-
近年来药物和个人护理品(PPCPs),即人们在日常生活中使用的各种药物和个人护理用品,在水环境保护方面受到越来越多的关注。大量研究发现PPCPs在水环境中的检出浓度通常在ng·L−1至mg·L−1的范围内,对水生生态系统和人类健康构成潜在的风险[1],包括急性和慢性毒性、内分泌干扰效应以及抗生素抗性基因的产生与传播等[2]。抗生素是目前广泛使用的药物之一,医疗和兽用中常用的抗生素包括β-内酰胺类、氨基糖苷类、大环内酯类、四环素类、喹诺酮类和磺胺类等多个大类[3]。其中,喹诺酮类抗生素由于具有广谱抗菌性、抗菌能力强、吸收效果好和价格低廉等优点而被广泛使用。喹诺酮类抗生素发展至今共有四代,目前使用最多的是第三代产品,包括氧氟沙星、恩诺沙星和环丙沙星等[4],从第三代起由于在C-6位上多了氟原子又称作氟喹诺酮类抗生素(FQs)。FQs是当前人类和兽用医疗中的重要抗菌剂[5],相较于前两代,第三、四代FQs具有更好的抗菌效果和更低的副作用,因此使用广泛。由于这类化合物化学结构稳定,无法被人体和动物体完全吸收代谢,会有显著部分随新陈代谢排出体外[6]。因此,这些抗生素可以通过未经处理的市政污水、工业废水以及养殖场的污水而进入环境中[7]。此外,许多研究表明传统的污水处理厂不能将FQs完全降解,剩余部分会随着污水和污泥进入环境中[8-11]。近年来,随着对抗生素在水和土壤等环境中积累而导致天然细菌种群中抗生素耐药性的产生与传播方面的认识,环境抗生素污染对生态系统和人类健康的威胁引起了人们的广泛关注[12-13]。已有研究表明在地表水中能够检测出FQs[14],也有研究发现FQs可以在污水处理厂污泥[15-17]、粪肥施用后的农田土壤中累积[18]。因此,有效去除生活污水、工业废水以及养殖场污水中的FQs是控制其环境污染风险的重要途径。
-
针对废水中FQs的去除,当前的研究中已经建立了多种方法,包括物理吸附[19-20]、生物降解[16]和化学降解[21]。吸附是最普遍的物理过程之一,可以用于去除水中的FQs[22-23]。前人已经研究了不同的吸附剂,如天然沸石[24]、二氧化钛纳米颗粒[25]和磁性介孔碳材料[26]对FQs的吸附性能。吸附只是污染物的相转移,无法实现污染物的最终分解。FQs是一类较难被生物降解的抗生素,对微生物具有一定的生物毒性与繁殖抑制性[27],因此废水中的FQs在传统生物处理工艺中不能被有效降解[13]。为了提高废水中FQs的去除效率,需要发展高效的化学处理方法。
高级氧化工艺(AOPs)是一类成熟有效的废水处理方法[28-29],主要通过产生多种具有强氧化能力的反应性物质(如活性自由基等)来降解有机污染物。常见的反应性物质在酸性条件下的氧化还原电位高低顺序为:·OH > SO4·- > FeVI > O3 > H2O2 > MnVII > HClO > ClO2[30]。在这些反应性物质中,·OH是一种仅次于氟的强氧化剂,能够将有机污染物氧化甚至矿化[31],同时·OH的攻击没有选择性,通常以106—109 mol·L−1·s−1的速率攻击有机分子的可氧化部分[32],并可最终将有机污染物矿化为二氧化碳和水[33- 34]。光化学氧化、催化湿式氧化、声化学氧化、臭氧氧化、电化学氧化和芬顿氧化等AOPs已被开发并部分应用于直接矿化有机污染物或改善有机污染物的生物降解性[35-36]。已有研究表明,AOPs能够有效处理抗生素废水,并在去除FQs方面具有一定的应用前景[37-39]。光降解和光催化降解[40-42]、二氧化氯氧化[7]、臭氧氧化[43-45]、高锰酸盐氧化[46-47]、高铁酸盐氧化[48-50]和芬顿氧化[51-52]等已被证实能够有效去除FQs。其中,光催化法虽然能够达到很好的去除效率,但光能利用率较低且受催化剂、紫外线波长及反应器的限制;二氧化氯单独氧化的效果并不理想,需要在催化剂存在的条件下才能达到较好的处理效果;臭氧发生器投资较大,运行费用高,并且其高能耗已成为制约推广应用的重要因素;高铁酸盐价格昂贵且溶液不稳定,限制了其在污水处理方面的发展。与其它AOPs相比,芬顿工艺因其适用范围广、抗干扰能力强、操作简单以及有机物降解矿化迅速等优势而得到广泛应用[53-54]。
-
芬顿氧化反应最初由H.J.H. Fenton在1894年提出,他发现在含有酒石酸或醋酸的水溶液中一定量的亚铁盐能够与某些特定的氧化剂发生反应,同时过量的亚铁盐和氧化剂会降低反应效果[55]。芬顿氧化被认为是金属催化的均相氧化反应,其中亚铁离子(Fe2+)作为催化剂[56],在酸性条件(pH值为2—4)下,通过催化过氧化氢(H2O2)分解产生大量的·OH来降解污染物,是一种安全、高效和经济的高级氧化技术,能够将污染物矿化为二氧化碳、无机盐和水[57]。
芬顿氧化的反应机理较为复杂,包括20多个链式基元化学反应[58],主要的反应机理由反应(1)和(2)组成,芬顿氧化对有机污染物的降解能力由反应(1)产生·OH的能力决定,由于催化剂Fe2+的存在,·OH可以持续产生,反应(2)可以再生具有催化性能的Fe2+[59],但前者的反应速率约是后者的6000倍[60],导致Fe2+和Fe3+不能形成有效循环,从而使溶液中的Fe3+含量随反应的进行持续增高。Fe3+在pH值大于3的环境中会以Fe(OH)3形式沉淀,形成大量的铁污泥,导致铁物种的严重流失和催化活性的降低,甚至阻断芬顿反应的进行,此外,这些铁污泥也带来分离和处置的问题[61]。
作为一种简单而灵活的方法,芬顿氧化反应可通过自由基链反应在均相或异相系统中发生,从而在没有任何特殊反应物或设备的情况下产生·OH[28, 62]。传统的芬顿氧化也存在一些缺点,如水溶液的pH值须在特定的酸性范围内,而大多数有机废水的pH值不在此范围内。废水和天然水体的pH值通常在6.5—8.5,为了使芬顿工艺中达到最佳pH范围需要加酸将pH值调整为2—4并在处理后再加碱调节至中性,这会增加试剂消耗和操作成本;其次,芬顿氧化产生的大量铁污泥需要处置以避免二次污染的风险。为了克服这些缺陷,传统的芬顿氧化工艺被不断改进,出现了包括改性铁芬顿法、电芬顿法、光芬顿法、微波芬顿法等类芬顿法,这些方法以不同的方式引发·OH的产生,其核心的降解机理都是利用·OH的氧化能力。
-
芬顿反应的局限性之一是催化剂(Fe2+)再生非常缓慢,而光芬顿法在一定程度上缓解了这一问题。光芬顿氧化于1960年首次被提出,由于它对高含量有机物的高效降解能力而成为广受欢迎的一种高级氧化技术[63]。在结合紫外线(λ < 400 nm)和芬顿试剂的光芬顿降解过程中,Fe2+催化H2O2转化为·OH,此外,光提供的能量可以加速Fe3+还原为Fe2+,进而催化H2O2分解并产生额外的·OH[64]。这是因为水中的主要Fe3+物种,Fe(OH)2+,具有光敏性(吸收波长高达400 nm),吸收光后可以再生为Fe2+,在系统中形成铁循环,从而加速有机污染物的降解[64]。另一方面,H2O2的直接光解也会产生·OH,也参与有机污染物的降解。与传统的芬顿氧化相比,Fe2+和光的协同催化作用可产生更多的·OH,增强了光芬顿过程中有机污染物的氧化降解,并减少Fe2+的消耗和铁污泥的产生。
光芬顿氧化通常在酸性或中性条件下进行,在紫外光照射下的主要反应如(3)和(4)所示[65],反应(3)中Fe3+在紫外线照射下与H2O2反应生成·OH和Fe2+,随后进行反应(1),实现Fe3+和Fe2+之间的快速转化[66]。此外,如反应(5)所示,存在于弱酸性溶液中的Fe(III)羟基络合物还有Fe(HO2)2+,Fe(OH)2+是Fe3+在光照条件下还原为Fe2+的中间体,可在紫外光和可见光区吸收相当多的光,这些络合物经过光还原产生Fe(II)和·OH,如反应(6)所示[67]。此外,使用太阳光或紫外线的光芬顿处理技术还会导致污染水体中微生物的“失活”,有利于处理水后续的饮用或灌溉等[68]。
光芬顿工艺中主要的光源有太阳光和紫外线。在不利用太阳光的情况下,光照装置是光芬顿工艺的核心组成部分[69]。可以利用UVA (315 nm < λ < 400 nm)、UVB (285 nm < λ < 315 nm)或UVC (λ < 285 nm)作为光源,不同强度和波长的光照对有机污染物的降解有显著影响[70]。紫外线辐照装置在光芬顿工艺的应用中面临着许多挑战,例如使用寿命短、能耗高、环境污染风险以及经济成本高[71]等。最近的研究表明利用太阳光作为光芬顿工艺的光源处理废水也可以取得较为理想的效果[72]。Pliego等比较了传统芬顿和太阳光芬顿工艺在去除水溶液中舍曲林(一种抗抑郁药)的性能,发现太阳光芬顿工艺对总有机碳(TOC)的去除率比传统的芬顿工艺提高了2.5倍,具有明显的优势[73]。作为一种免费的可再生能源,太阳光在光芬顿工艺中的应用具有较好的前景,但会受到当地气候条件的显著限制。
综上所述,光芬顿系统利用紫外线或太阳光提供的能量来加速Fe3+还原为Fe2+,从而可以减少Fe2+的输入和铁污泥的产生,提高有机污染物的降解效率。与此同时,光芬顿法存在光能利用率低、运行成本高和光反应器设计复杂等缺点,且反应过程受光强的影响较大。此外,光芬顿氧化并未能完全克服溶液需处于酸性pH环境和生成大量铁污泥等缺点,因此限制了这一工艺的大规模应用[53, 74]。
已有大量研究表明基于芬顿氧化的方法,比如经典的芬顿氧化、电芬顿、光芬顿、光电芬顿和使用非均相催化剂的类芬顿等,对FQs有较好的去除效果[51, 75-79]。同时,这类抗生素在经过芬顿氧化后微生物降解性能得到提高,并能够被部分矿化[80-82]。表1总结了芬顿和光芬顿氧化处理氟喹诺酮类抗生素的操作条件以及降解效率。
Santos等使用芬顿氧化降解诺氟沙星,发现在最佳处理条件下(Fe2+/H2O2 = 0.142, pH = 3.0)诺氟沙星在反应60 min后的矿化效率为50%,通过对比实验发现H2O2浓度对降解效率的影响最大,同时诺氟沙星的矿化速率低于降解速率,表明有稳定的中间产物生成[83]。Giri等使用芬顿氧化降解环丙沙星,在Fe2+/H2O2比约为0.125时去除率最高可达74.4%,而当Fe2+/H2O2 > 0.125时,环丙沙星的去除率略有下降[77]。环丙沙星在降解过程中有两个不同的速率阶段:最初的去除速率较快,然后以几乎恒定的速率被降解[77]。这是由于Fe2+的快速消耗,·OH的产生速率在反应初期较快,后期由于Fe2+的缓慢再生限制了·OH的生成速率,进而控制了环丙沙星的降解速率。吴健等研究表明,在紫外光(254 nm,30 W)辐照下,当pH 4.0、H2O2浓度为23.50 mmol·L−1、Fe2+浓度为720 μmol·L−1时,诺氟沙星的降解速率最快,且芬顿反应对TOC去除率明显滞后于光芬顿反应,表明紫外光的引入可以显著提高有机物的矿化[88]。诺氟沙星的TOC去除率低于降解率,说明其在降解中生成了一些稳定的有机中间产物[88]。
目前大多数研究仍集中于在实验室研究合成废水中FQs的芬顿及光芬顿降解,而在实际处理中将芬顿工艺应用于大型污水处理厂中可能会受到各种限制。与其它能耗高的AOPs相比,芬顿工艺的pH环境要求较为严格,扩大芬顿反应的pH范围会降低工艺成本。Wang等向降解诺氟沙星的芬顿体系加入羟胺以加速Fe3+与Fe2+的氧化还原循环(同时对·OH无明显抑制),从而提高了反应速率并扩大了有效pH范围[89]。光芬顿法中也有研究针对此限制作出改善,通过在废水中添加配体或络合剂,在中性pH下实现有机物的光芬顿氧化[90]。多羧酸化合物,例如柠檬酸盐,草酸盐,马来酸盐和乙二胺-N,N′-二琥珀酸(EDDS)[91-94],均可与铁形成络合物,使其保持在溶液中,并在太阳紫外线辐照下发生快速的光化学反应。此外,在应用芬顿法去除FQs时还需要考虑成本[95-96],主要包括试剂消耗和能耗,这会随废水成分和预期的降解/矿化效率而变化。同时,处理后废水严格的pH控制要求(导致试剂消耗增加)以及铁污泥的处置也会导致处理成本的增加。近期研究表明,利用电芬顿或其它替代氧化剂对FQs进行降解可降低处理成本[97-98]。为了提高芬顿工艺应用的可行性,可以将其与常规生物处理技术结合使用,以同时实现高矿化效率和良好的经济可行性。芬顿反应过程中处理条件的不同会直接影响到降解效率,为了使FQs的氧化效率达到最佳,确定其最佳的处理条件是必要的。
-
芬顿和光芬顿氧化工艺的关键是反应条件,包括溶液初始pH值、Fe2+浓度和H2O2的浓度,不同条件下有机污染物的降解效率差别显著。这些关键参数决定了反应系统中·OH的产生和消耗,并相应的控制了有机污染物的降解速率。
-
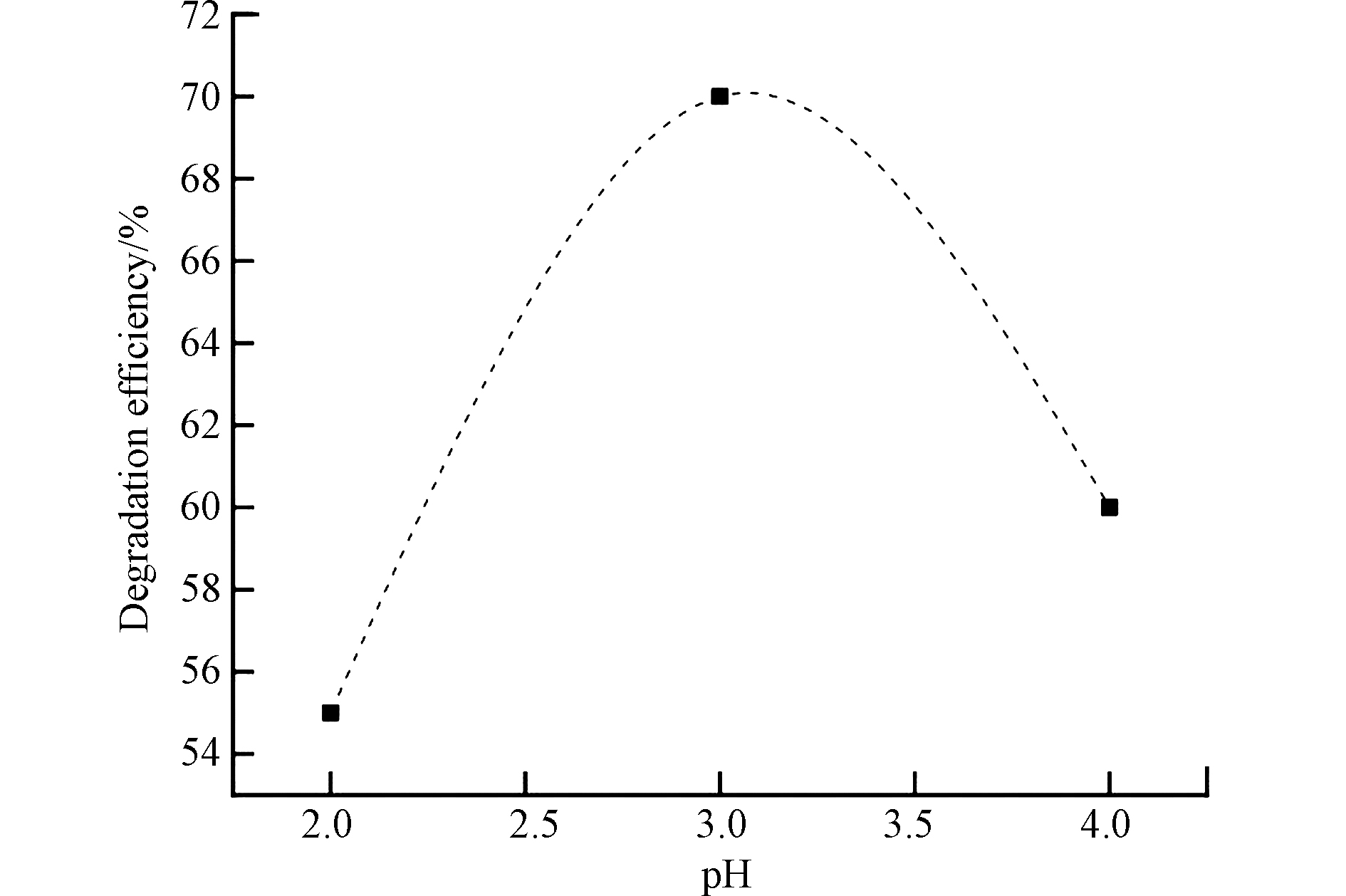
溶液的初始pH值是影响芬顿和光芬顿降解工艺最重要的一个因素,它可以影响·OH的产率以及Fe2+的浓度,从而影响最终的处理效率[99]。图1显示了芬顿氧化环丙沙星过程中降解效率随pH值的变化[85],当pH值为3.0时,环丙沙星的降解率达到最佳,而过低或过高的pH值会降低降解率。芬顿反应在pH值范围为2—4时可以达到最佳的处理效率[67]。当溶液处于酸性条件时,可溶性Fe2+的浓度最大,可以催化氧化H2O2生成大量的·OH[70]。由于Fe2+催化剂的失活,在过低pH值和过高pH值条件下都无法有效处理废水中的有机污染物。在光芬顿反应中,氧化效率最佳的pH值范围为2—4.5。首先,在此pH值范围内,高溶解度的Fe(OH)2+是主要的氢氧化铁络合物,其与游离的Fe3+、Fe(OH)2+和Fe2(OH)24+相比具有更高的光反应活性[100]。与此同时,在pH值高于5.0的溶液中Fe3+容易沉淀形成无定形氢氧化铁污泥,进而阻挡了反应器中紫外线的通透[101-102],·OH的产量会随之下降,影响光芬顿的降解效率。
在芬顿和光芬顿体系中,当溶液pH值较低时,Fe2+会形成[Fe(Ⅱ)(H2O)6]2+,与[Fe(Ⅱ)(OH)(H2O)5]+相比,其与H2O2的反应较慢导致产生的·OH量变少,同时,大量的H+会抑制Fe3+和H2O2之间的反应,并形成稳定的[H3O2]+,使H2O2更加稳定,从而降低其与Fe2+的反应性[103]。此外,·OH与H+会发生反应(7)[104],在低pH值下,H+对·OH的清除作用变得更强,导致·OH产量下降最终使有机污染物的降解效率变低[105]。
当溶液的pH值较高时,溶液中Fe2+的水解和沉淀作用增强,导致其催化能力下降[106],Fe3+更容易形成Fe(OH)3沉淀,从而阻断Fe3+和Fe2+之间的相互转化[107]。在此条件下H2O2会加速分解为H2O和O2,也会导致有机污染物降解率的下降[108]。另外,·OH的氧化电位随着溶液pH值的增加而降低[109],也会降低芬顿反应中有机污染物的氧化效率。
-
不同的芬顿过程遵循类似的氧化机制:Fe2+催化H2O2分解,生成高氧化性的·OH,进而氧化降解有机污染物。因此,在芬顿和光芬顿氧化中,Fe2+和H2O2的剂量是决定反应速率和处理成本的重要因素。图2显示了芬顿体系中Fe2+与H2O2摩尔比对环丙沙星芬顿降解率的影响[85],Fe2+和H2O2之间存在最优的剂量比,其中一种物质的不足或者过量都会导致芬顿和光芬顿的氧化效率下降。
Fe2+催化剂是影响芬顿和光芬顿工艺中有机污染物降解效率的重要参数之一。通常,随着Fe2+浓度的增加,有机污染物的降解效率会增加[110],然而,当铁盐浓度过高时,去除增量可能很小。过量的铁盐会导致溶液电导率的增加和需要处理的铁污泥量增加[111]。芬顿反应从Fe2+与H2O2之间产生·OH的反应开始,随着有机污染物的氧化,Fe2+的浓度迅速下降(反应1),因此,需要通过还原Fe3+形成Fe2+来达到平衡并使Fe2+浓度达到稳态(反应2)。在反应过程中·OH的浓度变化可分为两个阶段,在初始阶段,·OH的产生更快,导致更高的降解效率,有机污染物的氧化主要发生在这一阶段;在第二阶段,由于Fe2+和H2O2的逐渐消耗,·OH的产生速率显著降低。在H2O2相对不足的情况下,芬顿体系中过量的Fe2+不仅增加处理成本和铁污泥的产生,还会增强Fe2+对·OH的清除作用,给有机污染物的降解带来负面影响[112]。
在有足够量催化剂(Fe2+)存在的情况下,加入H2O2会相应地产生·OH。由于H2O2的氧化能力与·OH相比较弱,当H2O2过量后再继续增加其剂量将不会显著提升有机污染物的降解效率。此外,H2O2可以和·OH发生反应,H2O2过量时会增加·OH的消耗量,因此会降低污染物的降解率,甚至对污染物的降解起到抑制作用。同时,过量的H2O2会由于其自分解带来的O2脱气使铁污泥悬浮[113-114]。因此,适当的Fe2+和H2O2剂量对于芬顿和光芬顿反应中有机污染物的降解至关重要。针对目标污染物的去除,通常可以通过正交实验来优化芬顿试剂的剂量,也可以通过建立相应的基元反应模型,预测最优处理条件并通过实验进行验证。
-
在光芬顿法中,紫外线能量主要用于Fe3+到Fe2+的光还原和H2O2的光解,也可能导致有机污染物的光降解。因此,不同类型的光源(波长与光强)会影响有机污染物在光芬顿氧化处理中的降解效率。
紫外光波长可以显著影响·OH的直接形成以及Fe3+到Fe2+的光还原。研究表明,当紫外光波长从360 nm降低到313 nm时,Fe3+光还原为Fe2+的量子产率从0.017增大到0.14[100]。Guo等的实验结果显示光芬顿工艺在波长为365 nm时对甲苯的降解和矿化效率比在波长为254 nm时高得多,这是因为随着波长的增加,Fe3+到Fe2+的还原反应增强,产生更多的·OH[115]。然而,H2O2对于320 nm以上的光完全不吸收,过高的波长导致H2O2无法直接光解产生·OH,从而降低光芬顿的降解效率[116]。
紫外光源的功率(决定光强)直接影响Fe3+的光还原速率和H2O2的光解速率,进而影响有机污染物的降解。当使用相同波长的紫外光时,光源功率的增加会提高有机污染物的去除率,这是因为紫外线功率的增加提高了Fe3+到Fe2+的光再生速率,促进了芬顿反应中·OH的生成,并且由H2O2光解产生·OH的速率也会提高[117]。
-
绝大部分氟喹诺酮类抗生素为分子结构上带有羧基和哌嗪环侧链上氨基的两性化合物。通过高效液相色谱与质谱联用鉴定这类化合物在芬顿和光芬顿降解过程中的中间产物,可以推测出相应的降解路径和可能的降解机理。Xiao等报道了环丙沙星在芬顿氧化过程中的12种产物并提出3种可能的降解机理:哌嗪环氧化脱烷,脱氟和芳香环上的羟基化以及脱羧反应[78]。Pi等确定了氧氟沙星在芬顿降解过程中的9种产物,并提出了可能发生的哌嗪环和恶嗪基降解反应机理[84]。
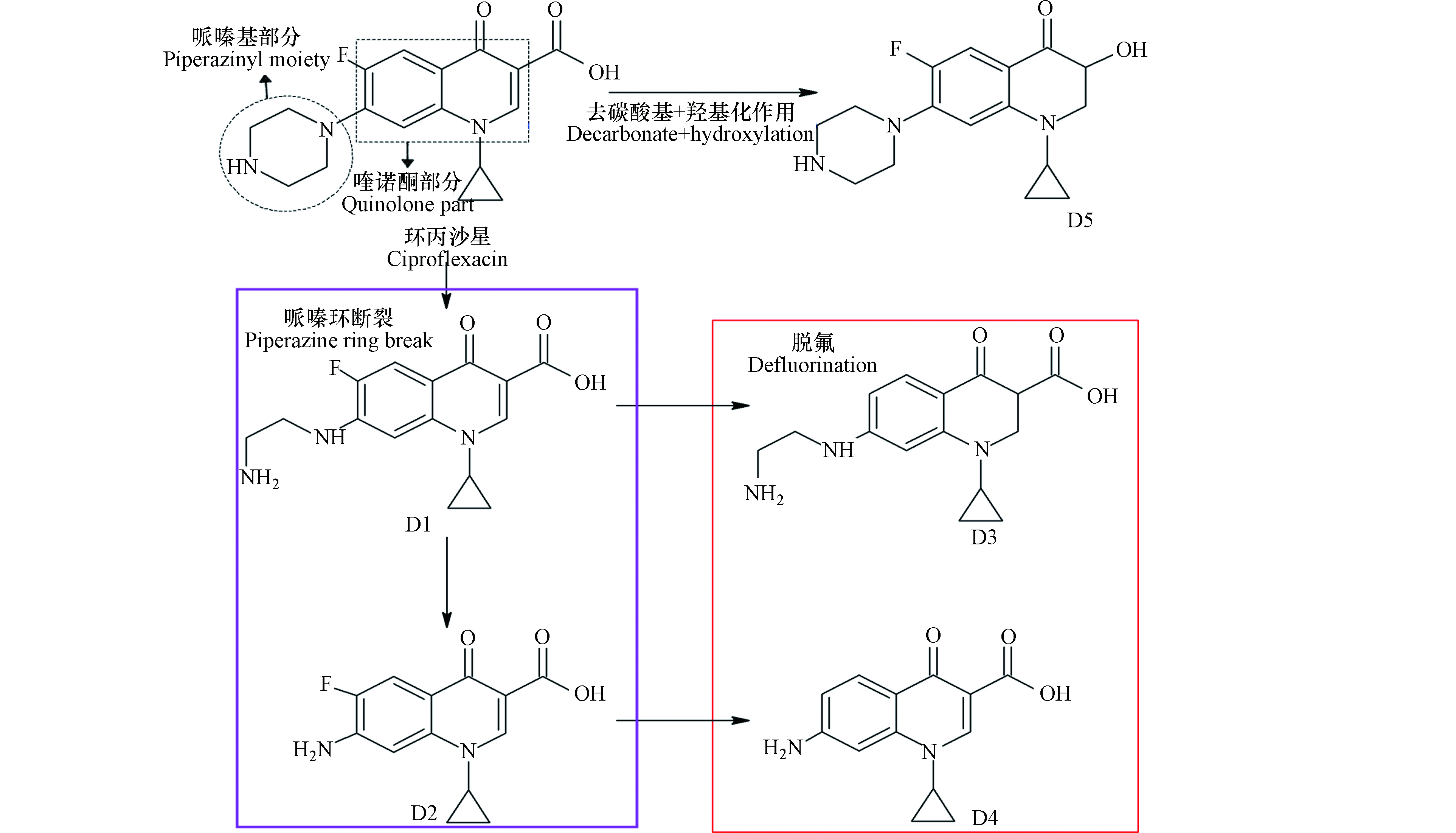
如图3所示,环丙沙星在芬顿氧化过程中哌嗪环首先发生裂解,形成脱乙烯基产物D1 [77]。据文献报道,多种AOPs均可导致氟喹诺酮类抗生素哌嗪环的裂解[118]。哌嗪环裂解后续的降解和脱氟反应产生降解产物D2和D3。脱氟后,降解产物D2可以进一步部分转化为分子量更低的降解产物D4。除此之外,环丙沙星分子发生脱羧基和羟基化后可以形成降解产物D5。在上述产物中,核心的喹诺酮结构和环丙基基团保持完整[85]。
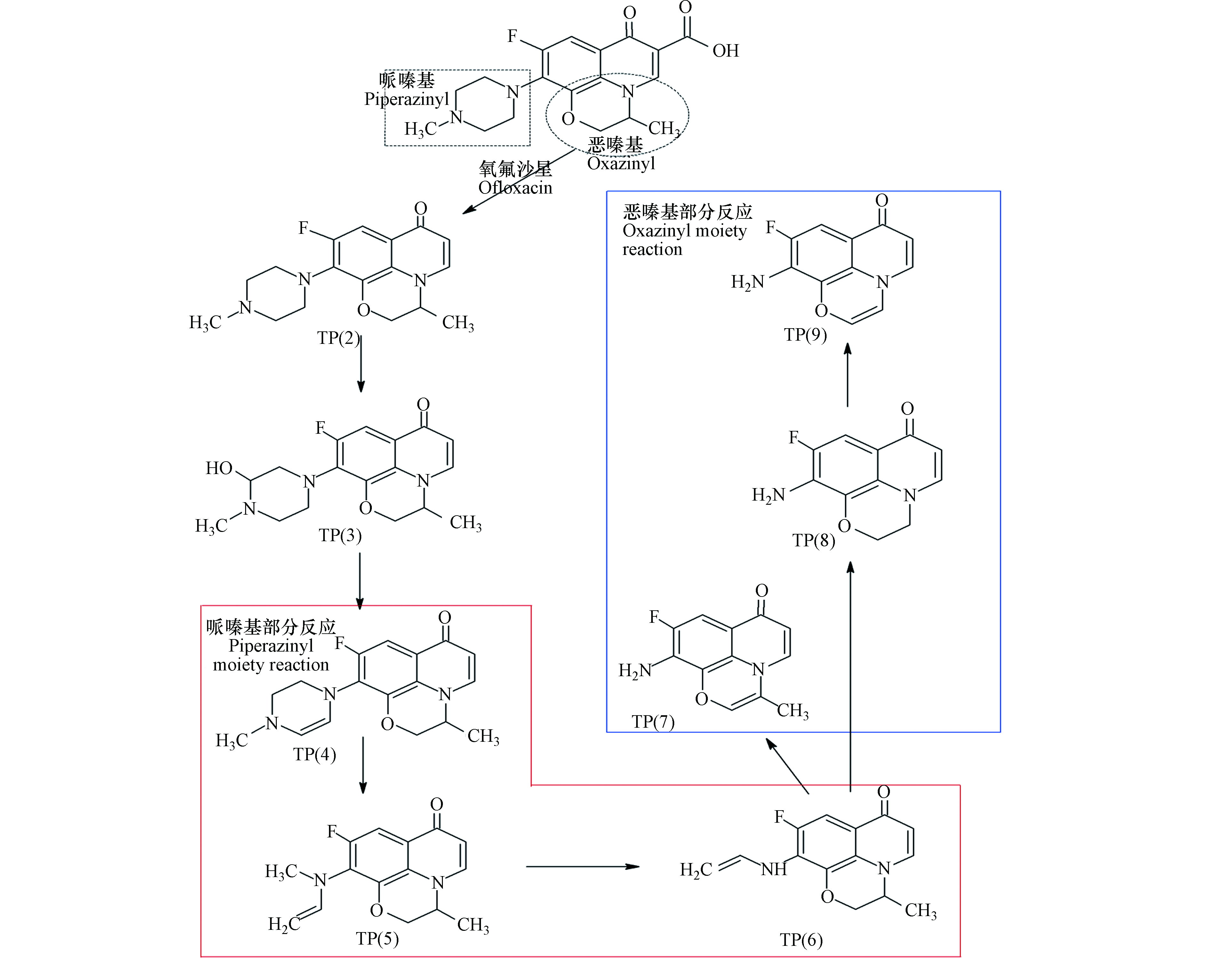
图4显示了氧氟沙星的芬顿氧化路径,其中TPs(4—6)代表氧氟沙星在芬顿氧化过程中哌嗪环取代基形成的转化产物。当·OH攻击哌嗪环取代基时,会形成两个不同的降解途径。TP(3)为在甲基处反应后的转化产物,生成的以碳为中心的自由基有助于消除甲基,从而发生酸催化的水解反应,可以认为是在哌嗪环取代基上添加了羟基,此外,TP(3)可能会受到额外的·OH攻击而形成TP(4)。甲基的供电子能力能够影响降解产物的生成,其中甲基可以从哌嗪环取代基上解离,TP(4)通过消除甲基而转变为TP(5),并通过失去C和N来打开哌嗪基环。此外,可以通过进一步消除甲基来生成TP(6)。最后,当·OH攻击恶嗪环取代基时,可能有助于消除甲基,或协助形成TPs(7—9)的乙烯键[84].
FQs在芬顿氧化过程中会共同发生的反应有哌嗪环断裂、脱氟和脱羧反应。由于FQs中存在两个强吸电子取代基,即羧基和氟,容易被·OH攻击,因此在芬顿氧化过程中会发生脱羧和脱氟反应。由于氟原子的诱导效应,使哌嗪环承受一个角应力,进而导致哌嗪环部分降解(乙烯基丢失),已经有文献显示使用不同种类的AOPs均可实现哌嗪环部分的降解[44, 118]。在降解过程中·OH发挥了重要作用,而哌嗪环是分子结构中最容易被氧化的部分,它会首先受到亲电子的·OH的攻击[119]。哌嗪环受·OH攻击后主要发生两个降解反应:生成以碳为中心的自由基,进而在甲基上进行酸催化的水解反应;哌嗪基的另一部分会被·OH直接氧化导致哌嗪环裂解[120]。哌嗪环部分的完全降解和脱氟可以同时发生,产生两种不同的降解产物,脱氟以及哌嗪环降解后的产物可以部分转化为分子量更低的产物。除此之外,脱羧和羟基化可以形成新的降解产物。对于没有哌嗪环的FQs,芬顿和光芬顿过程中的主要反应路径包括脱氟、脱氧和烷基氨基环的链断裂[121]。此外,氧氟沙星存在其它FQs没有的恶嗪基基团,此基团也会受到·OH的攻击而发生裂解,这是因为恶嗪基上的C7—C8的长度相对较大,易被攻击导致其被破坏[122]。
总体而言,FQs的母体喹诺酮环在芬顿降解过程中保持稳定,脱氟和脱羧的不同顺序最终将导致形成不同的降解产物。到目前为止,导致FQs脱氟和脱羧反应顺序差异的根本原因尚未被揭示,仍有待深入研究[123]。
-
反应动力学研究有助于定量认识芬顿和光芬顿氧化中影响有机污染物降解速率的主要参数,如溶液的pH值、H2O2和Fe2+的剂量、反应温度等。更重要的是,反应动力学参数可以为处理工艺条件的设计和优化提供科学依据。
芬顿和光芬顿氧化过程中FQs的降解速率由其与·OH之间的二阶反应速率常数(k)决定,而k值通常可以利用竞争动力学方法确定。Luo等选择了检出率高的抗生素FQs和磺胺类药物作为模型化合物,通过竞争动力学实验确定了几种化合物与·OH反应的二阶速率常数,并构建了定量结构活性关系模型(QSAR),该模型共有三个分子结构描述符,即CH2RX片段数量(C-006)、C原子上的正原子静电荷(qc+)和分子偶极矩(μ)[124]。表2为几种FQs与·OH反应的二阶速率常数及3种分子结构的具体数值,根据表中数据可以得知在同样的反应条件下,恩诺沙星的降解效率最高,因为其具有较多的CH2RX结构,碳原子上的正电荷较少,同时分子偶极矩较高。由于·OH会与具有活性氢的官能团发生夺氢反应,因此带有CH2RX基团的化学物质容易受到·OH的攻击,从而导致其与·OH的反应速率较快;带有正电荷的碳原子给电子能力较弱,由于·OH是亲电试剂,因此具有这些碳原子的化学物质几乎不会受到·OH的攻击,从而导致·OH反应性降低。μ是相对于正电荷和负电荷中心的位移,并携带有关分子中电荷分布的信息,μ值较高的化学物质更倾向于与·OH反应。
-
芬顿和光芬顿氧化均能实现FQs的有效降解和矿化,其中光芬顿氧化的降解和矿化效率要高于芬顿氧化。因为FQs在降解过程中会生成稳定的中间降解产物,而不是被完全矿化,有必要评估处理后水体的生态毒理效应。
Gupta和Garg通过活性污泥进行呼吸抑制试验考察了环丙沙星废水在经芬顿氧化处理前后的毒性,结果表明,经芬顿处理后废水毒性降低,对微生物底物利用的抑制率由67%降低至39%[85]。在6 h后,未经处理和处理过的废水的氧气吸收率分别为9.8 mg·L−1·h−1和18.1 mg·L−1·h−1,表明芬顿氧化可以有效降低环丙沙星的毒性[85]。
大型蚤急性毒性试验结果显示太阳光芬顿法处理后的氧氟沙星废水(含8.5 mg·L−1溶解性有机碳)相比未经处理的废水出现了毒性增强现象[125]。进一步的分析表明,增加的毒性作用来自于废水中存在的溶解有机碳经过通过太阳光芬顿处理产生的副产物,而不是来自氧氟沙星的转化中间体[126]。此外,氧氟沙星废水经太阳光芬顿法处理后与初始废水相比对不同植物(高粱、番茄、小白菜)的毒性均显著降低[51, 121, 126]。
综上所述,FQs在芬顿工艺中产生的副产物不会增加溶液的毒性,经过芬顿和光芬顿处理后的溶液生态毒性显著下降,对生态系统的危害可以得到有效控制。
-
FQs具有诸多优点而在医疗和禽畜养殖业中广泛使用,但其大量进入环境会带来潜在的生态和公共健康风险。芬顿和光芬顿作为高效且经济的废水高级氧化技术,在控制工业废水以及养殖场污水中FQs等抗生素污染中具有重要的实用价值。已有研究对芬顿和光芬顿氧化的反应机理与影响因素、FQs在芬顿和光芬顿氧化过程中降解的产物、路径以及动力学获得了较为全面的认识。为了进一步提高芬顿及光芬顿处理的效果、降低运营成本和环境风险,未来研究和应用中应优先考虑以下问题:
(1)芬顿反应的降解效率主要由·OH的产量决定,而Fe2+作为催化剂影响·OH的产生。因此,通过不同的方法促进芬顿体系中Fe2+与Fe3+之间的循环,可以显著提高污染物的降解效率并减少铁污泥的产生。
(2)光芬顿法整体而言对光能利用率较低,使得能耗较大,成本增高。因此,提高工艺中对紫外线能量的利用率以及完善基于太阳光的光芬顿法对于减少能源消耗和降低处理成本具有重要的意义。
(3)单独使用芬顿或光芬顿法处理含FQs等抗生素废水的费用通常较高,可以将其作为预处理方法提高FQs等抗生素的生物降解性,后续与微生物法联用以降低废水处理成本。
(4)已开展的AOPs研究集中在第三代喹诺酮类抗生素的降解上,但随着其大量和长时间的使用,人类及牲畜会出现不同程度的抗药性,使其治疗效果下降。未来第四代喹诺酮类抗生素的用量将越来越大,因此有必要研究芬顿和光芬顿法对第四代喹诺酮类抗生素的处理效果与降解机理。
芬顿及光芬顿法降解氟喹诺酮类抗生素研究进展
Recent development in the degradation of fluoroquinolones by Fenton and photo-Fenton processes
-
摘要: 氟喹诺酮类抗生素的广泛使用导致其在环境中被广泛检出。现有的污水处理工艺无法有效去除废水中的氟喹诺酮类抗生素,而高效的降解方法对于控制氟喹诺酮类抗生素的污染至关重要。芬顿法能够降解大多数难处理有机污染物,同时具有适用范围广、抗干扰能力强、操作简单和污染物降解迅速等优点。本文综述了芬顿和光芬顿反应降解氟喹诺酮类抗生素的最新进展、相关的反应机理和主要工艺条件(如pH值、芬顿试剂剂量和光源等)对降解效率的影响,以及典型氟喹诺酮类抗生素的芬顿及光芬顿降解的产物与降解路径。随着对环境中抗生素污染危害的认识,未来有必要加强对芬顿及光芬顿这类高效、低成本处理技术的研究,为氟喹诺酮类及其它类型抗生素的危害消减提供理论依据与技术支持。Abstract: Fluoroquinolones have been widely detected in the environment due to their widespread use. Existing technologies employed in wastewater treatment facilities cannot effectively remove fluoroquinolones, while highly efficient removal methods are important for controlling their pollution. Fenton process can effectively degrade most refractory organic pollutants and has the advantages of wide applicability, robust performance, simple operation, and rapid degradation of organic pollutants. This review summarizes the recent development in the degradation of fluoroquinolones by Fenton and photo-Fenton processes, the relevant reaction mechanism, and the effect of major operating parameters (including solution pH, dosage of Fenton’s reagent, and light source) on the treatment performance, as well as the degradation products and pathways of representative fluoroquinolones by Fenton and photo-Fenton processes. With the improved understanding on the environmental risk of antibiotic pollution, it is necessary to strengthen the research on efficient and low-cost treatment technologies, such as Fenton and photo-Fenton processes, to provide theoretical basis and technological support for eliminating the risk of fluoroquinolones and other types of antibiotics.
-

-
表 1 氟喹诺酮类抗生素的芬顿和光芬顿氧化
Table 1. Fenton and photo-Fenton oxidation of fluoroquinolones
氧化方式
Oxidation method目标物
Target pollutant初始浓度
Initial concentration最佳操作条件
Optimum operating conditions降解效率
Degradation efficiency参考文献
Reference芬顿氧化 环丙沙星 15 mg·L−1 [Fe2+]/[H2O2] = 0.125; pH = 3.5 74.4% [77] 诺氟沙星 15 mg·L−1 [Fe2+] = 0.8 mmol·L−1; [H2O2] =
5.64 mmol·L−1; pH = 3.060% [83] 氧氟沙星 24.93 mmol·L−1 [Fe2+] = 0.03 mmol·L−1; [H2O2] =
15.0 mmol·L−1; pH = 4.093.1% [84] 环丙沙星 100 mg·L−1 [H2O2]/[Fe2+] = 10; pH = 3.0 70% [85] 光芬顿氧化 氧氟沙星 100 μg·L−1 [Fe2+] = 5 mg·L−1; [H2O2] = 75 mg·L−1;
pH = 2.8—2.978.6% [51] 环丙沙星 0.15 mmol·L−1 [Fe2+] = 0.5 mmol·L−1; [H2O2] =
10 mmol·L−1; pH = 3.0100% [86] 恩诺沙星 0.15 mmol·L−1 [Fe2+] = 0.5 mmol·L−1; [H2O2] =
10 mmol·L−1; pH = 3.0100% [86] 环丙沙星 15 mg·L−1 [H2O2] = 5.0 mmol·L−1; [Fe2+] =
0.05 mmol·L−1; pH = 4.0100% [87] 表 2 部分FQs与·OH反应的二阶速率常数与相应的QSAR模型基础数据[124]
Table 2. The second-order rate constants of the reactions between selected FQs and ·OH and their property data for the corresponding QSAR model[124]
目标物
Target pollutant二阶速率常数/(L·mol−1·s−1)
Second-order rate
constantCH2RX片段数量(C-006)
Number of CH2RX fragment碳原子上的正原子静电荷(qc+)
Most positive net atomic
charge on C atom分子偶极矩(μ)
Molecular dipole moment环丙沙星 (3.32 ± 0.40) × 1010 4.0 1.049 58.89 诺氟沙星 (3.63 ± 0.44) × 1010 5.0 1.268 59.01 氧氟沙星 (1.57 ± 0.26) × 1010 5.0 0.8547 57.77 恩诺沙星 (4.16 ± 0.22) × 1010 5.0 1.040 59.97 -
[1] KUMAR A, KHAN M, ZENG X, et al. Development of g-C3N4/TiO2/Fe3O4@SiO2 heterojunction via sol-gel route: A magnetically recyclable direct contact Z-scheme nanophotocatalyst for enhanced photocatalytic removal of ibuprofen from real sewage effluent under visible light [J]. Chemical Engineering Journal, 2018, 353: 645-656. doi: 10.1016/j.cej.2018.07.153 [2] NARVÁEZ J A L, MAHECHA P V. Exploring the potential of using the residues of lulo (Solanum quitoense) for the production of biopolymers [J]. Acta Agronomica, 2012, 61(1): 93-94. [3] KÜMMERER K. Antibiotics in the aquatic environment – A review – Part I [J]. Chemosphere, 2009, 75(4): 417-434. doi: 10.1016/j.chemosphere.2008.11.086 [4] 张君, 封丽, 田隽, 等. 氟喹诺酮类在环境中的分布及去除研究进展 [J]. 环境科学与技术, 2019, 42(S1): 77-84. ZHANG J, FENG L, TIAN J, et al. Research progress on the distribution and removal of fluoroquinolones in the environment [J]. Environmental Science And Technology, 2019, 42(S1): 77-84(in Chinese).
[5] ČVANČAROVÁ M, MOEDER M, FILIPOVÁ A, et al. Biotransformation of fluoroquinolone antibiotics by ligninolytic fungi – Metabolites, enzymes and residual antibacterial activity [J]. Chemosphere, 2015, 136: 311-320. doi: 10.1016/j.chemosphere.2014.12.012 [6] GOLET E M, XIFRA I, SIEGRIST H, et al. Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil [J]. Environmental Science and Technology, 2003, 37(15): 3243-3249. doi: 10.1021/es0264448 [7] WANG P, HE Y L, HUANG CH. Oxidation of fluoroquinolone antibiotics and structurally related amines by chlorine dioxide: Reaction kinetics, product and pathway evaluation [J]. Water Research, 2010, 44(20): 5989-5998. doi: 10.1016/j.watres.2010.07.053 [8] XU W, ZHANG G, LI X, et al. Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China [J]. Water Research, 2007, 41(19): 4526-4534. doi: 10.1016/j.watres.2007.06.023 [9] GÖBEL A, THOMSEN A, MCARDELL C S, et al. Extraction and determination of sulfonamides, macrolides, and trimethoprim in sewage sludge [J]. Journal of Chromatography A, 2005, 1085(2): 179-189. doi: 10.1016/j.chroma.2005.05.051 [10] MCARDELL C, MOLNAR E, SUTER M, et al. Occurrence and fate of macrolide antibiotics in wastewater treatment plants and in the Glatt Valley Watershed, Switzerland [J]. Environmental Science & Technology, 2003, 37: 5479-86. [11] GOLET E M, ALDER A C, GIGER W. Environmental exposure and risk assessment of fluoroquinolone antibacterial agents in wastewater and river water of the glatt valley watershed, Switzerland [J]. Environmental Science & Technology, 2002, 36(17): 3645-3651. [12] HUBER M M, GÖBEL A, JOSS A, et al. Oxidation of pharmaceuticals during ozonation of municipal wastewater effluents: A pilot study [J]. Environmental Science & Technology, 2005, 39(11): 4290-4299. [13] SIRÉS I, BRILLAS E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review [J]. Environment International, 2012, 40(1): 212-229. [14] SPELTINI A, STURINI M, MARASCHI F, et al. Fluoroquinolone antibiotics in environmental waters: Sample preparation and determination [J]. Journal of Separation Science, 2010, 33(8): 1115-1131. [15] LILLENBERG M, YURCHENKO S, KIPPER K, et al. Simultaneous determination of fluoroquinolones, sulfonamides and tetracyclines in sewage sludge by pressurized liquid extraction and liquid chromatography electrospray ionization-mass spectrometry [J]. Journal of Chromatography A, 2009, 1216(32): 5949-5954. doi: 10.1016/j.chroma.2009.06.029 [16] LI B, ZHANG T. Biodegradation and adsorption of antibiotics in the activated sludge process [J]. Environmental Science & Technology, 2010, 44(9): 3468-3473. [17] JIA A, WAN Y, XIAO Y, et al. Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant [J]. Water Research, 2012, 46(2): 387-394. doi: 10.1016/j.watres.2011.10.055 [18] SPELTINI A, STURINI M, MARASCHI F, et al. Analytical methods for the determination of fluoroquinolones in solid environmental matrices [J]. TrAC Trends in Analytical Chemistry, 2011, 30(8): 1337-1350. doi: 10.1016/j.trac.2011.04.011 [19] GU C, KARTHIKEYAN K G. Sorption of the antimicrobial ciprofloxacin to aluminum and iron hydrous oxides [J]. Environmental Science & Technology, 2005, 39(23): 9166-9173. [20] LI H, ZHANG D, HAN X, et al. Adsorption of antibiotic ciprofloxacin on carbon nanotubes: pH dependence and thermodynamics [J]. Chemosphere, 2014, 95: 150-155. doi: 10.1016/j.chemosphere.2013.08.053 [21] HU L, MARTIN H M, STRATHMANN T J. Oxidation kinetics of antibiotics during water treatment with potassium permanganate [J]. Environmental Science & Technology, 2010, 44(16): 6416-6422. [22] YANG Y, OK Y S, KIM K H, et al. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review [J]. Science of the Total Environment, 2017, 596-597: 303-320. doi: 10.1016/j.scitotenv.2017.04.102 [23] KIM H, HWANG Y S, SHARMA V K. Adsorption of antibiotics and iopromide onto single-walled and multi-walled carbon nanotubes [J]. Chemical Engineering Journal, 2014, 255: 23-27. doi: 10.1016/j.cej.2014.06.035 [24] ÖTKER H M, AKMEHMET B I. Adsorption and degradation of enrofloxacin, a veterinary antibiotic on natural zeolite [J]. Journal of Hazardous Materials, 2005, 122(3): 251-258. doi: 10.1016/j.jhazmat.2005.03.005 [25] VAN W E M, SEYMOUR M D, PETERSON J W. Interaction of the fluoroquinolone antibiotic, ofloxacin, with titanium oxide nanoparticles in water: Adsorption and breakdown [J]. Science of the Total Environment, 2012, 441: 1-9. doi: 10.1016/j.scitotenv.2012.09.067 [26] JIANG C, ZHANG X, XU X, et al. Magnetic mesoporous carbon material with strong ciprofloxacin adsorption removal property fabricated through the calcination of mixed valence Fe based metal-organic framework [J]. Journal of Porous Materials, 2016, 23(5): 1297-1304. doi: 10.1007/s10934-016-0188-x [27] MICHAEL I, RIZZO L, MCARDELL C S, et al. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review [J]. Water Research, 2013, 47(3): 957-995. doi: 10.1016/j.watres.2012.11.027 [28] KLAVARIOTI M, MANTZAVINOS D, KASSINOS D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes [J]. Environment International, 2009, 35(2): 402-417. doi: 10.1016/j.envint.2008.07.009 [29] MALATO S, MALDONADO M I, BLANCO J, et al. Decontamination and disinfection of water by solar photocatalysis: recent overview and trends [J]. Catalysis Today, 2009, 147(1): 1-59. doi: 10.1016/j.cattod.2009.06.018 [30] FENG M, WANG Z Y, DIONYSIOU D D, et al. Metal-mediated oxidation of fluoroquinolone antibiotics in water: A review on kinetics, transformation products, and toxicity assessment [J]. Journal of Hazardous Materials, 2018, 344: 1136-1154. doi: 10.1016/j.jhazmat.2017.08.067 [31] LITTER M I. Introduction to photochemical advanced oxidation processes for water treatment [J]. Environmental Photochemistry Part II, 2005, 2: 325-366. [32] ANDREOZZI R, CAPRIO V, INSOLA A, et al. Advanced oxidation processes (AOP) for water purification and recovery [J]. Catalysis Today, 1999, 53(1): 51-59. doi: 10.1016/S0920-5861(99)00102-9 [33] DUESTERBERG C K, WAITE T D. Process optimization of Fenton oxidation using kinetic modeling [J]. Environmental Science & Technology, 2006, 40(13): 4189-4195. [34] CHENG M, ZENG G M, HUANG D L, et al. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review [J]. Chemical Engineering Journal, 2016, 284: 582-598. doi: 10.1016/j.cej.2015.09.001 [35] BOKARE A D, CHOI W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes [J]. Journal of Hazardous Materials, 2014, 275: 121-135. doi: 10.1016/j.jhazmat.2014.04.054 [36] SHARMA A, AHMAD J, FLORA S J S. Application of advanced oxidation processes and toxicity assessment of transformation products [J]. Environmental Research, 2018, 167: 223-233. doi: 10.1016/j.envres.2018.07.010 [37] ADAMS C, WANG Y, LOFTIN K, et al. Removal of antibiotics from sur face and distilled water in conventional water treatment processes [J]. Journal of Environmental Engineering, 2002, 128(3): 253-260. doi: 10.1061/(ASCE)0733-9372(2002)128:3(253) [38] ARSLAN A I, DOGRUEL S. Pre-treatment of penicillin formulation effluent by advanced oxidation processes [J]. Journal of Hazardous Materials, 2004, 112(1): 105-113. [39] ELMOLLA E S, CHAUDHURI M. The feasibility of using combined TiO2 photocatalysis-SBR process for antibiotic wastewater treatment [J]. Desalination, 2011, 272(1): 218-224. [40] AN T, YANG H, LI G, et al. Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water [J]. Applied Catalysis B: Environmental, 2010, 94(3): 288-294. [41] AN T, YANG H, SONG W, et al. Mechanistic considerations for the advanced oxidation treatment of fluoroquinolone pharmaceutical compounds using TiO2 heterogeneous catalysis [J]. Journal of Physical Chemistry A, 2010, 114(7): 2569-2575. doi: 10.1021/jp911349y [42] PAUL T, MILLER P L, STRATHMANN T J. Visible-light-mediated TiO2 photocatalysis of fluoroquinolone antibacterial agents [J]. Environmental Science & Technology, 2007, 41(13): 4720-4727. [43] DEWITTE B, DEWULF J, DEMEESTERE K, et al. Ozonation of ciprofloxaci n in water: HRMS identification of reaction products and pathways [J]. Environmental Science & Technology, 2008, 42(13): 4889-4895. [44] LIU C, NANABOINA V, KORSHIN G V, et al. Spectroscopic study of degradation products of ciprofloxacin, norfloxacin and lomefloxacin formed in ozonated wastewater [J]. Water Research, 2012, 46(16): 5235-5246. doi: 10.1016/j.watres.2012.07.005 [45] CARBAJO J B, PETRE A L, ROSAL R., et al Continuous ozonation treatment of ofloxacin: Transformation products, water matrix effect and aquatic toxicity [J]. Journal of Hazardous Materials, 2015, 292: 34-43. doi: 10.1016/j.jhazmat.2015.02.075 [46] XU Y, LIU S, GUO F, et al. Evaluation of the oxidation of enrofloxacin by permanganate and the antimicrobial activity of the products [J]. Chemosphere, 2016, 144: 113-121. doi: 10.1016/j.chemosphere.2015.07.083 [47] KULKARNI R M, HANAGADAKAR M S, MALLADI R S, et al. Experimental and theoretical studies on the oxidation of lomefloxacin by alkaline permanganate [J]. Desalination and Water Treatment, 2016, 57(23): 10826-10838. doi: 10.1080/19443994.2015.1037797 [48] JIANG J Q, ZHOU Z, PAHL O. Preliminary study of ciprofloxacin (cip) removal by potassium ferrate(Ⅵ) [J]. Separation and Purification Technology, 2012, 88: 95-98. doi: 10.1016/j.seppur.2011.12.021 [49] ZHOU Z, JIANG J Q. Reaction kinetics and oxidation products formation in the degradation of ciprofloxacin and ibuprofen by ferrate(VI) [J]. Chemosphere, 2015, 119: S95-S100. doi: 10.1016/j.chemosphere.2014.04.006 [50] BARIŞÇI S, ULU F, SILLANPÄÄ M. et al The usage of different forms of ferrate (Ⅵ) ion for amoxicillin and ciprofloxacin removal: density functional theory based modelling of redox decomposition [J]. Journal of Chemical Technology & Biotechnology, 2016, 91(1): 257-266. [51] MICHAEL I, HAPESHI E, MICHAEL C, et al. Solar photo-Fenton process on the abatement of antibiotics at a pilot scale: Degradation kinetics, ecotoxicity and phytotoxicity assessment and removal of antibiotic resistant enterococci [J]. Water Research, 2012, 46(17): 5621-5634. doi: 10.1016/j.watres.2012.07.049 [52] ÖZCAN A, ATILIR ÖZCAN A, DEMIRCI Y. Evaluation of mineralization kinetics and pathway of norfloxacin removal from water by electro-Fenton treatment [J]. Chemical Engineering Journal, 2016, 304: 518-526. doi: 10.1016/j.cej.2016.06.105 [53] WANG N, ZHENG T, ZHANG G S, et al. A review on Fenton-like processes for organic wastewater treatment [J]. Journal of Environmental Chemical Engineering, 2016, 4(1): 762-787. doi: 10.1016/j.jece.2015.12.016 [54] CHEN L, MA J, LI X C, et al. Strong enhancement on Fenton oxidation by addition of hydroxylamine to accelerate the ferric and ferrous iron cycles [J]. Environmental Science & Technology, 2011, 45(9): 3925-3930. [55] FENTON H J H, LXXIII. Oxidation of tartaric acid in presence of iron [J]. Journal of the Chemical Society, Transactions, 1894, 65(0): 899-910. doi: 10.1039/CT8946500899 [56] TEKIN H, BILKAY O, ATABERK S, et al. Use of Fenton oxidation to improve the biodegradability of a pharmaceutical wastewater [J]. Journal of Hazardous Materials, 2006, 136: 258-65. doi: 10.1016/j.jhazmat.2005.12.012 [57] 何东芹, Fenton类高级氧化反应在污泥脱水和污染物降解中的作用机制[D]. 合肥: 中国科学技术大学, 2017. HE D Q. The mechanism of Fenton-like advanced oxidation reactions in sludge dewatering and pollutant degradation [D]. Hefei: University of Science and Technology of China, 2017.
[58] DUESTERBERG C K, MYLON S E, WAITE T D. pH effects on iron-catalyzed oxidation using Fenton’s reagent [J]. Environmental Science & Technology, 2008, 42(22): 8522-8527. [59] BABUPONNUSAMI A, MUTHUKUMAR K. A review on Fenton and improvements to the Fenton process for wastewater treatment [J]. Journal of Environmental Chemical Engineering, 2014, 2(1): 557-572. doi: 10.1016/j.jece.2013.10.011 [60] ONG W, CHENG M, MA J, et al. Decomposition of hydrogen peroxide driven by photochemical cycling of iron species in clay [J]. Environmental Science & Technology, 2006, 40(15): 4782-4787. [61] NIE Y, HU C, QU J, et al. Efficient photodegradation of Acid Red B by immobilized ferrocene in the presence of UVA and H2O2 [J]. Journal of Hazardous Materials, 2008, 154(1): 146-152. [62] WANG J, WANG S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review [J]. Journal of Environmental Management, 2016, 182: 620-640. doi: 10.1016/j.jenvman.2016.07.049 [63] MACHULEK A, MORAES J E F. VAUTIER-GIONGO C., et al Abatement of the inhibitory effect of chloride anions on the photo-Fenton process [J]. Environmental Science & Technology, 2007, 41(24): 8459-8463. [64] AVETTA P, PENSATO A, MINELLA M, et al. Activation of persulfate by irradiated magnetite: Implications for the degradation of phenol under heterogeneous photo-Fenton-like conditions [J]. Environmental Science & Technology, 2015, 49(2): 1043-1050. [65] CLARIZIA L, RUSSO D, DI SOMMA I, et al. Homogeneous photo-Fenton processes at near neutral pH: A review [J]. Applied Catalysis B: Environmental, 2017, 209: 358-371. doi: 10.1016/j.apcatb.2017.03.011 [66] MAEZONO T, TOKUMURA M, SEKINE M, et al. Hydroxyl radical concentration profile in photo-Fenton oxidation process: Generation and consumption of hydroxyl radicals during the discoloration of azo-dye Orange II [J]. Chemosphere, 2011, 82(10): 1422-1430. doi: 10.1016/j.chemosphere.2010.11.052 [67] IGNATELLO J J, OLIVEROS E, MACKAY A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry [J]. Critical Reviews in Environmental Science and Technology, 2006, 36(1): 1-84. doi: 10.1080/10643380500326564 [68] RAHIM P S, ABDUL A A R, WAN D W M A. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters [J]. Journal of Industrial and Engineering Chemistry, 2015, 21: 53-69. doi: 10.1016/j.jiec.2014.05.005 [69] LIU R, XU Y, CHEN B. Self-assembled nano-FeO(OH)/reduced graphene oxide aerogel as a reusable catalyst for photo-Fenton degradation of phenolic organics [J]. Environmental Science & Technology, 2018, 52(12): 7043-7053. [70] BRILLAS E, SIRÉS I, OTURAN M A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry [J]. Chemical Reviews, 2009, 109(12): 6570-6631. doi: 10.1021/cr900136g [71] JO W K, TAYADE R J. New generation energy-efficient light source for photocatalysis: LEDs for environmental applications [J]. Industrial & Engineering Chemistry Research, 2014, 53(6): 2073-2084. [72] LIU J, WU J Y, KANG C L, et al. Photo-Fenton effect of 4-chlorophenol in ice [J]. Journal of Hazardous Materials, 2013, 261: 500-511. doi: 10.1016/j.jhazmat.2013.07.040 [73] PLIEGO G, XEKOUKOULOTAKIS N, VENIERI D, et al. Complete degradation of the persistent anti-depressant sertraline in aqueous solution by solar photo-Fenton oxidation [J]. Journal of Chemical Technology & Biotechnology, 2014, 89(6): 814-818. [74] PLIEGO G, ZAZO J A, GARCIA M P, et al. Trends in the intensification of the Fenton process for wastewater treatment: an overview [J]. Critical Reviews in Environmental Science and Technology, 2015, 45(24): 2611-2692. doi: 10.1080/10643389.2015.1025646 [75] BARHOUMI N, LABIADH L, OTURAN M A, et al. Electrochemical mineralization of the antibiotic levofloxacin by electro-Fenton-pyrite process [J]. Chemosphere, 2015, 141: 250-257. doi: 10.1016/j.chemosphere.2015.08.003 [76] YAHYA M S, OTURAN N, EL K K, et al. Oxidative degradation study on antimicrobial agent ciprofloxacin by electro-fenton process: Kinetics and oxidation products [J]. Chemosphere, 2014, 117: 447-454. doi: 10.1016/j.chemosphere.2014.08.016 [77] GIRI A S, GOLDER A K. Ciprofloxacin degradation from aqueous solution by Fenton oxidation: Reaction kinetics and degradation mechanisms [J]. RSC Advances, 2014, 4(13): 6738-6745. doi: 10.1039/c3ra45709e [78] XIAO X, ZENG X, LEMLEY A T. Species-dependent degradation of ciprofloxacin in a membrane anodic Fenton system [J]. Journal of Agricultural and Food Chemistry, 2010, 58(18): 10169-10175. doi: 10.1021/jf101943c [79] GONG Y, LI J, ZHANG Y, et al. Partial degradation of levofloxacin for biodegradability improvement by electro-Fenton process using an activated carbon fiber felt cathode [J]. Journal of Hazardous Materials, 2016, 304: 320-328. doi: 10.1016/j.jhazmat.2015.10.064 [80] YAHYA M S, EL KARBANE M, OTURAN N, et al. Mineralization of the antibiotic levofloxacin in aqueous medium by electro-Fenton process: kinetics and intermediate products analysis [J]. Environmental Technology, 2016, 37(10): 1276-1287. doi: 10.1080/09593330.2015.1111427 [81] ANNABI C, FOURCADE F, SOUTREL I, et al. Degradation of enoxacin antibiotic by the electro-Fenton process: Optimization, biodegradability improvement and degradation mechanism [J]. Journal of Environmental Management, 2016, 165: 96-105. doi: 10.1016/j.jenvman.2015.09.018 [82] GARCIA S S, GARRIDO J A, RODRÍGUEZ R M, et al. Mineralization of flumequine in acidic medium by electro-Fenton and photoelectro-Fenton processes [J]. Water Research, 2012, 46(7): 2067-2076. doi: 10.1016/j.watres.2012.01.019 [83] SANTOS L V D S, MEIRELES A M, LANGE L C. Degradation of antibiotics norfloxacin by Fenton, UV and UV/H2O2 [J]. Journal of Environmental Management, 2015, 154: 8-12. [84] PI Y, FENG J, SONG M, et al. Degradation potential of ofloxacin and its resulting transformation products during Fenton oxidation process [J]. Chinese Science Bulletin, 2014, 59(21): 2618-2624. doi: 10.1007/s11434-014-0293-7 [85] GUPTA A, GARG A. Degradation of ciprofloxacin using Fenton's oxidation: Effect of operating parameters, identification of oxidized by-products and toxicity assessment [J]. Chemosphere, 2018, 193: 1181-1188. doi: 10.1016/j.chemosphere.2017.11.046 [86] BOBU M, YEDILER A, SIMINICEANU I, et al. Comparison of different advanced oxidation processes for the degradation of two fluoroquinolone antibiotics in aqueous solutions [J]. Journal of Environmental Science and Health, Part A, 2013, 48(3): 251-262. doi: 10.1080/10934529.2013.726805 [87] SUN S P, GUO H Q, KE Q, et al. Degradation of antibiotic ciprofloxacin hydrochloride by photo-Fenton oxidation process [J]. Environmental Engineering Science, 2009, 26(4): 753-759. doi: 10.1089/ees.2008.0076 [88] 吴健, 熊振湖. UV/Fenton法对诺氟沙星的降解与矿化 [J]. 天津城市建设学院学报, 2008, 14(4): 259-262. WU J, XIONG Z H. Degradation and mineralization of norfloxacin by UV/Fenton method [J]. Journal of Tianjin Institute of Urban Construction, 2008, 14(4): 259-262(in Chinese).
[89] WANG C, YU G, CHEN H, et al. Degradation of norfloxacin by hydroxylamine enhanced fenton system: Kinetics, mechanism and degradation pathway [J]. Chemosphere, 2021, 270: 129408. doi: 10.1016/j.chemosphere.2020.129408 [90] GIRI A S, GOLDER A K. Ciprofloxacin degradation in photo-Fenton and photo-catalytic processes: Degradation mechanisms and iron chelation [J]. Journal of Environmental Sciences, 2019, 80: 82-92. doi: 10.1016/j.jes.2018.09.016 [91] NOGUEIRA A A, SOUZA B M, DEZOTTI M W C, et al. Ferrioxalate complexes as strategy to drive a photo-Fenton reaction at mild pH conditions: A case study on levofloxacin oxidation [J]. Journal of Photochemistry and Photobiology A: Chemistry, 2017, 345: 109-123. doi: 10.1016/j.jphotochem.2017.05.020 [92] PERINI J A L, TONETTI A L, VIDAL C, et al. Simultaneous degradation of ciprofloxacin, amoxicillin, sulfathiazole and sulfamethazine, and disinfection of hospital effluent after biological treatment via photo-Fenton process under ultraviolet germicidal irradiation [J]. Applied Catalysis B: Environmental, 2018, 224: 761-771. doi: 10.1016/j.apcatb.2017.11.021 [93] SEIBERT D, DIEL T, WELTER J B, et al. Performance of photo-Fenton process mediated by Fe (III)-carboxylate complexes applied to degradation of landfill leachate [J]. Journal of Environmental Chemical Engineering, 2017, 5(5): 4462-4470. doi: 10.1016/j.jece.2017.08.043 [94] MIRALLES CUEVAS S, AUDINO F, OLLER I, et al. Pharmaceuticals removal from natural water by nanofiltration combined with advanced tertiary treatments (solar photo-Fenton, photo-Fenton-like Fe(III)–EDDS complex and ozonation) [J]. Separation and Purification Technology, 2014, 122: 515-522. doi: 10.1016/j.seppur.2013.12.006 [95] LIU X, ZHOU Y, ZHANG J, et al. Insight into electro-Fenton and photo-Fenton for the degradation of antibiotics: Mechanism study and research gaps [J]. Chemical Engineering Journal, 2018, 347: 379-397. doi: 10.1016/j.cej.2018.04.142 [96] SHARMILA V G, KUMAR S A, BANU J R, et al. Feasibility analysis of homogenizer coupled solar photo Fenton process for waste activated sludge reduction [J]. Journal of Environmental Management, 2019, 238: 251-256. [97] HUANG A, ZHI D, TANG H, et al. Effect of Fe2+, Mn2+ catalysts on the performance of electro-Fenton degradation of antibiotic ciprofloxacin, and expanding the utilizing of acid mine drainage [J]. Science of the Total Environment, 2020, 720: 137560. doi: 10.1016/j.scitotenv.2020.137560 [98] CHEN Y, WANG A, ZHANG Y, et al. Electro-Fenton degradation of antibiotic ciprofloxacin (CIP): Formation of Fe3+-CIP chelate and its effect on catalytic behavior of Fe2+/Fe3+ and CIP mineralization [J]. Electrochimica Acta, 2017, 256: 185-195. doi: 10.1016/j.electacta.2017.09.173 [99] SUN J H, SUN S P, FAN M H, et al. Oxidative decomposition of p-nitroaniline in water by solar photo-Fenton advanced oxidation process [J]. Journal of Hazardous Materials, 2008, 153(1): 187-193. [100] FAUST B, HOIGNÉ J. Photolysis of Fe(Ⅲ)-hydroxy complexes as sources of OH radicals in clouds, fog and rain [J]. Atmospheric Environment. Part A. General Topics, 1990, 24: 79-89. doi: 10.1016/0960-1686(90)90443-Q [101] KIM Y K, HUH I R. Enhancing biological treatability of landfill leachate by chemical oxidation [J]. Environmental Engineering Science, 1997, 14: 73-79. doi: 10.1089/ees.1997.14.73 [102] KIM S M, VOGELPOHL A. Degradation of organic pollutants by the photo-Fenton-process [J]. Chemical Engineering & Technology, 1998, 21(2): 187-191. [103] KAVITHA V, PALANIVELU K. Destruction of cresols by Fenton oxidation process [J]. Water Research, 2005, 39(13): 3062-3072. doi: 10.1016/j.watres.2005.05.011 [104] HASSAN A K, RAHMAN M M, CHATTOPADHAY G, et al. Kinetic of the degradation of sulfanilic acid azochromotrop (SPADNS) by Fenton process coupled with ultrasonic irradiation or L-cysteine acceleration [J]. Environmental Technology & Innovation, 2019, 15: 100380. [105] PIGNATELLO J J. Dark and photoassisted iron(3+)-catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide [J]. Environmental Science & Technology, 1992, 26(5): 944-951. [106] FAN X, HAO H, WANG Y, et al. Fenton-like degradation of nalidixic acid with Fe3+/H2O2 [J]. Environmental Science and Pollution Research, 2013, 20(6): 3649-3656. doi: 10.1007/s11356-012-1279-0 [107] GUEDES A M F M, MADEIRA L M P, BOAVENTURA R A R, et al. Fenton oxidation of cork cooking wastewater—overall kinetic analysis [J]. Water Research, 2003, 37(13): 3061-3069. doi: 10.1016/S0043-1354(03)00178-7 [108] AMIMI M, QOURZAL S, BARKA N, et al. Methomyl degradation in aqueous solutions by Fenton's reagent and the photo-Fenton system [J]. Separation and Purification Technology, 2008, 61(1): 103-108. doi: 10.1016/j.seppur.2007.09.017 [109] BOSSMANN S, OLIVEROS E, GÖB S, et al. New evidence against hydroxyl radicals as reactive intermediates in the thermal and photochemically enhanced fenton reactions [J]. Journal of Physical Chemistry A, 1998, 102: 5542-5550. [110] WANG Q, TIAN S, NING. Degradation mechanism of methylene blue in a heterogeneous Fenton-like reaction catalyzed by ferrocene [J]. Industrial & Engineering Chemistry Research, 2014, 53(2): 643-649. [111] GOGATE P R, PANDIT A B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions [J]. Advances in Environmental Research, 2004, 8(3): 501-551. [112] PANIZZA M, CERISOLA G. Electro-Fenton degradation of synthetic dyes [J]. Water Research, 2009, 43(2): 339-344. doi: 10.1016/j.watres.2008.10.028 [113] KIM J S, KIM H Y, WON C H, et al. Treatment of leachate produced in stabilized landfills by coagulation and Fenton oxidation process [J]. Journal of the Chinese Institute of Chemical Engineers, 2001, 32: 425-429. [114] LAU I W C, WANG P, FANG H H. Organic removal of anaerobically treated leachate by Fenton coagulation [J]. Journal of Environmental Engineering, 2001, 127(7): 666-669. doi: 10.1061/(ASCE)0733-9372(2001)127:7(666) [115] GUO W, LI T, CHEN Q, et al. The roles of wavelength in the gaseous toluene removal with •OH from UV activated Fenton reagent [J]. Chemosphere, 2021: 129998. [116] HERNEY RAMIREZ J, VICENTE M A, MADEIRA L M. Heterogeneous photo-Fenton oxidation with pillared clay-based catalysts for wastewater treatment: A review [J]. Applied Catalysis B: Environmental, 2010, 98(1): 10-26. [117] FENG J, HU X, YUE P L, et al. Discoloration and mineralization of Reactive Red HE-3B by heterogeneous photo-Fenton reaction [J]. Water Research, 2003, 37(15): 3776-3784. doi: 10.1016/S0043-1354(03)00268-9 [118] ZHANG X, LI R, JIA M, et al. Degradation of ciprofloxacin in aqueous bismuth oxybromide (BiOBr) suspensions under visible light irradiation: A direct hole oxidation pathway [J]. Chemical Engineering Journal, 2015, 274: 290-297. doi: 10.1016/j.cej.2015.03.077 [119] BABIĆ S, PERIŠA M, ŠKORIĆ I. Photolytic degradation of norfloxacin, enrofloxacin and ciprofloxacin in various aqueous media [J]. Chemosphere, 2013, 91(11): 1635-1642. doi: 10.1016/j.chemosphere.2012.12.072 [120] GUINEA E, BRILLAS E, CENTELLAS F, et al. Oxidation of enrofloxacin with conductive-diamond electrochemical oxidation, ozonation and Fenton oxidation. A comparison [J]. Water Research, 2009, 43(8): 2131-2138. doi: 10.1016/j.watres.2009.02.025 [121] RODRIGUES S C, MANIERO M G, RATH S, et al. Degradation of flumequine by the Fenton and photo-Fenton processes: Evaluation of residual antimicrobial activity [J]. Science of the Total Environment, 2013, 445-446: 337-346. doi: 10.1016/j.scitotenv.2012.12.079 [122] GUO H, LI Z, LIN S, et al. Multi-catalysis induced by pulsed discharge plasma coupled with graphene-Fe3O4 nanocomposites for efficient removal of ofloxacin in water: Mechanism, degradation pathway and potential toxicity [J]. Chemosphere, 2021, 265: 129089. doi: 10.1016/j.chemosphere.2020.129089 [123] GOU Y, CHEN P, YANG L, et al. Degradation of fluoroquinolones in homogeneous and heterogeneous photo-Fenton processes: A review [J]. Chemosphere, 2021, 270: 129481. doi: 10.1016/j.chemosphere.2020.129481 [124] LUO X, WEI X, CHEN J, et al. Rate constants of hydroxyl radicals reaction with different dissociation species of fluoroquinolones and sulfonamides: Combined experimental and QSAR studies [J]. Water Research, 2019, 166: 115083. doi: 10.1016/j.watres.2019.115083 [125] MICHAEL I, HAPESHI E, MICHAEL C, et al. Solar Fenton and solar TiO2 catalytic treatment of ofloxacin in secondary treated effluents: Evaluation of operational and kinetic parameters [J]. Water Research, 2010, 44(18): 5450-5462. doi: 10.1016/j.watres.2010.06.053 [126] MICHAEL I, HAPESHI E, ACEÑA J, et al. Light-induced catalytic transformation of ofloxacin by solar Fenton in various water matrices at a pilot plant: Mineralization and characterization of major intermediate products [J]. Science of the Total Environment, 2013, 461-462: 39-48. doi: 10.1016/j.scitotenv.2013.04.054 -



 下载:
下载: