-
汞化合物的应用非常广泛,它们广泛应用于化工、制药、冶金、电子仪器、军事等高新技术领域,还可用于制造科学测量仪器(如压力表、温度计等)、电子电器产品、化工产品、催化剂、汞灯、电极等。然而,由于其在工业和制造业中被大量使用,其造成的环境污染越来越严重[1-2]。与其它污染物不同的是,汞不能被生态系统本身的物理、化学或生物手段降解,而是以生物和非生物两种形式在自然界中迁移和积累。由于其毒性极强,易于渗透,难以代谢,在生物体内富集后容易造成长期伤害[3-5]。例如,汞离子可以在人体内长期存在,严重危害中枢神经系统、消化系统和肾脏等。此外,其对呼吸系统,皮肤、血液和眼睛等也有较大危害[6-7]。因此,开发一种能够快速、简洁、准确检测汞离子的方法具有十分重要的意义。
传统的汞离子检测方法主要有原子吸收光谱法(AAS)、原子发射光谱法(AES)、电感耦合等离子质谱法(ICP-MS)、高效液相色谱法(HPLC)、X-射线荧光光谱法(XRF)以及电化学方法等,这些方法具有灵敏度高、特异性好、应用广泛等优点[8-10]。但这些方法往往需要昂贵的设备,并且样品的预处理比较复杂。与传统的检测方法相比,荧光探针法不仅操作简便、检测速度快、特异性强,而且还特别适合于在细胞微环境中进行检测[11-15]。
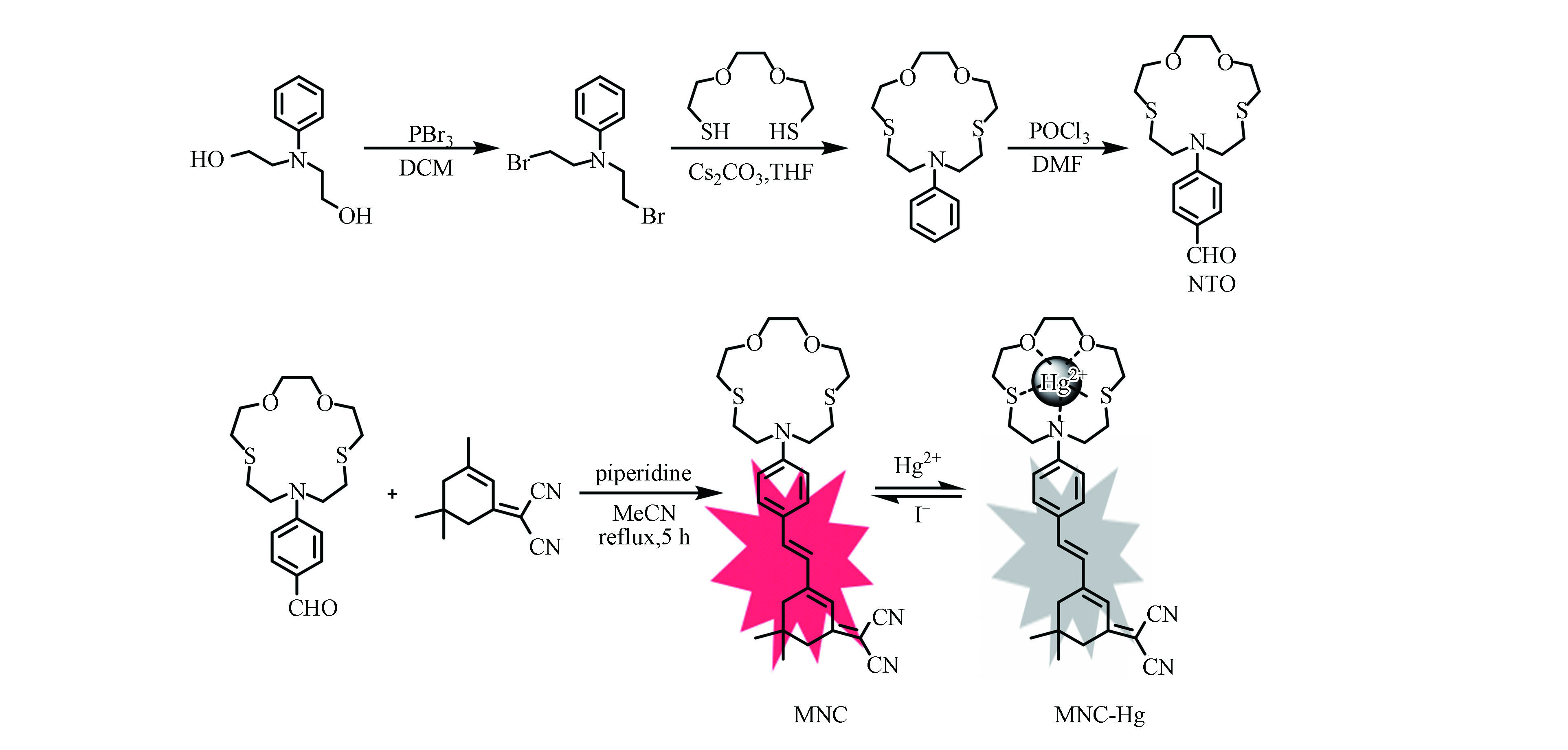
本文以10-苯基-1,4-二氧杂-7,13-二硫杂-10-氮杂环戊烷为汞离子检测单元、(3,5,5-三甲基环己-2-烯亚基)丙二腈为荧光团,构建了一个具有红色荧光信号的比色型汞离子荧光探针,并研究了该探针对汞离子的响应以及其在生物体中的荧光成像应用。
全文HTML
-
除非另有说明,所有实验过程均根据标准Schlenk技术在氮气氛围下进行[16],所有的药品和试剂均购于武汉欣申试化工科技有限公司,无需进一步纯化,溶剂使用时均经脱水处理。目标化合物经核磁氢谱和质谱表征,核磁氢谱经Bruker Mercury Plus 400 MHz核磁共振波谱仪测试完成,并以四甲基硅烷(TMS)作为化学位移值的内标,质谱经Trace 1300-ISQ质谱仪测试完成。
-
将探针(1.57 mg,0.003 mmol)溶于二甲基亚砜(DMSO,3 mL)中,得到储备溶液(10−3 mol·L−1)。金属阳离子Cu2+、Zn2+、Mn2+、Ni2+、Cd2+、Pd2+、Pb2+、Co2+、Hg2+、Na+、K+、Mg2+、Ca2+、Li+、Al3+分别溶解在蒸馏水中以制备浓度为10−2 mol·L−1的溶液。其中紫外-可见吸收光谱是经日立U-3310紫外分光光度计测试得到,荧光光谱是通过CARY ECLIPSE荧光光谱仪测试得到。
-
在Olympus FV1000-IX81共聚焦激光扫描显微镜下获得细胞图像。其中细胞培养方法如下:在添加了10%胎牛血清(FBS)的Dulbecco’s modified Eagle’s medium(DMEM)培养基中培养Hela细胞,并在含5%CO2的加湿大气中孵育。将细胞洗涤3次,然后与新鲜培养基一起孵育,用含有DMEM的血清将探针稀释至10−5 mol·L−1,并与细胞共孵育30 min。再将细胞洗涤3次,用含DMEM的血清将Hg2+稀释至10−4 mol·L−1,并与细胞共孵育30 min。
-
在博视精达BD-YGD-1倒置荧光显微镜下获得斑马鱼图像。其中斑马鱼的处理方法如下:购买来自南京一树梨花公司的斑马鱼鱼卵,将鱼卵在28 ℃的保温箱中孵化,并及时补充水,孵化后无需添加饲料并继续养3 d后直接使用。
-
称取虾肉0.5 g于5 mL浓硝酸中后在60 ℃下酸解过夜。酸解结束后80 ℃加热至完全溶解,减压蒸馏除去所有溶剂。分次用蒸馏水将硝解液残渣溶解、离心,收集上层清液后用NaOH溶液调至pH = 7.4,将体积定容至150 mL得到预处理的虾样品溶液。
-
探针的合成方法如图1所示,首先根据文献方法合成具有汞离子识别功能的单元NTO[17-19],将其与(3,5,5-三甲基环己-2-烯亚基)丙二腈以1∶1溶解在乙腈中,加入少量哌啶。回流反应5 h,待溶液变成血红色后停止加热。经旋转蒸发仪除去溶剂,通过柱层析纯化(PE:DCM = 1:2),以较高收率得到具有红色荧光的目标探针MNC。其结构通过核磁共振和电喷雾质谱进行了表征。1H NMR (400 MHz, CDCl3) δ =7.38−7.40 (d, 2H, Ar-H), 7.68−7.02 (d, 1H, =CH), 6.81 (s, 1H, =CH), 6.75 (d, 1H, =CH), 6.62−6.64 (d, 2H, Ar-H), 3.82 (t, 4H, -CH2), 3.65−3.71 (m, 8H, -CH2),2.91 (t, 4H, -CH2), 2.77(t, 4H, -CH2), 2.57 (s, 2H, -CH2), 2.45 (s, 2H, -CH2), 1.07 (s, 6H, -CH3). ESI MS (m/z):calcd. for C29H37N3O2S2, 523.23; found 524.15 [M+H]+。
1.1. 药品与合成
1.2. 光谱测试
1.3. 细胞成像
1.4. 活体成像
1.5. 虾样品预处理
1.6. 探针的合成与表征
-
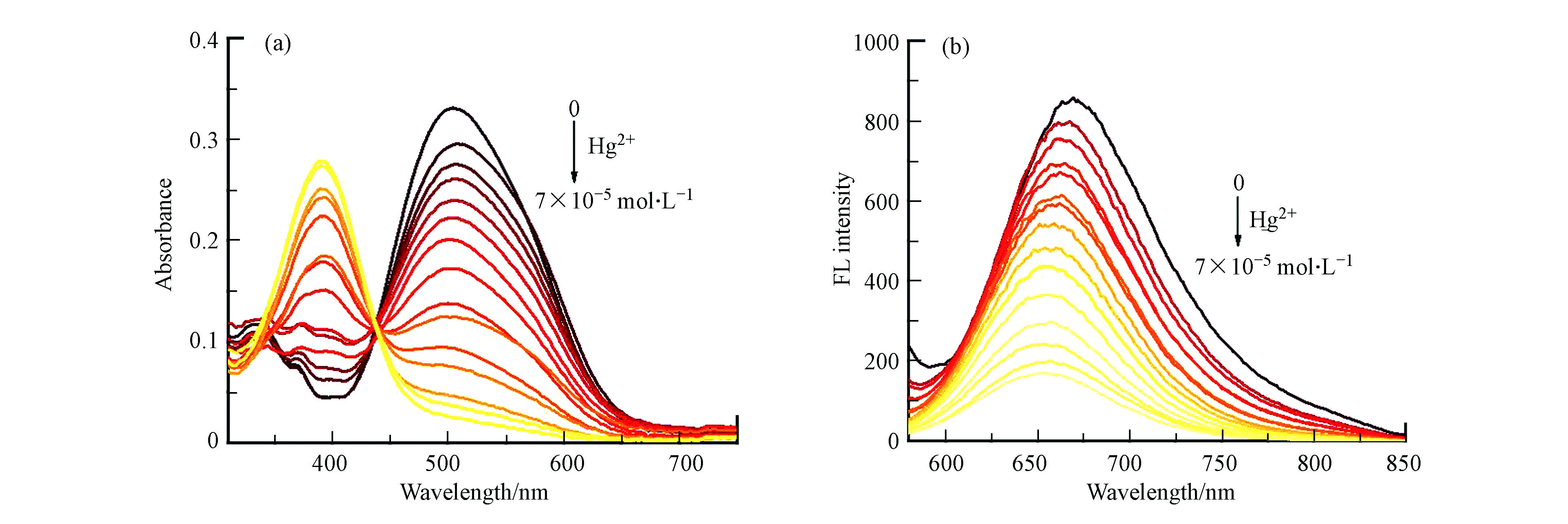
首先利用紫外-可见吸收光谱和荧光光谱研究了探针MNC在DMSO-H2O(V/V = 5:95)溶液中对各种金属离子如Cu2+、Zn2+、Mn2+、Ni2+、Cd2+、Pd2+、Pb2+、Co2+、Hg2+、Na+、K+、Mg2+、Ca2+、Li+、Al3+的选择性。如图2所示,探针MNC的紫外吸收和荧光发射分别位于520 nm和672 nm处。加入Hg2+后,紫外吸收光谱和荧光光谱发生明显改变。最大吸收波长从520 nm处蓝移至400 nm处(图2a),溶液颜色从红色转变成黄色(图2b)。在荧光光谱中,672 nm处的荧光发射峰明显减弱(图2c)。特别值得提及的是,当加入其它金属离子时,溶液的颜色和荧光变化较小。这一系列实验结果表明,该探针不仅能够通过颜色变化高选择性识别Hg2+,而且还可以通过荧光变化特异性检测Hg2+。此外,探针在较宽pH范围内(3.0 — 10.0)均具有高选择性(图2d)。通过计算拟合发现,探针MNC和Hg2+的络合比为1∶1,结合常数ka = 1.6×105 (mol·L−1)−1,检测限DL为8.33×10−5 mol·L−1。
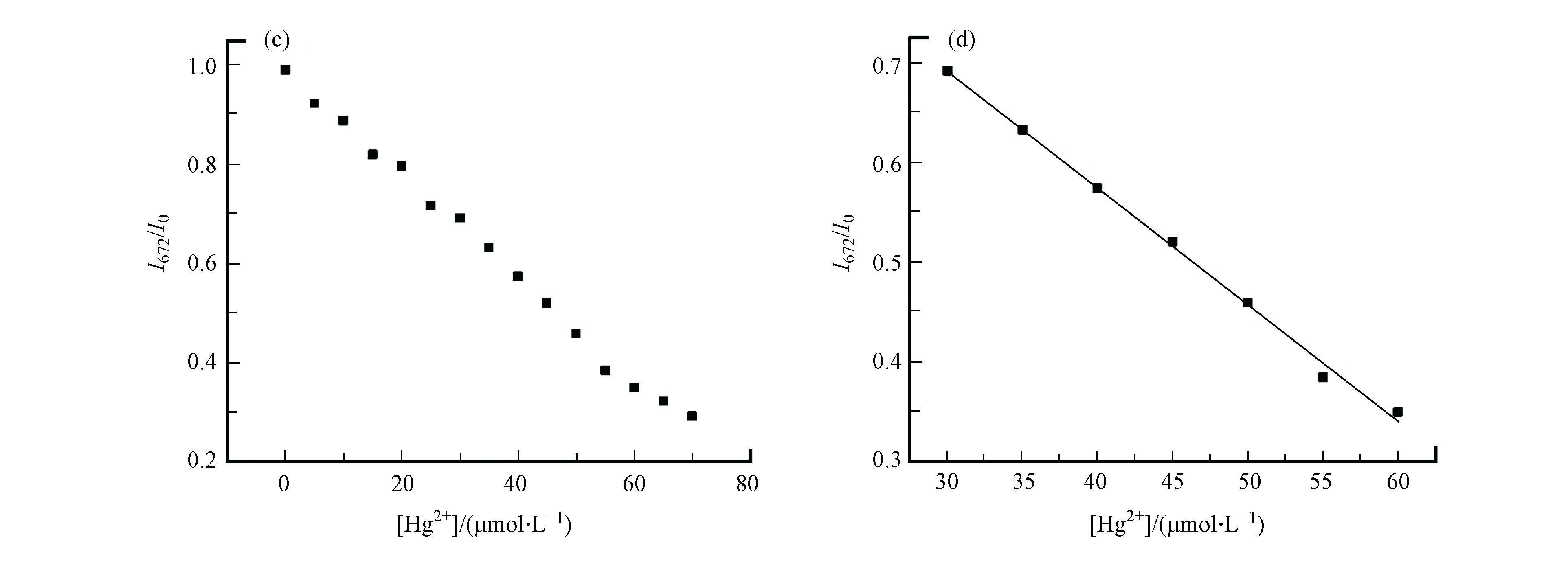
为了进一步探究探针MNC对Hg2+的响应,研究了探针MNC在DMSO-H2O(V/V = 5:95)溶液中加入不同浓度Hg2+(0 — 7×10−5 mol·L−1)后的紫外-可见吸收光谱与荧光光谱。在紫外吸收光谱(图3a)中,随着Hg2+的加入,探针MNC在520 nm的吸收峰逐渐减弱,同时在400 nm处的吸收峰逐渐增强,等吸收点出现在447 nm处。在荧光光谱(图3b)中,Hg2+的加入会导致MNC在672 nm的荧光强度逐渐减弱。在一定范围内探针MNC的最大发射峰强度和Hg2+浓度(3×10−5 — 6×10−5 mol·L−1)之间呈现出良好的线性相关关系(R2 = 0.9966)(图3c、d),表明探针MNC具有定量检测Hg2 +浓度的潜力。
利用碘离子(I−)研究了探针MNC(10−5 mol·L−1)与Hg2+(2×10−4 mol·L−1)之间的络合与解离。研究结果显示,随着KI的加入,络合物MNC-Hg的紫外-可见吸收光谱在400 nm的吸收峰逐渐减弱,并伴随着520 nm处的吸收峰逐渐增强(图4a)。在荧光光谱中,672 nm处的荧光强度随着KI的加入(0 — 1.1×10−4 mol·L−1)逐渐增强(图4b、c)。结果表明,探针MNC对Hg2+的络合能够在I−离子存在下发生可逆的络合与解离,该可逆过程为的Hg2+定量检测提供了一个合适的方法。值得注意的是,KI的浓度在一定范围内(3×10−5 — 8×10−5 mol·L−1)与络合物MNC-Hg的荧光强度呈现良好的线性关系(R2 = 0.9892)(图4d),进一步表明该探针能够用于Hg2+的定量检测。
-
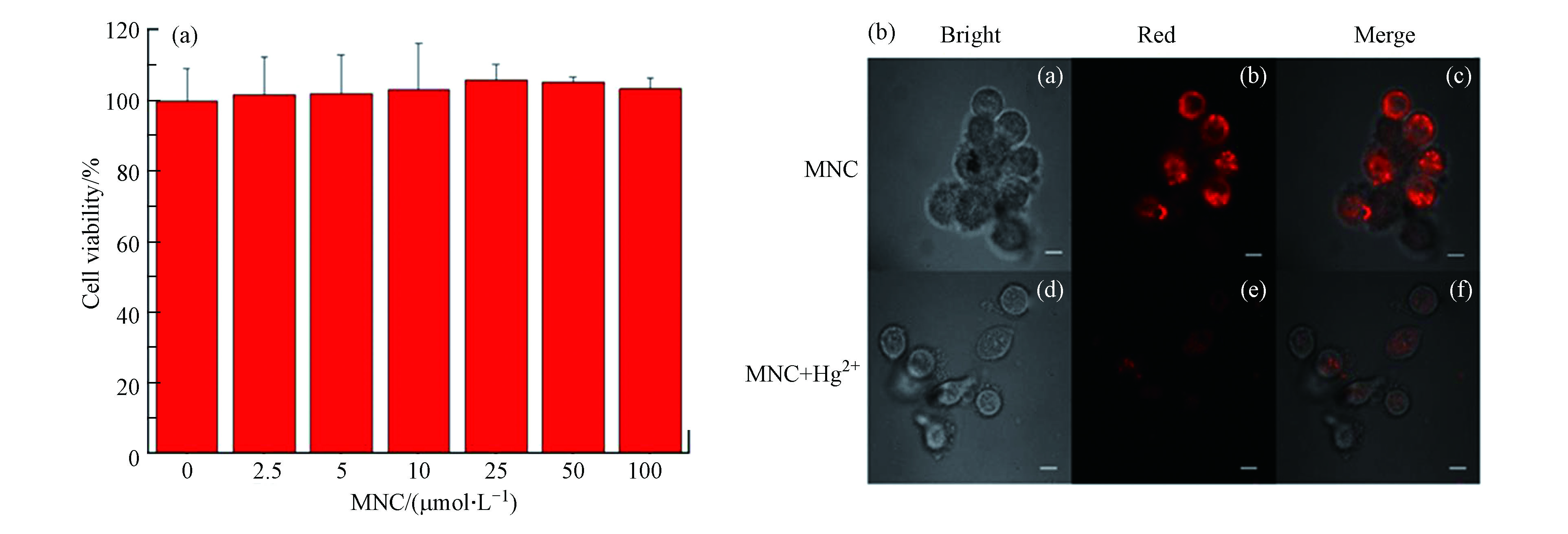
探针MNC表现出了良好的Hg2+检测能力,随后考察了探针MNC在细胞中检测Hg2+的可行性。首先,采用MTT实验验证了探针的细胞毒性。采用不同浓度的探针与Hela细胞共孵化2 h,观察细胞存活情况(图5a),结果显示在MNC浓度为2.5×10−6、5×10−6、10−5、2.5×10−5、5×10−5、10−4 mol·L−1 的环境中细胞存活率都接近100%,表明探针MNC的细胞毒性极低。随后,利用探针MNC对Hela细胞中的Hg2+进行荧光成像。在激光共聚焦显微镜(LSCM)下,探针MNC在Hela细胞中显示出红色荧光,与Hg2+孵育后红色荧光减弱(图5b),表明探针MNC可以用于细胞中Hg2+的可视化检测。
-
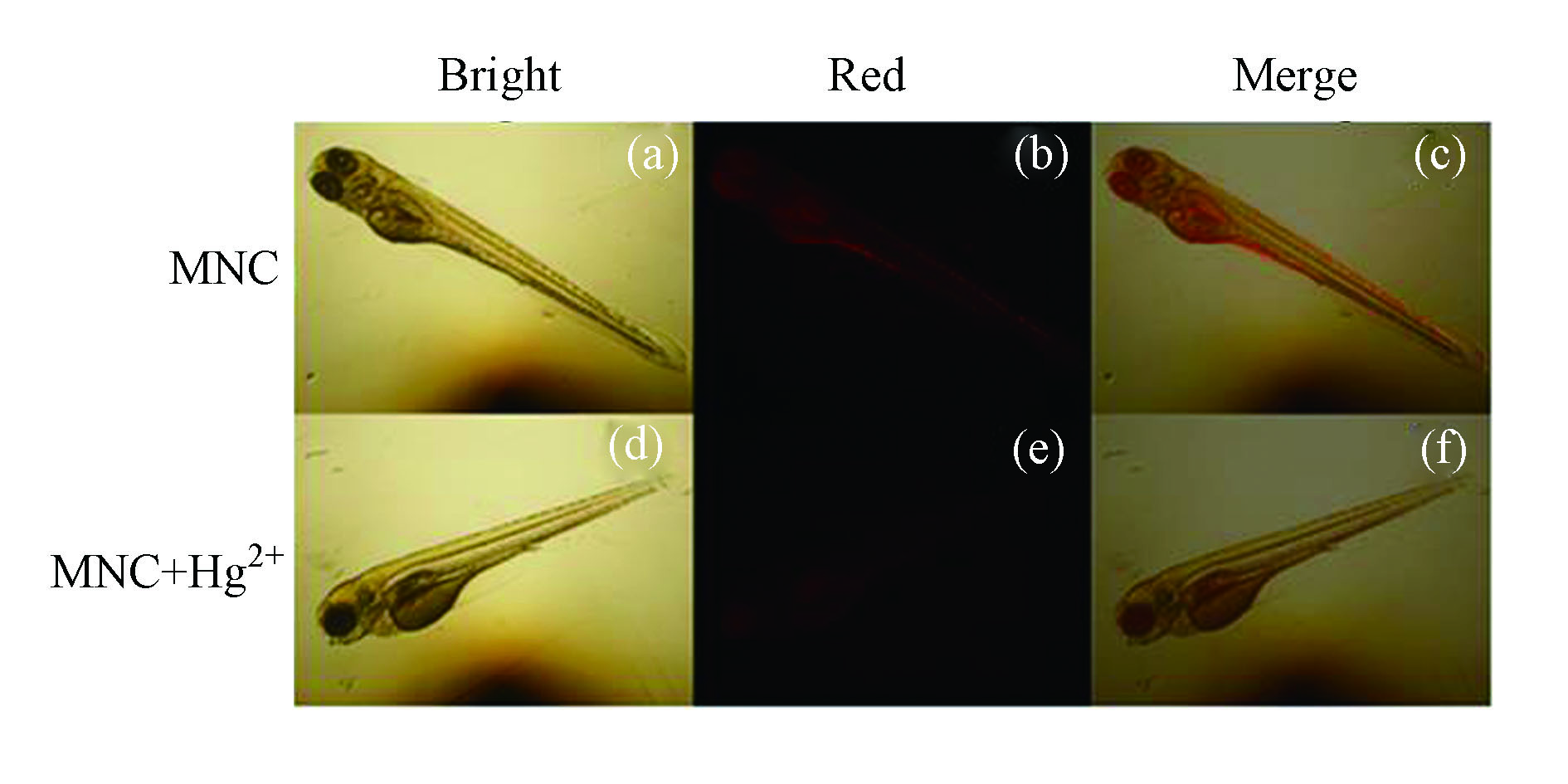
随后,进一步探索了该探针在斑马鱼活体中对Hg2+的检测能力。将斑马鱼幼体用探针MNC(10−6 mol·L−1)共培养1 h后,用蒸馏水洗去浮色后置于倒置荧光显微镜下观察。可以发现斑马鱼幼体的眼睛和腹部有明亮的红色荧光(图6a— c),表明探针MNC能够在活体水平上进行成像。进一步将以上斑马鱼与Hg2+(5×10−6 mol·L−1)共培养20 min后,再置于显微镜下观察发现,与仅用探针培养的斑马鱼相比,Hg2+处理过的斑马鱼幼体内的荧光强度明显减弱(图6d — f),这是由于斑马鱼体内的探针MNC与汞离子络合所致。这些研究结果表明,探针MNC可以用于斑马鱼体内检测Hg2+。
-
鉴于探针MNC具备定量检测Hg2+的能力,随后将其应用于虾样品中Hg2+含量的测定。将预处理后的样品与N,N-二甲基甲酰胺(DMF)按照体积比95:5混合,加入不同浓度的Hg2+,再加入探针MNC(10−5 mol·L−1)混合均匀后,测量其荧光光谱。结果显示,探针MNC的荧光强度与Hg2+浓度呈较好的线性关系(R2 = 0.9818)。通过拟合计算出回收率在82.87% — 122.25%之间,相对标准偏差(RSD)小于13.3%,相对误差小于22.25%。虽然存在一定的误差,但Hg2+的添加量与实测值基本一致,表明探针MNC可以用于虾中Hg2+含量的初步定量分析。
2.1. 探针检测
2.2. 细胞成像
2.3. 活体成像
2.4. 虾样品中Hg2+检测
-
本文介绍了一例新的比色型Hg2+荧光探针,它由含有苯甲醛基的氧硫杂冠醚和具有红光发射的(3,5,5-三甲基环己-2-烯亚基)丙二腈组成,该探针能够高选择性检测Hg2+,且荧光强度与Hg2+浓度呈现良好的线性相关,能够用于虾样品中Hg2+的定量检测分析。特别值得提及的是,该探针还可以用于活细胞与斑马鱼中Hg2+的可视化检测。下一步的研究将集中在将该探针用于更多实际样品中Hg2+的检测,建立定量检测分析平台。



 下载:
下载: