-
随着合成化学工业的发展,水体中检出的各类微量有机污染物也日渐增多,包括不同种类的农药、内分泌干扰物、药物和个人护理品等。传统的饮用水和污水处理工艺通常无法有效去除这些微量污染物。基于紫外(UV)的高级氧化技术(UV/H2O2)是去除微量有机污染物的有效方法[1-2],目前在国内饮用水厂和污水处理厂已有一定的应用。然而,常规UV光源(254 nm)下的高级氧化工艺依赖于化学氧化剂的投加来产生HO·等活性物种,这涉及到氧化剂的储存、运输以及残余药剂的去除等问题,增加了操作的复杂性和处理成本。真空紫外线(VUV)技术是近年来的一个研究热点。与常规UV高级氧化工艺相比,VUV/UV高级氧化的优点在于不需要化学氧化剂,水分子在VUV辐照下的光解就可以产生高浓度的HO·,是一种很有前景的绿色高级氧化技术。另一方面,由于VUV能量高,仅5 mm的水层即可使VUV辐射强度衰减约90%,因此产生的HO·也主要集中在灯管附近很薄的水层内。此时,HO·的利用率及相关影响因素决定了VUV/UV高级氧化技术的实际效率。在已有研究中,大量基于VUV/UV工艺的研究均是在序批式反应器内进行的,其中自由基可与目标污染物及背景物质充分接触反应。然而,实际应用中的VUV/UV反应器通常为过流式,这可能导致HO·在整个反应器内分布不均匀,表现出与序批式反应器不同的效果[3]。目前,在过流式条件下水中常见的背景物质对VUV/UV工艺降解微量污染物的影响研究还较少。此外,不同于常规高级氧化工艺,VUV光解水产生HO·的过程受到可吸收185 nm辐射的无机离子的显著影响,但对该蔽光效应的量化分析还比较缺乏。基于此,本研究探究了过流式VUV/UV反应器中氯离子(Cl–)、碳酸氢根离子(HCO3–)、硝酸盐(NO3–)和溶解性有机物(dissolved organic matter,DOM)对典型微量污染物阿特拉津(atrazine)降解的影响,并计算不同水质条件下VUV/UV工艺降解目标污染物所需的单位能耗。本研究可为VUV/UV技术在实际条件下的推广应用提供参考。
-
阿特拉津(ATZ)购于梯希爱(上海)化成工业发展有限公司,纯度大于98%。氯化钠、碳酸氢钠和硝酸钠购于国药集团化学试剂有限公司,均为分析纯。甲酸、乙腈购于西格玛-阿拉丁公司,纯度为色谱纯。用于配制溶解性有机物溶液的腐殖酸(HA)购于北京伊诺凯科技有限公司,纯度大于90%。目标污染物ATZ的初始浓度固定为2.5 μmol·L−1,考察的Cl–和HCO3–浓度设置为1.0、2.5和5.0 mmol·L−1,NO3–浓度设置为10.0和20.0 mg·L−1(以氮计),DOM浓度设置为1.0、5.0和10.0 mg·L−1(以碳计)。反应溶液均用去离子水配制。
-
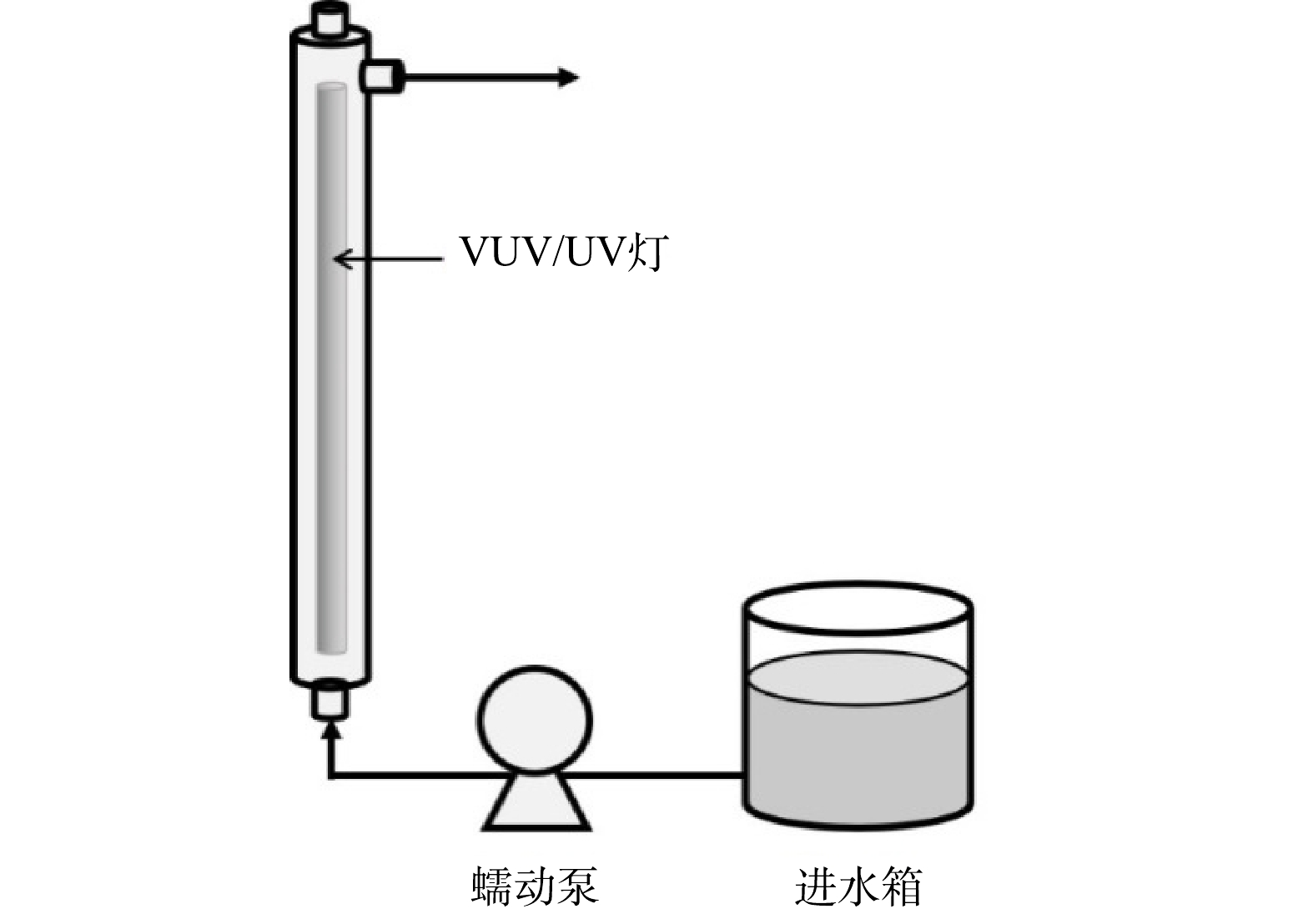
污染物的降解实验在高为450 mm、内径为50 mm、有效体积为750 mL的管式反应器中进行。反应器竖直放置,中心固定一根长为436 mm、外径为20.5 mm、功率为21 W的VUV/UV灯(GCL436T5VH/4,莱劭思,美国)。整个反应装置示意图如图1。
在进行辐照实验前,至少提前10 min开启VUV/UV灯,使其输出达到稳定,并同时在反应器内循环冷却水以防灯管发热影响输出功率。进水箱中的反应溶液通过蠕动泵与反应器下端的进水口相连,进水模式为过流式,即溶液只通过1次反应器后从上端出口流出,在此处取样,进行后续分析。通过控制蠕动泵的转速来改变进入反应器的流量(1.5~9.0 L·min−1),进而改变每次进水在反应器内的水力停留时间(HRT)(5~30 s)以及接受的UV辐射剂量(70.5~423 mJ·cm−2)。每次调整流量后先将反应器内的溶液排空,再进行新流量下的降解实验。在每个进水流量下,将该流量所对应的HRT认定为溶液通过反应器时所接收辐照的时间。实验温度控制在室温(25±2 ℃)。以2 μmol·L−1的ATZ为感光剂在同型号的UV灯(GCL436T5L/4,莱劭思,美国)下直接光解,确定反应器内的平均UV强度为14.1 mW·cm−2。
-
目标污染物ATZ的浓度由装有C18色谱柱(安捷伦,150 mm×2.1 mm,3 μm,美国)的高效液相色谱HPLC(安捷伦,1200系列,美国)来测定,测定条件如下:流动相由乙腈和0.2%甲酸水组成(体积比为75:25),流速为0.8 mL·min−1,柱温为40 ℃,检测波长为234 nm,进样体积为50 μL。NO3–和NO2–的浓度用哈希试剂测定(方法编号分别为10020和10019) 。DOM的浓度用TOC分析仪(岛津,TOC-VCPH,日本)测定。pH由pH计(赛多利斯,PB-10,德国)测定。
-
水和不同溶质对185 nm VUV有不同的吸收能力,根据式(1)可以计算水和溶质对VUV光子的吸收比例。
式中:fi为物质对185 nm VUV光子的吸收比例;ai为物质i在185 nm处的单位吸光度,cm−1;εi为物质在185 nm处的摩尔吸收系数,L·(mol·cm)−1;Ci为物质的浓度,mol·L−1;atotal为水中所有物质在185 nm处的单位吸光度,cm−1。
-
过流式VUV/UV反应器中Cl–浓度对ATZ降解的影响结果如图2所示。由图2可见,水中Cl–的存在可抑制VUV/UV工艺对ATZ的降解,且Cl–浓度越高,对ATZ降解抑制程度越高。当辐照时间为30 s时,在Cl–浓度为1.0、2.5和5.0 mmol·L−1的溶液中,ATZ的去除率由空白组中的57%分别降至42%、39%和33%(图2(a))。对各Cl–浓度下不同辐照时间时ATZ的降解率进行拟一级反应动力学拟合,发现拟合程度较好(R2 > 0.96)。拟一级降解速率常数kapp,VUV/UV从空白组中的0.029 4 s−1分别降低至0.021 5、0.017 7和0.015 0 s−1;而ATZ在UV下发生直接光降解的速率常数kapp,UV为0.012 5 s−1。这表明1.0、2.5和5.0 mmol·L−1 Cl–对VUV贡献的ATZ降解速率常数kapp,VUV(即kapp,VUV/UV–kapp,UV)的抑制程度分别为47%、69%和85%。
Cl–对ATZ降解的抑制可归因于以下2点。一方面,Cl–在185 nm处的摩尔吸收系数较大(ε=3 500 L·(mol·cm−1))[4],根据式(1)计算可得1.0、2.5和5.0 mmol·L−1的Cl–在185 nm处的单位吸光度分别为3.5、8.8和17.5 cm−1,而水的单位吸光度为1.8 cm−1(25 ℃)[5]。这表明,当水溶液中含有1.0、2.5和5.0 mmol·L−1的Cl–时,VUV/UV发出的185 nm处VUV光子分别只有34%、17%和9%被水吸收(图2(b)),VUV光解水产生的HO·浓度相对于空白组中的浓度显著降低,这是导致ATZ降解受到抑制的主要原因。另一方面,Cl–在VUV辐照下会产生Cl·(式(2)),而Cl·又会与Cl–快速反应生成Cl2–·(式(3)),Cl·氧化性强,而Cl2–·氧化性较弱,二者与ATZ的二级反应速率常数分别为6.9×109 L·(mol·s)−1和1.0×107 L (mol·s)−1 [6],Cl·与ATZ的反应活性甚至超过HO·与ATZ的反应活性(后者的二级反应速率常数2.3×109 L (mol·s)−1 [7],表明ATZ可能会因被Cl·氧化而降解。由图2(b)可见,水溶液中Cl–存在时由VUV贡献的ATZ降解速率常数kapp,VUV的下降程度低于VUV光子被Cl–吸收的比例(如5.0 mmol·L−1时的85% vs.空白组的91%),说明Cl–在VUV辐射下产生的活性氯自由基(Cl·、Cl2–·等)确实在ATZ降解过程中发挥了作用。需要注意的是,虽然Cl–可能会与水中的HO·结合生成ClHO–·,但这个反应是可逆的,且逆反应速率高于正反应速率(式(4)),所以二者的结合产物ClHO–·在产生后会立即解离而再次生成HO·,因此Cl–对HO·的影响可以忽略[8-12]。本研究中VUV/UV辐照下Cl–对ATZ降解的抑制程度要略高于YANG等在细管流反应器内得到的结果[13],表明反应器内的流态对降解可能也有一定的影响。总体而言,Cl–对VUV/UV工艺降解水中微量污染物的影响程度主要取决于污染物自身特性。当目标污染物的UV直接光解速率低且与活性氯自由基反应慢时,Cl–对VUV光子的竞争可能导致污染物(如三氯乙基磷酸酯[14])降解速率超过90%以上的降幅。而当目标污染物与活性氯自由基的反应速率快且超过其与HO·的反应速率时,Cl–在VUV辐射下产生的活性氯自由基会促进污染物(如尿素[15])的降解。
-
过流式VUV/UV反应器中HCO3–浓度对ATZ的降解影响结果如图3所示。与上述Cl–存在时的结果类似,当水中HCO3–浓度较高时,ATZ的降解受到了明显的抑制。当HCO3–浓度为1.0、2.5和5.0 mmol·L−1、辐照时间为30 s时,ATZ的去除率分别为49%、35%和29%(图3(a))。对上述各HCO3–浓度下不同辐照时间的降解数据进行拟一级动力学拟合,得到的降解速率常数kapp,VUV/UV分别为0.024 0、0.014 8和0.011 5 s−1 (R2 > 0.98)。
HCO3–对VUV/UV辐照下ATZ降解的影响可从蔽光效应和自由基反应两个方面解释。HCO3–在185 nm处的摩尔吸光系数(ε = 290 L·(mol·cm)−1)[4]远低于Cl–,相应地,1.0、2.5和5.0 mmol·L−1的HCO3–在185 nm处的单位吸光度分别为0.29、0.73和1.45 cm−1,会分别吸收14%、29%和45%的VUV光子(图3(b)),导致水分子在VUV辐射下产生HO·的途径受到抑制。但不同于Cl–存在的情况,高浓度(如5.0 mmol·L−1)HCO3–对VUV光子的竞争吸收程度远小于其对VUV贡献的ATZ降解的抑制程度(分别为45%和100%),表明此时HCO3–对HO·的清除作用不可忽略。事实上,HCO3–对HO·的二级反应速率常数为8.5×106 L·(mol·s)−1,当HCO3–浓度为1.0、2.5和5.0 mmol·L−1时,HCO3–对HO·的消耗速率为8.5×103、2.1×104和4.3×104 s−1,分别为ATZ对HO·消耗速率(5.8×103 s−1)的1.5、3.7和7.4倍。这表明当HCO3–浓度较低(如1.0 mmol·L−1)时,ATZ降解速率的降低可以主要用HCO3–对VUV的蔽光效应来解释;而当HCO3–浓度较高(如5.0 mmol·L−1以上)时,除了蔽光效应外,HCO3–对HO·较强的清除作用也是导致ATZ降解速率显著降低的重要原因。5.0 mmol·L−1的HCO3–可以完全抑制由VUV贡献的ATZ降解,此时ATZ只有在UV下的直接光降解途径。虽然HCO3–在VUV辐射下以及在清除HO·的过程中会产生新自由基CO3–·(式(5)),但CO3–·与ATZ的二级反应速率常数为4.0×106 L·(mol·s)−1 [16],与HO·的相比低了1 000倍以上,因此CO3–·与ATZ的反应在该体系中可以忽略。此外,水体碱度的增加也会带来pH的升高。当HCO3–的浓度分别为1.0、2.5和5.0 mmol·L−1时,溶液的pH分别为7.8、8.2和8.5,而HO·的氧化还原电位在高pH条件下有所下降[17],这也是导致ATZ降解速率降低的原因之一。本研究中HCO3–对VUV/UV工艺降解ATZ的影响程度同样高于YANG等在细管流反应器中的结果[13],但HCO3–对不同种类的微量有机污染物的降解均体现出明显的抑制效果,究其原因在于CO3–·与大部分的目标污染物的反应活性都偏低[18],难以表现出其额外的氧化作用。
-
在过流式VUV/UV反应器中,NO3–浓度对ATZ的降解影响结果如图4所示。NO3–同样可抑制ATZ的降解。当辐照时间为30 s、NO3–为10 mg·L−1和20 mg·L−1时,ATZ的降解率从空白组中的57%分别降至42%和40%(图4(a))。对上述2个浓度(10 mg·L−1和20 mg·L−1)下不同辐照时间的降解率进行拟一级动力学拟合,得到的降解速率常数kapp,VUV/UV从空白组中的0.029 4 s−1分别降低至0.021 1 s−1和0.019 0 s−1 (R2>0.95)。
NO3–在VUV辐照下对ATZ降解的影响归因于一下几点。首先,NO3–在185 nm处的摩尔吸光系数(ε = 480 0 L·(mol·cm)−1)[4]高于Cl–,10 mg·L−1和20 mg·L−1 NO3–对VUV光子的吸收比例分别为66%和79%(图4(b)),高于相应条件下VUV贡献的ATZ降解速率常数kapp,VUV的下降程度(分别为49%和62%),说明ATZ除了被VUV辐照水分子产生的HO·氧化外还存在其他的降解途径。事实上,NO3–在UV辐照下可以产生NO2·和HO·(式(6)),但该反应在254 nm辐照下的量子产率较小,而在185 nm辐照下的量子产率较大,所以VUV辐照下NO3–产生的NO2·和HO·会在一定程度上补充水中氧化性自由基。已有研究[19-20]表明,UV高级氧化过程中NO3–的存在可能会促进水中目标污染物的降解,其原因同样在于NO3–在低波长(< 240 nm)辐照下产生了氧化性自由基。本研究中NO3–对VUV/UV辐照下ATZ降解的抑制程度低于YANG等的研究结果[13],其原因主要在于后者采用的NO3–浓度更高。
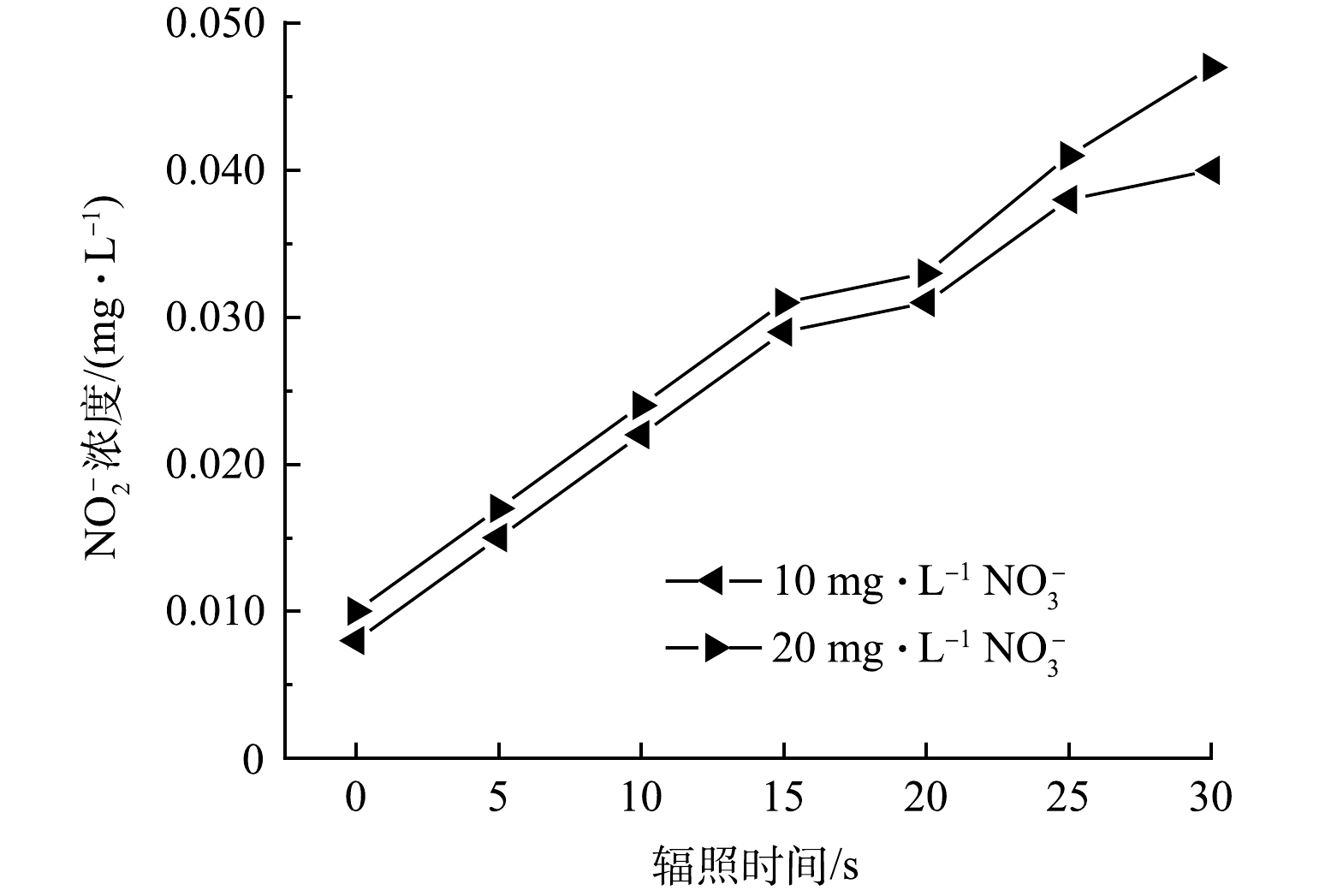
另一方面,VUV辐照水分子除了产生HO·外,还会同时产生还原性物种如水合电子eaq–,进而将NO3–还原为NO2–。另外,NO3–的直接VUV光解也会产生NO2–(式(7))。但NO2–在产生的同时又能被HO·快速氧化为NO3–(式(8))。因此在探究NO3–影响ATZ降解的过程中,监测了NO3–和NO2–的浓度变化。由图5可见,反应溶液中NO2–的浓度随辐照时间的延长而线性增加。10.0 mg·L−1 NO3–在VUV/UV辐照30 s后,NO2–浓度从反应起始的0.008 mg·L−1升高至0.040 mg·L−1,净生成量为0.032 mg·L−1;而20.0 mg·L−1的NO3–在相同的辐照条件下,NO2–浓度从反应起始的0.010 mg·L−1升高至0.047 mg·L−1,净生成量为0.037 mg·L−1。而NO3–的浓度基本没有发生变化,10.0 mg·L−1和20.0 mg·L−1 NO3–在被VUV/UV辐照30 s (相应的UV剂量为423 mJ·cm−2)后,两者的浓度均只降低了0.1 mg·L−1。这一结果表明在NO3–浓度为10.0 mg·L−1和20.0 mg·L−1时,NO2–生成速率的限制因素并不是NO3–的浓度,而可能是反应体系中还原性物种和氧化性物种的浓度。溶液中的eaq–可以和溶解氧快速发生反应被清除,HO·则容易将NO2–氧化为NO3–。这两方面原因共同导致在上述反应条件下NO2–的生成量不高。本研究中NO2–的生成量要显著低于HAN等在平行光束仪下反应皿中得到的结果[21],说明实际反应器内溶液的流态可能会加快NO2–向NO3–的转化。亚硝酸盐是一种致癌物,在多国的饮用水标准中都有明确的限值。例如,我国饮用水标准中规定NO2–浓度不得超过1.0 mg·L−1(以N计),而在欧盟的标准中,这一限值更低至0.1 mg·L−1(以N计)。本实验中选用的NO3–浓度为我国饮用水标准中规定的NO3–上限。实验结果表明,将VUV/UV作为饮用水深度处理工艺以去除水中微量有机污染物时,在UV剂量为400 mJ·cm−2左右时,生成的NO2–不会有超标的风险。
-
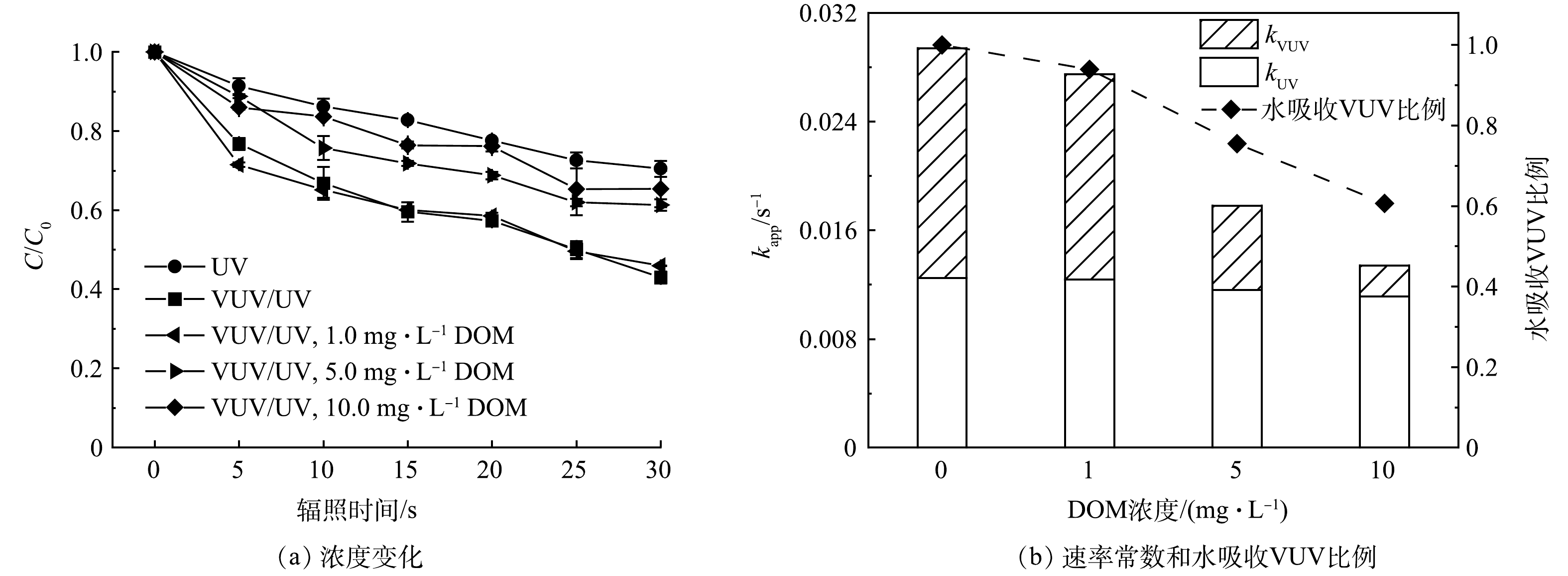
在过流式VUV/UV反应器中,DOM浓度对ATZ降解的影响结果如图6所示。与空白组相比,DOM浓度为1.0 mg·L−1时,对ATZ的降解几乎没有影响,而当DOM浓度增大至5.0和10.0 mg·L−1时,ATZ的降解明显受到抑制。且DOM浓度越高,ATZ降解被抑制的程度越大。VUV/UV辐照30 s,在1.0、5.0和10.0 mg·L−1 DOM的影响下,ATZ的去除率分别为53%、39%和35%(图6(a))。对上述3个DOM浓度不同HRT时的降解数据进行拟一级动力学拟合,得到的降解速率常数kapp,VUV/UV分别为0.027 6、0.018 7和0.014 8 s−1(R2 > 0.96)。
DOM对VUV/UV工艺降解微量有机污染物的影响也有多条途径的参与。首先是蔽光效应,DOM成分(如腐殖酸、黄腐酸等)中通常含有大量的共轭和芳香结构,在254 nm波长下通常具有较高的吸光度,本研究中测得1.0、5.0和10.0 mg·L−1 DOM溶液对UV(254 nm)的单位吸光度分别为0.022、0.073和0.109 cm−1。溶液吸光度的增大会导致反应器内的平均UV强度降低,因此,引入水介质因子FW(式(9))对具有一定吸光能力的溶液所接收的平均UV强度进行校正(式(10))。在DOM为1.0、5.0和10.0 mg·L−1时,溶液的平均UV强度分别降低至空白组的97%、92%和88%,这会导致ATZ的UV直接光降解速率常数kapp,UV降低。而DOM在185 nm处的单位吸光度要高于其在254 nm处的单位吸光度,有文献报道作为DOM标准物质的SRNOM在185 nm处的摩尔吸光系数为1402 L·(mol·cm)−1(25 ℃)[22],即0.117 L·mg−1·cm−1。参考该数值,本研究中1.0、5.0和10.0 mg·L−1的DOM在185 nm下的单位吸光度分别为0.117、0.585和1.170 cm−1,分别可以竞争吸收6%、25%和39%的VUV光子(图6(b)),因此VUV光解水产生的HO·浓度降低。值得一提的是,部分DOM在UV辐照下可以转变为激发态DOM*,并同时产生单线态氧1O2等活性物种[23-24],可能会促进目标污染物的降解[25-26]。本研究中DOM浓度为1.0 mg·L−1时,ATZ的降解并未受到抑制,可能与DOM在UV辐照下激发产生上述活性氧化物种的促进作用有关。而另一方面,DOM是一种反应容量极大的HO·清除剂,其二级反应速率常数一般在104 L·(mg·s)−1级别[27-28],具体数值与DOM的结构及组成有关。因此DOM的存在会使得溶液中HO·浓度降低,从而导致ATZ被HO·氧化降解的途径受到一定程度的抑制。
式中:A为实际水样在254 nm处的吸光度;Eavg为高透光率溶液条件下反应器内的平均UV强度,mW·cm−2;E'avg为实际水样条件下反应器内的平均UV强度,mW·cm−2。
-
采用目标污染物降解90%所需的电能(EEO,kWh·m−3)对VUV/UV工艺在不同水质条件下的能耗进行评估,其计算公式如式(11)所示,计算结果如表1所示。
式中:P表示紫外灯的输出功率,kW;Q表示进水溶液的体积流速,m3·h−1;kapp表示目标污染物的降解速率常数,s−1;V表示反应器有效辐照体积,L。
在空白组中,单独UV辐照下ATZ降解的速率常数为0.012 5 s−1,计算得到相应的EEO为1.43 kWh·m−3,而在VUV/UV辐照下ATZ降解的速率常数提高至0.029 4 s−1,相应的EEO降低至0.61 kWh·m−3。计算可知UV和VUV对ATZ降解的贡献率分别为43%和57%。由表1可见,VUV/UV辐照下,5.0 mmol·L−1 HCO3–对ATZ降解效率的影响最大,具体而言,5.0 mmol·L−1 HCO3–对HO·较强的清除作用使得VUV对ATZ降解的贡献几乎可以忽略不计,如ATZ在5.0 mmol·L−1 HCO3–的背景下降解的EEO为1.56 kWh·m−3,与ATZ在单独UV辐照下降解的EEO相当。Cl–和DOM也会显著影响VUV/UV工艺降解ATZ的效率,在5.0 mmol·L−1 Cl–和10.0 mg·L−1 DOM的背景下,ATZ降解的EEO分别升高至1.19 kWh·m−3和1.21 kWh·m−3,与ATZ在空白组中降解的EEO相比,增加了95%和98%。我国生活饮用水卫生标准(GB 5749-2022)中规定的氯离子、硝酸盐和总有机碳的质量浓度上限分别为250(即7.0 mmol·L−1)、20和5.0 mg·L−1。上述3个上限条件中,硝酸盐的影响最弱,在20 mg·L−1 NO3–的背景下,ATZ降解的EEO为0.94 kWh·m−3,与空白组中的EEO相比增加了54%。我国北方地区水源水中的无机离子浓度要高于南方地区,因此,VUV/UV工艺在基础水质良好而微量污染物风险较高的南方地区饮用水处理中具有更好的应用前景。
-
1)过流式VUV/UV反应器中ATZ的降解受到水中Cl–、HCO3–、NO3–和DOM的抑制,当这些背景物质分别为5.0 mmol·L−1、5.0 mmol·L−1、20.0 mg·L−1和10.0 mg·L−1时,在辐照时间为30 s的条件下,ATZ的去除率由空白组中的57%分别下降至33%、29%、40%和35%。
2) 3种无机阴离子(Cl–、HCO3–和NO3–)均对185 nm的VUV辐射有一定的吸收能力,因此,会对VUV产生蔽光效应,其中NO3–>Cl–>HCO3–。但Cl–和NO3–在VUV辐照下产生的活性自由基如Cl·和Cl2–·、NO2·和HO·能在一定程度上补充水中氧化性自由基,使得由VUV贡献的ATZ降解速率常数kapp,VUV被抑制的比例低于VUV光子被相应阴离子竞争吸收的比例,且在本研究中NO3–在VUV辐照下产生的NO2–不超过国家生活饮用水标准限值。HCO3–对VUV的蔽光作用较弱,而HCO3–对ATZ降解的抑制作用主要是由于HCO3–对HO·较强的清除作用导致的。低浓度DOM对ATZ的降解几乎没有抑制作用,这与DOM在VUV/UV辐照下可能产生活性物种有关,而高浓度DOM对ATZ的降解有显著抑制主要是由于DOM对HO·的清除作用导致的。
3)水中复杂的背景物质会使VUV/UV工艺降解微量有机污染物时的能耗增加,在5 mmol·L−1 Cl–、5 mmol·L−1 HCO3–、20 mg·L−1 NO3–和10 mg·L−1 DOM的影响下,ATZ降解的EEO从空白组中的0.61 kWh·m−3分别升高至1.19、1.56、0.94和1.21 kWh·m−3。因此VUV/UV工艺更适合用于基础水质良好而微量污染物风险较高的饮用水深度处理中。
背景水质对过流式VUV/UV反应器降解水中阿特拉津的影响
Effect of water matrices on atrazine degradation in a flow-through VUV/UV reactor
-
摘要: 为探究连续流进水模式下水中复杂的背景物质对真空紫外/紫外 (VUV/UV) 高级氧化工艺效率的影响,采用过流式VUV/UV反应器,考察了水中不同浓度的氯离子 (Cl–) 、碱度 (HCO3–) 、硝酸盐 (NO3–) 和溶解性有机物 (DOM) 对微量污染物阿特拉津 (ATZ) 降解的影响。结果表明,上述背景组分对ATZ的VUV/UV降解均表现出一定的抑制作用,辐照时间为30 s时,ATZ去除率从空白组中的57%分别最多下降至33%、29%、40%和35%,且过流式条件下的抑制程度与文献中序批式反应器中的略有不同。污染物去除率下降的原因在于,一方面,三种无机阴离子都对VUV辐射有一定的蔽光效应,NO3–强于Cl–强于HCO3–;另一方面,VUV辐照下Cl–产生的Cl·和Cl2•–、NO3–产生的NO2·和HO·都能补充水中氧化性自由基浓度,使得VUV贡献的ATZ降解速率常数的抑制程度低于VUV光子被阴离子竞争吸收的比例,但HCO3–对HO·较强的清除作用则导致了ATZ的降解速率常数的快速下降。低浓度DOM在VUV/UV辐照下可能产生的活性物种抵消了其对VUV/UV辐射的蔽光效应,但高浓度DOM对HO·的清除作用仍使其对ATZ降解产生了显著的抑制。在所有考察的水质条件下,ATZ降解所需的单位能耗EEO介于0.61~1.56 kWh·m-3。Abstract: In order to explore the impact of complex water matrices on VUV/UV advanced oxidation efficiency under continuous flow mode, the degradation of atrazine (ATZ) in water with additional chloride (Cl–), bicarbonate (HCO3–), nitrate (NO3–) and dissolved organic matter (DOM) at different concentrations were investigated in a flow-through VUV/UV reactor. The results showed that all these water constituents had some inhibition effects on ATZ degradation. The ATZ removal decreased from 57% in deionized water to 33%, 29%, 40% and 35% under the largest investigated matrix concentrations at an irradiation time of 30 s. These inhibition extents were somewhat different from those reported in batch reactors in literatures. The reduction of ATZ removal could be ascribed to following reasons. On the one hand, all the three anions have a certain shielding effect on VUV irradiation, following the order: NO3– > Cl– > HCO3–. On the other hand, reactive species under VUV irradiation, such as Cl· and Cl2•– produced from chloride, NO2· and HO· formed by nitrate, could supplement the concentration of oxidative radicals in water and contribute to ATZ degradation, which resulted in a lower reduction of ATZ degradation contributed by VUV than the proportion of VUV photons absorbed by the anions. In contrast, the strong scavenging of HO· by HCO3– led to a significant reduction of ATZ degradation. The reactive species generated from DOM at low contents could counteract the shielding effect, while DOM at high concentration still had a prominent inhibition on ATZ degradation due to its remarkable scavenging of HO·. The specific energy consumption under the investigated water matrices ranged between 0.61~1.56 kWh·m−3.
-

-
表 1 过流式VUV/UV反应器中ATZ在不同水质条件下降解的能耗EEO
Table 1. The energy consumption of ATZ degradation under different matrices in the flow-through VUV/UV reactor
背景物质 浓度 kapp/s-1 EEO/(kWh·m-3) Cl– 1.0 mmol·L-1 0.021 5 0.83 2.5 mmol·L-1 0.017 7 1.01 5.0 mmol·L-1 0.015 0 1.19 HCO3– 1.0 mmol·L-1 >0.024 0 0.75 2.5 mmol·L-1 0.014 8 1.21 5.0 mmol·L-1 0.011 5 1.56 NO3– 10.0 mg·L-1 0.021 1 0.85 20.0 mg·L-1 0.019 0 0.94 DOM 1.0 mg·L-1 0.027 6 0.65 5.0 mg·L-1 0.018 7 0.96 10.0 mg·L-1 0.014 8 1.21 -
[1] 胡晋博, 李梦凯, 蔡恒文, 等. 不同光源UV/H2O2工艺降解四环素动力学[J]. 环境工程学报, 2021, 15(8): 2618-2626. doi: 10.12030/j.cjee.202012105 [2] 詹露梦, 李文涛, 李梦凯, 等. 过流式UV/H2O2反应器中阿特拉津降解动力学的测定及模拟评估[J]. 环境工程学报, 2021, 15(3): 982-991. doi: 10.12030/j.cjee.202008067 [3] 邵婉婷, 王文龙, 杜烨, 等. 双波长紫外线(VUV/UV)对有机污染物强化去除特性与原理[J]. 环境科学研究, 2021, 34(6): 1397-1406. doi: 10.13198/j.issn.1001-6929.2020.11.28 [4] FURATIAN L, MOHSENI M. Influence of major anions on the 185 nm advanced oxidation process - Sulphate, bicarbonate, and chloride[J]. Chemosphere, 2018, 201: 503-510. doi: 10.1016/j.chemosphere.2018.02.160 [5] WEEKS J L, MEABURN G, GORDON S. Absorption coefficients of liquid water and aqueous solutions in far ultraviolet[J]. Radiation Research, 1963, 19(3): 559-567. doi: 10.2307/3571475 [6] YE B, LIU Z Y, ZHU X Q, et al. Degradation of atrazine (ATZ) by ammonia/chlorine synergistic oxidation process[J]. Chemical Engineering Journal, 2021, 415(14): 128841. [7] DE LAAT J, BERGER P, POINOT T, et al. Modeling the oxidation of atrazine by H2O2/UV. Estimation of kinetic parameters[J]. Ozone-Science & Engineering, 1997, 19(5): 395-408. [8] JAYSON G G, PARSONS B J, SWALLOW A J. Some simple, highly reactive, inorganic chlorine derivatives in aqueous-solution - their formation using pulses of radiation and their role in mechanism of fricke dosimeter[J]. Journal of the Chemical Society-Faraday Transactions, 1973, 1(9): 1597-1607. [9] ANASTASIO C, MATTHEW B M. A chemical probe technique for the determination of reactive halogen species in aqueous solution: Part 2 - chloride solutions and mixed bromide/chloride solutions[J]. Atmospheric Chemistry and Physics, 2006, 6: 2439-2451. doi: 10.5194/acp-6-2439-2006 [10] GUAN Y H, MA J, LI X C, et al. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system[J]. Environmental Science & Technology, 2011, 45(21): 9308-9314. [11] LIAO C H, KANG S F, WU F A. Hydroxyl radical scavenging role of chloride and bicarbonate ions in the H2O2/UV process[J]. Chemosphere, 2001, 44(5): 1193-1200. doi: 10.1016/S0045-6535(00)00278-2 [12] GREBEL J E, PIGNATELLO J J, MITCH W A. Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline waters[J]. Environmental Science & Technology, 2010, 44(17): 6822-6828. [13] YANG L X, LI M K, LI W T, et al. Bench- and pilot-scale studies on the removal of pesticides from water by VUV/UV process[J]. Chemical Engineering Journal, 2018, 342: 155-162. doi: 10.1016/j.cej.2018.02.075 [14] CHEN Y J, YE J S, CHEN Y, et al. Degradation kinetics, mechanism and toxicology of tris(2-chloroethyl) phosphate with 185 nm vacuum ultraviolet[J]. Chemical Engineering Journal, 2019, 356: 98-106. doi: 10.1016/j.cej.2018.09.007 [15] LONG L C, BU Y A, CHEN B Y, et al. Removal of urea from swimming pool water by UV/VUV: The roles of additives, mechanisms, influencing factors, and reaction products[J]. Water Research, 2019, 161: 89-97. doi: 10.1016/j.watres.2019.05.098 [16] CANONICA S, KOHN T, MAC M, et al. Photosensitizer method to determine rate constants for the reaction of carbonate radical with organic compounds[J]. Environmental Science & Technology, 2005, 39(23): 9182-9188. [17] XIAO Y J, ZHANG L F, ZHANG W, et al. Comparative evaluation of iodoacids removal by UV/persulfate and UV/H2O2 processes[J]. Water Research, 2016, 102: 629-639. doi: 10.1016/j.watres.2016.07.004 [18] WOJNAROVITS L, TOTH T, TAKACS E, et al. Rate constants of carbonate radical anion reactions with molecules of environmental interest in aqueous solution: A review[J]. Science of the Total Environment, 2020, 717: 137219. doi: 10.1016/j.scitotenv.2020.137219 [19] SUN P Z, PAVLOSTATHIS S G, HUANG C H. Photodegradation of veterinary ionophore antibiotics under UV and solar irradiation[J]. Environmental Science & Technology, 2014, 48(22): 13188-13196. [20] WANG Y F, RODDICK F A, FAN L H. Direct and indirect photolysis of seven micropollutants in secondary effluent from a wastewater lagoon[J]. Chemosphere, 2017, 185: 297-308. doi: 10.1016/j.chemosphere.2017.06.122 [21] HAN M Q, MOHSENI M. Impact of organic and inorganic carbon on the formation of nitrite during the VUV photolysis of nitrate containing water[J]. Water Research, 2019, 168: 115169. [22] SERRANO M A, MOHSENI M. Temperature dependence of the absorbance of 185 nm photons by water and commonly occurring solutes and its influence on the VUV advanced oxidation process[J]. Environmental Science:Water Research & Technology, 2018, 4(9): 1303-1309. [23] LESTER Y, SHARPLESS C M, MAMANE H, et al. Production of photooxidants by dissolved organic matter during UV water treatment[J]. Environmental Science & Technology, 2013, 47(20): 11726-11733. [24] MAIZEL A C, REMUCAL C K. Molecular composition and photochemical reactivity of size-fractionated dissolved organic matter[J]. Environmental Science & Technology, 2017, 51(4): 2113-2123. [25] DALRYMPLE R M, CARFAGNO A K, SHARPLESS C M. Correlations between dissolved organic matter optical properties and quantum yields of singlet oxygen and hydrogen peroxide[J]. Environmental Science & Technology, 2010, 44(15): 5824-5829. [26] WENK J, von GUNTEN U, CANONICA S. Effect of dissolved organic matter on the transformation of contaminants induced by excited triplet states and the hydroxyl radical[J]. Environmental Science & Technology, 2011, 45(4): 1334-1340. [27] GOLDSTONE J V, PULLIN M J, BERTILSSON S, et al. Reactions of hydroxyl radical with humic substances: bleaching, mineralization, and production of bioavailable carbon substrates[J]. Environmental Science & Technology, 2002, 36: 362-372. [28] WESTERHOFF P, MEZYK S P, COOPER W J, et al. Electron pulse radiolysis determination of hydroxyl radical rate constants with suwannee river fulvic acid and other dissolved organic matter isolates[J]. Environmental Science & Technology, 2007, 41(13): 4640-4646. -



 下载:
下载: