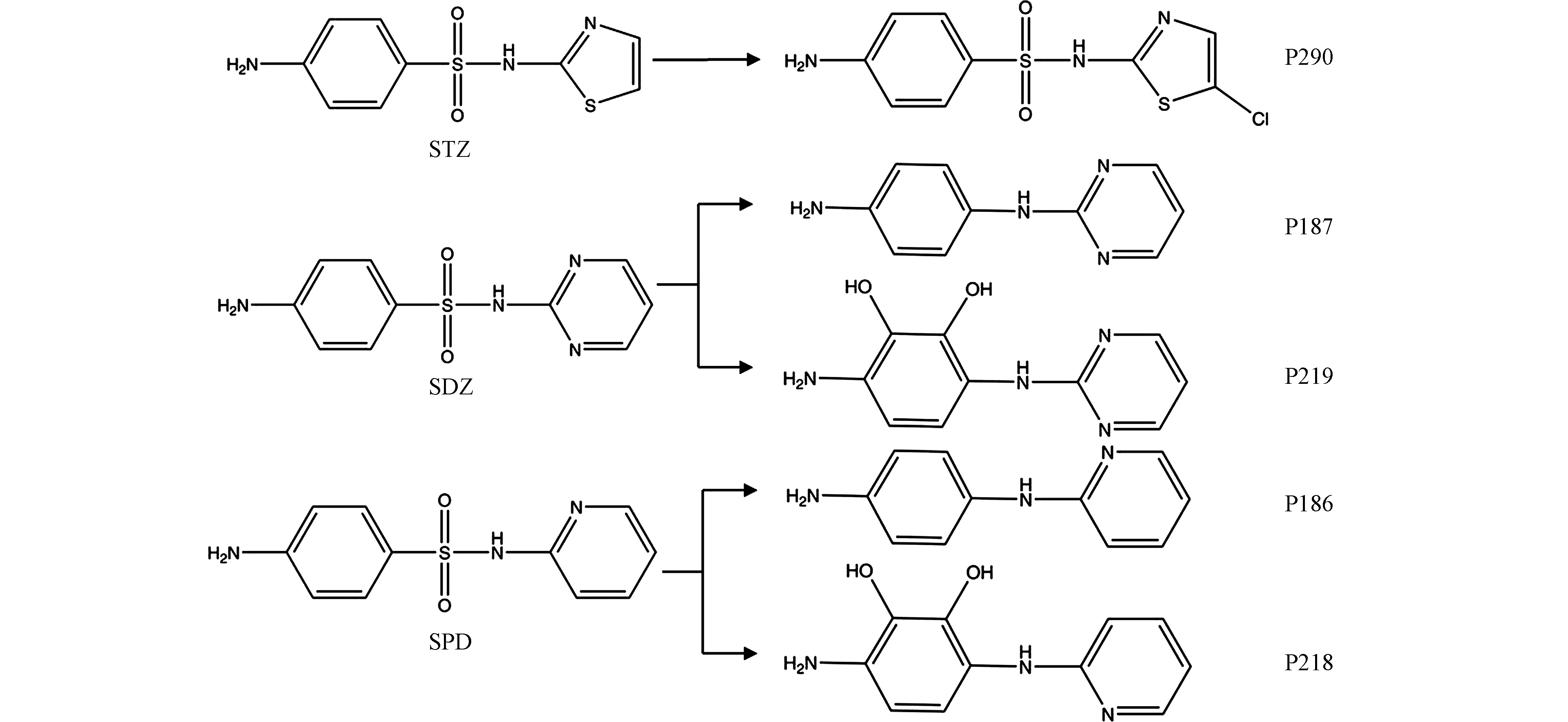
-
近年来,药品及个人护理品(pharmaceutical and personal care products, PPCPs)在世界范围内广泛使用,在地表水、废水甚至饮用水中检测到的频率不断增加,对生态环境和人类健康构成了严重危害[1-4]。高级氧化工艺(advanced oxidation processes, AOPs)可以产生大量自由基,被认为是去除PPCPs的有效技术[5-7]。紫外/过氧化氢(UV/H2O2)工艺是代表性的高级氧化工艺,其可以产生羟基自由基(·OH),对PPCPs有较好的去除效果[8]。但UV/H2O2工艺存在着药剂投加量大,成本高昂的缺点[9-10]。有研究发现,在氯化等消毒过程中PPCPs在一定程度上可以被去除[11]。但是,氯化消毒无法将顽固型PPCPs完全转化[12]。因此,在消毒过程中能够同时完成对PPCPs的去除的紫外/氯(UV/Cl)工艺近年来受到了广泛关注。作为一种高级氧化工艺,UV/Cl工艺不仅能够产生羟基自由基,还能生成Cl·和
$ {\rm{Cl}}_{\rm{2}}^{{\rm{ \cdot - }}} $ 等氯类自由基(reactive chlorine species, RCS),被认为是UV/H2O2工艺的替代品[13-18]。传统的研究通常只关注UV/Cl工艺中PPCPs的转化效率,缺少从反应机理以及转化产物毒性角度对不同环境中的UV/Cl工艺进行综合评价。有研究表明,PPCPs在氧化过程中有着较强的pH依赖性,且不同PPCPs差异明显[19-20],但现有研究未对其进一步解释。此外有研究发现,高级氧化工艺中众多PPCPs不能完全矿化,而是转化为毒性更高的中间产物[21-24]。基于此,通过探究不同条件下PPCPs的转化机理,评估其转化产物毒性情况,对实际水处理工艺的选择以及饮用水安全有重要意义。
磺胺类药物(sulfonamides, SAs)是一种典型的PPCPs,因其价格低、性质稳定,在防止细菌感染方面效果显著,一直以来就广泛被用做抗菌药物。磺胺类药物近年来在地表水、废水甚至饮用水中检测到的频率不断增加,已达到ng·L−1到μg·L−1的级别[25-26]。因此,本文选取3种磺胺类药物为目标PPCPs,探究UV/Cl工艺消毒过程中磺胺类药物的转化机理和产物毒性。
全文HTML
-
3种磺胺类药物磺胺噻唑(sulfathiazole, STZ)、磺胺嘧啶(sulfadiazine, SDZ)、磺胺吡啶(sulfapyridine, SPD)均为分析纯(>98%),购自中国J&K化工有限公司。硝基苯(nitrobenzene, NB)、硫酸钠(Na2SO3, ACS级)和次氯酸钠溶液(NaClO, ACS级,5%游离氯)购自美国Sigma-Aldrich公司。过氧化氢(H2O2, 30%)、盐酸(HCl, 37%—40%)、氢氧化钠(NaOH)和磷酸盐缓冲液(phosphate buffer saline, PBS)购自国药集团化学试剂有限公司。用于高效液相色谱的甲醇、乙炔、异丙醇和甲酸购自德国默克公司。
通过测定所测化学品对发光菌生长的抑制效果,进行了急性毒性试验。本文以发光杆菌为实验对象。发光菌冻干粉及辅助试剂(复苏稀释液、渗透压调节液)购自中国朗氏生物仪器有限公司。
-
以UV/Cl工艺为例,每隔一个月配制一次高浓度的NaClO溶液,用余氯测试仪测定其浓度。配制10 μmol·L−1的目标溶液(STZ、SDZ和SPD),量取800 mL倒入反应容器中,使用磷酸盐缓冲液和1 mol·L−1 NaOH以及1 mol·L−1 HCl来调节反应溶液的pH。反应时,在紫外灯照射条件下(254 nm的低压汞灯,辐照量为3.14 mW·cm−2)加入一定量NaClO溶液,测得初始浓度为14 μmol·L−1。以200 r·min−1的速度磁力搅拌反应溶液,控制温度为25 ℃。在每个取样点取样5 mL,然后用5 mmol·L−1 Na2SO3(Na2SO3/氯物质的量比为1.2)淬灭样品中余氯,再经0.45 μm的玻璃纤维滤膜过滤后,装入液相小瓶中待测。
为了研究氯类自由基在UV/Cl工艺中的作用,在相同的条件下进行了紫外对照实验和·OH屏蔽实验。紫外对照实验除未加入NaClO溶液外,其余实验条件均与UV/Cl实验相同。·OH屏蔽对照实验是在UV/Cl实验相同条件下加入一定量的探针化合物硝基苯,控制浓度为5 μmol·L−1,以屏蔽·OH在UV/Cl工艺中的作用。
-
选取明亮发光杆菌T3为实验菌种,分别比较了3种SAs在氯化、UV/H2O2和UV/Cl工艺中的毒性变化情况。向900 μL淬灭余氯后的3种SAs溶液中分别加入100 μL渗透压调节液,在SAs溶液使用前应先用0.22 μm的玻璃纤维滤膜过滤。将冷冻的发光菌冻干粉在25 ℃下放置15 min,加入0.55 mL的复苏稀释液。取50 μL活化的菌悬液加入到配置好的SAs中混合15 min。每个样品的毒性测试进行3次平行实验,抑制率可由公式(1)计算得出。
其中,I是样品的发光强度; I0是1000 μL渗透调节溶液的发光强度。
-
样品的TOC用加拿大奥罗拉公司TOC分析仪(型号1030)检测。溶液的pH值用瑞士Mettler-Toledo公司的pH计测定。使用高效液相色谱(Agilent Technologies 1200系列)测定SAs和硝基苯的浓度,色谱柱为安捷伦Eclipse XDB−C18柱(4.6 mm × 150 mm,5 μm粒径),流动相分别为超纯水/乙腈(60:40,V / V)和超纯水/乙腈(50:50,V / V),流速均为1.0 mL·min−1,柱温箱温度30 ℃,紫外检测器的波长分别为270 nm和262 nm。使用安捷伦1290超高液相色谱串联AB SCIEX TRIPLE TOF 5600高分辨质谱检测SAs在UV/Cl工艺中的主要转化产物,流动相为含0.1%甲酸的超纯水/乙腈(75:25, V / V)
,流速设置为0.6 mL·min−1。 -
·OH和RCS在反应中的相对贡献的计算基于自由基浓度的稳态假设前提。在已知·OH与NB反应的二阶动力学速率常数k·OH - NB[21]和·OH与布洛芬(ibuprofen,IBF)的二阶动力学速率常数k·OH - IBF[20]情况下,通过动力学实验确定NB和SAs的准一级动力学速率常数k′NB和k′SAs,以及紫外工艺中SAs反应的准一级动力学速率常数k′UV - SAs。在相同的条件下,在UV/H2O2工艺中转化SAs和IBF,通过公式(5)计算得到k·OH - SAs。最后,通过公式(2)—(4)计算得知·OH和RCS与SAs反应的准一级动力学速率常数k′·OH - SAs和k'RCS - SAs[20]。
1.1. 化学品和材料
1.2. 动力学实验
1.3. 毒性实验
1.4. 分析方法
1.5. 自由基相对贡献计算方法
-
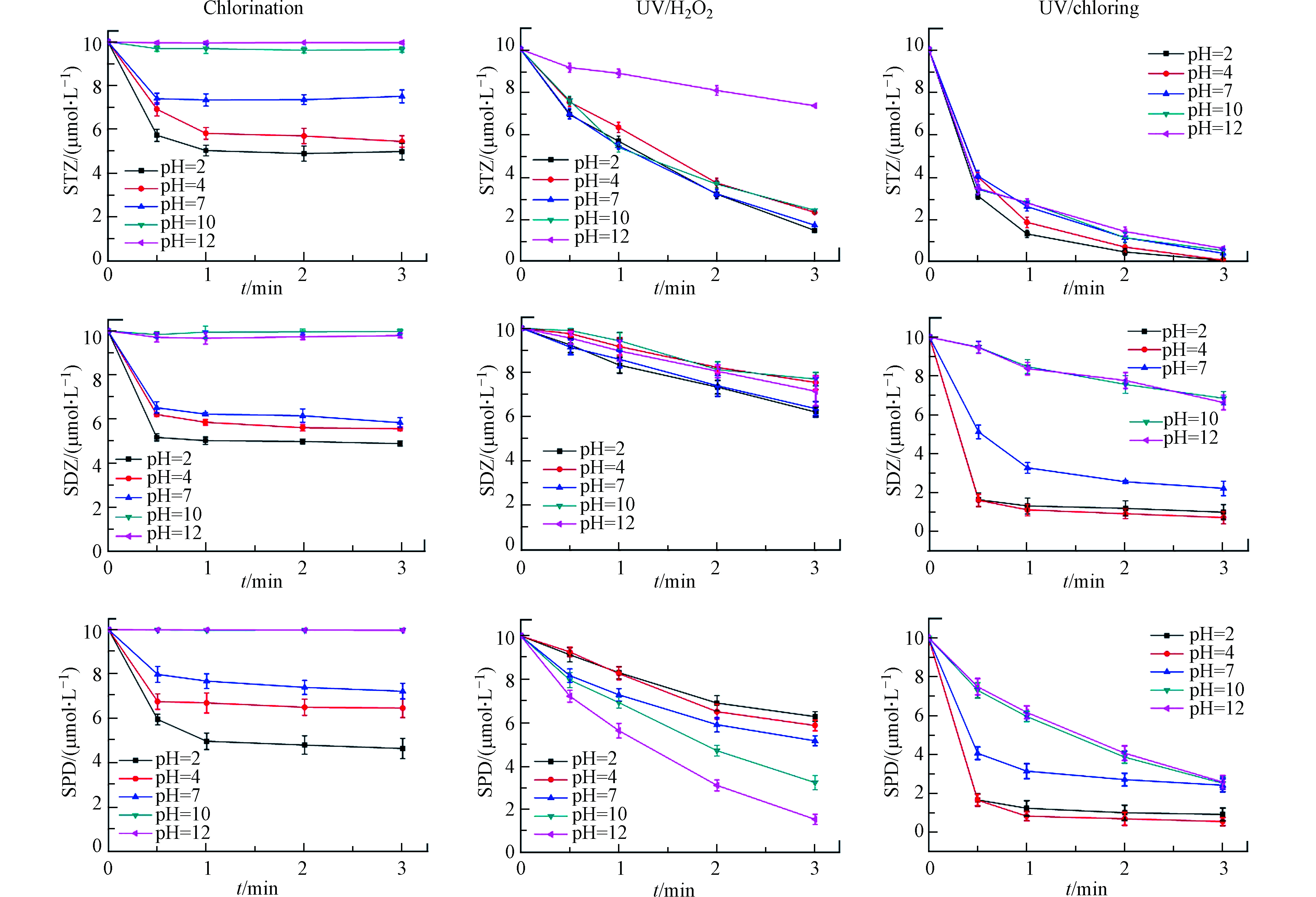
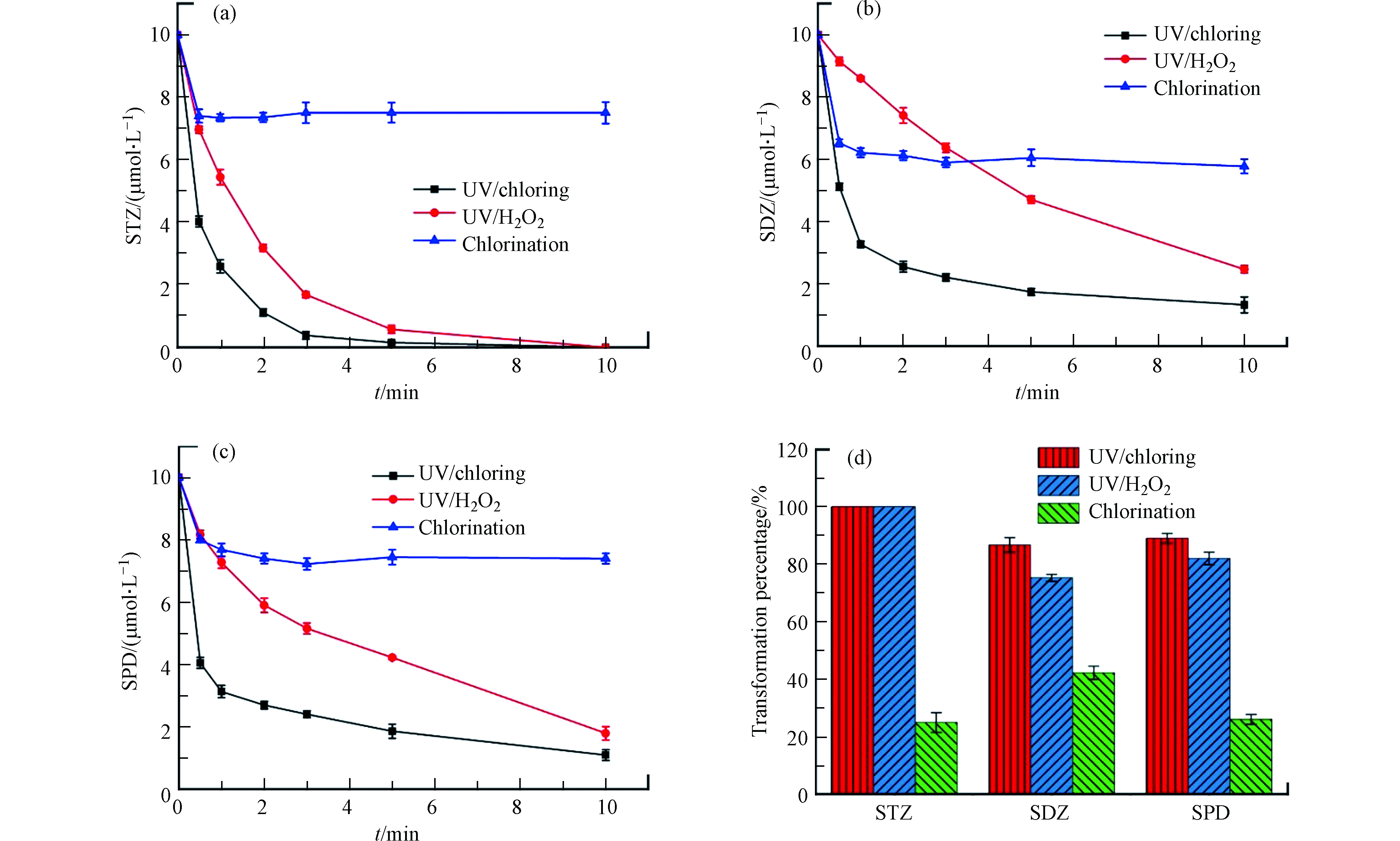
图1比较了pH 7时,氯化、UV/H2O2和UV/Cl工艺中SAs转化的动力学。氯化工艺中,3种SAs在反应开始1 min后浓度保持稳定,转化效率较低,最高转化率仅为42.2%。UV/H2O2和UV/Cl工艺的转化效果明显优于氯化工艺,在10 min内可以完全转化STZ,对SDZ和SPD的转化率达到70%以上,证明紫外光照和·OH以及RCS在SAs转化过程中发挥了重要作用。UV/H2O2和UV/Cl工艺对3种SAs转化效果的差别与SAs的结构有关,SDZ和SPD具有六元杂环,比STZ的五元杂环更难参与反应[27-28]。
-
本研究选取pH值为2、4、7、10和12初步探究pH对SAs转化影响,反应动力学如图2所示。氯化工艺中3种磺胺类药物的转化均随着pH的上升而逐渐降低。这是由于氯化工艺中反应组分比较单一,主要为HOCl/OCl−。pH的升高会使更多HOCl转化为OCl−,而HOCl与有机物反应的反应活性比OCl−更高[29-30]。
在UV/H2O2工艺中,pH对不同SAs转化的影响并不一致。STZ在pH 12时明显较差,在其它pH下无明显差异。推测是·OH在STZ转化过程中起到了主要的作用,·OH的活性在碱性条件下会受到明显抑制[31-32]。SDZ和SPD的转化与STZ有明显差异,表现为SDZ转化过程受pH影响不大,而SPD转化与pH呈正相关,显然实验结果与·OH的活性并不一致。因此,碱性条件下·OH不是3种SAs转化的主要反应组分。有研究发现,UV/H2O2工艺中存在具有氧化性的O2和
$ {\rm{HO}}_{\rm{2}}^ \cdot $ [31, 33-35].虽然溶液pH升高导致·OH受到抑制,但会促进O2和$ {\rm{HO}}_{\rm{2}}^ \cdot $ 的生成,如公式(6)—(8)所示。O2和$ {\rm{HO}}_{\rm{2}}^ \cdot $ 能够氧化SAs,降低溶液中SAs含量。从SDZ和SPD的结果中可以发现,O2和$ {\rm{HO}}_{\rm{2}}^ \cdot $ 对SAs转化的贡献甚至超过了·OH。综上所述,UV/H2O2工艺中SAs主要受到·OH、O2和$ {\rm{HO}}_{\rm{2}}^ \cdot $ 的作用,pH则会通过影响三者的含量而影响转化效率。UV/Cl工艺中呈现出了与氯化工艺相似的规律,但由于UV/Cl工艺中反应组分复杂,为了明确pH对UV/Cl工艺的影响机制,需要对UV/Cl工艺进一步研究,探究各反应组分的相对贡献。
-
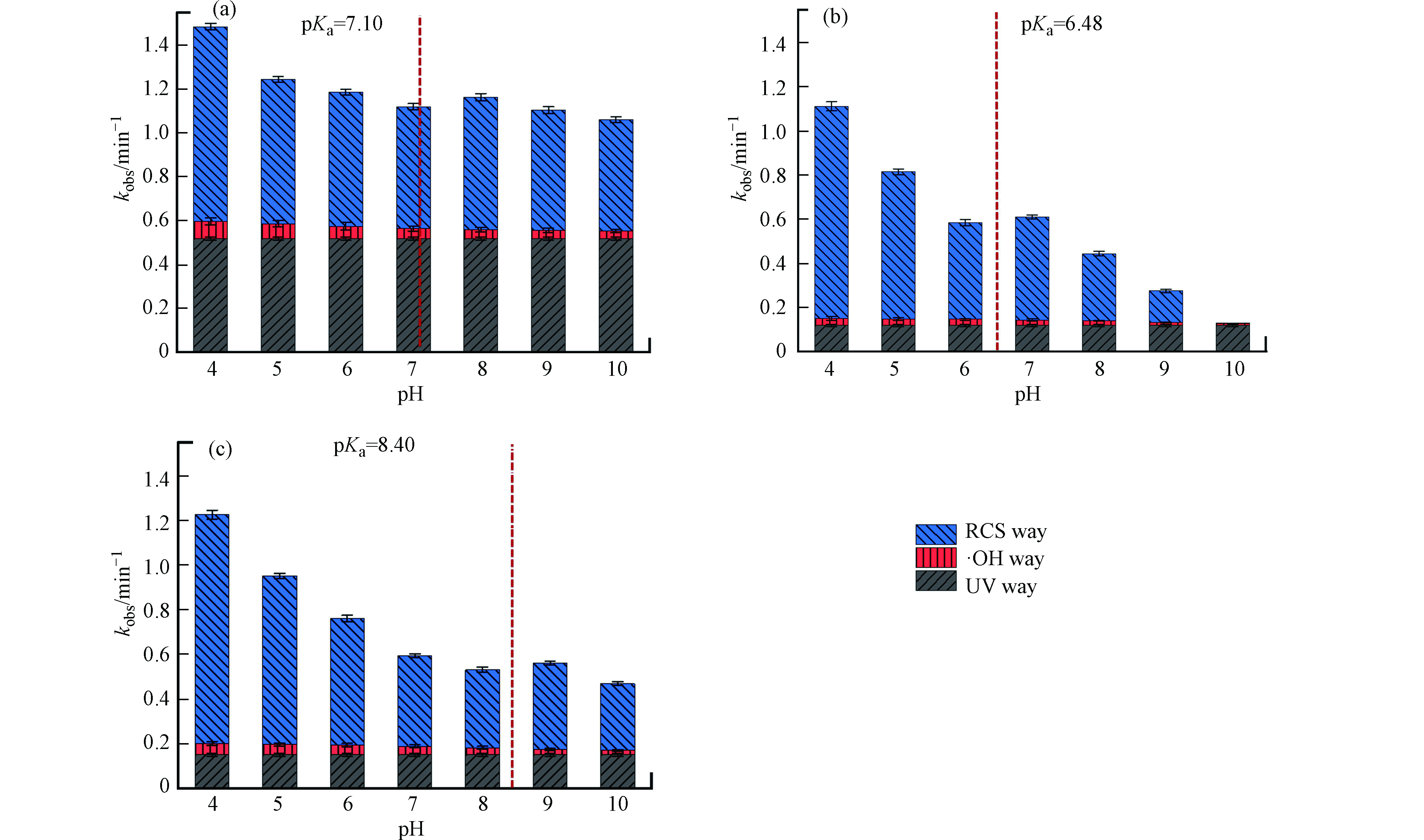
UV/Cl工艺中各反应组分在不同pH条件下的准一级动力学速率常数kobs如图3所示。紫外光照在转化过程中起到了重要作用,并且对于STZ的作用(kobs=0.519 min−1)明显优于SDZ (kobs =0.119 min−1)和SPD (kobs=0.154 min−1)。·OH在3种SAs转化过程中相对贡献最小,与UV/H2O2工艺的动力学结果一致。RCS在3种SAs转化过程中差异最为明显,且受pH影响较大。此前有研究指出,RCS有较强的pH依赖性。·Cl在与苯甲酸、氯苯和苯酚类物质反应时选择性和氧化性比·OH更高[36],
${\rm{Cl}}_{\rm{2}}^{{\rm{ \cdot - }}}$ 在与羟基、氨基和甲氧基的烯烃和芳香烃化合物反应时具有选择性[37],ClO·则与含甲氧基的芳香烃化合物反应迅速[38]。UV/Cl工艺中SAs的转化率与pH值呈现大致负相关,这是由于不同pH时UV/Cl工艺中反应组分的改变和SAs形态的变化。有研究发现,溶液中HOCl/OCl-均可与·OH和·Cl反应生成ClO·,如公式(9)—(12)所示[39-41]。pH升高促进HOCl转化为OCl−,而OCl−更易与·OH和·Cl发生反应(k2>k1,k4>k3),导致pH升高时高氧化性的·OH和·Cl转化为低氧化性的ClO·。
但随着pH升高,SAs的转化效率出现升高的现象。推测是由于pH升高时SAs从分子状态转变为负离子状态,或从正离子状态转变为分子状态,导致含电子量增加,从而更易被氧化。结合3种SAs的pKa值发现,转化率的升高总是发生在pKa值附近。因此综合两种因素推断,SAs的转化率会随pH的升高不断降低,但在当pH增加到物质pKa附近时会突然增加。由于两种因素不仅适用于SAs,因此这一现象在UV/Cl工艺中PPCPs转化时应当是普遍存在的。
-
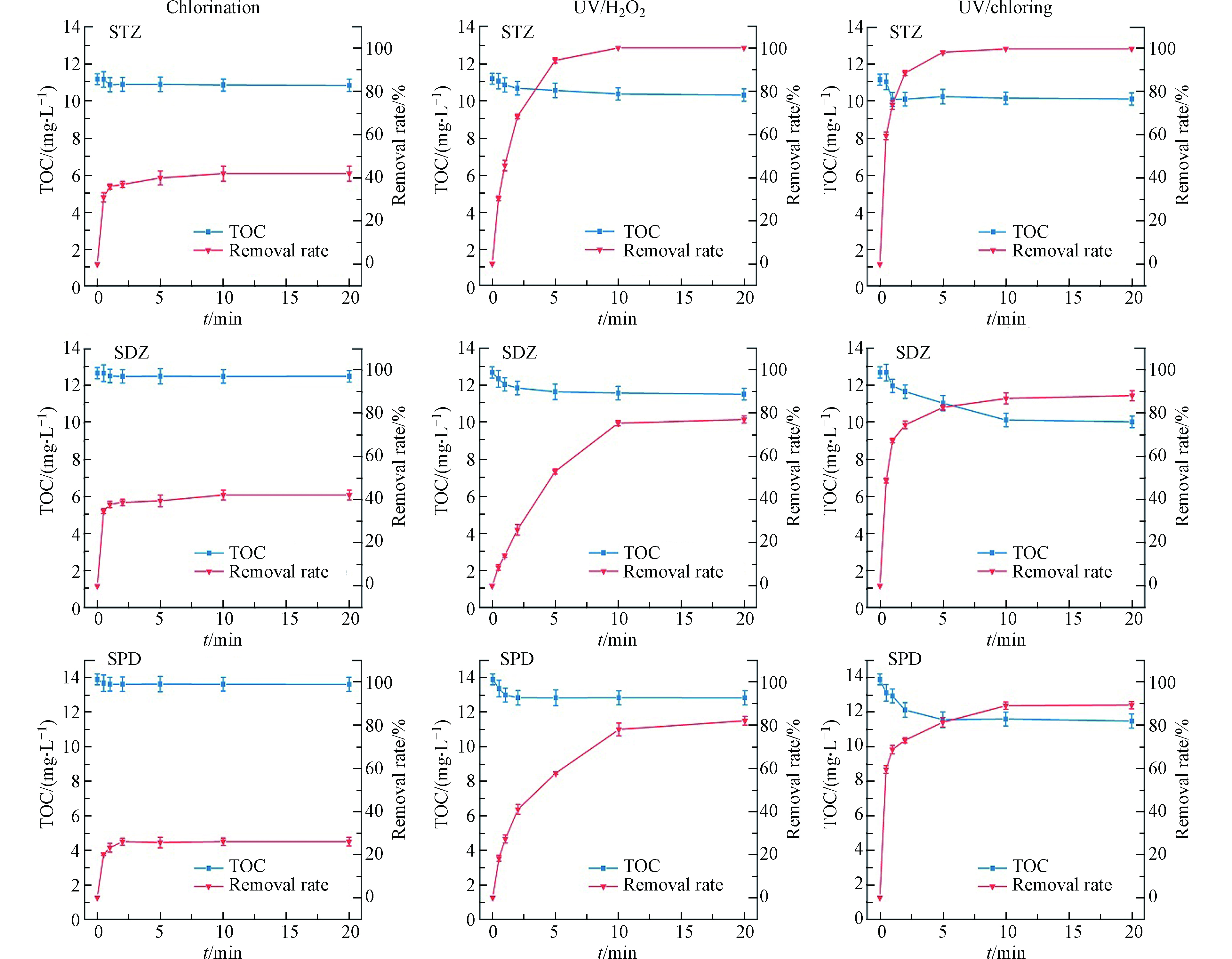
为探究3种工艺中磺胺类药物的矿化程度,本研究对3种工艺中反应时的总有机碳(total organic carbon, TOC)进行检测,如图4所示。TOC的降低代表工艺中磺胺类药物被矿化为无机分子,矿化程度越高则TOC下降更为明显。氯化工艺中3种SAs的矿化程度相似,STZ、SDZ、SPD溶液TOC分别下降了3.00%、1.36%和2.03%。
相较于氯化工艺,UV/H2O2和UV/Cl工艺对溶液TOC的削减效果更明显。STZ在UV/Cl和UV/H2O2工艺中矿化程度接近(TOC分别下降9.38%和9.27%),SDZ和SPD在UV/Cl工艺中矿化率(TOC分别下降21.06%和17.37%)则明显高于在UV/H2O2工艺中(TOC分别下降9.27%和7.66%)。然而,在SAs转化率达到70%时,3种工艺矿化程度均未达到10%,证明在本研究实验条件下,溶液中SAs大部分转化为有机中间产物,而未转化为无机分子。
-
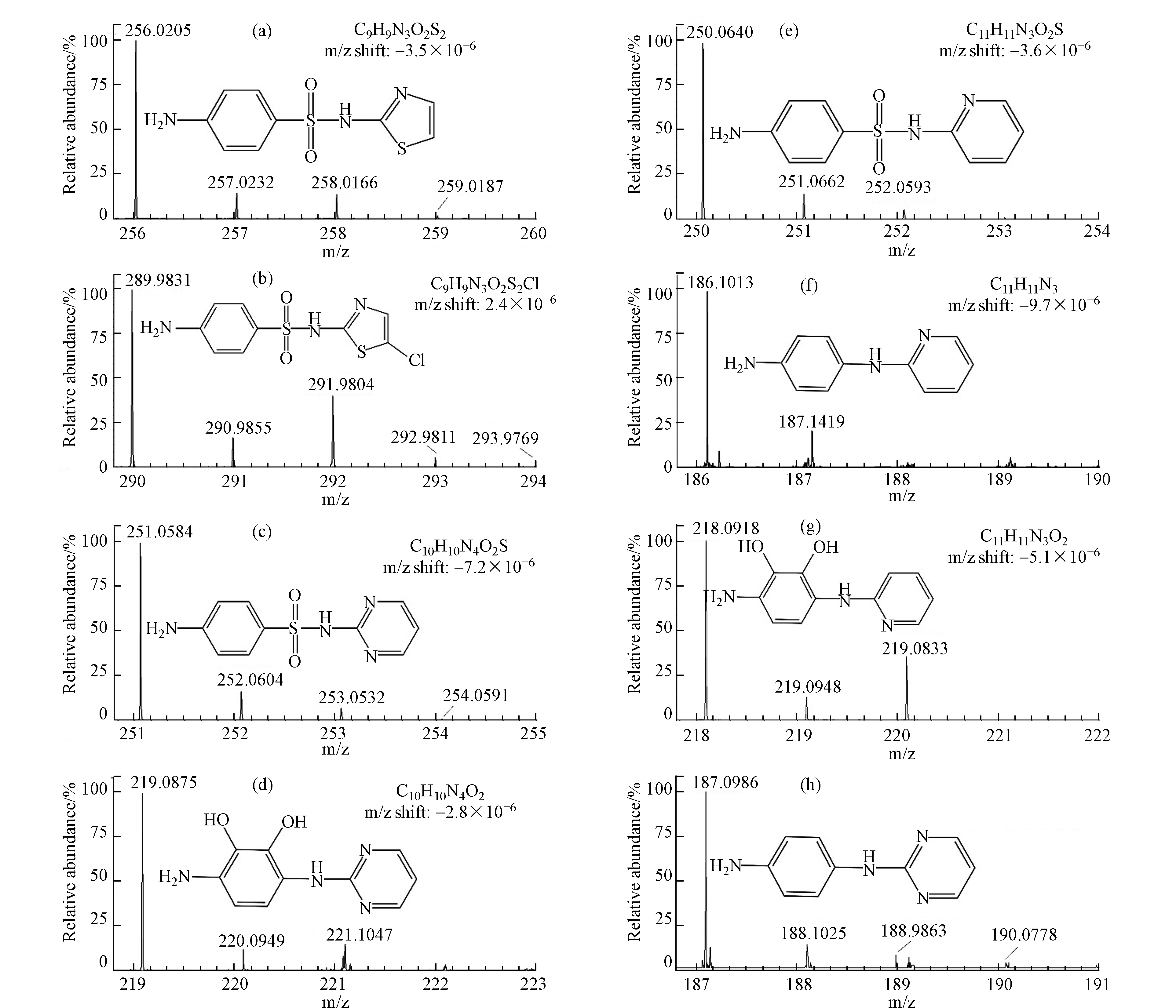
此前的研究中已被广泛报道了氯化和UV/H2O2工艺中SAs的中间产物的检测及转化途径的预测[23, 42-44],但未对UV/Cl工艺中SAs的转化产物进行研究。基于此,本研究通过对产物进行超高效液相色谱串联Q-TOF质谱检测(ESI正离子模式),通过高分辨率质谱的精确质荷比(m/z)确定物质分子式后,与数据库中物质进行对照,推测得到物质的相应结构式以及3种SAs在UV/Cl工艺中的转化途径。原物质与产物的质谱图如图5所示,分子式如表1所示。
在UV/Cl工艺中,STZ被检测到氯取代产物P290,与此前氯化工艺中SAs转化的研究结果一致[42]。SDZ与SPD均检测到脱磺酸基产物(分别为P187和P186),脱磺酸基产物在此前氧化工艺中SAs转化的研究中已有广泛报道,包括氯化[42]、二氧化氯[19]、基于硫酸盐的高级氧化[43]和光降解[45-46]。由于P187和P186在液相中具有特定的保留时间,因此在检测过程中确定为是转化产物,而不是质谱中的电离产物。此外,在SDZ和SPD转化中也检测到了产物P219和P218,m/z值比P187和P186高32个单位,因此推测为羟基化产物,但其具体产生原因目前尚不明确,有待进一步研究。
根据以上所检测出的转化产物,推测STZ、SDZ和SPD在UV/Cl工艺中的转化路径,如图6所示。SDZ和SPD由于其结构上相似的杂环取代基而产生相似的转化产物,这也证实了两者在UV/Cl工艺中的转化机制相似。虽然3种工艺都能在不同程度降低SAs浓度,但考虑到中间产物的存在,溶液依然可能存在较高毒性。有必要因此,探究不同工艺中溶液的毒性变化是极为必要的。
-
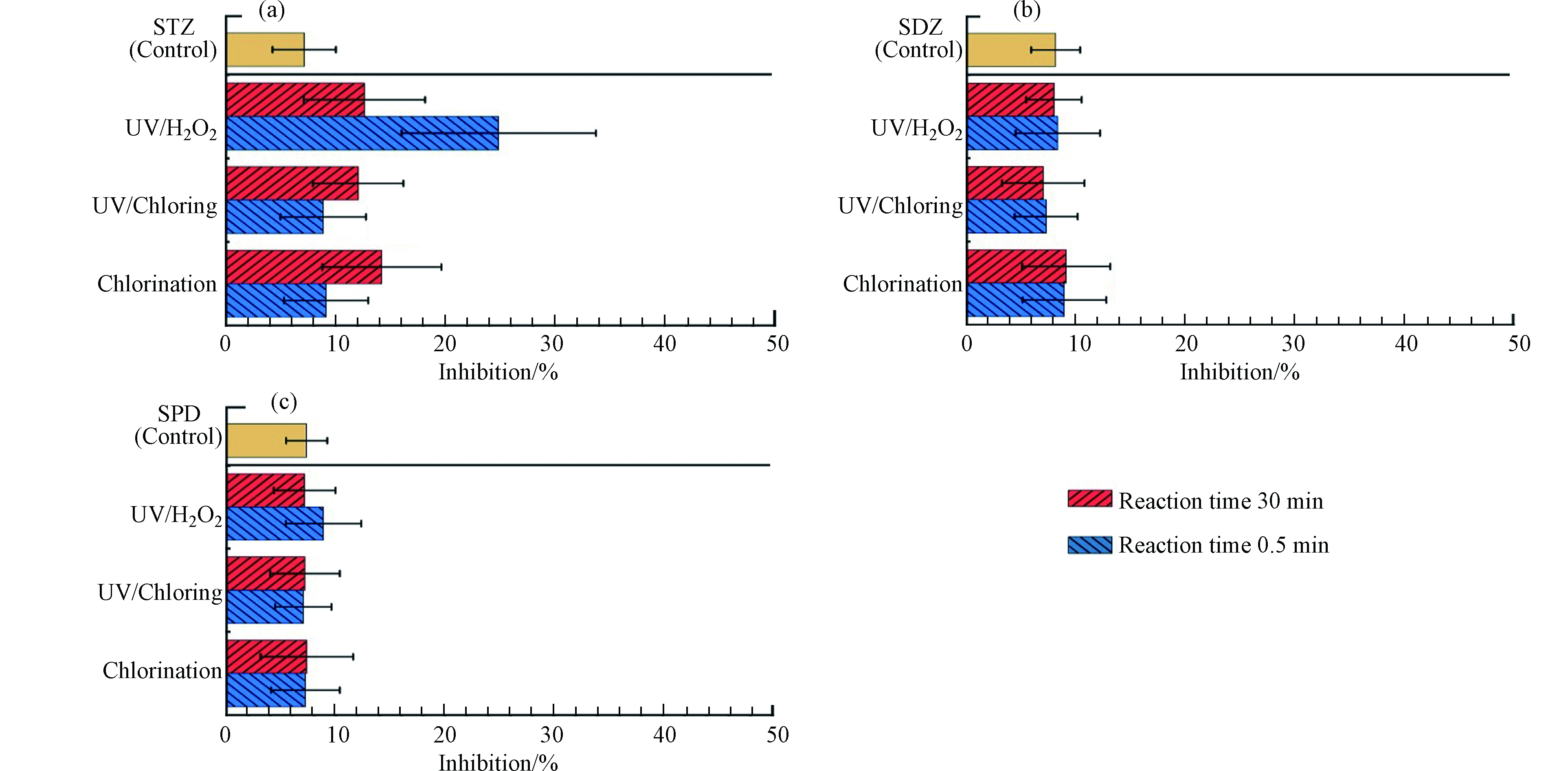
图7为氯化、UV/H2O2和UV/Cl工艺中3种SAs溶液的毒性情况,选取反应开始0.5 min表示反应初始阶段,反应开始30 min表示反应结束阶段。反应前STZ溶液的发光菌抑制率为7.13%。在氯化和UV/Cl工艺反应初始阶段,STZ溶液毒性略有提高,抑制率分别达到9.13%和8.86%,而在UV/H2O2工艺反应初始阶段,STZ溶液毒性上升更为明显,抑制率达到24.86%。3种工艺结束阶段,STZ溶液的毒性均较反应前溶液有所提高,抑制率分别达到14.21%、12.06%和12.62%。证明3种工艺STZ转化过程中,均生成了高毒性的中间产物。SDZ和SPD在毒性变化方面仍然存在相似的结果。在反应前SDZ和SPD溶液的发光菌抑制率分别为8.14%和7.33%。SDZ和SPD溶液经过氯化工艺后,发光菌抑制率分别提高到9.11%和7.34%,而经过UV/Cl工艺(6.99%和7.18%)和UV/H2O2工艺(7.99%和7.14%)后发光菌抑制率均有所下降。
UV/H2O2工艺中3种SAs转化过程表现出相似的毒性变化规律,即反应初始阶段毒性较高,反应结束阶段下降。UV/Cl工艺中的毒性变化规律有所区别,STZ溶液的毒性在反应结束阶段有所增加,而SDZ和SPD溶液在反应前后毒性几乎相等。通过对转化产物的研究,推测STZ溶液毒性升高是由于UV/Cl工艺降解STZ时产生的氯取代产物,这与磺胺甲嘧啶降解的毒性变化结果相似[23]。
2.1. 氯化、UV/H2O2和UV/Cl工艺的转化动力学
2.2. pH对氯化、UV/H2O2和UV/Cl工艺中SAs转化的影响
2.3. UV/Cl工艺中各反应组分对SAs转化的相对贡献
2.4. UV/Cl工艺中SAs的矿化程度
2.5. UV/Cl工艺中SAs的转化产物
2.6. 三种工艺处理后的溶液毒性评估
-
本文通过探究3种SAs的自由基反应机制、转化产物和溶液毒性演变,全面评估了UV/Cl工艺中SAs的转化,并与氯化工艺和UV/H2O2工艺进行了比较。UV/Cl工艺中SAs转化时,氯类自由基作用最为明显,因此对3种SAs的转化效果最好,但表现为转化效率更易受pH值影响。UV/Cl工艺中,SAs的转化率会随pH的升高不断降低,但在物质pKa值附近时会突然增加。3种工艺降解SAs的矿化程度较低,且溶液的毒性未明显降低,证明生成了具有毒性的中间产物。通过对UV/Cl工艺的转化产物检测,发现STZ的氯取代产物,以及SDZ和SPD的脱硫产物和羟基化产物,而STZ的氯取代产物导致反应后溶液的毒性升高。



 下载:
下载: