-
温室效应会引起全球气候变暖和极端高温天气。我国交通运输温室气体排放占总排放的9%~10%,道路车辆贡献了70%~80%的温室气体排放[1-4];而欧盟与美国交通运输温室气体排放约占27.34%与34.95%[5]。随着化石燃料的日益紧张,重型燃气车成为越来越多道路车辆运输行业的选择之一。世界各国制定一系列措施控制温室气体的排放。当前执行的重型国六及欧六E阶段排放标准规定了更为严格的各种污染物排放限值,其中与五阶段相比温室气体CH4的排放限值严格了近55%,为500 mg·kWh−1。N2O是第三大温室气体 (前面为CO2、CH4) ,可在空气中长期存留并传输至大气平流层进而破坏臭氧层,其单分子增温潜势是CH4的14倍、CO2的298倍。当前轻型车国六标准 (GB 18352-2016) 限值为20~30 mg·km−1。
随着重型车市场保有量的增加,N2O排放已引起越来越多关注。欧盟委员会在即将发布的欧七排放标准提案中,计划将N2O列入监管之中。在国六标准下,现行技术中部分催化器可能排放较高浓度的N2O。故国内相关学者预测未来的排放法规可能将N2O与CO2、CH4等温室气体共同加以限制[6]。燃气机原机排放中的N2O浓度很低,主要为三元催化转化器 (three-way catalytic converter,TWC) 中的副反应产生[7]。唐飞等[8]基于铑基催化器研究发现N2O生成存在低温与高温2种反应路径。在低温下,通过CO还原NO反应生成N2O,而NH3通过H2还原NO反应生成N2O。从微观层面分析,汪永等[9]在无O2条件下,利用傅里叶变换红外光谱研究了钯催化剂上NH3与NO的反应,发现N2O生成与反应温度相关,在低温时通过HON中间产物反应生成,而在高温下,通过N•和 NO结合而生成。CANT等[10]通过模拟汽车尾气研究了铂、钯、铑基单贵金属在CeO2/Al2O3 2种载体催化剂中N2O生成与温度关系,发现铂基催化剂与铑基催化剂N2O生成温度较窄,分别约为300 ℃与250 ℃,而在钯基催化剂中N2O在温度约200 ℃时及300~500 ℃ 2个温度范围内产生。欧盟委员会研究中心成员用整车预测排放模型系统 (portable emission measurement system,PEMS) 测试方法对重型车的排放进行研究,发现N2O与NH3呈现良好相关性,其排放特性与环境温度相关,不同车辆类型工况其排放特性也存在差异[11-13]。
当前燃气机尾气主要通过废气再循环 (exhaust gas recirculation,EGR) 系统与铂/铑/钯基三元催化器进行净化处理。目前,国内外对N2O的研究大多集中在形成机理,亟需了解现有技术下的排放水平以进行控制研究。而国家对燃气成分尚未制定市售统一标准,不同组分污染物排放水平及衍生副产物N2O的排放量尚未见有效数据支持。本研究在考虑市售液态、气态不同组分燃气特点的基础上,通过搭建台架测试平台重点研究当前排放水平重型燃气机在WHTC工况下的N2O排放特性,包括冷态与热态不同启动条件下的排放情况,以期对燃气机排放N2O的源头控制提供参考。
-
以采用“当量比燃烧+增压中冷+EGR”直列六缸10.52 L排量的某国六车用燃气机作为研究对象,燃气机额定功率是309 kW、额定转速为1900 r·min−1,最大扭矩在转速区间800~1400 r·min−1为1 920 N·m,发动机采用进气歧管燃料喷射方式。后处理选用以堇青石为载体,铂、铑、钯 3种贵金属按照比例3∶1∶6进行涂覆的三元催化器 (TWC) ,并在发动机与TWC间排气管路包裹10 mm以上厚度保温材料。
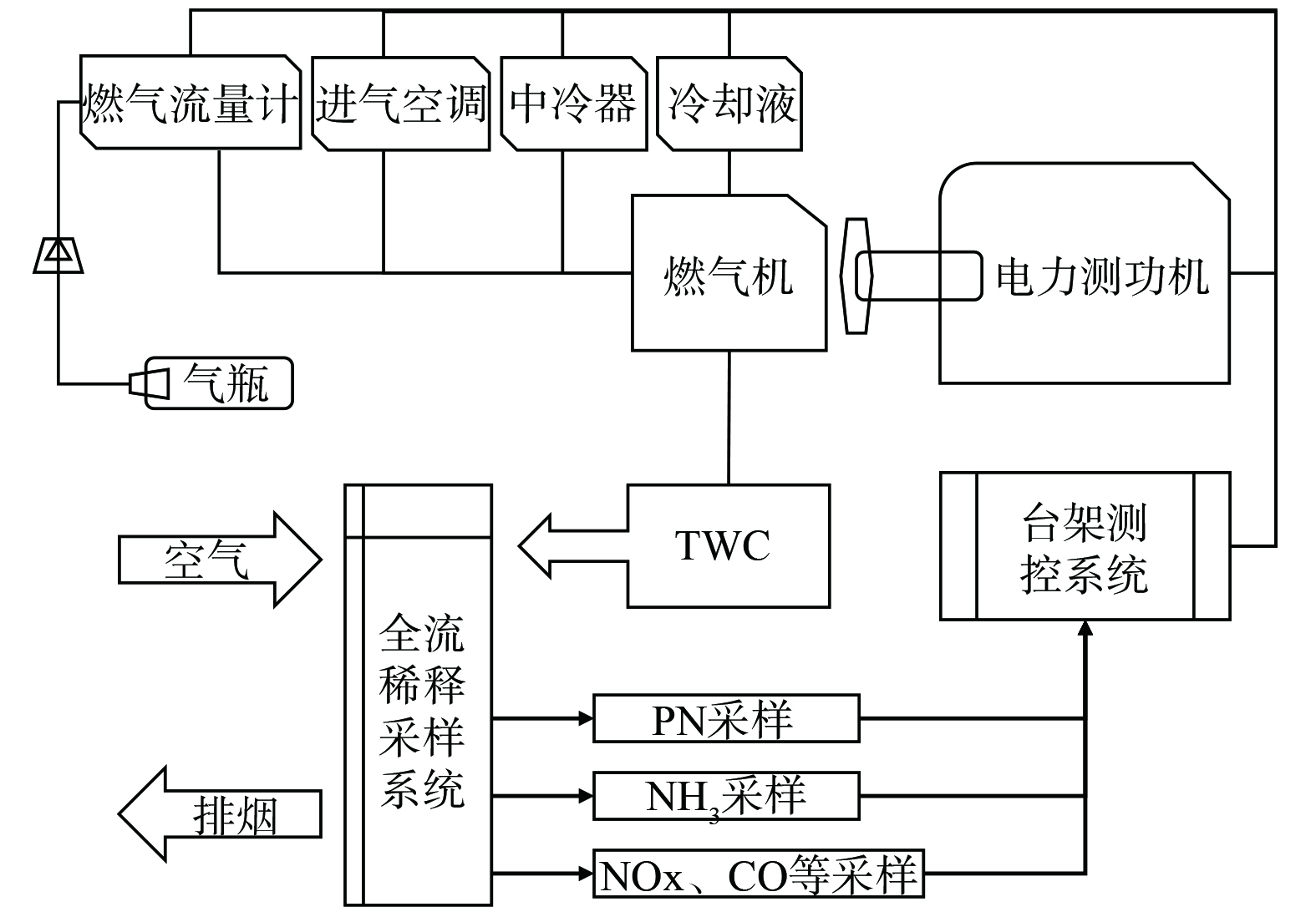
燃气通过压力调节器进行减压后经燃气流量计送入燃气机,使用AVL INDY S50-4/3001-1BV-1电力测功机、全流稀释采样系统、AVL AMAi60排放分析系统、AVL FTIR i60傅立叶变换红外吸收光谱仪、Toceil-CMF025燃气流量计、SensyFlow P空气流量计、SESAM i60 FT氨气分析仪、全室闭式进气空调系统等设备组成的测试系统,所搭建燃气机实验台架示意如图1所示。
-
进行台架测试前按照设计参数先对发动机进行边界条件设定及性能校核,设置发动机进气温度为24.5 ℃,进气相对湿度50%,空调压力100.2 kPa,发动机冷却水最高温度为95 ℃,中冷后最高出口温度为55 ℃,额定点排气背压为15 kPa。
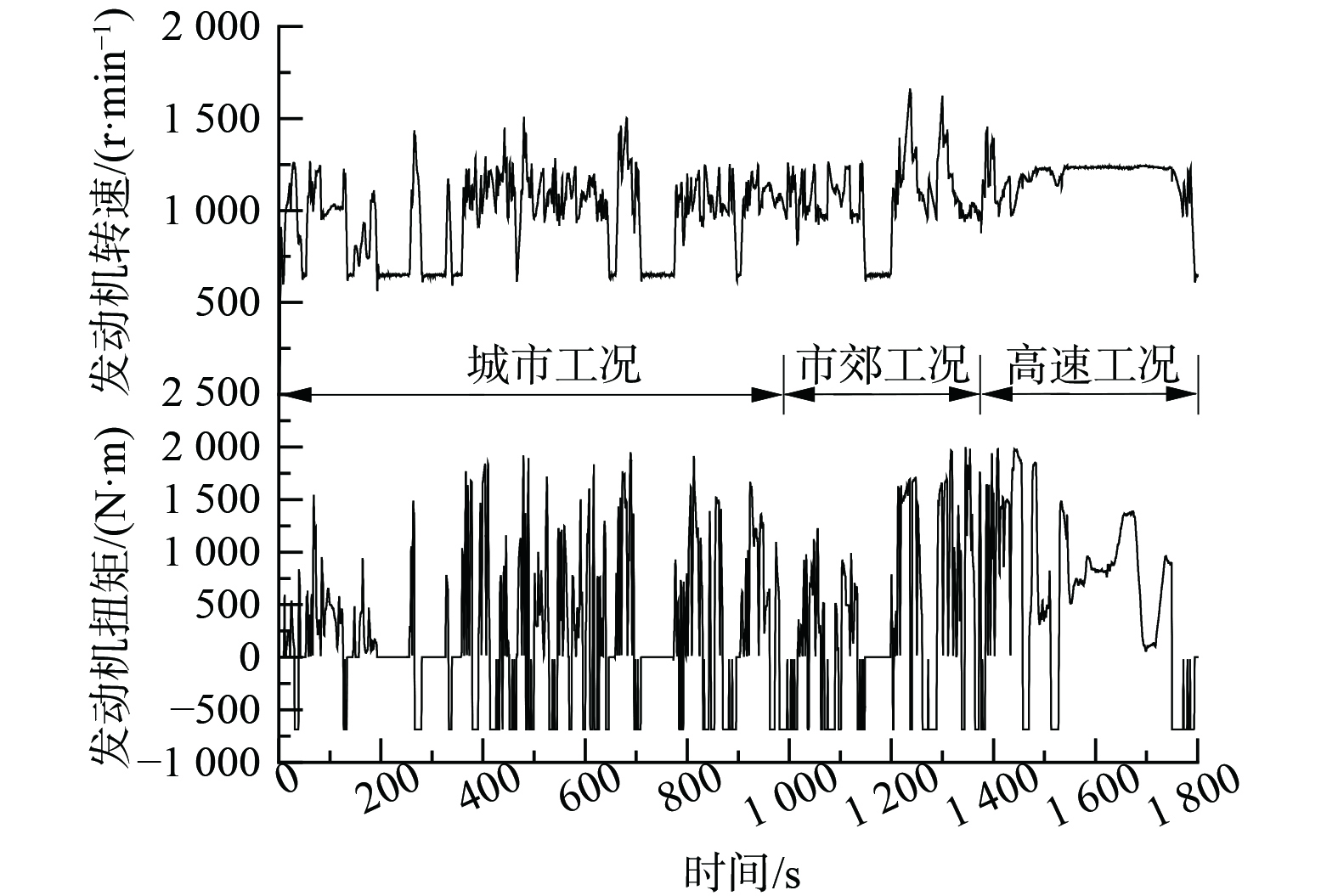
为减少外界因素的影响,选用GB 17691-2018 (以下简称“标准”) 重型柴油车污染物排放限值及测量方法全球统一瞬态实验循环 (World Harmonized Transient Cycle,WHTC) 进行实验。在市售液化天然气 (liquefied natural gas,LNG) 、市售压缩天然气 (compressed natural gas,CNG) 、低热值G25 3种不同燃气组分下进行冷热态排放测试[14],WHTC瞬态循环由1 800个逐秒变化的工况点组成 (图2) ,覆盖城市工况、市郊工况与高速3种统计路况,包含冷态、热态。冷热态WHTC之间发动机热浸10 min。更换燃气后进行1次WHTC自学习,待发动机在室温 (20~30 ℃) 下自然冷却6 h左右。发动机水温及TWC温度满足冷却要求后进行下一种燃气的冷热态排放。按照标准规定气态污染物浓度数据记录频率至少为2 Hz,其他数据记录频率至少为1 Hz,本次记录频率统一设置为10 Hz[14]。燃气主要组分见表1。
为确保结果的有效性,对实验室测试条件大气因子进行计算,满足0.93≤fa≤1.07则认为实验结果有效。计算公式如式 (1) 。
式中:Ps为干空气压力,kPa;Ta为发动机进气口处空气的绝对温度,K。
为控制实际循环与基准循环时间迟滞带来的偏差影响,使用最小二乘法对于WHTC循环下,转速、扭矩和功率进行基准值与实际值之间的线性回归分析,并对每条回归线计算y基于x估算值标准偏差 (standard error of estimate,SEE) 及相关系数r2,计算公式如式 (2) ,结果见表2。
式中:y为转速、扭矩、功率在测功机的实测值;a1为线性回归线的斜率;x为转速、扭矩、功率在按照WHTC循环计算的基准值;a0为线性回归线的截距;
-
发动机N2O、NH3的内部尾气净化是十分复杂的过程。燃气发动机有别于柴油机,国六普遍采用当量比燃烧,其尾气排放含有大量水蒸气、CH4。尾气组成对形成N2O过程中有重要影响。前期已有相关研究人员对N2O的生成机理进行探究,其生成过程主要涉及式 (7) 和 (8) 。其中,式 (7) 为第一反应路径,各种污染物在不同工况点氧化还原反应存在不同优先级,且N2O与NH3反应过程存在竞争关系[15-17]。N2O/NH3生成路径见以下公式。
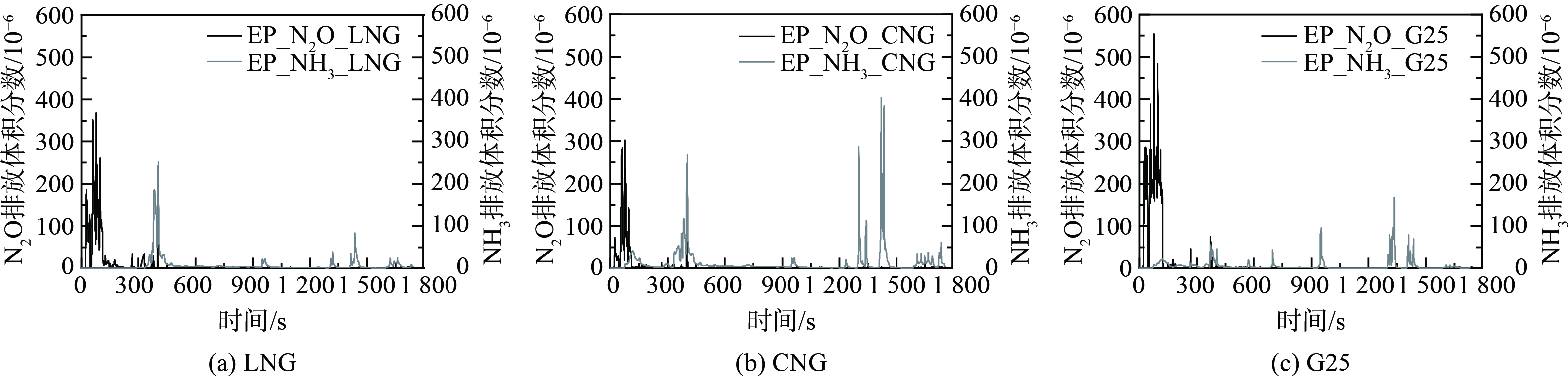
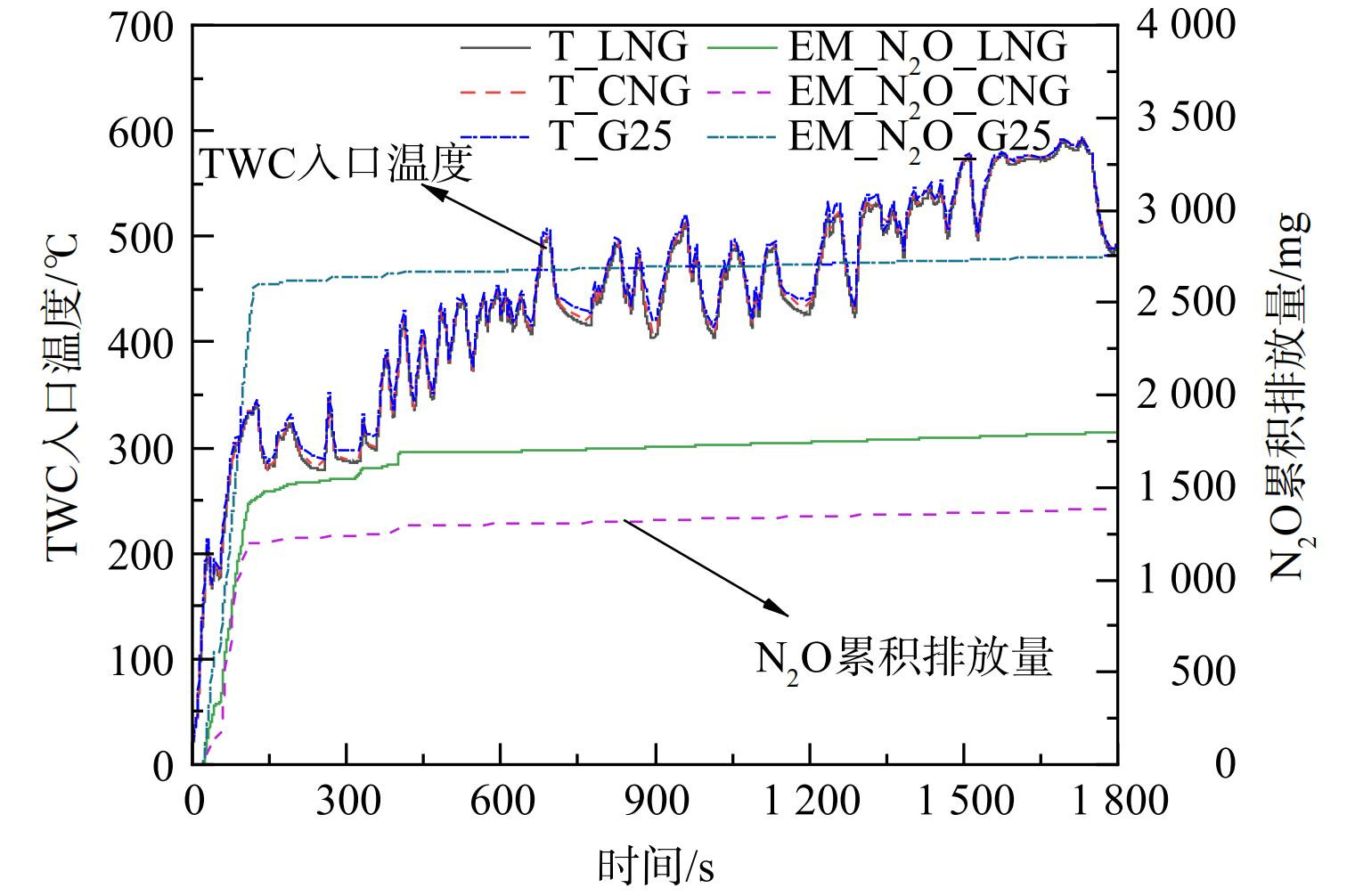
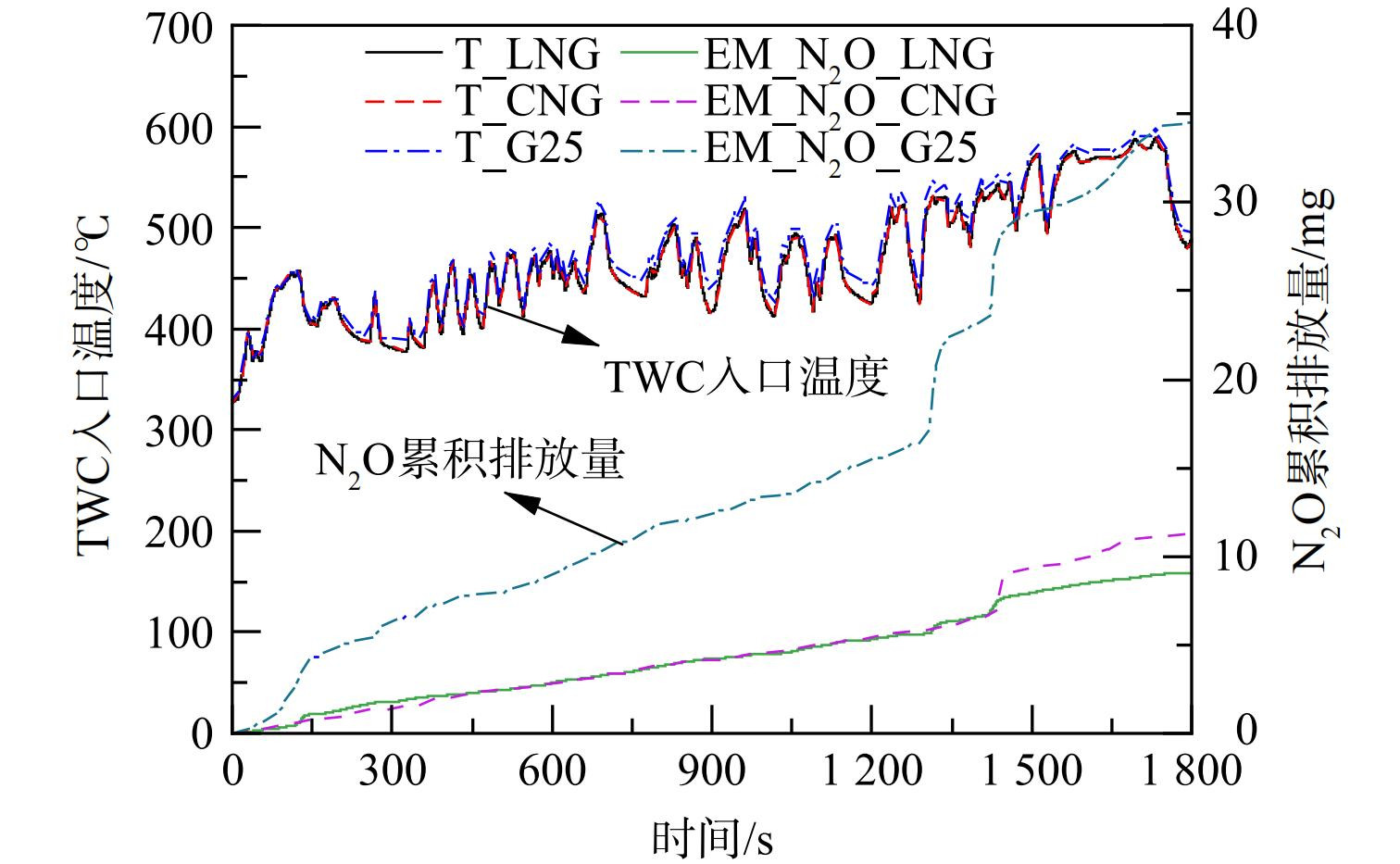
图3和图4分别为在冷态WHTC工况下,LNG、CNG热值、G25热值3种不同燃气组分条件下的N2O、NH3瞬态分布、TWC入口温度及N2O累积排放量。从瞬态排放曲线可知,在冷态全过程WHTC工况中,3种燃气N2O、NH3排放特性基本一致,2种污染物在启动初期及某些工况点出现高浓度排放。在冷态WHTC工况下的冷启动初期,发动机燃气燃烧不充分,此时的尾气中CO体积分数升高,N2O主要按照反应式 (7) 生成。在发动机冷态启动20 s后,N2O随着三元催化器进气温度的提高瞬速大量生成,在76 s之后,发动机转速扭矩瞬速降低,峰值与进气温度同时到达阶段峰值。其中,LNG峰值为368×10−6,CNG热值峰值为301×10−6,G25热值峰值为547×10−6。随着温度震荡升高,在140 s之后,N2O体积分数急剧降低,在300 s之后偶有波动性弱峰出现。其中,LNG与CNG热值最大峰值均出现401 s左右,分别为226×10−6、112×10−6;G25热值峰值出现在372 s附近,峰值高度63×10−6;同时检测到NH3出现不同程度峰值。由于这一阶段后处理入口温度为200~280 ℃,而水煤气与蒸汽重整生成H2往往在300 ℃以上,因此该阶段主要反应是式 (9) 中异氰酸酯基团的水解。这通常被认为是NH3形成的第二路径[18-19]。在450 s以后几乎无明显N2O生成,总量增长放缓,生成明显受到抑制,NH3选择性生成快速增加。在900 s之后,TWC入口温度稳定在400 ℃以上。OH等[18]研究NH3选择性时发现,在温度为400~500 ℃时,达到100%,NH3分别在970 s、1 340 s、1 440 s左右出现高度差异峰值,峰值出现时间基本一致。该阶段反应式 (5) 、 (6) 是主要反应,其中CNG燃气NH3体积分数最大为404×10−6,峰值出现时间段基本处于图2发动机急加速加负荷阶段,而高负荷、高温及高的缸压会加速H2产生促进NH3生成[9]。冷态WHTC循环累积N2O总量G25>LNG>CNG,且集中出现在城市工况冷启动阶段。
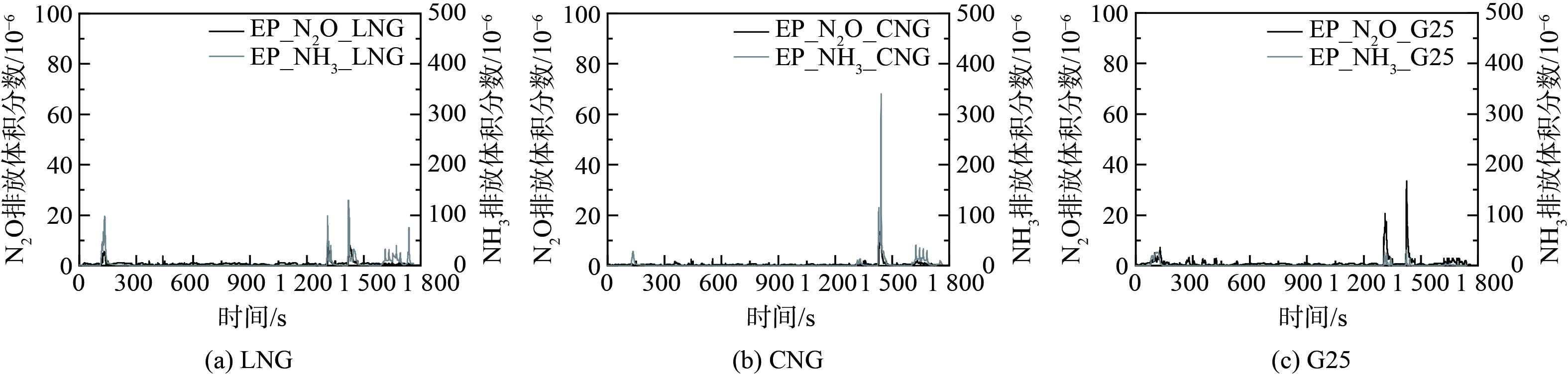
图5和图6为热态WHTC工况下N2O、NH3瞬态分布,TWC入口温度及N2O累积排放量。结果表明,在热态WHTC循环TWC入口起始温度均在325 ℃以上,进入循环后在30 s内短时间升温至400 ℃,之后维持在400 ℃以上。在整个循环过程中,大部分测点检测到的3种燃气N2O排放量均为极少量,较冷态WHTC工况相比峰值点数值小,峰值频次低。除CNG热值燃气出现高于30×10−6的1个峰值外,其他2种燃气有3个低峰值,且与NH3排放峰值出现时刻基本一致。LNG与G25热值在130 s左右出现第一弱峰,体积分数均为6~7×10−6;在1 330 s左右陆续出现第二弱峰,峰值高度分别为7×10−6、21×10−6;在1 440 s左右达到3种燃气产生的N2O体积分数均达到热态最大峰值,分别为8×10−6、13×10−6、34×10−6。热态WHTC工况可认为NH3生成主要是以式 (5) 、 (6) 进行,较冷态在循环工况初期NH3排放量减少。在1 200 s之后陆续出现检测到较明显的NH3生成,其中CNG燃气最大峰值达349×10−6,G25燃气排放浓度最低。从总量上看,N2O热态排放量约占冷态的1%,3种燃气WHTC循环累积N2O总量高低顺序与冷态一致。
-
不同温度下贵金属活性存在差异且对尾气成分转化效率有较大影响,贵金属钯能有效转化废气中CO、HC及NOx为CO2、H2O与N2,但高温 (500 ℃) 下会产生副产物N2O与NH3,且更有利于NH3生成[17, 20- 21];贵金属铂与铑在250~400 ℃下形成N2O。在相同条件下,贵金属铑生成浓度最高;贵金属铂活性点在高温下会被完全还原,NO完全进行解离,纯铂催化剂高温下N2O较难生成[7, 22]。因此,合理配比贵金属比例可有效对污染物排放平衡控制,以满足法规限值要求。
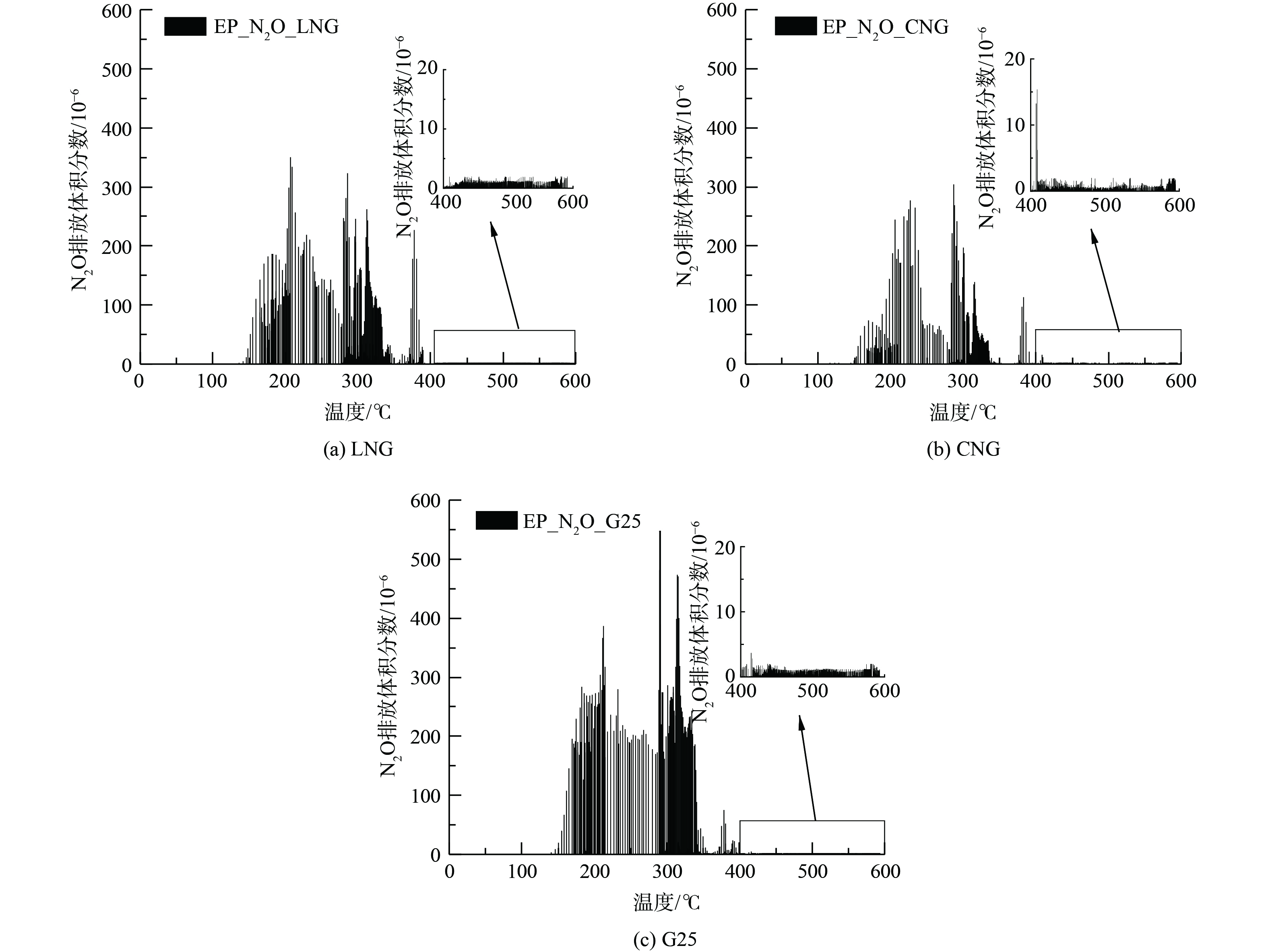
图7为不同燃气WHTC冷态循环N2O随温度分布情况。N2O与温度有密切关联,3种燃气主要N2O生成趋势一致。在冷态WHTC循环在160 ℃以下基本无N2O生成.从160 ℃起至220 ℃,N2O体积分数随温度升高瞬速产生到达第一峰值点,LNG、CNG、G25最高体积分数分别为349×10−6、276×10−6、385×10−6;随温度继续升高至约280 ℃,N2O体积分数缓慢降低但仍保持较高释放,平均排放体积分数均在30×10−6以上。当温度为280~350 ℃时,N2O生成存在多个峰值点,最高值出现在 (285±5 ) ℃,其中G25排放体积分数最高为547×10−6。这可能与贵金属铂与铑催化作用存在较大关系。在350~380 ℃温度区间N2O生成急剧减少。超过400 ℃后,随着温度继续升高N2O生成明显受到抑制。此时的N2O体积分数基本在5×10−6以下,贵金属钯在此过程中可能占主导作用。
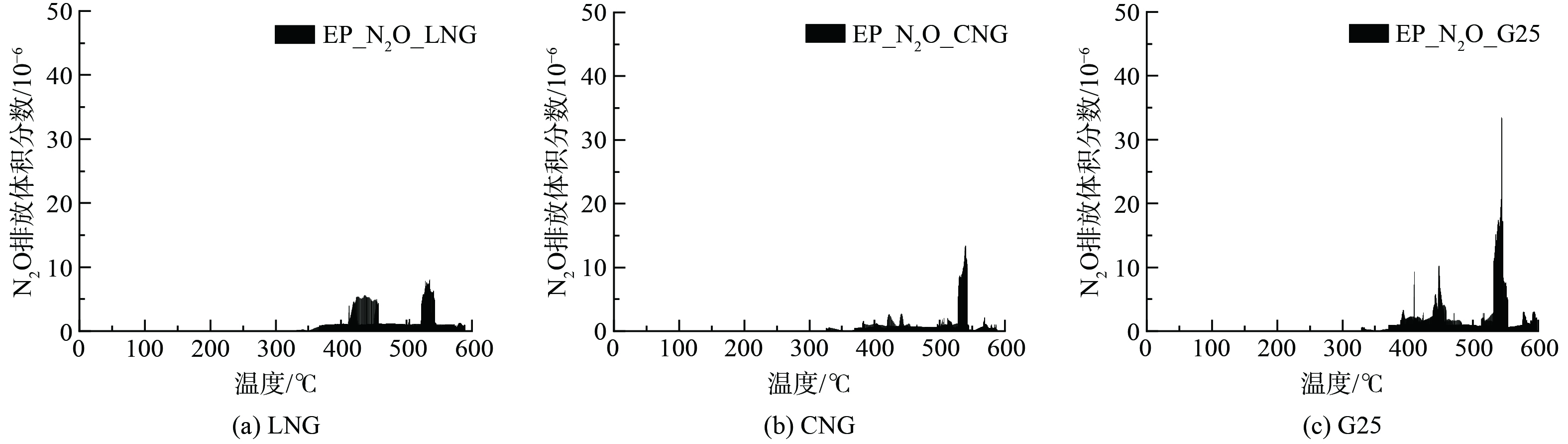
图8为不同燃气WHTC热态循环N2O随温度分布情况。热态WHTC循环起始温度高于325 ℃,整个热态范围N2O生成量较冷态相比明显减少,与冷态高温区相当量级。相比冷态,在热态400 ℃以下无明显N2O生成,结合图4发现热态TWC入口温度在30 s左右升至400 ℃。由于发动机是热机状态发动机燃烧工况稳定,因此N2O生成条件受抑制。在550 ℃有较低体积分数的N2O弱峰,且该温度范围基本对应图2、图4中WHTC工况高速扭矩工况。该工况时间段扭矩及排温波动相对较小,在高温下发生水煤气反应生成大量H2,反应式 (8) 可能主要在这一温度段进行。这也与BALL等[23]的发现一致。
-
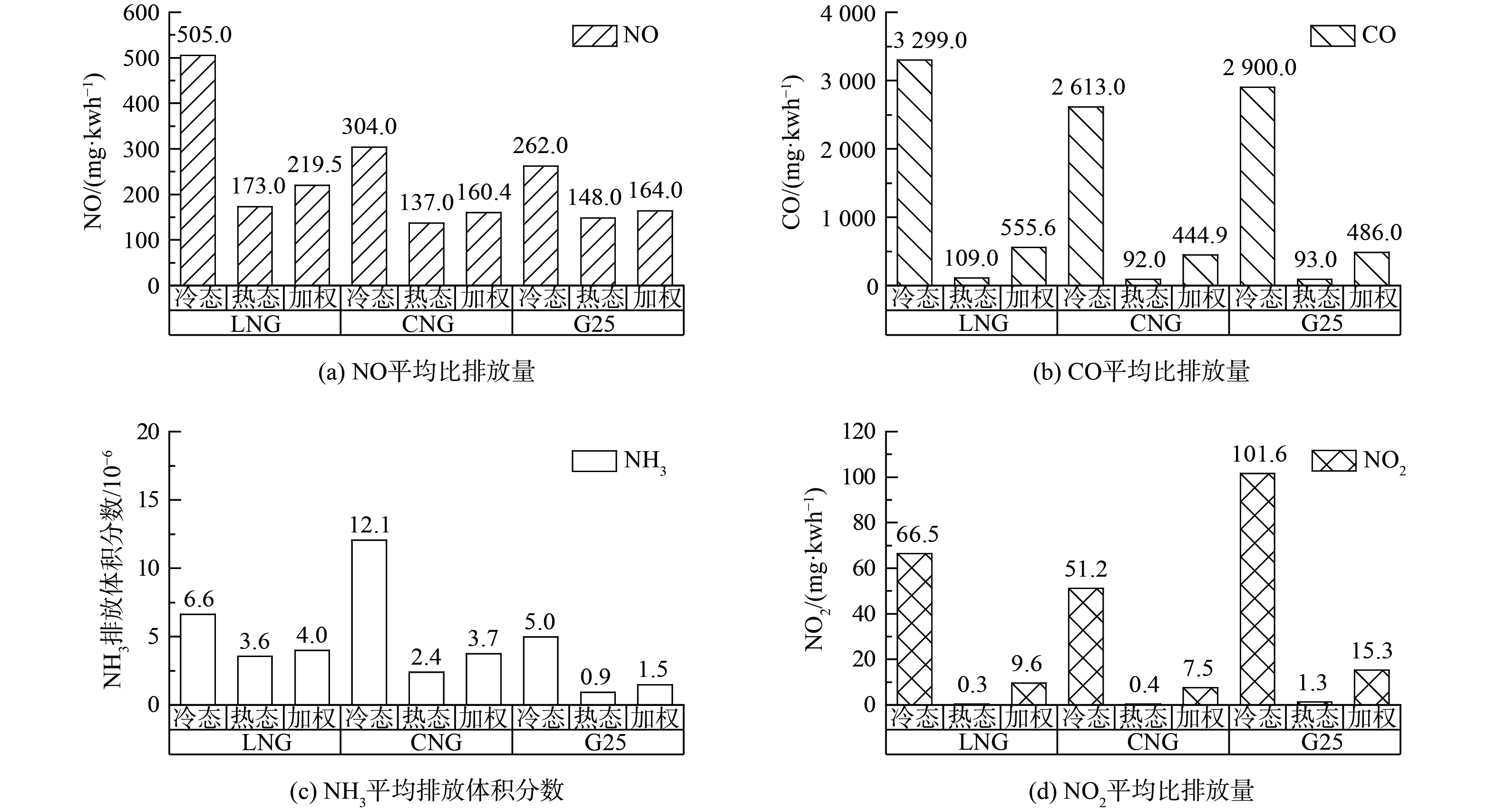
图9为瞬态WHTC工况各点加权平均获得各燃气NO、CO、N2O比排放量及NH3的平均排放体积分数。其中,NO、CO是参与N2O、NH3形成的关键污染物。冷态各污染物比排放量均高于热态。其中,NO平均比排放量、NH3平均排放体积分数的冷态与热态数值基本处于同一数量级,冷态结果高于热态2~5倍之间。而CO、N2O冷态比排放量高于热态30~100倍。N2O冷热态加权比排放量分别为9.6 mg·kWh−1、7.5 mg·kWh−1、15.3 mg·kWh−1;NH3的平均排放体积分数冷热态加权值分别为4.0×10−6、5.0×10−6、1.5×10−6。总体上,N2O平均比排放量G25>LNG>CNG,NH3平均排放体积分数CNG>LNG>G25,两者趋势相反。而污染物NO、CO比排放量高低与NH3、N2O无明显规律。
该发动机具有燃料自适应能力,在满足相同功率扭矩下燃气进气量根据ECU自动调节。表3为各燃气消耗量,相同燃气冷态消耗高于热态。这与燃烧初期冷态不完全燃烧,机体零部件润滑不充分,接触面转动阻力大有一定关系。各燃气消耗量G25>CNG>LNG,G25循环耗气量较LNG、CNG分别高23.8%、19.9%。从表1燃气组分看,G25、CNG含有较高体积分数 (13.75%、0.95%) 不可燃N2,这可能是高于LNG燃气量的主要原因之一。通过比对发现,不同燃气之间加权平均比排放污染物数值存在差异,高N2比例的低热值G25有利于降低NH3排放,但N2O平均比排放量较高,这说明燃气组分差异对N2O存在一定影响。
-
1) 基于WHTC循环工况使用3种不同组分燃气 (市售LNG、市售CNG、G25) 进行了相关实验后发现,N2O主要生成于WHTC城市工况发动机冷启动阶段,市郊工况及城市工况基本无明显N2O生成。
2) 在一定工况下,N2O 与NH3的生成存在竞争关系,后处理进气温度范围为160~400 ℃,急速加负荷工况、高于400 ℃时NH3选择性生成量增加,高温会抑制N2O排放。
3) TWC催化转化器中贵金属种类及比例对HC、CO、NO等反应生成N2O、NH3有重要影响,可通过配比优化贵金属比例平衡不同污染物气体排放浓度。
4) N2O与NH3在不同燃气比排放呈现浓度相反趋势,G25冷、热态排放均高于LNG、CNG,低热值气有利于抑制NH3生成,但不利于N2O排放控制。
重型燃气机铂/铑/钯基三元催化器N2O排放特性
N2O emission characteristics of Pt/Rh/Pd three-way catalyst for heavy duty gas engine
-
摘要: 以采用当量比燃烧+EGR+铂/铑/钯基TWC技术路线、且满足国六排放标准的重型燃气机为研究对象,基于全球统一瞬态实验循环 (WHTC) 工况对燃气机瞬态条件下不同燃气组分、排气温度、尾气组分的N2O排放特性进行定量研究。结果表明:N2O排放主要集中在冷态WHTC城市工况前140 s,热态WHTC总排放量约是冷态的1%;燃料组分对N2O生成有一定影响,含N2高的低热值G25燃气冷、热态N2O排放浓度均高于LNG、CNG,加权比排放量分别为15.3 mg·kWh−1、9.6 mg·kWh−1、7.5 mg·kWh−1;N2O生成与温度密切相关,主要生成区间为160~350 ℃,高温会抑制N2O生成;N2O与NH3的生成存在竞争关系,高于400 ℃时NH3生成量增加。本研究可为重型燃气机污染物N2O的源头控制提供参考。
-
关键词:
- 重型燃气机 /
- 三元催化转化器(TWC) /
- N2O /
- 排放特性
Abstract: Taking a heavy-duty gas engine using equivalent ratio combustion EGR Pt/Rh/Pd three-way Catalyst and meeting the National VI emission standards as the research object, this study quantitatively investigated the N2O emission characteristics of the gas engine under transient conditions with different gas compositions, exhaust temperatures, and tail gas compositions based on the global transient cycle WHTC. The results showed that N2O emissions were mainly concentrated in the first 140 seconds of the cold WHTC urban cycle, and the total N2O emissions of the hot WHTC are about 1% of the cold cycle. The fuel composition had a certain influence on N2O generation. The cold and hot N2O emission concentrations of G25 gas with high N2 content were higher than those of LNG and CNG, and the weighted specific emission was 15.3 mg·kWh−1, 9.6 mg·kWh−1, and 7.5 mg·kWh−1, respectively. N2O generation was closely related to temperature, and the main generation interval was between 160~350 ℃. High temperature inhibited N2O generation. There was a competitive relationship between N2O and NH3 generation, and the NH3 generation increased when the temperature was higher than 400 ℃. This study can provide data reference for the source control of N2O pollution in heavy-duty gas engine. -

-
表 1 燃气主要组分表
Table 1. Main components of gas
燃料组分 甲烷 乙烷 氮气 硫含量/(mg·m-³) LNG 99.83% 0.04% 0.11% <0.10 CNG 92.99% 3.97% 0.95% <1.00 G25 86.24% <0.01% 13.75% <10.00 表 2 大气因子及回归线偏差校对参数
Table 2. Calibration parameters of atmospheric factor and regression line deviation
校对项 WHTC冷态 WHTC热态 a1 a0 SEE r2 fa a1 a0 SEE r2 fa LNG 转速 0.981 19.393 19.321 0.983 1.003 0.983 18.474 16.971 0.987 1.006 扭矩 0.973 3.276 139.475 0.934 0.971 4.761 156.527 0.916 功率 0.985 0.457 18.187 0.926 0.984 0.6 19.644 0.915 CNG 转速 0.979 22.855 19.123 0.983 1.005 0.98 21.301 18.552 0.984 1.009 扭矩 0.974 3.15 138.98 0.934 0.97 5.293 152.063 0.92 功率 0.988 0.212 18.142 0.927 0.985 0.546 19.2 0.918 G25 转速 0.983 18.397 17.726 0.986 0.998 0.978 24.11 20.114 0.982 1.003 扭矩 0.971 4.413 147.031 0.924 0.953 19.859 147.193 0.921 功率 0.982 0.913 19.205 0.915 0.969 2.374 18.413 0.922 注:3种燃气WHTC循环相关偏差均满标准表C.2要求[14]。 表 3 燃气消耗量对比
Table 3. Comparison of gas consumption
kg·h−1 工况 冷热WHTC 热态WHTC 加权 LNG 11.695 11.49 11.519 CNG 12.172 11.845 11.891 G25 14.552 14.212 14.260 -
[1] 黄志辉, 纪亮, 尹洁, 等. 中国道路交通二氧化碳排放达峰路径研究[J]. 环境科学研究, 2022, 35(2): 385-393. doi: 10.13198/j.issn.1001-6929.2021.11.06 [2] 袁志逸, 李振宇, 康利平, 等. 中国交通部门低碳排放措施和路径研究综述[J]. 气候变化研究进展, 2021, 17(1): 27-35. [3] 刘俊伶, 孙一赫, 王克, 等. 中国交通部门中长期低碳发展路径研究[J]. 气候变化研究进展, 2018, 14(5): 513-521. [4] 生态环境部. 中华人民共和国气候变化第二次两年更新报告[R/OL][2019-07-01]. 2018. https://www.mee.gov.cn/ywgz/ydqhbh/wsqtkz/201907/P020190701765971866571.pdf [5] EEA. Greenhouse Gas Emissions from Transport in Europe[M]. Brussels: European Environment Agency, 2021. [6] 陈婷, 倪红, 谷雪景, 等. 中国移动源下阶段排放法规综述和分析[J]. 内燃机工程, 2018, 39(6): 24-30. doi: 10.13949/j.cnki.nrjgc.2018.06.003 [7] 郑婷婷, 王国栋, 顾绍晶, 等. 汽车尾气净化三效催化剂中N2O和NH3的生成及控制研究进展[J]. 化工进展, 2020, 39(6): 2399-2410. [8] 唐飞, 钱叶剑, 孟顺, 等. 当量比燃烧天然气发动机铑基三效催化器次生污染物研究[J]. 内燃机工程, 2021, 42(6): 70-79. doi: 10.13949/j.cnki.nrjgc.2021.06.010 [9] WANG Y, LI Y, WANG Z, et al. Hydrogen formation from methane rich combustion under high pressure and high temperature conditions[J]. International Journal of Hydrogen Energy, 2017, 42(20): 14301-14311. doi: 10.1016/j.ijhydene.2017.04.022 [10] CANT N W, ANGOVE D E, CHAMBERS D C. Nitrous oxide formation during the reaction of simulated exhaust streams over rhodium, platinum and palladium catalysts[J]. Applied Catalysis B Environmental, 1998, 17(1): 63-73. [11] TOMMASO S, ROBERTO G, D. M A, et al. Measuring emissions from a demonstrator heavy-duty diesel vehicle under Real-World Conditions-Moving Forward to Euro VII[J]. Catalysts, 2022, 12(2): 184-184. doi: 10.3390/catal12020184 [12] RICARDO S, ROBERTO G, TOMMASO S, et al. NH3 and N2O real world emissions measurement from a CNG heavy duty vehicle using on-board measurement systems[J]. Applied Sciences, 2021, 11(21): 10055-10055. doi: 10.3390/app112110055 [13] NEVALAINEN P, KINNUNEN N M, KIRVESLAHTI A, et al. Formation of NH3 and N2O in a modern natural gas three-way catalyst designed for heavy-duty vehicles: The effects of simulated exhaust gas composition and ageing[J]. Applied Catalysis A, General, 2018, 552: 30-37. doi: 10.1016/j.apcata.2017.12.017 [14] 生态环境部. GB 17691-2018重型柴油车污染物排放限值及测量方法(中国第六阶段)[S]. 北京: 中国环境出版社, 2018. [15] ODAKA M, KOIKE N, SUZUKI H. Influence of catalyst deactivation on N2O emissions from automobiles[J]. Chemosphere - Global Change Science, 2000, 2(3): 413-423. [16] ZHANG Q, LI M H, SHAO S D, et al. Ammonia emissions of a natural gas engine at the stoichiometric operation with TWC[J]. Applied Thermal Engineering, 2018, 130: 1363-1372. doi: 10.1016/j.applthermaleng.2017.11.098 [17] QIAN Y, WEI X, SUN Y, et al. Investigation of the formation characteristics of N2O and NH3 for stoichiometric natural gas engines with Pd-only catalyst,[J]. Fuel, 2022, 329: 125223-125233. doi: 10.1016/j.fuel.2022.125223 [18] OH S H, TRIPLETT T. Reaction pathways and mechanism for ammonia formation and removal over palladium-based three-way catalysts: Multiple roles of CO[J]. Catalysis Today, 2014, 231: 22-32. doi: 10.1016/j.cattod.2013.11.048 [19] ADAMS E C, SKOGLUNDH M, ELME T, et al. Water–gas-shift assisted ammonia formation over Pd/Ce/alumina[J]. Catalysis today, 2018, 307: 169-174. doi: 10.1016/j.cattod.2017.05.035 [20] 方晶晶,许林军,鲁毅钧,等. 铈改性Al2O3担载Pd催化剂的CO氧化性能研究[J]. 环境工程学报, 2009, 3(5): 947-950. [21] SAILESH N. BEHERA M S. Investigating the potential role of ammonia in ion chemistry of fine particulate matter formation for an urban environment[J]. Science of The Total Environment, 2010, 408(17): 3569-3575. doi: 10.1016/j.scitotenv.2010.04.017 [22] RENÈME Y, DHAINAUT F, GRANGER P. Kinetics of the NO/H2/O2 reactions on natural gas vehicle catalysts—Influence of Rh addition to Pd[J]. Applied Catalysis B Environmental, 2012, 111: 424-432. [23] BALL D, MOSER D, YANG Y, et al. N2O emissions of low emission vehicles[J]. Sae International Journal of Fuels & Lubricants, 2013, 6(2): 450-456. -



 下载:
下载: