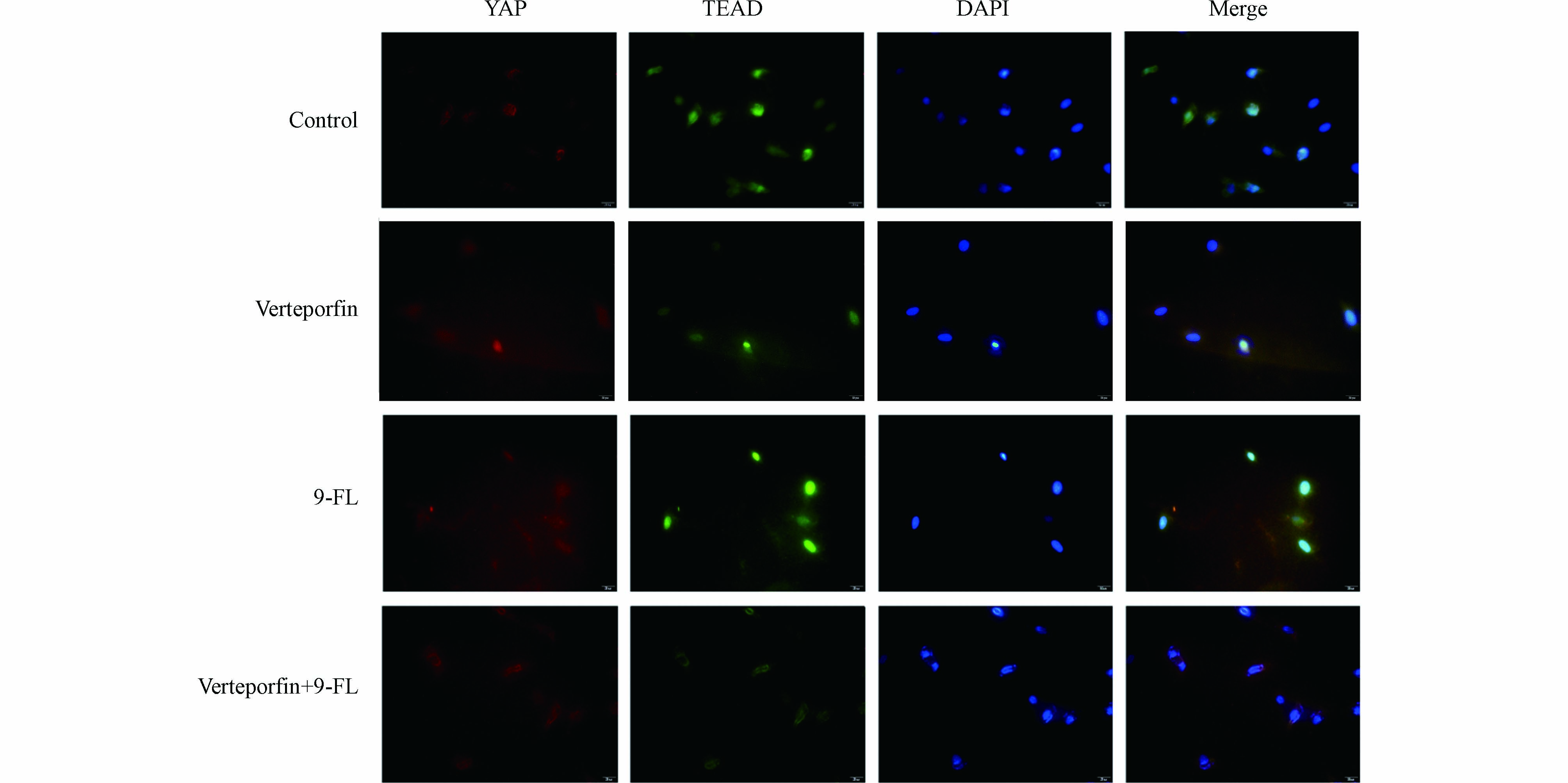
Hippo通路级联效应MST-LATS-YAP的失活已在多种癌症中检出. 据报道,Hippo通路中转录共激活因子YAP发生去磷酸化后会从细胞质转移到细胞核,通过与核转录因子TEADs的相互作用,直接或间接调控肿瘤转移和侵袭相关靶基因的表达,这些靶基因可诱导肿瘤细胞形成侵袭性假足,降低细胞间黏附,降解细胞外基质,引起上皮间质转化,或通过其他信号通路如丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)、TGF/Smad等间接促进肿瘤的侵袭转移[31, 40]. 为检测9-Fluorenone(9-FL)暴露是否影响了YAP与TEAD的相互作用,采用免疫荧光检测了YAP和TEAD的结合效果. 与对照组相比,在9-Fluorenone暴露后,YAP与TEAD的荧光重合效果较强. 为确证这一结果,在9-Fluorenone暴露前加入YAP和TEAD的阻断剂verteporfin[41]进行预处理后,发现YAP和TEAD的荧光重合强度明显减弱,证实9-Fluorenone暴露影响了YAP与TEAD的结合与相互作用(图4),该过程可能与肿瘤EMT的发生有关.
-
为明确9-Fluorenone暴露诱导肝癌细胞Hippo通路失活是否与其诱导的EMT和侵袭转移有关,采用YAP-TEAD抑制剂verteporfin预处理后检测了细胞侵袭及EMT标志基因E-cadherin和Fibronectin的转录表达,发现由高浓度9-Fluorenone暴露诱导的E-cadherin基因的mRNA水平降低以及Fibronectin基因的mRNA水平升高均得到了显著抑制,且verteporfin预处理后,细胞侵袭数目减少(图5). 结果证实,9-Fluorenone的促肿瘤侵袭迁移作用与Hippo-YAP通路失活有关.
前期有关硝基多环芳烃的促肿瘤研究结果发现,1-硝基芘、1-硝基萘与9-硝基蒽的促癌作用均与Hippo通路有关[32, 42]. 本研究结果与以上研究结论相似,这些研究均表明Hippo-YAP通路在多环芳烃衍生物的促肿瘤作用中至关重要. 此外,最新研究发现,Hippo通路组分在空气污染暴露诱导的肺部疾病中有重要作用. 如暴露于柴油废气颗粒的小鼠肺部和A549细胞中E-cadherin和p-YAP的表达均有下降[43];产前柴油尾气颗粒暴露通过Hippo信号通路失调,损害胎儿肺生长,导致胎儿肺发育不良[44]. 这些结果提示,Hippo通路失调可能是空气污染暴露损伤的重要过程,在大气毒理学研究中应及时关注Hippo信号通路的调控作用.
-
本研究通过肝癌细胞暴露模型发现,较高浓度9-Fluorenone暴露可促进肝癌细胞侵袭、迁移及EMT的发生,且Hippo-YAP通路在该过程中有重要作用. 9-Fluorenone暴露可通过诱导Hippo上游激酶MST、LATS以及YAP蛋白的去磷酸化,使得YAP进入细胞核内与TEAD结合,调控EMT的发生,进一步促进肝癌细胞侵袭转移. 本研究结果为含氧多环芳烃的促肿瘤活性研究提供了实验基础,对于全面评估多环芳烃及衍生物的生态和健康风险具有重要参考作用.
Role of Hippo pathway in promoting invasion and metastasis of liver cancer cells by oxygenated polycyclic aromatic hydrocarbons
- Received Date: 21/04/2023
- Accepted Date: 26/05/2023
- Available Online: 27/04/2024
-
Key words:
- oxygenated polycyclic aromatic hydrocarbons /
- invasion and metastasis /
- Hippo /
- EMT
Abstract: Due to the presence of oxygenated functional groups, oxygenated polycyclic aromatic hydrocarbons (oxy-PAHs) possess higher polarity, solubility, mobility, and biological reactivity than their parent PAHs, and are more widely distributed in the environment. Although numerous oxy-PAHs exhibit heightened genotoxicity, carcinogenicity, and developmental toxicity, limited research has been conducted to elucidate the underlying mechanisms responsible for their tumorigenic promotion. Notably, the Hippo-YAP pathway has emerged as a pivotal regulatory process governing cancer cell immunity, proliferation, and metastasis. In this study, the tumor promoting effect of oxy-PAHs (specifically 9-Fluorenone) that are commonly found in the environment, as well as the underlying Hippo-YAP regulation mechanism were investigated by using the liver cancer cell exposure model. The results showed that a high concentration of 9-Fluorenone exposure significantly enhanced the invasion, migration, and epithelial-mesenchymal transition (EMT) of HepG2 cells, and the Hippo-YAP pathway was identified as playing a crucial role in the tumor-promoting process. Specifically, 9-Fluorenone exposure can induce dephosphorylation of upstream kinases in the Hippo pathway, such as MST and LATS, and then induce dephosphorylation of YAP. Consequently, YAP is able to bind to TEAD in the nucleus, thereby regulating the occurrence of EMT and ultimately promoting the invasion and metastasis of cancer cells. This study contributes valuable toxicological evidence supporting the tumor-promoting effect of oxy-PAHs and holds significant reference value for the comprehensive assessment of the ecological and health risks associated with PAHs and their derivatives.




 DownLoad:
DownLoad:
