-
随着国家在水环境治理政策支持力度的加大,国内江河湖泊水质已得到明显提升,但部分地表水水质仍还未能达到国家《地表水环境质量标准》(GB 3838-2002)中的III级标准,呈现出微污染特征[1-2]。强化混凝工艺因其具有成本低廉及操作简便等优点已成为微污染地表水处理中广泛使用的技术之一,可有效去除水体悬浮胶体颗粒及天然有机物,提升水质[3-6]。通常认为,决定混凝效果的核心是混凝剂[3, 7-9]。传统的混凝剂主要是铝盐及铁盐等。人们为了进一步提高无机混凝剂的混凝性能,还开发了基于铝铁盐的复合混凝剂[10-13]。然而无论是单一铝铁盐混凝剂,还是复合混凝剂,其在实际应用中残留的金属离子对环境均存在二次污染的风险[14-17]。
因此,混凝剂除需具备良好的混凝性能,同时还应具有绿色环保等重要特征。钛盐是+4价无机盐,具有较高电荷有着良好的混凝效果及环保特点;采用钛盐的混凝体系中产生的污泥还可用于制备TiO2,从而实现资源再利用并减少环境负担[18]。聚硅酸(PSA)具有绿色和低成本特征,已被研究证明其与钛盐复合使用的高效性,硅钛复合混凝剂具有优良的电中和与粘结架桥作用和环境友好特点[18-20]。近年来锌基混凝剂因其无毒、环保等特征而受到广泛关注[10, 11, 21-22]。此外,锌离子对水体中天然有机物腐殖酸(HA)具有较强的吸附性能,易生成Zn-HA复合物[23],可提高对有机微污染物的混凝去除效率。然而,基于PSA、钛盐及锌盐的无机复合混凝剂材料及其在针对有机微污染地表水净化处理中的应用至今还鲜有报道。
本文开发了一种新型的复合聚硅酸钛锌混凝剂(PSTZ),应用胶体滴定、扫描电子显微镜(SEM)、透射电子显微镜(TEM)、X射线衍射(XRD)和傅立叶变换红外(FTIR)进行材料结构表征。采用高岭土悬浊液和HA水溶液为模拟水样,详细考察了复合混凝剂中各组成含量及pH对PSTZ混凝性能及絮体结构的影响,同时与聚硅酸钛(PST)和聚硅酸锌(PSZ)进行性能对比;通过对其表观混凝性能、ζ电位及絮体特性等系统研究,对其协同混凝机制进行了深入探讨。除模拟水样外,还考察了PSTZ对采自南京羊山湖实际地表水的混凝效果,并与传统混凝剂聚合氯化铝(PACl)进行对比,进一步验证PSZT强化混凝的有效性。
-
Na2SiO3·9H2O、Ti(SO4)2、ZnSO4·7H2O、H2SO4及NaOH等,均为分析纯试剂,购自上海凌峰化学试剂有限公司;聚乙烯硫酸钾(酯化度为96%)、腐殖酸钠(NaHA)及PACl([Al2(OH)nCl6-n]m,n=3.6—5,m<10,Al2O3含量≥28%)购自阿拉丁有限公司;高岭土(平均粒径约4.18 μm)和甲苯胺蓝O购自国药控股化学试剂有限公司。除对实际地表水处理外,其他实验中均使用去离子水。
-
用稀硫酸将Na2SiO3溶液调至pH 4.0,在20 ℃下搅拌1.5 h,制备得到PSA。将一定量Ti(SO4)2和(或)ZnSO4固体溶于去离子水后,加入上述PSA溶液中,在室温下搅拌熟化一定时间,分别可制得PST、PSZ和PSTZ。采用相同方法,通过调节不同进料Si/Ti和Si/Zn摩尔比(详见表1),制得一系列不同配比的复合混凝剂。
采用中心复合设计的响应曲面法对PSTZ制备工艺进行优化[24],以混凝效果为输出结果,并使用Design-Expert software (DX10)完成数据分析。
混凝剂的电荷密度(CDs)通过胶体滴定法获得[25],并测得混凝剂溶于去离子水后pH值。利用傅立叶红外光谱仪(Bruker IFS 66/S型)和X射线衍射(ARL X'TRA)对复合混凝剂进行结构表征。此外,还通过扫描电镜(FEI Quanta 250)和透射电镜(JEOL JEM-2100)观察复合混凝剂表面形貌。
-
本文选取高岭土和HA分别模拟地表水中典型的无机胶体颗粒和水溶性有机物。以(30.0±1.0) NTU的高岭土悬浮液及(5.0±0.1) mg·L−1的HA水溶液分别为模拟水样。为了消除某些共存离子的干扰,使用去离子水制备所有水样。实际微污染地表水取自南京市羊山湖。该原水的初始浊度和UV254分别为:(22.5±2.5) NTU和(0.160±0.010) a.u.。
混凝前新鲜配制复合混凝剂母液,浓度为0.3% wt(以SiO2计)。在室温下,采用程控六联混凝实验搅拌仪(TA6-1,武汉恒岭科技有限公司)进行混凝测试。具体实验过程如下:(1)在300 r·min−1快速搅拌条件下加入一定量复合混凝剂,投加量均以SiO2计;(2)保持300 r·min−1快速搅拌5 min后,50 r·min−1慢搅12 min;(3)静置沉淀20 min。实验结束后,取玻璃杯液面下2 cm处上层清液,进行后续水质测定,同时小心取出玻璃杯底部沉淀絮体进行絮体结构的观察与分析。模拟水样pH使用H2SO4或NaOH稀溶液调节。
-
对高岭土悬浮液及HA水溶液混凝效果分别采用残留浊度和残留HA浓度衡量。水样浊度采用浊度仪测量(WGZ-200S,上海昕瑞仪器有限公司);HA浓度采用标准曲线法,通过UV-1800光谱仪(日本岛津公司)测定溶液UV254计算。需要指出的是,尽管膜过滤被广泛应用于去除较小的HA絮体[26-27],但是在本工作中,为准确评估混凝剂的实际混凝效果,不采用滤膜过滤直接检测,从而避免膜过滤作用对检测的干扰。
-
混凝实验后,采用Malvern Model Nano-Z Zetasizer(UK)测定上清液ζ电位。
-
将从烧杯底部取出的絮体分散于玻璃培养皿中,置于XTL-3400 光学显微镜下并利用CK-300 数码照相机(上海蔡康光学仪器有限公司)对絮体进行拍照,所得照片利用图片分析软件(Imagepro® Plus 6.0)进行絮体结构分析,得到了絮体的特征长度(l, μm)以及二维分形维数(D2)值。其中,l定义为穿过絮体中心的最长直径值,D2则由絮体投影面积(A, μm2)与l之间的指数关系获得,其计算公式如下[28-29]:
致密的絮体通常具有较高的D2值,而具有疏松和分支结构的絮体具有较低的D2值。
上述实验中的每一项测量都重复3次,最终结果为3次测量的平均值(
$\overline S $ ),误差棒表示标准偏差(σ),在95%置信度下置信区间为($\overline S $ -2σ,$\overline S $ +2σ)。 -
通过控制合成过程中Si/Ti和Si/Zn的进料摩尔比,成功制备了8种不同复合混凝剂,如表1所示,其中根据响应曲面法获得的最佳投料比制得PSTZ2。分别对其CD、水解行为、分子结构及表面形貌进行了检测。
-
不同复合混凝剂CD采用胶体滴定法测得并列于表1中。对于PST系列混凝剂,由于Ti(Ⅳ)具有较高的电荷,Ti含量较高的PST具有较高的CD值[30-31]。通过比较相同Si/Ti物质的量比下的PSTZs,发现Zn的引入进一步提高了复合混凝剂的CD值。
由于实际测得的混凝剂CD及混凝效果与其水解产物有关[32],通过检测复合混凝剂溶于去离子水后pH值进一步研究其水解行为,不同复合混凝剂水溶液pH值,如表1所示。由于Ti的水解反应,pH随着Ti的比例增加而降低。值得一提的是,PSTZ2和PSTZ3的pH分别高于PSTZ1和PST4,这可能是由于Zn的存在影响了Ti与Si的水解和聚合过程[21],导致了溶液pH升高。
-
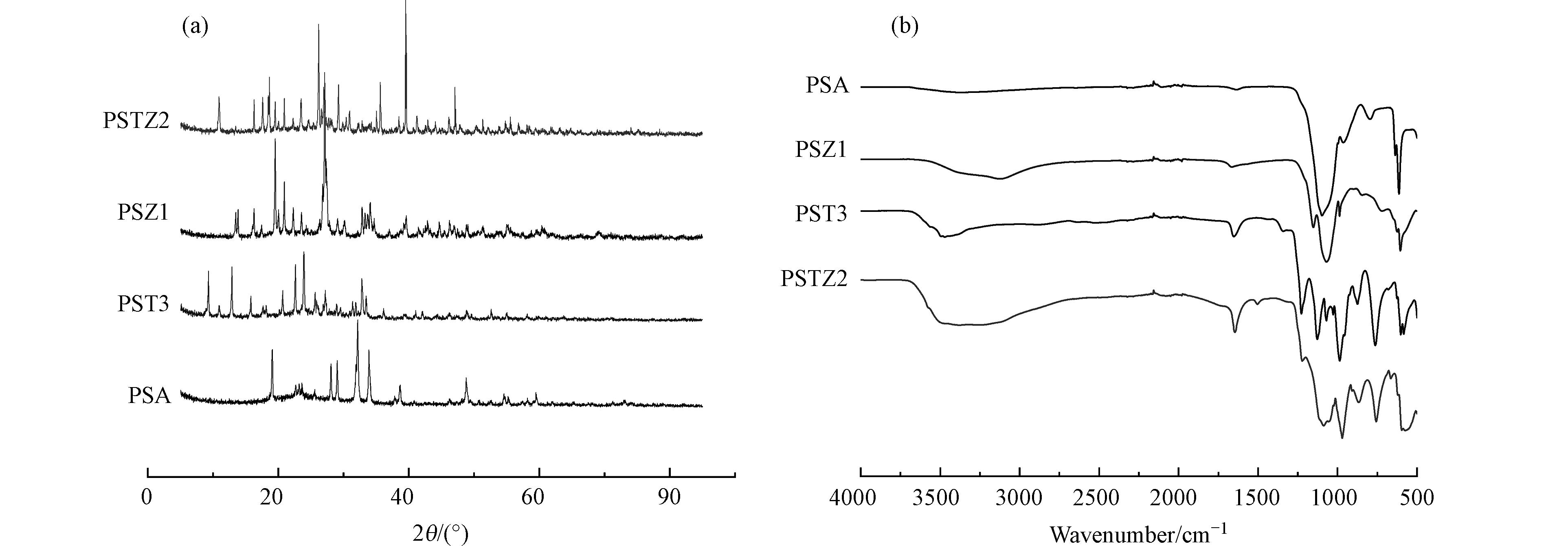
通过XRD对复合混凝剂相组成进行分析,图1a对比了PSTZ2,PST3和PSZ1与PSA的XRD图谱。在其XRD图谱中观察到的不同衍射峰,分别代表了PSTZ2,PST3,PSZ1和PSA的晶体特征。PSTZ2的特征峰(2θ=10.90°、17.58°、18.44°、20.90°、23.48°、26.16°、27.08°、29.22°、30.94°、39.52°)不完全与PST3(2θ=9.28°、12.88°、22.62°、23.88°、25.64°、27.18°、32.80°)、PSZ1 (2θ=13.84°、16.26°、19.52°、20.02°、20.88°、23.56°、26.80°、27.10°、27.38°、32.90°、33.68°)和PSA(2θ=19.06°、23.62°、28.06°、29.02°、32.14°、33.88°)相同,4种药剂的结构成分具有差异;其中,PSTZ2有相对其他混凝剂更多的特征峰,说明其多晶相的特点[10],并且几乎没有特征峰可通过XRD标准卡片进行分辨,这表明PSTZ2生成了更多且复杂的新型物相结构,并非原材料简单的混合[10, 21],而是由Ti、Zn及Si等元素复合组成的新型多晶化合物。
同时采用FTIR表征了上述4种复合混凝剂,结果如图1b所示。由图1可知,PSTZ2在波数为3200—3600 cm−1范围内,存在一个较宽的吸收峰,这是由PSTZ2中与Ti、Zn及Si相连的—OH和样品吸附水分子中的—OH伸缩振动引起[21, 33],此外PSTZ2在1646 cm−1的尖峰为吸附水、配位水及结晶水的弯曲振动吸收峰[18, 21, 34],归因于Ti的强水解性和强吸水性[34],与PST3谱图1651 cm−1处基本吻合。在PST3谱图中750—1230 cm−1处出现了多个尖强吸收峰,分别为Si—O—Si、T—O—Ti、Si—O—Ti等基团特征峰[18, 34, 35],而在PSTZ2谱图中1088 cm−1处出现了相对于PST3较宽弱的吸收峰,这是由于引入的Zn形成了一系列Zn—O键结构逐渐弱化了上述尖强吸收峰[11, 33],取而代之为各种复杂吸收峰的叠加。在572 cm−1和575 cm−1处PSTZ2出现的Si—O吸收峰与PSA、PST3及PSZ1谱图相比出现了不同程度的红移,可以解释为Ti—O及Zn—O等键结构影响了Si—O的伸缩振动。PSTZ2在1505 cm−1处出现的新峰可能是由于生成的特殊Ti—O—Zn基团的特征峰,进一步说明PSTZ2结构中Si、Ti及Zn不是简单的混合。因此,通过XRD和FTIR分析可以说明Si、Ti及Zn等元素之间发生相互作用,生成了新的复杂多晶化合物,并且这种无机复合混凝剂的多晶结构被认为具有增强的粘结架桥作用[22]。
-
PSA、PSZ1、PST3和PSTZ2表面形貌分别采用SEM和TEM进行直接观察。图2a-2d为不同混凝剂SEM照片,相比于PSA具有明显光滑的沟槽表面结构,Zn和Ti的分别引入使得PSZ1和PST3表面更加致密(图2a,2b和2c),PSZ1呈现出更为清晰的层状结构。
Wei等[36]研究发现,混凝剂的层状结构更容易在混凝过程中通过架桥形成网状结构,从而能有效促进混凝性能。而PST3则呈现出不规则多孔的粗糙表面结构,比表面增大,有更多的结合位点,从而增强了其吸附能力。PSTZ2(图2d)同时呈现了PSZ1和PST3的表面结构特征,即兼具有层状和多孔表面结构,但是,PSTZ2比PST具有更明显的层状表面和更大的孔结构。导致PSTZ2结构变化的可能原因是Si、Ti及Zn相互之间存在协同相互作用,从而形成了新的复合化合物。
为了更好地探究混凝剂在水溶液中的形态,在TEM下直接观察了PSA、PSZ1、PST3和PSTZ2在溶液中的结构形态,如图2e-2h所示。由于Zn的存在,PSZ1(图2f)和PSTZ2(图2h)比PSA(图2e)和PST3(图2g)表面结构更为密实,这与SEM结果一致。Ti的不同水解产物分散并嵌入整个复合混凝剂结构中(图2g和2h),增加了结合位点并增强了电中和作用。
-
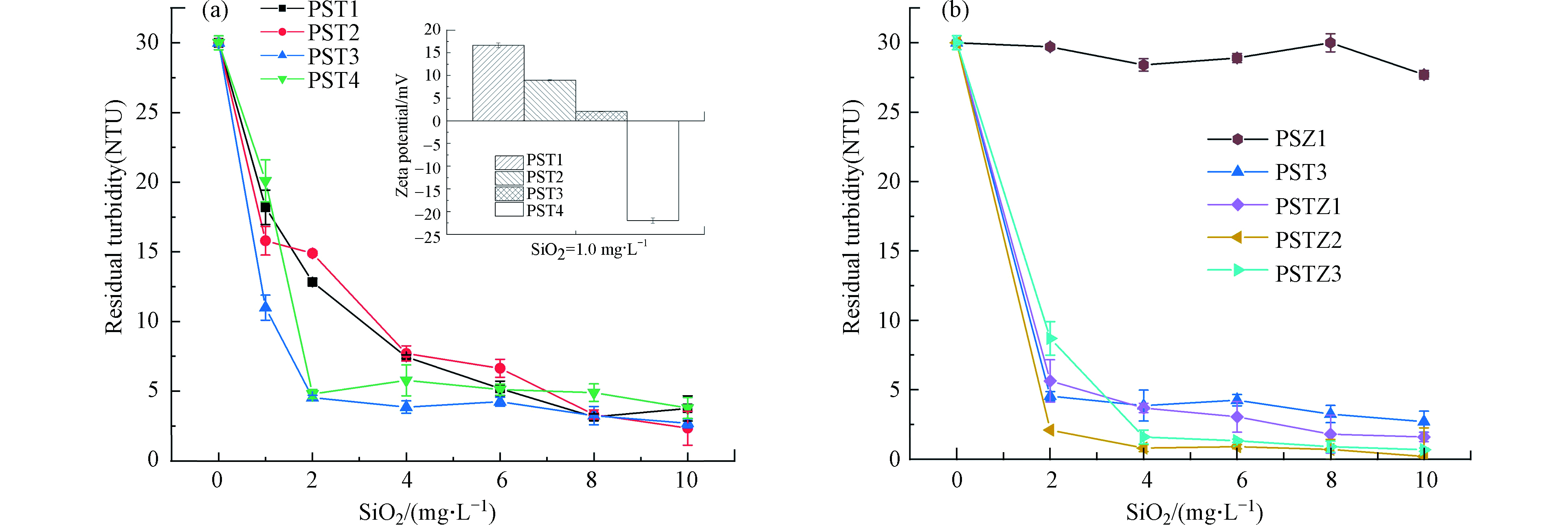
PST对高岭土悬浮液的混凝效果如图3a所示,水样残留浊度随混凝剂投加量增大先快速下降,随之逐渐趋于不变,PSTs表现出良好的混凝去浊性能。当PSTs的投加量均为1.0 mg·L−1时,PST4由于较低CD(表1)投加量不足导致电中和作用不充分而混凝效果较差,且絮体表面仍带有负电性(图3a插图)。但是,CD较高的PST1(表1)表现出的混凝性能却低于PST2和PST3,这是由于含有较多Ti时,大量金属离子会吸附在胶粒表面使其带正电荷,ζ电位升高(图3a插图),与同样带正电的PSTs混凝剂相排斥[11];此外,较多Ti造成水解生成过多的质子,造成混凝性能下降[34]。Huang等[18]研究发现钛硅混凝剂中Si的比例过多或过少都会导致混凝效果都欠佳;Moussas等[12]在铁硅混凝剂中也发现Si含量过少会导致生成的絮体较小,混凝效果下降。因此,只有在适中的金属与Si元素物质的量比时,其复合混凝剂的电中和和粘结架桥协同作用才能有效发挥[12, 18, 36]。从图3a可以看出,PST系列混凝剂中Si/Ti物质的量比为1:0.53的PST3表现出最佳的混凝效果,进一步证明适中的Si/Ti摩尔比制得的钛硅混凝剂混凝性能优良。值得注意的是,PST1、PST2、PST3在投加量为1.0 mg·L−1时ζ电位已大于0(图3a插图),且随着混凝剂投加量的增大,混凝效果进一步增强(图3a),这说明这时粘结架桥作用在混凝过程也起到了重要作用[13];而粘结架桥作用被认为是降低水体浊度的有效机制[37],因此,当投加量大于6.0 mg·L−1时,PST1、PST2、PST3、PST4体系中的残留浊度趋于相近。
PSTZ1、PSTZ2、PSTZ3与PSZ1、PST3的混凝性能如图3b所示,PSTZ1、PSTZ2、PSTZ3表现出优于PST3的混凝性能,其中最优条件下,Si/Ti/Zn摩尔比为1∶0.53∶1.23时制得的PSTZ2混凝效果最佳,再次验证了响应曲面法结果的可靠性。此外,PSTZ2的CD高于PST3,而PST3投加量为1.0 mg·L−1时,ζ电位已大于0 mV(图3a插图),由于混凝效果没有受到电荷反转的影响,PSTZ2混凝性能的增强可归因于由于引入Zn而加强的网捕卷扫作用[22, 38]。而PSTZ3的CD低于PST3,但当投加量大于4.0 mg·L−1时仍具有优于PST3的混凝性能,进一步证明了Zn具有电中和以外的其他显著促进作用,如:网捕卷扫作用。
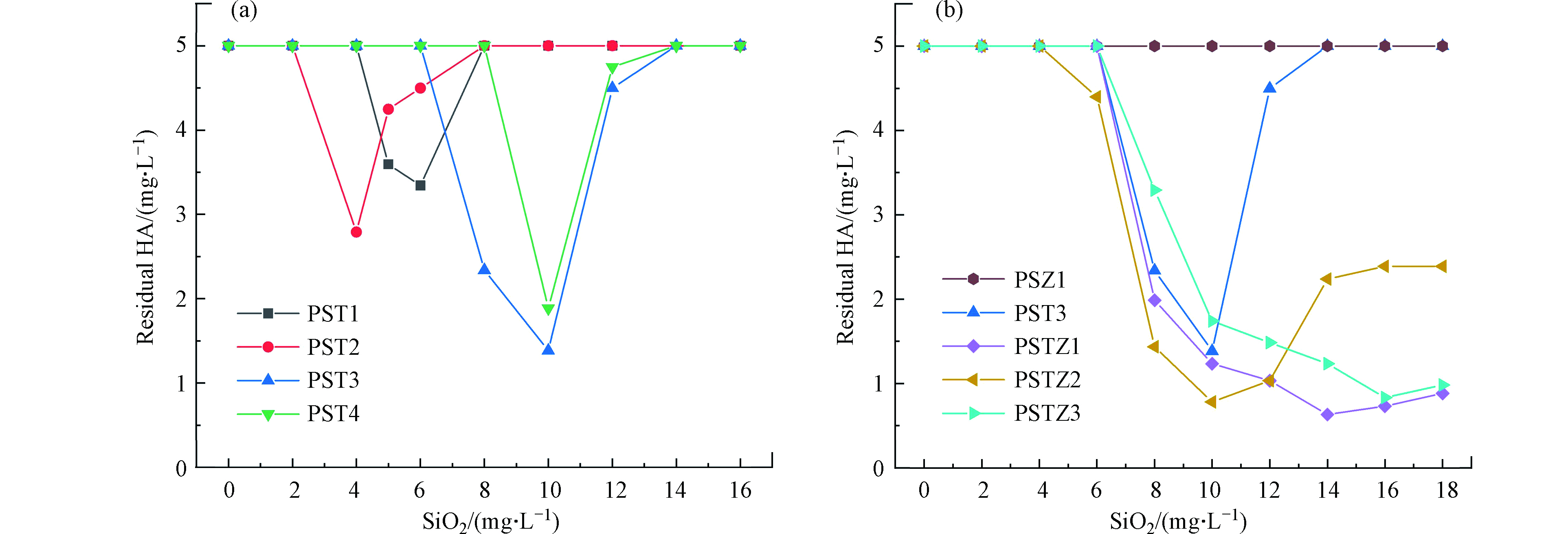
对于腐殖酸溶液,与处理高岭土悬浮液的混凝效果随混凝剂投加量趋势不同,随着PST与PSTZ投加量的增大,残留HA浓度先减少后增大,说明电中和作用在混凝中起到重要作用[39],投加量过大引起的混凝效果恶化归因于再稳定效应[28, 40]。在图4a中,PST1和PST2的最佳投加量较低,但其相应的污染物去除率却低于PST3和PST4。表1显示PST1和PST2有着较高的CD,但是Si的比例相对较低,从而导致PSA的粘结架桥作用较弱,而粘结架桥作用可以显著增强HA的去除[18, 37]。此外,在保证良好粘结架桥作用基础上,具有相对较高CD的PST3比PST4具有更好的混凝性能(图4a),与此前混凝高岭土悬浊液以及前人报道结果[18]一致。总之,由于电中和及粘结架桥作用均为混凝HA水溶液的重要机制,而PST混凝剂的CD是由带不同电性的Si与Ti含量比所决定,因此采用适中Si/Ti比例制得的复合混凝剂有着适中的CD值,并相应具有最佳混凝性能。
进一步比较PSTZ1-PSTZ3及PSZ1与PST3混凝效果(图4b),当投加量小于10.0 mg·L−1时,无论PSTZs还是PST3,残留HA浓度随混凝剂CD的增加而降低(表1及图4b),进一步证明电中和效应对去除水体中天然有机物起着重要作用[40-41]。但是,当投加量大于10.0 mg·L−1时,引入Zn明显增强了PSTZ混凝性能,说明除电中和作用外,Zn提高网捕卷扫作用[22, 38]。杨青青[11]研究也证明Zn的引入有助于浊度和天然有机物的去除。但CD过低的PSZ1单独使用对高岭土悬浮液和腐殖酸溶液的混凝性能均较差,进一步旁证了电中和效应发挥着重要作用。
-
为了进一步探讨PSTZ各元素混凝作用,在不同pH条件下,比较了PST3、PSZ1和PSTZ2混凝性能,同时检测了混凝后上清液ζ电位以及絮体性质(l和D2),列于表2中。
对于高岭土悬浮液,在不同pH条件下,PSTZ2均表现出优于PST3和PSZ1的混凝效果。相比于酸性和碱性条件,在近中性pH时,PSTZ2有着较低的残留浊度,较大的絮体尺寸,絮体结构也更为密实[42],且最佳投加量也较低,具有更佳的混凝性能。这是由于在近中性条件下,PSTZ2不仅有着高效的电中和作用,其水解产物具有良好的粘结架桥作用,能够有效降低水体浊度;但在酸性条件下这些无机盐水解产物较难形成[39];此外,酸性溶液中存在过多的质子,因此所需混凝剂投加量加大,混凝性能下降[34];同时还导致水体ζ电位大于0(表2)。而在碱性条件下,高岭土胶体颗粒表面负电性增强可更为稳定地分散在水中难以去除;此外,当pH>9.0时,会生成系列带负电性的Ti水解产物(如:Ti(OH)5-)[19, 39],从而导致电中和作用被削弱,需消耗更多的混凝剂来压缩双电层,使得最佳投加量时流出液pH降低,上清液ζ电位小于0。PST3混凝性能随pH变化趋势与PSTZ2相似,这进一步说明硅钛在PSTZ混凝过程中起到重要作用,Xu等[35]也证实硅钛混凝剂在接近pH中性下效果最好。
然而PSZ1在近中性和酸性pH条件下,对高岭土悬浮液混凝效果不明显,这可能是由于Zn无法有效水解生成氢氧化物,同时预实验结果显示,即使PSZ投加量高达20.0 mg·L−1,对除浊的效果也十分有限。但是PSZ1却在碱性条件下混凝效果显著提高,尽管PSZ1对高岭土颗粒的表面电荷影响较小,但PSZ1混凝后的絮体尺寸相较于PSTZ2和PST3混凝后絮体更大(表2),表现出良好的除浊效果[28, 37],这是由于Zn的引入会有效提高网捕卷扫作用[22, 38]。Zn的这一网捕卷扫增强效应,也使得相同投加量下时PSTZ2混凝后絮体尺寸大于PST3;此外,从PST3,PSZ1和PSTZ2混凝后上清液ζ电位看,Zn的加入还有效减轻了Ti水解产物Ti(OH)5-的负面影响[39],因此,PSTZ2具有更佳的混凝效果。
此外,在酸性和近中性pH下,最佳投加量下PSTZ2和PST3混凝后上清液的ζ电位接近于零,说明电中和在混凝中起着重要作用[40- 41]。PSTZ2的效果优于相同投加量下的PST3,且PSTZ2的絮体l更大,表明Zn改善了絮体特征,除浊效果更佳。这些发现进一步证实了Zn主要是通过网捕卷扫增强了混凝性能,而不是电中和效应。
对于腐殖酸溶液,HA是一种地表水中常见的水溶性天然有机物,含有大量阴离子性含氧官能团[43]。根据表2,PSTZ2在不同pH条件下依然表现出优于PST3和PSZ1的HA去除效果,且表现出明显的pH依赖性[44-45];但与混凝高岭土悬浊液不同的是,pH值越低,PSTZ2混凝剂的投加量越低,絮体尺寸越大,表现出更佳的净化效果。酸性条件下,HA中的负离子含氧官能团被质子化,它们更容易与O—Ti—O骨架结合[39],提高电中和效率,从而减少混凝剂的投加量,提高去除性能。此外,在酸性和近中性pH条件下,最优点处上清液ζ均接近于零,说明HA水溶液的混凝主要遵循简单电中和机制[9, 14]。但在碱性条件下,最优投加量下上清液ζ小于0,去除机理主要是电荷补丁机制[9]。
而PSZ1与其在混凝高岭土悬浮液中结果相似,在近中性pH和酸性条件下均无明显去除作用,而在碱性条件下对HA去除效果显著增强,同时混凝后HA上清液ζ接近于0。这是由于Zn对HA具有很强的吸附能力,易于形成Zn-HA络合物[33],且随着pH升高HA去质子化程度增高,A−与Zn结合能力进一步增强[33];Zn与HA之间的缔合作用使得絮体的表面电荷被猝灭,导致ζ上升。此外,由于PSZ1在碱性条件下还具有良好的网捕卷扫作用,使得最终对HA去除性能显著增强。此外,尽管PSTZ2与PST3在不同pH条件下具有相近的最佳投加量,但PSTZ2均表现出更佳的去除效果,这是由于Zn的引入易于与HA结合形成络合物,且其网捕卷扫作用也得以增强,使得絮体尺寸变大且密实程度增大(表2);同时Zn还抑制限制了Ti的水解,减轻Ti(OH)5-的负面作用[39]。
-
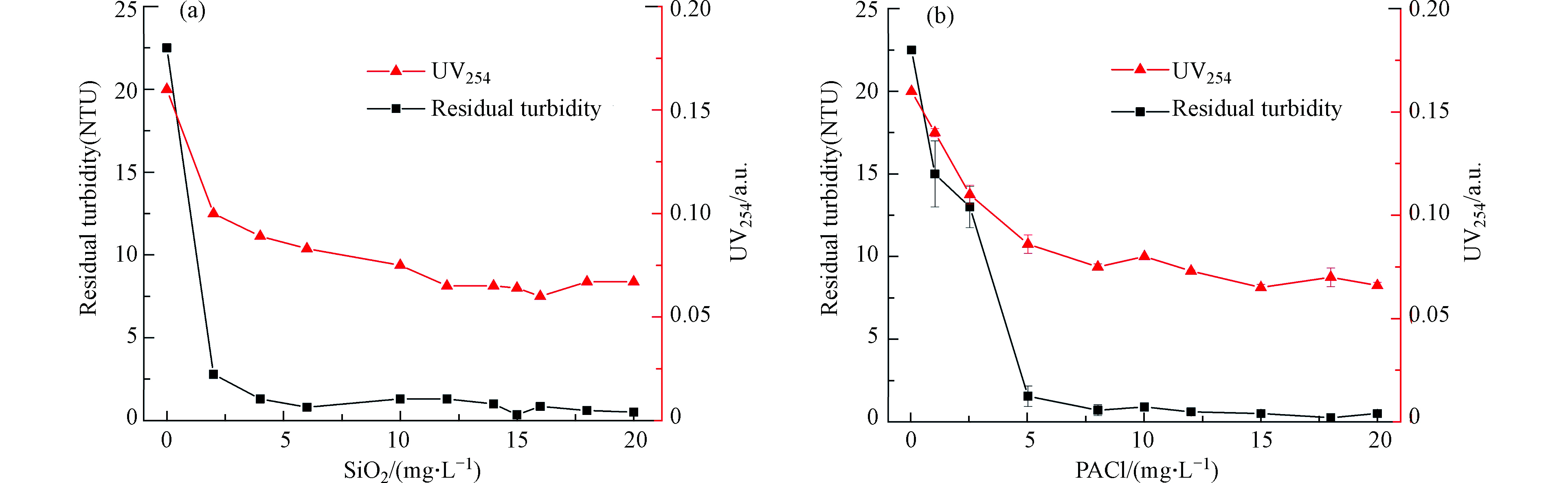
将PSTZ2应用于净化实际微污染地表水,并与传统混凝剂PACl进行比较,如图5所示。图5表明PSTZ2和PACl均能有效净化该实际地表水,且均具有较宽的混凝有效范围;在最佳投加量条件下,残留浊度接近于0,并可去除60%以上水体UV254。此外,PSTZ2相比于PACl具有较低的混凝剂最佳投加量,且PSTZ2具有较高的混凝效率。
从实际成本上看,以达到有效混凝效果(残留浊度<2.5NTU)计算,即当PSTZ2和PACl投加量分别为2.0 mg·L−1与5.0 mg·L−1时,PSTZ和PACl处理1吨水的价格分别约为31 元和30 元,说明PSTZ2具有与传统混凝剂相近的使用成本。但是,相比于PACl,PSTZ中钛、锌离子生物毒性低,安全性高,具有显著的绿色环保特征;此外,经PSTZ混凝后的底泥还可通过回收进一步制备光催化剂TiO2,具有再利用的潜在价值,因此PSTZ作为一种绿色高效的无机复合混凝剂,表现出优于传统混凝剂的综合性能与应用价值。
-
本论文以聚硅酸、钛盐及锌盐为原材,开发了一种绿色高效的无机复合混凝剂——聚硅酸钛锌混凝剂。其结构表征证明PSTZ是由Ti、Zn及Si等元素复合组成的新型多晶化合物,而不是原材料的简单混合。PSTZ相较于PST和PSZ,表现出对高岭土悬浮液和HA水溶液更好的混凝性能。且PSTZ具有适中的Si/Ti摩尔比时,可更为充分地发挥Ti电中和作用,以及Si粘结架桥效应;具有更佳的电中和和粘结架桥作用,表现出优良的混凝效果。同时Zn的引入不仅可与HA形成络合物,还能抑制Ti的水解所引起的负面影响,并表现出良好的网捕卷扫作用,从而进一步增强了PSTZ的混凝性能。针对实际微污染地表水的净化,PSTZ2与传统混凝剂PACl相比,具有相似的混凝效果和应用成本,但PSTZ显著的绿色环保特性及可再利用的潜在价值,证实了该新型复合混凝剂的高效性及优越性。尽管该复合混凝剂还需通过处理不同的实际污废水来进一步验证其有效性,但PSTZ在未来水处理应用有着重要的应用前景。
新型复合聚硅酸钛锌混凝剂的合成、表征及混凝性能
Synthesis, characterization, and coagulation performance of a novel polysilicic-titanium-zinc composite coagulant
-
摘要: 采用聚硅酸(PSA)、钛盐及锌盐以不同摩尔比合成了一系列新型聚硅酸钛锌(PSTZ)混凝剂,并使用胶体滴定、扫描电子显微镜、透射电子显微镜、X射线衍射和傅立叶变换红外对药剂进行了结构表征。以高岭土悬浊液和腐殖酸(HA)水溶液为模拟水样,详细考察了各组成含量及pH对PSTZ混凝性能及絮体结构的影响,同时与聚硅酸钛(PST)和聚硅酸锌(PSZ)进行性能对比。结果表明,由于PSTZ具有层状和多孔的表面结构,PSTZ混凝性能优于PST和PSZ,形成絮体尺寸也大;进一步研究发现具有适中Si/Ti物质的量比(1:0.53)的PSTZ混凝剂具有更佳的电中和与粘结架桥作用;对Zn离子而言,特别是在pH 9.0—10.0下,易于与HA形成Zn-HA络合物,同时减轻Ti水解产物Ti(OH)5-的负面影响,进一步增强PSTZ混凝性能。此外,PSTZ在近中性pH条件下对高岭土悬浊液混凝性能最佳,而在酸性条件下对HA水溶液净化效果最优,这是由于PSTZ对不同污染物具有不同混凝机制所造成的。除模拟水样外,还考察了PSTZ对实际微污染地表水的混凝效果,并与传统混凝剂聚合氯化铝(PACl)进行对比,PSTZ表现出优于PACl的混凝性能,进一步证实了该复合混凝剂的有效性。该工作提供了一种绿色环保、可回收再利用的无机复合混凝剂,可有效地净化微污染地表水,提高用水安全。
-
关键词:
- 聚硅酸钛锌复合混凝剂 /
- 微污染地表水 /
- 强化混凝 /
- 混凝机理
Abstract: A series of new composite coagulants, polysilicic-titanium-zinc (PSTZ) was synthesized by polysilicic acid (PSA), titanium salt, and zinc salt with different molar ratios, which was characterized by colloidal titration, scanning electron microscopy, transmission electron microscopy, x-ray diffraction and fourier transform infrared spectra. The kaolin suspension and humic acid (HA) aqueous solution were used as simulated water samples, and the effects of the composition contents in PSTZ and pH on the coagulation performance and floc structure were investigated in detail, which were compared with those of polysilicic-titanium (PST) and polysilicic-zinc (PSZ). PSTZ exhibited better coagulation performance than PST and PSZ due to its porous surface structural characteristics, and the size of the flocs formed by PSTZ was larger. PSTZ with moderate Si/Ti molar ratio of 1: 0.53 had better charge neutralization and adsorption-bridging effects; besides, especially at the pH of 9.0—10.0, Zn could adsorb HA to form Zn-HA complexes, and simultaneously alleviate the negative effect of Ti hydrolysis product Ti(OH)5-, causing the improved coagulation performance of PSTZ. In addition, PSTZ showed better effects at the natural pH in coagulation of kaolin suspension but at the acidic condition in removal of HA due to their different coagulation mechanisms in removal of various pollutants. In addition to simulated water, the performance of PSTZ in coagulation of a real surface water sample was also investigated and compared with a conventional coagulant, polyaluminum chloride (PACl). The coagulation performance of PSTZ was better than that of PACl, further confirming the effectiveness of this composite coagulant. This work provides a green, environmentally friendly, recyclable inorganic composite coagulant, which can effectively purify micro-polluted surface water and improve water safety. -

-
图 3 (a) PSTs和(b) PSTZs混凝高岭土悬浮液后的残留浊度.
Figure 3. The performance of (a) PSTs and (b) PSTZs in coagulation of kaolin suspensions, the inset of Fig. 3a is the zeta potentials of supernatants coagulated by PSTs with the dose of 1.0 mg·L−1.
表 1 不同元素摩尔比制得的PST、PSTZ及PSZ的电荷密度以及pH
Table 1. Charge density and pH of PST, PSTZ, and PSZ prepared with different molar ratio of elements
混凝剂
Coagulants硅∶钛∶锌
Si∶Ti∶Zn a电荷密度/(mmol·L−1)
Charge densitypH b PST1 1∶2∶0 3.591 3.68 PST2 1∶1∶0 3.192 3.85 PST3 1∶0.53∶0 1.710 4.02 PST4 1∶0.25∶0 0.570 4.07 PSTZ1 1∶0.53∶0.5 2.394 4.07 PSTZ2 1∶0.53∶1.23 2.622 4.59 PSTZ3 1∶0.25∶1.23 0.741 4.24 PSZ1 1∶0∶1.23 0.456 5.75 a 物质的量比;b 混凝剂溶于离子水后的溶液pH值,混凝剂浓度为2.0 mg·L−1.
a Molar ratio; b The pH value of the solution after the coagulant with the concentration of 2.0 mg·L−1 was dissolved in ionized water.表 2 PSTZ2、PST3和PSZ1在各pH下对不同模拟废水的混凝性能和絮体特征
Table 2. Coagulation performance and floc properties of PSTZ2, PST3 and PSZ1 for coagulation of various synthetic wastewaters at different pH levels
模拟废水
Wastewater初始pH
Initial pH混凝剂
Coagulants最优投加量/(mg·L−1)
Optimal dosea残留浊度/NTU
Residual
turbidity溶液pH
Effluent pHζ电位/mV
Zeta potential平均粒径/μm
Average二维分形维数
D2高岭土悬浮液 3.14 PSTZ2 12.0 2.80 3.05 4.3±0.7 260±20 1.773±0.054 PST3 12.0 2.85 3.00 4.7±0.4 96±15 1.723±0.044 PSZ1b — — — — — — 5.90 PSTZ2 2.0 1.10 4.35 2.6±0.7 349±12 1.807±0.022 PST3 2.0 2.70 4.40 3.2±0.6 269±17 1.910±0.052 PSZ1b — — — — — — 9.93 PSTZ2 9.0 2.99 3.81 −8.2±1.0 200±14 1.844±0.065 PST3 12.0 2.78 3.58 −10.4±1.6 152±9 1.707±0.048 PSZ1 9.0 2.53 5.68 −34.4±2.8 230±22 1.882±0.081 模拟废水
Wastewater初始pH
Initial pH混凝剂
Coagulants最优投加量/(mg·L−1)
Optimal dosea残留腐殖酸/(mg·L−1)
Residual HA溶液pH
Effluent pHζ电位/mV
Zeta potential平均粒径/μm
Average二维分形维数
D2腐殖酸溶液 3.06 PSTZ2 2.0 1.69 3.06 −1.6±0.5 25.3±1.2 1.803±0.023 PST3 2.0 2.39 3.04 −0.7±0.9 14.2±0.9 1.739±0.037 PSZ1b — — — —
—
— 5.97 PSTZ2 8.0 1.40 3.77 −0.2±1.4 17.5±0.3 1.756±0.013 PST3 8.0 1.75 3.73 0.0±2.2 11.8±2.2 1.748±0.045 PSZ1b — — — — — — 9.91 PSTZ2 10.0 2.24 3.88 −11.0±1.7 10.0±1.2 1.706±0.045 PST3 10.0 2.49 3.86 −18.7±2.2 9.9±0.4 1.694±0.026 PSZ1 16.0 1.97 7.36 0.2±1.6 13.1±1.3 1.757±0.047 a 最优投加量是指当残留浊度首先低于3.00 NTU或残留HA首先低于2.50 mg·L−1时混凝剂的剂量;b 在酸性和近中性pH条件下,PSZ1的絮体ζ电位均为负,且混凝性能不佳.
a The optimal coagulant dose referred to the one at which the residual turbidity was first lower than 3.00 NTU or the residual HA was first lower than 2.50 mg·L−1;
b Under acidic and near-neutral pH conditions, the zeta potentials of flocs produced by PSZ1 were all negative and its coagulation performance was low. -
[1] 中华人民共和国生态环境部, 2019中国生态环境状况公报[EB], 2020. [2] 中华人民共和国生态环境部, GB 3838-2002, 地表水环境质量标准[S], 2002. [3] BRATBY J. Coagulation and flocculation in water and wastewater treatment, thirded[M]. IWA Publishing, London, 2016. [4] PIRSAHEB M, SHARAFI K, KARAMI A. Evaluating the performance of inorganic coagulants (poly aluminum chloride, ferrous sulfate, ferric chloride and aluminum sulfate) in removing the turbidity from aqueous solutions [J]. International Journal of Pharmacy & Technology, 2016, 8(2): 13168-13181. [5] VERMA A K, DASH R R, BHUNIA P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters [J]. Journal of Environmental Management, 2012, 93(1): 154-168. [6] WEI H, GAO B Q, REN J, et al. Coagulation/flocculation in dewatering of sludge: A review [J]. Water Research, 2018, 143: 608-631. doi: 10.1016/j.watres.2018.07.029 [7] JIANG J. The role of coagulation in water treatment [J]. Current Opinion in Chemical Engineering, 2015, 8: 36-44. doi: 10.1016/j.coche.2015.01.008 [8] LEE C S, ROBINSON J, CHONG M F. A review on application of flocculants in wastewater treatment [J]. Process Safety and Environmental Protection, 2014, 92: 489-508. doi: 10.1016/j.psep.2014.04.010 [9] YANG R, LI H, HUANG M, et al. A review on chitosan-based flocculants and their applications in water [J]. Water Research, 2016, 95: 59-89. doi: 10.1016/j.watres.2016.02.068 [10] FU Y, ZHANG J, WANG Y, et al. Resource preparation of poly-Al-Zn-Fe (PAZF) coagulant from galvanized aluminum slag: Characteristics, simultaneous removal efficiency and mechanism of nitrogen and organic matters [J]. Chemical Engineering Journal, 2012, 203: 301-308. doi: 10.1016/j.cej.2012.07.045 [11] 杨青青. 聚硅酸硫酸锌铁絮凝剂的制备及其在含藻水中的应用[D]. 重庆: 重庆大学, 2015. YANG Q Q. The preparation of poly-silicate-sulfate-Zn-Fe flocculant and application in the algae water[D]. Chongqing: University of Chongqing, 2015(in Chinese).
[12] MOUSSAS P A, ZOUBOULIS A I. A study on the properties and coagulation behaviour of modified inorganic polymeric coagulant-Polyferric silicate sulphate (PFSiS) [J]. Separation and Purification Technology, 2008, 63: 475-483. doi: 10.1016/j.seppur.2008.06.009 [13] WAN Y, HUANG X, SHI B Y, et al. Reduction of organic matter and disinfection byproducts formation potential by titanium, aluminum and ferric salts coagulation for micro-polluted source water treatment [J]. Chemosphere, 2019, 219: 28-35. doi: 10.1016/j.chemosphere.2018.11.117 [14] GUIBAL E, VAN M. DEMPSEY B A, et al A review of the use of chitosan for the removal of particulate and dissolved contaminants [J]. Separation and Purification Technology, 2006, 41(11): 2487-2514. [15] RIZZO L, GENNARO A, GALLO M, et al. Coagulation/chlorination of surface water: A comparison between chitosan and metal salts [J]. Separation and Purification Technology, 2008, 62(1): 79-85. doi: 10.1016/j.seppur.2007.12.020 [16] BOLTO B, GREGORY J. Organic polyelectrolytes in water treatment [J]. Water Research, 2007, 41(11): 2301-2324. doi: 10.1016/j.watres.2007.03.012 [17] OKUDA T, NISHIJIMA W, SUGIMOTO M, et al. Removal of coagulant aluminum from water treatment residuals by acid [J]. Water Research, 2014, 60: 75-81. doi: 10.1016/j.watres.2014.04.041 [18] HUANG X, GAO B, WANG Y, et al. Coagulation performance and flocs properties of a new composite coagulant: Polytitanium-silicate-sulfate [J]. Chemical Engineering Journal, 2014, 245: 173-179. doi: 10.1016/j.cej.2014.02.018 [19] HUSSAIN S, AWAD J, SARKAR B, et al. Coagulation of dissolved organic matter in surface water by novel titanium (Ⅲ) chloride: Mechanistic surface chemical and spectroscopic characterization [J]. Separation and Purification Technology, 2019, 213: 213-223. doi: 10.1016/j.seppur.2018.12.038 [20] WANG D, TANG H. Modified inorganic polymer Flocculant-PFSi [J]. Water Research, 2001, 35: 3418-3428. doi: 10.1016/S0043-1354(01)00034-3 [21] ZENG Y, PARK J. Characterization and coagulation performance of a novel inorganic polymer coagulant-poly-zinc-silicate-sulfate [J]. Colloids and Surface A-Physicochemical and Engineering Aspect, 2009, 334: 147-154. [22] ZHU G, WANG Q, YIN J, et al. Toward a better understanding of coagulation for dissolved organic nitrogen using polymeric zinc-iron-phosphate coagulant [J]. Water Research, 2016, 100: 201-210. doi: 10.1016/j.watres.2016.05.035 [23] GENC-FUHRMAN H, MIKKELSEN P S, LEDIN A. Simultaneous removal of As, Cd, Cr, Cu, Ni and Zn from stormwater using high-efficiency industrial sorbents: Effect of pH, contact time and humic acid [J]. Science of the Total Environment, 2016, 566/567: 76-85. doi: 10.1016/j.scitotenv.2016.04.210 [24] WATSON M, TUBIC A, AGBABA J, et al. Response surface methodology: Process and product optimization using designed experiments, second ed[M]. John Wiley & Sons, New York, 2002. [25] ZHANG W X, YANG H, DONG L, et al. Efficient removal of both cationic and anionic dyes from aqueous solutions using a novel amphoteric straw-based adsorbent [J]. Carbohydrate Polymers, 2012, 90(2): 887-893. doi: 10.1016/j.carbpol.2012.06.015 [26] CHOI K Y J, DEMPSEY B A. In-line coagulation with low-pressure membrane filtration [J]. Water Research, 2004, 38(19): 4271-4281. doi: 10.1016/j.watres.2004.08.006 [27] WANG Z, PENG S, NAN J, et al. Quantitative analysis of cake characteristics based on SEM imaging during coagulation-ultrafiltration process [J]. Environmental Science and Pollution Research International, 2019, 26: 36296-36307. doi: 10.1007/s11356-019-06678-7 [28] WU H, LIU Z, YANG H, et al. Evaluation of chain architectures and charge properties of various starch-based flocculants for flocculation of humic acid from water [J]. Water Research, 2016, 96: 126-135. doi: 10.1016/j.watres.2016.03.055 [29] CHAKRABORTI R K, GARDNER K H, ATKINSONA J F, et al. Changes in fractal dimension during aggregation [J]. Water Research, 2003, 37: 873-883. doi: 10.1016/S0043-1354(02)00379-2 [30] ZHAO Y, SHON H, PHUNTSHO S, et al. Removal of natural organic matter by titanium tetrachloride: the effect of total hardness and ionic strength [J]. Journal of Environmental Management, 2014, 134: 20-29. doi: 10.1016/j.jenvman.2014.01.002 [31] ZHAO Y, GAO B, SHON H, et al. The effect of second coagulant dose on the regrowth of flocs formed by charge neutralization and sweep coagulation using titanium tetrachloride TiCl4 [J]. Journal of Hazardous Materials, 2011, 198: 70-77. doi: 10.1016/j.jhazmat.2011.10.015 [32] ZHAO Y, GAO B, QI Q, et al. Cationic polyacrylamide as coagulant aid with titanium tetrachloride for low molecule organic matter removal [J]. Journal of Hazardous Materials, 2013, 258: 84-92. [33] 张鹏, 王雨露, 赵冬琴, 等. 聚磷氯化铁镁钛混凝剂的制备与表征 [J]. 环境化学, 2018, 37(12): 2677-2687. doi: 10.7524/j.issn.0254-6108.2018012104 ZHANG P, WANG Y L, ZHAO D Q, et al. Preparation and characterization of polychlorinated ferric magnesium titanium(PFMTC) [J]. Environmental Chemistry, 2018, 37(12): 2677-2687(in Chinese). doi: 10.7524/j.issn.0254-6108.2018012104
[34] 陈伟. 铁钛混凝剂的制备及在除藻和控制藻源膜污染中的应用研究[D]. 重庆: 重庆大学, 2016. CHEN W. The preparation of Fe-Ti based coagulant and its application in algae removal and membrane fouling controlling[D]. Chongqing: University of Chongqing, 2016(in Chinese).
[35] XU B, ZHANG Y J, LI X, et al. A simple preparation route for polysilicate titanium salt from spent titanium solutions [J]. Water Science and Technology, 2019, 80: 1347-1356. doi: 10.2166/wst.2019.383 [36] WEI Y, DING A, DONG L, et al. Characterization and coagulation performance of an inorganic coagulant-poly-magnesium-silicate-chloride in treatment of simulated dyeing wastewater [J]. Colloids and Surface A-Physicochemical and Engineering Aspect, 2015, 470: 137-141. [37] TANG Y N, HU X Y, CAI J, et al. An enhanced coagulation using a starch-based coagulant assisted by polysilicic acid in treating simulated and real surface water [J]. Chemosphere, 2020, 259: 127464. doi: 10.1016/j.chemosphere.2020.127464 [38] WEI Y X, DONG X Z, DING A M, et al. Characterization and coagulation-flocculation behavior of an inorganic polymer coagulant-poly-ferric-zinc-sulfate [J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 58: 351-356. doi: 10.1016/j.jtice.2015.06.004 [39] WANG X, GAN Y, GUO S, et al. Advantages of titanium xerogel over titanium tetrachloride and polytitanium tetrachloride in coagulation: A mechanism analysis [J]. Water Research, 2018, 132: 350-360. doi: 10.1016/j.watres.2017.12.081 [40] WEI H, REN J, LI A, et al. Sludge dewaterability of a starch-based flocculant and its combined usage with ferric chloride [J]. Chemical Engineering Journal, 2018, 349: 737-747. doi: 10.1016/j.cej.2018.05.151 [41] LIU Z, HUANG H, LI A, et al. Flocculation and antimicrobial properties of a cationized starch [J]. Water Research, 2017, 119: 57-66. doi: 10.1016/j.watres.2017.04.043 [42] 王毅力, 卢佳, 杜白雨, 等. 聚合氯化铁-腐殖酸(PFC-HA)絮体的不同拓扑空间下分形维数的研究 [J]. 环境科学学报, 2008, 28(4): 606-615. doi: 10.3321/j.issn:0253-2468.2008.04.003 WANG Y L, LU J, DU B, et al. Fractal dimension of polyferric chloride humic acid(PFC-HA) flocs in different topological spaces [J]. Acta Scientiae Circumstantiae, 2008, 28(4): 606-615(in Chinese). doi: 10.3321/j.issn:0253-2468.2008.04.003
[43] XIAO F, XIAO P, WANG D S. Influence of allochthonous organic matters on algae removal: Organic removal and floc characteristics [J]. Colloids and Surface A-Physicochemical and Engineering Aspect, 2019, 583: 123995. [44] GREGOR J, NOKES C, FENTON E. Optimising natural organic matter removal from low turbidity waters by controlled pH adjustment of aluminium coagulation [J]. Water Research, 1997, 31(12): 2949-2958. doi: 10.1016/S0043-1354(97)00154-1 [45] NARKIS N, REBHUN M. Stoichiometric relationship between humic and fulvic acids and flocculants [J]. Journal American Water Works Association, 1977, 69(6): 325-328. doi: 10.1002/j.1551-8833.1977.tb06752.x -



 下载:
下载: