-
生物炭是生物质在限氧条件下制备的一类含碳固体物质,因其在土壤改良、污染物固定、提高农产品产质量等方面的广泛应用而备受研究者关注[1-4]。但生物炭对污染物的吸附容量受其特性的限制(如表面官能团含量、孔隙结构及比表面积、pH值、阳离子交换量、Zeta电位等),此外传统生物炭还存在诸多不足,如对污染物的吸附容量较低、含有少量污染物(重金属)、施用后难以从环境中分离等,这些因素限制了生物炭的推广和应用。值得注意的是,生物炭改性可有效改变其对污染物的吸附及降解。如硝酸改性可以增加生物炭羧基和负电荷含量,使其对U(Ⅵ)的吸附量提高40倍[5];重金属改性生物炭能够增加生物炭中持久性自由基的浓度和类型,增强过硫酸盐自由基对多氯联苯的降解[6];而硫铁改性生物炭能有效降低污染稻田土中Cd的生物可利用性,减少Cd在不同水稻组织中的积累,同时增加水稻总叶绿素含量以及根、茎和籽粒生物量[7]。然而有研究报道了HF和HCl改性会减少对Cd的吸附;过渡金属改性生物炭后其持久性自由基浓度的减少抑制了有机物的降解;与此同时还有研究表明不同化学剂量的KOH改性可能导致生物炭性质出现极大差别[8]。因此不同方法改性对生物炭性质有何影响?性质改变后对污染物的吸附与降解是促进还是抑制?促进或抑制的机制如何?这些问题需亟待解决。
本文在阐述改性方法对生物炭性质影响的基础之上,综述了生物炭改性对其吸附与降解污染物的影响机制,并提出改性生物炭需进一步研究的相关科学问题。这将为科学改性生物炭提供选择依据,有助于全面理解改性生物炭的环境效应及促进生物炭的应用推广。
全文HTML
-
生物炭改性按其炭化操作先后顺序可分为先改性后炭化,先炭化后改性,或者是炭化改性同步进行。目前研究人员开发了多种技术进行生物炭改性,主要包括物理改性、化学改性和生物改性。
-
常见的物理改性方法有蒸汽活化、紫外辐射、球磨、冻融循环等。改性方法对生物炭特性的影响见表1。
蒸汽活化是一种常见的物理改性方法,因生物炭热解过程产生的不完全燃烧产物被去除而能增加其比表面积。同时水分子中的氧可以被交换到炭表面自由活性位点,产生的氢气能与表面的碳反应生成表面氢配物(方程1—3)[4]。如生物炭经蒸汽活化后其比表面积和孔径增大,含氧官能团数量减少[9];冻融循环能使浸入生物炭内部的水在冰冻时通过冻胀力作用使生物炭孔径和比表面积增大,同时促进生物炭中溶解性物质的淋出[10];球磨生物炭在机械力的作用下其颗粒能够达到纳米级,其颗粒尺寸的减小促使比表面积的增大,同时内部孔隙结构在机械力的作用下也会更为发达[11];而紫外改性则会诱导生物炭表面发生氧化反应从而增加生物炭中的—COOH、—OH等官能团数量[12]。因此,物理改性可增加生物炭的孔隙结构、比表面积和含氧官能团。
-
化学改性是生物炭改性中最常见的方法,根据改性试剂的不同可分为酸改性、碱改性、有机试剂改性、金属盐或金属氧化物改性、纳米材料改性、等离子体改性等[4, 14-16]。
-
酸改性是指将生物质或生物炭浸泡或悬浮于酸性溶液中,经洗涤,再干燥或炭化后便可获得改性生物炭[4]。目前常用的酸改性试剂主要有HNO3、H2SO4、H3PO4、HCl、柠檬酸、酒石酸和乙酸等(表2)。不同酸改性试剂对生物炭特性有不同的影响,如HNO3改性因引起生物炭表面氧化而导致—COOH和—NO3的丰富度增加,同时降低灰分含量[5, 17];H3PO4除了作为脱水剂和扩孔剂扩大孔隙体积和比表面积外,还能与生物炭表面发生反应生成—P=O和—P=OOH官能团[18];H2SO4改性则与生物炭上芳香烃发生磺化反应而引入—SO3H[19];而HCl改性能有效去除生物炭中的矿物组分[20]。与无机酸相比,有机酸对生物炭的改性可通过酯化作用增加—COOH含量,同时还具有生物炭质量损失较小、改性作用强度较弱、对环境友好以及成本低等优势[21]。
综上所述,酸改性对生物炭特性的影响主要体现如下:①降低灰分含量。酸改性试剂能促进生物炭中矿物成分溶解,从而降低生物炭中的灰分含量。如,小分子有机酸和HCl能够去除生物炭中K+、Ca2+、Mg2+、Fe3+、Al3+等矿物元素从而使生物炭灰分含量降低[4]。②改变比表面积和孔隙结构。酸改性试剂对生物炭比表面积及孔隙结构的作用主要与4个方面相关,其一是酸试剂能促进可溶性物质的溶解,从而提高生物炭的孔隙率[4];其二是产生大量CO和H2O使得生物炭孔隙结构更为发达,增大比表面积[22];其三是降低生物质炭化过程中纤维素的结晶度,改善其孔隙结构[23];其四是有机酸等进入生物炭内部,占据生物炭的微孔,导致比表面积降低[21, 24]。③表面元素组成发生变化。一方面因生物炭中有机碳流失使得C含量降低而O含量增加,另一方面因生物炭中灰分含量的降低而导致C相对含量增加。此外,生物炭表面含氧官能团的增加也能提高其O含量。④改变表面官能团的数量和种类。如HNO3的氧化作用可增加生物炭表面的—COOH数量,H3PO4、H2SO4则可引入—SO3H、—P=OOH、—P=O等官能团,因此酸改性可有效增加生物炭表面的含氧官能团数量和种类。
-
碱改性可分为两种,一是将生物炭或生物质浸泡到碱液中,后洗涤、干燥或炭化得到改性生物炭;二是将生物质或生物炭与碱直接混合在限氧条件下加热改性。常见的碱改性试剂有KOH和NaOH(表3)。KOH改性能引起生物炭表面结构的显著变化,如KOH改性污泥生物炭因K可以插入碳基晶格而导致生物炭膨胀,以及KOH的脱灰作用可导致生物炭比表面积增大[26-27];而NaOH会与碳基质发生作用提高生物炭的孔隙率并增加比表面积,同时还能增加—OH、—COOH数量和阳离子交换量(cation exchange capacity,CEC)[28]。需要注意的是,碱改性对生物炭比表面积的影响主要取决于碱性试剂种类及试剂与原料的质量比。如KOH与生物炭的质量比为1时,海藻生物炭比表面积扩大了近10倍,超过1时比表面积逐渐减小,当KOH添加过量时,其比表面积与原始生物炭相当,因为KOH过渡活化会导致碳结构坍塌而使比表面积减小[8]。
综上所述,碱改性对生物炭特性的影响主要体现在5个方面:①改变元素组成。碱改性试剂与生物炭的进一步作用会引起生物炭中各元素含量发生变化。②降低灰分含量。碱改性可以去除生物炭中的部分灰分,从而使生物炭的灰分含量减低[27]。③改变生物炭的孔隙结构和比表面积。如,适量KOH改性能清除生物炭孔隙结构中的堵塞物,同时K能嵌入碳基晶格导致其膨胀,这都有利于比表面积和孔隙结构的增加[29]。④增加表面官能团。NaOH改性可通过氧化作用增加生物炭的—OH和—COOH数量[8]。⑤增加CEC。碱改性增加的表面含氧官能团使生物炭表面带负电,负电荷的增多能够导致CEC增加[28].
-
常见的有机改性试剂有壳聚糖、聚乙烯亚胺、甲醇、戊二醛、尿素等,有机试剂改性在改变生物炭性质方面有重要的作用,能够引起生物炭多方面性质的变化(表4)。Sajjadi等[30] 用尿素改性松木生物炭,研究表明尿素中的—NH2和C=O经氢键作用与生物炭表面的—OH和—COOH官能团结合,从而增加了生物炭的表面官能团。另外,甲醇改性稻壳生物炭可以去除生物炭中的有机物,降低其环境风险[31];壳聚糖因富含—NH2和—OH,改性过程中与生物炭表面的—OH和—COOH结合,同时提高生物炭官能团数量和亲水性[32];而聚乙烯亚胺富含伯、仲胺基团和较强的反应活性,可与生物炭表面的—OH、—COOH、—CHO发生交联反应接枝于生物炭表面,提高生物炭的含氮官能团数量[33-34]。
有机试剂改性对生物炭理化特性的影响主要体现在4个方面:①改变表面官能团。如甲醇改性生物炭会与生物炭表面官能团发生反应,使酯基含量升高,而羰基则在反应中被消耗,其含量也随之降低[31];壳聚糖因含有丰富的—OH和—NH2,改性后可增加生物炭表面的—OH和—NH2含量[35];聚乙烯亚胺改性过程中发生的交联反应也会引入含氮基团[36]。②影响生物炭表面的芳香性、极性和亲水性。有机试剂O、H、N等元素含量丰富,改性后H、N、O等含量的增加可提高生物炭的H/C、(O+N)/C和O/C摩尔比,导致生物炭的芳香性降低,极性和亲水性升高,同时有机试剂改性后增加的含氮或含氧官能团也使得生物炭具有较高的极性和亲水性。如Shi等[35]用壳聚糖改性小麦秸秆生物炭,改性后有效降低生物炭的芳香性,提高生物炭的极性和亲水性。③去除生物炭中的有机物,降低生物炭的环境风险。生物炭中含有如多环芳烃、二噁英和呋喃等有害化合物,有机试剂的改性可以促进其溶解,通过预处理的去除可以降低生物炭的环境风险[37]。④改变元素组成。如壳聚糖改性能提高O、H、N含量[35],聚乙烯亚胺能提高H、N含量[34],而尿素改性能提高N元素含量[38]。
-
常见的金属氧化物或金属盐改性试剂有KMnO4、FeSO4、FeCl3、TiO2、MgCl2、AlCl3、ZnCl2。金属氧化物或金属盐改性可以增加生物炭中金属元素含量,金属元素的掺杂会使生物炭性质发生明显改变(表5),但改性生物炭的性质取决于改性试剂和生物质。如FeSO4/FeCl3改性可在生物炭表面生成γ-Fe2O3或Fe3O4从而使生物炭具有磁性[39];Xia等[40]用ZnCl2改性沼气渣生物炭,发现ZnCl2改性能促进生物炭微孔的发育致使生物炭比表面积增加;KMnO4改性能使油菜秸秆生物炭表面氧化从而增加生物炭表面含氧官能团数量[41];TiO2改性则可提高生物炭的光降解效率[42]。此外金属氧化物或金属盐类改性会堵塞生物炭的部分孔隙,但金属氧化物的比表面积高于低比表面积生物炭,对低比表面积生物炭而言,改性后其比表面积主要由金属氧化物或金属盐类贡献,其比表面积会增加,而高比表面积生物炭因其孔隙堵塞而导致比表面积降低[43]。
金属氧化物或金属盐改性对生物炭特性的影响主要有以下几个方面:①提高生物炭磁性。铁盐改性可在生物炭表面生成Fe3O4或γ-Fe2O3来增强生物炭的磁性,便于后期的分离和循环利用[44]。②改变生物炭的孔隙结构和比表面积。如ZnCl2改性可作为脱水剂降低炭化温度,从而抑制焦油的产生并促进气孔的形成和纤维膨胀[36],进而导致生物炭的孔隙结构和比表面积的增大;KMnO4改性能促进生物炭微孔的发育从而增加比表面积[41]。③影响自由基的产生。如TiO2改性生物炭在紫外光照射下能促进改性生物炭产生更多的·OH自由基[42];另外Fe3+、Cu2+、Ni2+、Zn2+等重金属离子在低浓度时能促进持久性自由基的产生,在高浓度时消耗持久性自由基,因此金属离子浓度的变化会影响生物炭中持久性自由基的浓度及类型[6]。④引入非金属杂原子,改变其他化学性质。金属盐改性会引入非金属杂原子,生物炭中非金属元素的掺杂会改变其化学性质。如N的掺杂可以引起生物炭中N掺杂位点电子结构发生变化而改变生物炭表面的酸碱性质,同时增强生物炭的极性[45],S的掺杂可破坏共价碳电子系统的电荷平衡,还能影响生物炭的多孔结构[46-47]。
-
纳米金属颗粒、石墨烯、氧化石墨烯、碳纳米管等纳米材料具有比表面积大,吸附污染物能力强等特点[51-53],但其成本高,具有一定的环境风险,纳米材料对生物炭的改性在保留纳米材料优异性能的同时,还具备降低纳米材料的环境风险和经济成本、抑制其发生团聚、防止被空气氧化等特点[25]。如纳米零价铁改性玉米秸秆生物炭可有效防止纳米零价铁的团聚,同时纳米零价铁堵塞了部分微孔而导致生物炭比表面积减小[51];石墨烯改性木材生物炭可提高生物炭的比表面积和石墨化程度;氧化石墨烯因有较高的比表面积和丰富的含氧官能团,对竹子生物炭的改性能导致生物炭表面含氧官能团的增加[54];而碳纳米管具有高比表面积和低负电性,对山核桃和甘蔗渣生物炭的改性导致其比表面积增大,Zeta电位降低[53]。
因此纳米材料改性生物炭能有效改变其性质(表6),其主要的影响有:①改变比表面积和孔隙体积。纳米材料本身的比表面积大,改性后纳米材料可分散于生物炭表面或稳定于生物炭孔隙内部,可使生物炭的比表面积和孔体积增加或减小。②降低Zeta电位。如氧化石墨烯呈高负电性,改性后可降低生物炭的Zeta电位。③增加表面官能团。氧化石墨烯表面含有丰富的含氧官能团,改性后能增加生物炭表面的—OH、—COOH等官能团数量[47]。
-
等离子体是由部分电子被剥夺后的原子及原子团经电离后由正负离子组成的离子化气体状物质[16, 56]。常见的等离子体改性气体有HCl、Cl2、H2S、O2等,等离子体与生物炭相互作用能产生自由基、离子和亚稳态等高度活跃的物质[57]。等离子体改性同样能引起生物炭孔隙结构、比表面积、表面官能团等性质发生变化(表7),如O2等离子体改性核桃壳生物炭因对孔隙中颗粒的去除而导致孔体积和比表面积增大,对生物炭表面的氧化还能提高O含量[58];而HCl等离子体改性农作物秸秆生物炭则会与生物炭表面发生反应而增加C—Cl等含氯官能团[16]。此外等离子体改性还具有节能、产量高、不产生有害副产物等特点。
等离子体改性对生物炭性质的影响主要体现在:①增加表面官能团。根据选择气体和放电环境的不同,在改性过程中气体会与生物炭发生氧化或还原反应从而增加官能团数量,如H2S等离子体改性使含硫基团和羧基数量增加[59],Cl2等离子体改性生物炭能成功引入C—Cl官能团[56]。②改变比表面积。O2等离子体改性生物炭能形成更多的微孔、中孔和大孔,从而使比表面积增加[58];而HCl等离子体改性因生物炭的部分孔隙被HCl气体堵塞,同时产生的自由基会轰击生物炭并与之发生反应,使生物炭孔壁坍塌,导致比表面积减小[16];此外等离子体还具有清洁生物炭表面的作用,孔隙中细微颗粒杂物的去除也能增加生物炭的比表面积。③改变元素组成。如Cl2等离子体改性能使C含量增加,O含量降低[56]。
-
生物炭内含多种元素能为微生物提供营养物质,其发达的孔隙结构亦是微生物适宜的栖息环境,此外生物炭的酸碱缓冲能力能降低外部酸碱环境改变对微生物的影响,在生物炭和微生物的协同作用下可增强对污染物的去除效果。如生物炭能为鲁菲不动杆菌提供栖息场所和营养物质,促进繁殖,改性后显示出对阿特拉津的高效降解[60];消化污泥微生物经固定化技术负载于生物炭表面后,能提高对水体中氨氮的去除效果[61],另外据有关研究报道,生物质经厌氧消化预处理后能改变生物炭的性质,预处理后制备的生物炭Zeta电位降低,比表面积、pH值、Ca、Mg、K等无机元素升高,对磷酸盐和Pb都具有高吸附容量,与活性碳相当[62-63]。
1.1. 物理改性
1.2. 化学改性
1.2.1. 酸改性
1.2.2. 碱改性
1.2.3. 有机试剂改性
1.2.4. 金属氧化物或金属盐改性
1.2.5. 纳性米材料改性
1.2.6. 等离子体改性
1.3. 生物改性
-
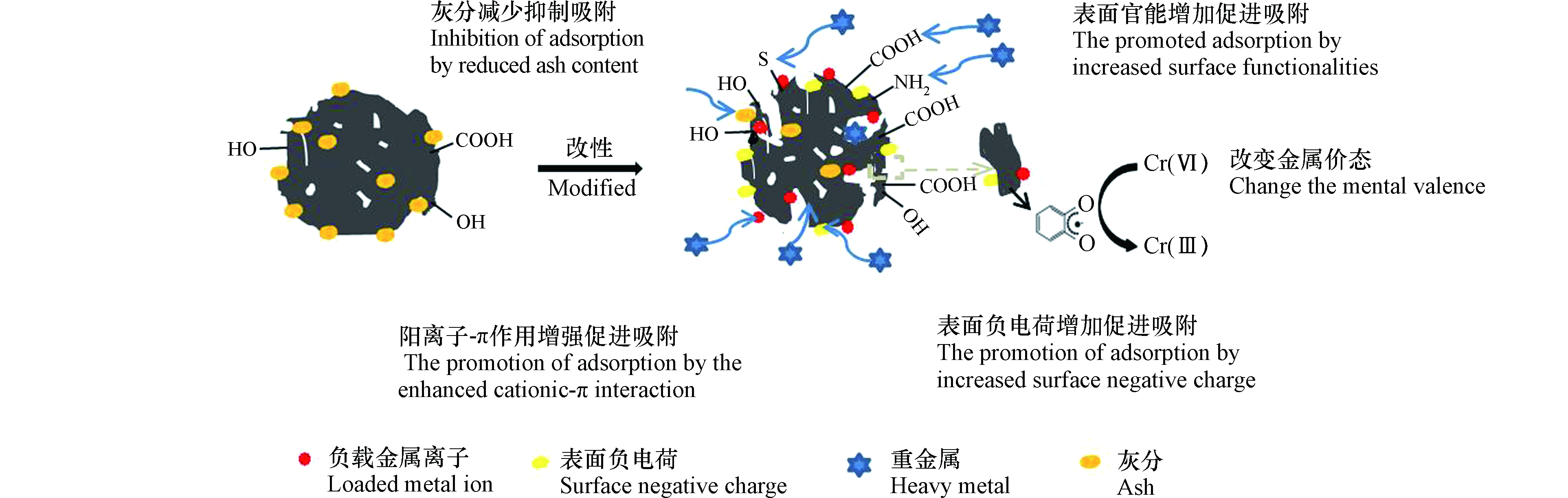
生物炭对重金属的吸附作用主要有静电作用、沉淀作用、表面络合作用、阳离子-π作用等[47]。因生物炭改性过程中其表面元素含量、官能团组成、比表面积以及灰分含量等均发生不同程度的改变,从而影响生物炭对重金属的固持。如,HNO3改性麦秆生物炭与未改性麦秆生物炭相比,其对U(Ⅵ)的吸附量提高40倍[5],这是HNO3改性使得生物炭表面—COOH官能团含量增加和Zeta电位电位降低,而促使U(Ⅵ)与生物炭表面络合与静电作用增强所致。KMnO4改性能促进生物炭微孔发育,提高比表面积和含氧官能团数量,对Cd的吸附容量增加主要是由负载锰氧化物所增加的吸附位点、比表面积、阳离子交换量和阳离子-π键合作用贡献,其中负载锰氧化物所形成的吸附位点占主导地位[41]。另外研究显示生物炭中S含量的增加亦会促进Hg在生物炭表面生成HgS沉淀,促进对土壤中Hg的吸附,降低Hg在土壤中的迁移性[64]。与之相反,利用HCl和HF对米糠生物炭能使生物炭矿物组分含量降低,从而使矿物组分与重金属之间的沉淀作用减少,进而降低米糠生物炭对Cd的吸附容量[65]。
此外,生物炭中持久性自由基能够参与重金属的氧化还原过程,而改性作用能调节生物炭中持久性自由基的类型及数量。如铁盐改性有利于生物炭石墨化程度的发展和以碳为中心持久自由基的产生,持久性自由基的存在促进电子的转移,进而将Cr(Ⅵ)还原为Cr(Ⅲ),其中由持久性自由基还原贡献的占比为18%[66]。
综上,改性对生物炭与重金属相互作用的影响(图1)主要有3个方面:①促进对重金属的固持。改性后—COOH、—NH2、—OH等官能团的增加加强了表面络合作用使重金属的吸附量增加;改性后生物炭比表面积的增加提升了对重金属的吸附容量;S含量的增加能增强生物炭对重金属Hg的亲和力而使生物炭对Hg的吸附量增加;还有生物炭 Zeta电位的降低可导致静电作用增强而提升对重金属的吸附量。②抑制对重金属的吸附。改性致使矿物组分的去除削弱了生物炭与重金属之间的沉淀作用,对重金属的吸附表现出抑制作用。③参与重金属氧化还原反应导致其价态改变。改性产生的持久性自由基作为电子穿梭体能促进重金属价态的改变。
-
众所周知生物炭对有机污染物的吸附机理包括疏水效应、π-π相互作用、氢键作用、孔隙填充效应等[67]。生物炭改性会使其理化特性发生改变,如孔隙结构更为发达、表面积增加、表面官能团更为丰富等,这将会改变其对有机污染物的吸附容量。如甲醇改性促进稻壳生物炭中有机物的溶解,增加了生物炭比表面积和含氧官能团丰富度,与四环素π-π相互作用的增强使其对四环素的吸附量提升1倍[31];污泥生物炭经NaOH和HNO3分步改性可提高其比表面积和孔隙率,孔隙结构的发展有效降低空间位阻效应,孔隙填充作用的增强促进对四环素的吸附[68]。Tan等[69]用KOH改性玉米秸秆生物炭,改性作用使玉米秸秆生物炭表面含氧官能团增加,H/C降低,由于阿特拉津与生物炭间π-π相互作用和H键作用的增强,生物炭对阿特拉津的吸附增加。另外还有研究发现TiO2改性生物炭减弱了生物炭与磺胺甲恶唑的π-π相互作用,对其吸附量减少[42],而H3PO4改性生物炭可增加生物炭的疏水性,对亲水性有机物的吸附量减少[22]。
此外腐殖酸改性生物炭后可分布于生物炭表面,生物炭表面部分活性位点被占据,生物炭中持久性自由基对环丙沙星的降解效率降低[70]。此外,近年来持久性自由基作为电子穿梭体活化过硫酸盐自由基降解持久性有机物的研究也发现金属(Fe3+、Cu2+、Ni2+、Zn2+)和酚类化合物(对苯二酚、邻苯二酚、苯酚)存在时对生物炭中持久性自由的产生有重要影响,重金属和酚类能够调节生物炭中持久性自由基的浓度和类型,过渡金属在低浓度时能从酚类化合物中接受电子,有利于持久性自由基的产生,在高浓度时则消耗持久性自由基,进而影响过硫酸盐自由基对有机物的降解[6]。
另外,还有研究表明生物炭经生物技术改性后,生物炭与微生物的混合作用能增强对有机物的降解。如鲁菲不动杆菌改性生物炭后能有效降解阿特拉津[60]。
综上所述,改性对生物炭-有机污染物间相互作用的影响有促进对有机污染物吸附、抑制对有机污染物吸附、促进对有机污染物降解和抑制对有机污染物降解4个方面(图2)。①改性促进生物炭对有机污染物的吸附机制主要体现在:改性后表面官能团的增加增强了生物炭与有机污染物间的π-π相互作用和H键作用,对有机污染物的吸附量增加;此外比表面积和空隙率的增加降低了空间位阻效应,导致孔隙填充作用增强。②抑制生物炭对有机污染物的吸附:改性导致π-π相互作用减弱,对有机污染物的吸附量随之减少;改性提升了生物炭的疏水性,对亲水性有机物的吸附量减少。③改性促进对有机污染物的降解:改性提高生物炭中持久性自由基的浓度可促进过硫酸盐自由基对有机污染物的降解;生物改性后微生物与生物炭的协同作用可增强对有机物的降解。④改性抑制对有机物的降解:高浓度金属离子改性时可消耗持久性自由基,抑制过硫酸盐自由基对有机物的降解;改性试剂占据的活性位点同样能降低持久性自由基的活性,进而抑制对有机污染物的降解。
2.1. 改性对生物炭与重金属相互作用的影响机制
2.2. 生物炭改性对有机污染物吸附和降解的影响机制
-
改性生物炭的研究近年来不断取得进展,其运用有着广阔的前景,但改性生物炭目前的应用还存在一些限制,在以下方面的研究还需继续完善:
(1) 改性生物炭的环境风险。如强酸强碱在改性过程中可能会导致二次污染的产生,过渡金属改性产生的持久性自由基可能会危害人体健康,改性生物炭在土壤中对微生物的影响及其与微量元素的关系尚不明确等,这些使用风险都可能会产生负面影响,不利于改性生物炭的推广应用。
(2) 改性生物炭规模化应用存在阻碍。目前改性生物炭的研究很多还处于实验室研究阶段,而且研究体系相对单一,但实际环境是一个复杂多元体系,改性生物炭在实际环境中表现如何,还受许多因素限制,应该在改性方法及其机理、规模化应用、降低成本方面加强研究。
(3) 改性生物炭应用缺乏动态评估。改性生物炭运用到环境后,其与污染物的相互作用、性质变化、环境效应等动态评估不应被忽视。



 下载:
下载:


