-
重金属在环境中是一般不能被降解的,采矿、冶金、机械制造、化工、电子等行业的重金属废水,若不经处理直接排入环境中,会对生态环境和人体健康造成重大危害,重金属污染已成为全球性重大环境问题之一[1-4]. 锌、铜、锰和镍等重金属虽是人体必需微量元素,但此类重金属在体内长时间累积也可能引起心血管、肺、神经和内分泌等多方面问题 [4-6]. 汞(Hg)、镉(Cd)、铅(Pb)、铬(Cr)和砷(As)等非生命活动所必需的“五毒”重金属,对人、动植物和微生物更是具有显著毒性[4-7]. 人们长期饮用含砷(As)地下水,会引发皮肤癌、黑脚病及其他神经系统疾病(如孟加拉国砷污染事件)[4-6];饮用铅(Pb)离子超标的水,会导致人身器官损害、痴呆、骨萎缩等疾病(如血铅事件)[7]等;镉在体内蓄积,会造成肾损伤,进而导致骨软化症(日本著名的公害病──痛痛病)[8-12];汞金属进入人体内,会导致汞中毒,如20世纪50年代,日本九州水俣湾发生的“水俣病”事件 [11-15]. 据不完全估计,全世界平均每年向环境排放重金属达千万吨:Cu约340万吨,Pb约500万吨,Ni约100万吨等[16]. 重金属污染的水体和土壤环境修复已成为当前环境保护领域的重点、热点和难点问题.
纳米零价铁(nano scale zero valent iron,nZVI)是最早用于环境治理的纳米材料之一,其原材料来源丰富、反应产物环境友好,适用于水体中重金属的治理与修复. nZVI去除水中重金属的研究开始于2000年,科学家们以聚合树脂、硅胶等作为载体,制备了粒径在10—30 nm的负载型铁纳米材料,并应用于水溶液中Cr(Ⅵ)和Pb(Ⅱ)的固定和修复,实验证明具有良好的修复效果[17]. 此后,韩国科学家在2005年报道了nZVI异位处理地下水中的As污染,结果表明nZVI对水体中砷的去除率可达95%以上 [18]. 2006年用nZVI去除水溶液中镍离子的研究表明,每克铁对于镍的去除容量达到0.13 g Ni·g−1 Fe,吸附容量比所有的吸附材料都高[19]. 此后,有关nZVI去除重金属的研究层出不穷 [20-30]. 至今,已有多篇文献报道了nZVI用于土壤及地下水卤代有机污染的原位修复研究 [31-38],但尚未有关于重金属污染土壤、地下水的实际场地修复的报道.
2014年,本研究团队首次进行了nZVI技术处理含重金属工业废水的中试研究[39],针对我国水体重金属污染控制现实需求,在nZVI处理工业废水(特别是重金属工业废水)工艺、过程及机理上进行创新型拓展,为纳米材料对含重金属工业废水的有效处置提供了解决方案. 本文重点总结了nZVI技术在工业含重金属废水处理中的原理、技术和最新进展,为本领域的研究者提供参考.
-
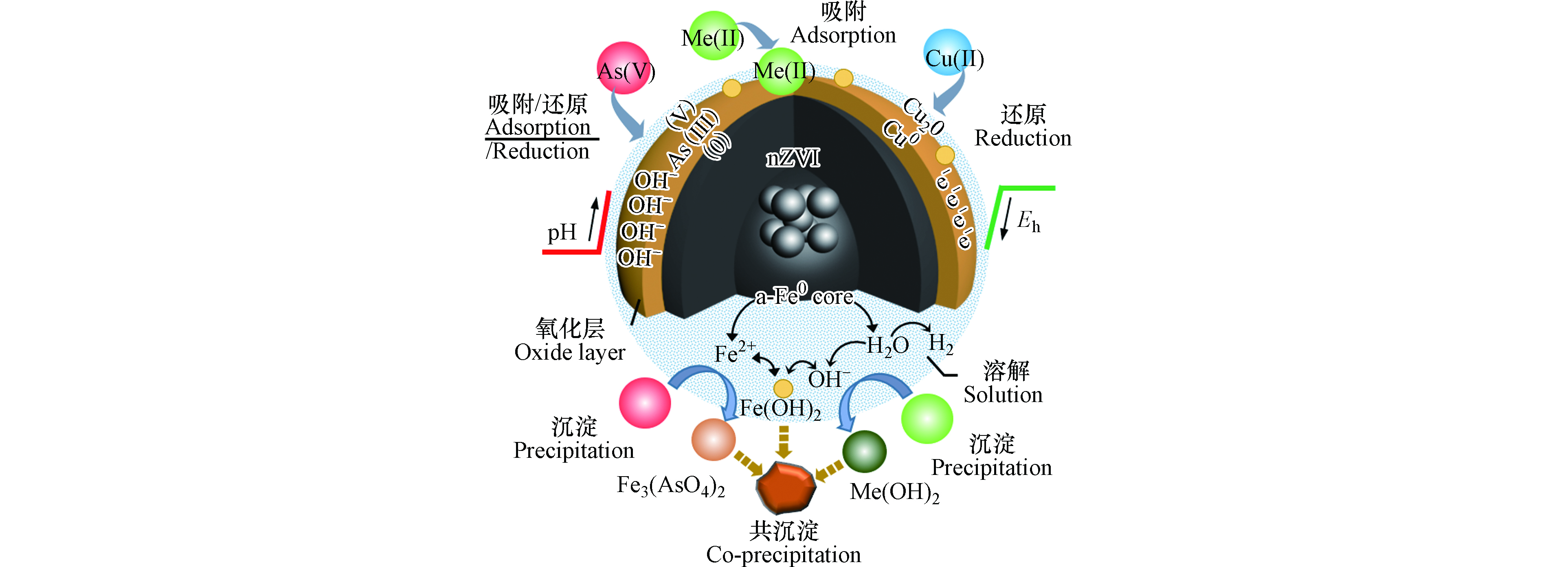
已有研究表明,不论是采用硼氢化钠化学还原氯化铁(硫酸亚铁)、氢气还原氧化铁等化学还原方法制备的nZVI,还是化学气相沉积、微米铁机械球磨方法等制备的nZVI,均具有核-壳结构[40-42],即内核为零价铁,壳层由多种铁氧化物组成,厚度为几个nm,因此nZVI兼具纳米材料和零价铁材料的双重特性. 在纳米零价铁去除重金属的过程中,壳层氧化铁不但能够作为污染物的活性位点,同时由于具有缺陷及导电性等特质,可提供电子转移通道[43-46],使部分重金属污染物被还原.
在水处理过程中,nZVI在水中发生系列反应,生成Fe2+、Fe(OH)2、FeOOH、Fe2O3 、Fe3O4 等一系列腐蚀产物[47-51],如式(1—9). 水中的重金属离子与nZVI可发生以下反应:(1)在nZVI表面快速形成表面络合物;(2)与铁氧化物共沉淀;(3)金属离子通过nZVI壳层的缺陷到达核-壳结构零价铁的界面,继而还原为零价金属[46,49].
研究表明,纳米零价铁去除水中重金属的主要作用机理包括:吸附、还原、沉淀及共沉淀(图1). nZVI的双纳米相结构为水相中的重金属离子的分离和转化提供有效且独特的解决方案[50-53]. 研究团队基于实验室小试中nZVI对重金属废水良好的处理效果,进一步构建“反应-分离-回用”式纳米零价铁反应器,用于实际废水的中试和工程应用研究,实验室小试、中试、工程应用中nZVI对于重金属的去除机理相同[39,54-58].
-
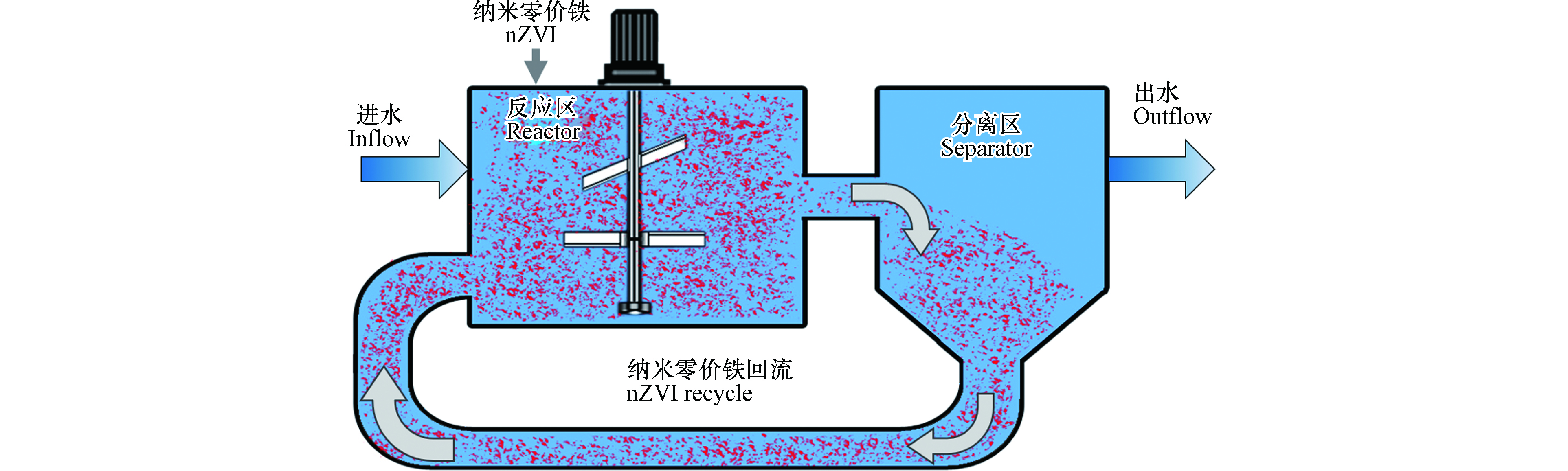
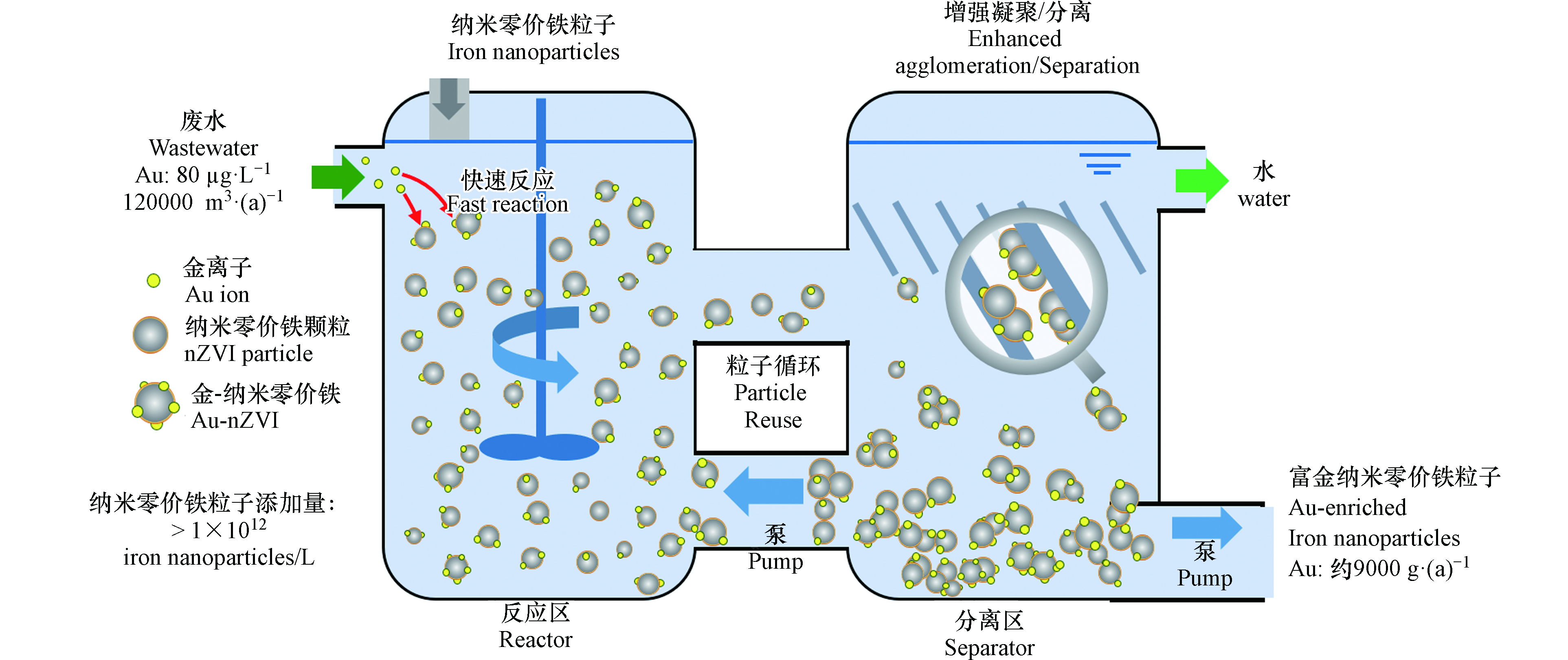
纳米零价铁进行重金属工业废水处理时,反应需在适宜的反应器内完成. 为此,笔者所在的研究团队设计了纳米零价铁反应器(nano iron reactor, NIR),将其用于实际工业废水处理的中试和工程应用[39,54-58],开拓了nZVI环境应用新领域. NIR反应器由反应区、分离区和污泥回流系统组成,采用全混合搅拌釜式反应器(continuous stirred tank reactor, CSTR)作为反应区,竖流式沉淀池作为分离区,沉降于分离区底部的污泥通过蠕动泵或离心泵进行回流. 在中试试验中,另配有纳米零价铁投药泵和在线pH 、氧化还原电位计检测记录仪等(图2).
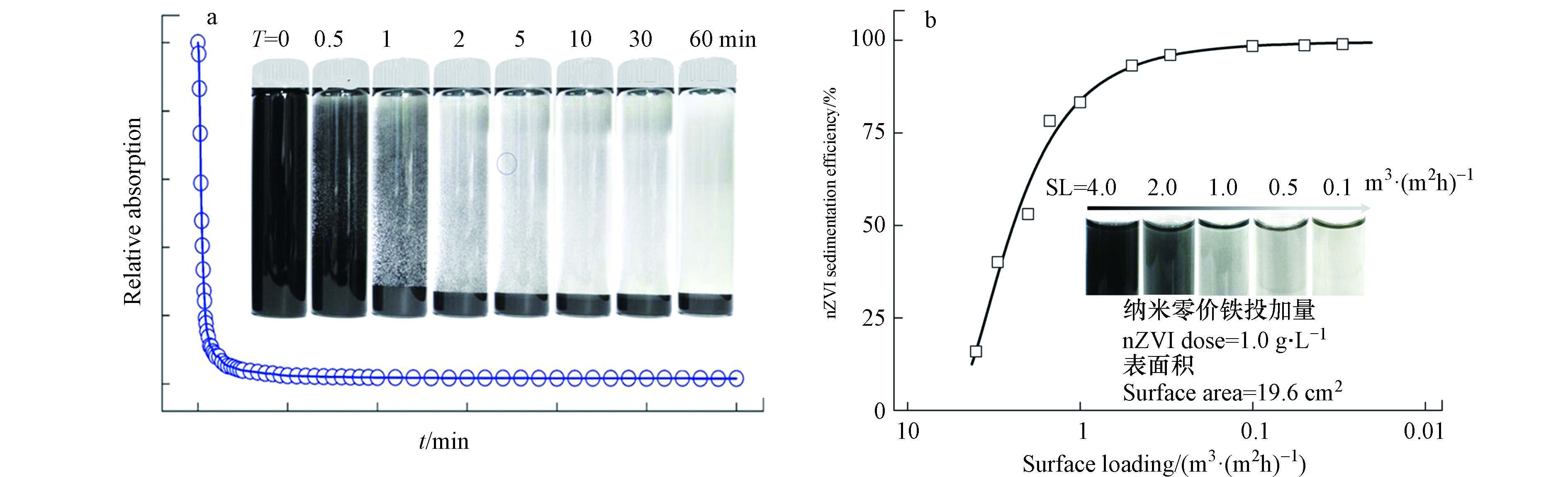
纳米零价铁与水经预分散后由蠕动泵泵入反应区. 待处理废水由离心泵从现场废水调节池抽提至纳米零价铁反应器内,管阀及流量计控制进水流量[57-58]. 在纳米零价铁反应器进行含重金属工业废水去除时,需要考虑水力停留时间、系统运行稳定性、反应后产物固液分离等对于反应效率的影响. 在处理含有强氧化性的重金属且浓度波动剧烈的工业废水时,需要预先设定反应区Eh控制范围并在线监测反应区其数值实时变化. 当Eh升高并突破控制范围时及时补充新鲜nZVI,使出水水质控制在排放标准范围以内 [39,54-58]. 在分离区中,纳米零价铁能够团聚成微米级尺寸的团聚体,通过重力沉降进行固液分离. 实验室研究证明,5 g·L−1的纳米零价铁,10 min的静态沉降效率可达96%,1 h的静态沉降效率达99%(图3A). 且纳米零价铁的浓度越高,静态沉降速度就越快;动态沉降的效果与表面水力负荷有关,由图3B可见,沉淀池表面负荷越低,纳米零价铁的沉降效果越好.
-
研究团队选取含铜线路板废水(江苏昆山)、含砷冶炼废水(湖北大冶)、含铅含锌制酸废水(湖南水口山)及含铜含砷冶炼废水(江西贵溪)作为处理对象,用纳米零价铁技术进行了中试及工程应用研究[39,54-58]. 重金属工业实际废水具有成分复杂、重金属浓度高、波动大等缺点(主要原水水质特征见表1),各废水中均同时存在多种重金属,主要为铜、砷、锌、铅、镍. 该类废水多为强酸性,其中部分废水中同时存在较高的盐分及氨氮等杂质,因此采用传统的石灰、硫化钠、吸附剂等材料无法同步去除废水中多种重金属阳离子和含氧阴离子。但纳米零价铁处理工艺在此类废水的中试及工程应用中均表现出了优异的性能.
-
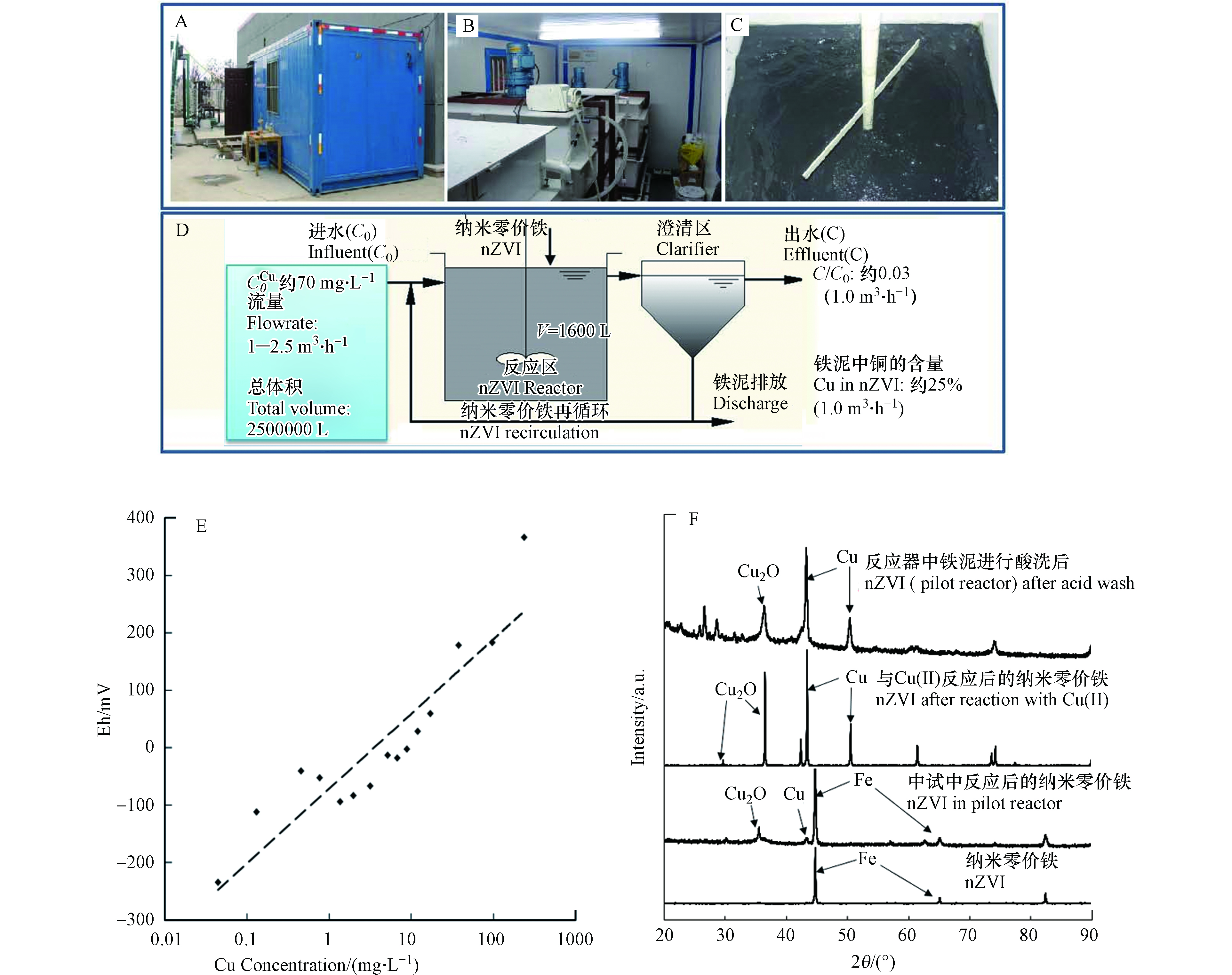
2014年,研究团队首先报导了纳米零价铁反应器(NIR)技术处理江苏昆山某高浓度含铜线路板废水的中试结果,证明了该技术处理含铜废水的可行性[38]. 整个中试共使用55 kg纳米零价铁,处理了250 t含铜废水([Cu(Ⅱ)]~70 mg·L−1). 在中试规模上,首次研究了纳米零价铁处理高浓度含铜废水效果及关键影响因素,通过改变进水容量,重点考察了该反应器水力停留时间(HRT)对装置长期运行的影响;此外,还考察了进出水铜浓度变化、反应区pH、Eh变化、去除负荷变化等因素(图4). 结果证明在流量为1.0 m3·h−1(HRT=1.6 h),nZVI投量为0.23 kg·m−3时,铜的去除率最高,可达97%. 废水中平均铜浓度从进水的52.5 mg·L−1降至出水的1.7 mg·L−1,且出水水质稳定(图4D). 该研究表明通过“反应—分离—回用”处理流程,nZVI在反应器内充分循环反应,除铜负荷高达343 mg Cu·g−1 nZVI,反应区nZVI容积负荷高达 1876 g-Cu·(m3·d)−1,且污泥中铜含量高达25%,具有回收价值.
nZVI对废水中铜(重金属)的去除负荷(removal capacity, mg Cu·g−1 nZVI,)可通过式10计算(以铜为例):
其中,
CuinT ,CuoutT 和FeT分别为流入、流出NIR的总铜量及纳米零价铁的总投量.中试结果发现,混合液氧化还原电位(Eh)和相应出水中Cu(Ⅱ)浓度具有较强的相关性:当出水中Cu(Ⅱ)浓度提高时(呈数量级升高时),对应时刻反应区内混合液Eh同样出现升高趋势;而当Eh降低并低于−0.2 V时, 对应出水中Cu(Ⅱ)浓度低于0.1 mg·L−1(图4E). 因此,通过监测反应区Eh变化,来判断反应器内的运行情况,尤其适用于含强氧化性重金属且浓度波动较大的工业废水.
采用 XRD对中试纳米零价铁反应产物进行了成分分析,如图4F所示. 新鲜纳米零价铁仅出现α-Fe的3个特征衍射峰,而含铜废水中试处理后获得泥样的XRD峰谱中出现新的特征衍射峰,经 PDF卡片对比确认为Cu2O和Cu0两种物质,溶液化学小试研究也出现同样情况. (图4F). 上述结果表明,水中Cu(Ⅱ)被纳米零价铁还原生成了零价铜和赤铜矿(Cu2O). 与采用加碱沉淀或硫化物沉淀形成的氢氧化铜或硫化铜相比,金属或氧化亚铜形式的铜具有更低的生物可利用性[59]. 换言之,采用纳米零价铁进行含铜废水处理,其固体产物或污泥更加稳定.
此外,对中试过程中产生的污泥进行了铜含量分析,以评估其回收价值. 研究发现,当进水流量为1.5 m3·h−1或更低时,产生的污泥中铜含量占污泥干重的10%(W/W)以上,当进水流量为1.0 m3·h−1时,污泥中铜含量高达25%. 一般而言,自然界铜矿工业品位(Cu,质量分数)达到0.5%及以上即具有开采价值. 这些铜矿石再经过一系列复杂的选矿过程后,铜含量富集,成为铜精矿. 根据国家标准[60],铜精矿按铜品位分为5个等级,其中一级品铜含量不低于32%,五级品铜含量不低于13%. 按此标准,本次中试获得的污泥属于铜精矿中的二级品,具有相当高的回收价值. 而采用传统加碱沉淀法处理产生的含铜污泥,通常因为污泥量大、铜含量低(一般低于5%)而成为危险废物,由专业危险废物处置中心进行安全处置,从而又产生了一笔不小的处置费用[20]. 本研究还对中试获得的含铜污泥进行酸洗,以浓缩提纯铜还原产物. 如图3F所示,经过盐酸加热酸洗后,污泥中残余零价铁被完全溶解,剩余固体主要成分为CuO和Cu2O. 可见,通过简单酸洗操作,即可将纳米零价铁处理后含铜污泥进一步富集提纯.
-
对湖北某地含砷冶炼废水,采用了两级纳米零价铁反应器在中试规模上研究了nZVI工艺处理含砷复杂多金属冶炼废水的可行性[54,58](图5A). 两级NIR是在一级反应器的基础上,增加了二级反应区,将单级循环与两级间回流相结合,同时联合曝气混凝沉淀处理工艺,提高废水中重金属的去除率和nZVI的利用率. 中试进水流量为400 L·h−1(各级 HRT=4 h),共使用75 kg的nZVI,处理35 m3冶炼废水. 研究表明,两级纳米零价铁反应器可同步稳定去除废水中砷、铜、锌、镍等7种重金属污染物,平均砷、铜去除率皆可达到99.9%,总体出水中各重金属浓度均低于0.1 mg·L−1,达到当地排放标准. 从而证明,采用两级纳米零价铁反应器联合“曝气混凝沉淀”处理工艺能够进一步加强了废水中重金属去除能力.
本中试研究中,纳米零价铁总投量为75 kg,两级纳米零价铁反应器去除/截留砷的质量为17.9 kg,去除负荷为 239 mg-As·(g-nZVI)−1. 通过文献研究比较发现,本次中试获得的除砷负荷较其他吸附材料平均高出几十甚至上百倍[61-69],如活性氧化铝(~15 mg·g−1)[62-63]、活性炭(3—30 mg·g−1)[64-65]、 二氧化钛(~30 mg·g−1)[66-67]等. 废水中大部分砷在第一级反应器内被去除(砷去除率为94%),若按一级反应器纳米零价铁浓度占总投量的 60%—80%计(第二级反应器中部分纳米零价铁被回流至第一级),则第一级反应器中纳米零价铁的除砷负荷可以高达 300—400 mg-As·(g-nZVI)−1. 假设纳米零价铁的比表面积为 30 m2·g−1,砷在氧化铁外壳(FeOOH)上的吸附密度为 2.6 位点·(nm2)−1[68], 则砷在纳米零价铁表面的吸附容量约为几十 mg-As·(g-nZVI)−1,仅为本次中试获得的除砷负荷的10%. 可见,纳米零价铁较高的除砷负荷并非仅由吸附作用达到.
对中试过程中获得的纳米零价铁污泥进行了XPS表征(图5C). 第一级、第二级纳米零价铁反应器中获得的泥样分别简称nZVI1和nZVI2,新鲜纳米零价铁简称nZVIF. 新鲜纳米零价铁表面元素主要为铁、氧和碳等. 经与冶炼废水反应后,在nZVI1表面出现了As(As3d,44 eV)、Cu(Cu2p,932 eV)、Cr(Cr2p,576 eV)、Pb(Pb4f,138 eV)等重金属元素特征峰,其相对含量高低与进水中含量高低一致:As>Cu>Cr>Pb. 此外,在nZVI2表面同样检出了As,但其余重金属元素(Cu、Cr、Pb)特征峰并不明显,可能是因为其在 nZVI2 中的相对含量较低.
一级反应器污泥中砷含量高达10%,主要是通过单级反应器内循环和两级反应器之间纳米零价铁回流实现了重金属的富集. 污泥产物的XPS固相表征显示,在一级反应器污泥中同时存在3种价态的砷,即As(Ⅴ)以及其两种还原产物As(Ⅲ)、As(0),而在二级反应器污泥中As(0)较少. 进一步证明二级反应器中纳米零价铁回流至一级反应器当中. 对污泥的XRD表征表明,纳米零价铁与水中As(Ⅴ)反应后产生了Fe3(AsO4)2沉淀,进一步证实纳米零价铁较高的除砷负荷是多种去除机理协同作用的结果(即吸附、还原、沉淀及共沉淀作用)[65].
-
对湖南水口山的制酸废水,研究团队进行了中试试验,比较了传统的石灰中和沉淀法和纳米零价铁“反应-分离-回用”处理系统对铅、锌等重金属离子的去除效率差异[56]. 制酸废水具有较强的酸性 (pH=2.5)及高浓度的铅、锌等重金属离子(平均铅浓度高达610 mg·L−1,锌的浓度高达195 mg·L-1),经石灰中和处理后出水中仍残留铅19 mg·L−1、锌18 mg·L−1,远超排放标准(Pb:0.1 mg·L−1,Zn:1.0 mg·L−1). 研究采用“石灰沉淀+NIR+混凝沉淀”工艺对该废水进行了处理研究(图6A). 其中石灰沉淀法采用该厂原有处理设备,包括一个中和反应池和一个辐流式沉淀池(Unit Ⅰ). 经过石灰沉淀处理后,大部分废水储于调节池(Unit Ⅱ)并经泵抽提至该厂综合废水处理站(生物处理),少部分废水经pH调节后(pH调至6.0)进入纳米零价铁反应器. 采用一级纳米零价铁反应器对废水进一步处理,控制进水流量为400 L·h−1,每天向反应区投加两次纳米零价铁(每次2—3 kg),出水中铅锌浓度均低于0.1 mg·L−1,且出水水质未受进水波动影响. 研究表明,采用纳米零价铁处理技术,可弥补传统石灰法重金属废水处理工艺不足,提高出水水质以达到日趋严格的排放标准.
本次中试共采用33.5 kg零价铁处理了27.6 m3的废水,纳米零价铁平均投加量为1.2 g·L−1,每天连续运行10—12 h,进行了连续6 d的实验. 研究表明,在流量为0.4 m3·h−1,纳米零价铁投量为 1.2 kg·m−3(纳米零价铁用量为33.5 kg,废水处理量为27.6 m3)条件下,出水中铅、锌浓度均低于 0.1 mg·L−1,且出水水质未受进水波动影响,表现出较强抗冲击负荷能力,且反应产物粒径大(是石灰处理产物的5倍)、易沉降分离. 这是由于纳米零价铁-水体系同时具有相对温和的pH(8.6)环境、OH−缓释特性及晶种效应,从而可实现废水中Pb(Ⅱ)、Zn(Ⅱ)等多种重金属离子的同步去除. 综上所述,NIR技术适用于高浓度铅、锌废水深度处理,有效弥补现行传统重金属废水处理工艺(如石灰沉淀法)不足,出水可稳定达标排放[27].
图6B可见,废水经石灰中和沉淀处理后Pb(Ⅱ)、Zn(Ⅱ)去除率均大于90%,出水中Pb(Ⅱ)、Zn(Ⅱ)平均浓度分别为19 mg·L−1和18 mg·L−1,仍无法达到相应排放标准(Pb:0.1 mg·L−1,Zn:1.0 mg·L−1). 当废水水质出现较大波动时,石灰法出水中Pb(Ⅱ)、Zn(Ⅱ)浓度也随之波动. 例如,当原水中Pb(Ⅱ)浓度超过1500 mg·L−1时,相应石灰法出水中Pb(Ⅱ)浓度增加至100 mg·L−1(图6B). 结果表明,石灰法可去除废水中大部分Pb(Ⅱ)、Zn(Ⅱ)等重金属离子,但无法保证出水水质稳定达标. 图6B可见,石灰法出水中残余的Pb(Ⅱ)、Zn(Ⅱ)、Cu(Ⅱ)、Cd(Ⅱ)等重金属离子在纳米零价铁反应器内得到有效去除,处理后出水中4种重金属浓度均降至0.1 mg·L−1以下,去除率大于99%,且整个中试运行过程中出水水质稳定达标. 后续混凝沉淀过程主要去除了纳米零价铁反应器出水中残余的悬浮物和铁离子. 此外,石灰法出水直接进行混凝沉淀处理时,出水中重金属离子浓度并未显著降低(如处理后Zn(Ⅱ)浓度仍有近5 mg·L−1),表明纳米零价铁反应器在去除Pb(Ⅱ)、Zn(Ⅱ)等重金属污染物时起到了关键作用(而非混凝沉淀过程).
纳米零价铁-水体系具有pH自我调节功能(图6C). 在纳米零价铁反应器中纳米零价铁浓度为2—5 g·L−1时,反应区pH保持在8—9范围内. 因此,利用纳米零价铁的自我pH调节功能,保持反应区pH稳定在弱碱性环境,从而有效减缓因进水水质波动而可能造成的不利影响. 其次,纳米零价铁可有效去除水中低浓度重金属离子. 纳米零价铁对水中Pb(Ⅱ)、Zn(Ⅱ)的去除机理包括吸附、还原、沉淀及共沉淀作用. 在几种作用协同作用下,纳米零价铁可有效同步去除水中多种低浓度(10—100 mg·L−1)重金属离子,而石灰或NaOH则无法达到类似效果. 此外,利用反应器不断“反应—分离—回用”纳米零价铁,使废水中低浓度的重金属在反应器污泥上得到富集,其中铅和锌含量分别占1.9%和1.1%. 最后,纳米零价铁反应产物具有优良沉降性能. 与石灰法沉淀池出水相比,纳米零价铁反应器沉淀区上清液浊度较低,相应重金属总浓度低于0.5 mg·L−1. 这是由于纳米零价铁在去除 Pb(Ⅱ)、Zn(Ⅱ)过程中扮演了晶种的角色,与重金属的反应、沉淀及生长提供了巨大的表面,从而形成尺寸大、密实、易沉降 的反应产物,并通过重力沉降方式实现快速固液分离.
综上,在现有石灰法预处理基础上,通过增加纳米零价铁深度处理单元,实现了强酸性高浓度铅、锌废水高效处理并稳定达标排放.
-
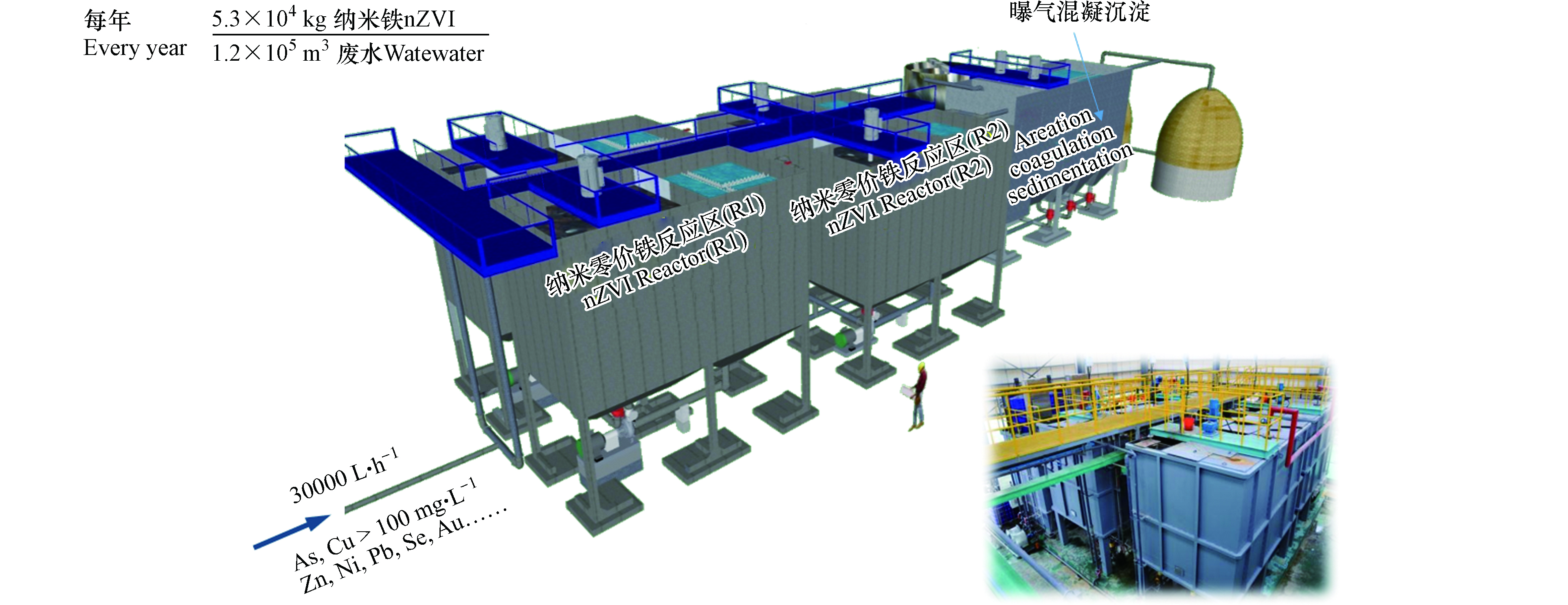
纳米零价铁技术也已经应用于江西贵溪某铜矿含铜、含砷冶炼生产废水处理工程[57-58]. 该废水酸性强(pH=1)、盐度高(~15%)、重金属浓度高,其中Cu(Ⅱ)浓度高达8000 mg·L−1,As(Ⅴ)浓度高达2000 mg·L−1. 废水首先经石灰中和沉淀预处理,再经pH调节(pH调至6—7)后作为本次工程进水. 本次工程中(图7),进水流量平均为30 m3·h−1,各级反应区HRT为2 h,纳米零价铁平均投量为 0.4—0.5 kg·m−3(单月累计使用4400 kg纳米零价铁处理9520 m3废水). 该系统长期平稳运行(3 年以上),废水中砷、铜平均浓度分别从预处理后的110 mg·L−1、103 mg·L−1降至0.29 mg·L−1、0.16 mg·L−1;纳米零价铁除砷、除铜负荷分别达245 mg·g−1 (As) nZVI和226 mg·g−1 (Cu)nZVI,总体重金属去除负荷超过 500 mg·g−1(重金属) nZVI.
提取该工程中的剩余污泥进行了分析,检测到As、Cu、Fe、Na、O及C含量较多,并检查到少量的Pb的存在.其中铜的含量达到10%、砷为8%,值得进一步回收. 最终产物的 形态主要为单质铜(Cu)、氧化亚铜(Cu2O)、Fe3(AsO4)2沉淀及铁的腐蚀产物(Fe3O4). 工程规模引用nZVI技术去除废水工业中的重金属,原理与实验室小试、中试相同[58].
-
对于含金冶炼废水,也在工程应用规模上证明了纳米零价铁工艺的可行性,研究证明,nZVI可以从重金属冶炼废水中进行贵金属金的回收[69]. 这是有关用nZVI进行水中痕量黄金回收的工程应用案例报道. 废水中金的回收分为3个步骤,从废水溶液中去除、在纳米铁颗粒表面富集和铁泥中金的回收,步骤如示意图8所示:在反应器中,废水中的金离子首先被纳米铁颗粒去除,在反应器和分离装置中进一步团聚,产生的铁泥经过脱水后得到金. nZVI主要通过以下3种原理对金进行回收:(1)nZVI是一种有效的还原剂,5.5—25000 μg·L−1浓度范围内的溶解性的金都可以被有效回收;(2)nZVI对金的富集能力主要取决于nZVI的持久反应活性、适宜的粒径和独特的铁-水化学作用等因素;(3)nZVI晶种效应,铁纳米颗粒作为晶种促进金纳米颗粒的生成. 晶种效应有效增大反应产物粒径并促进金-nZVI的沉淀分离.
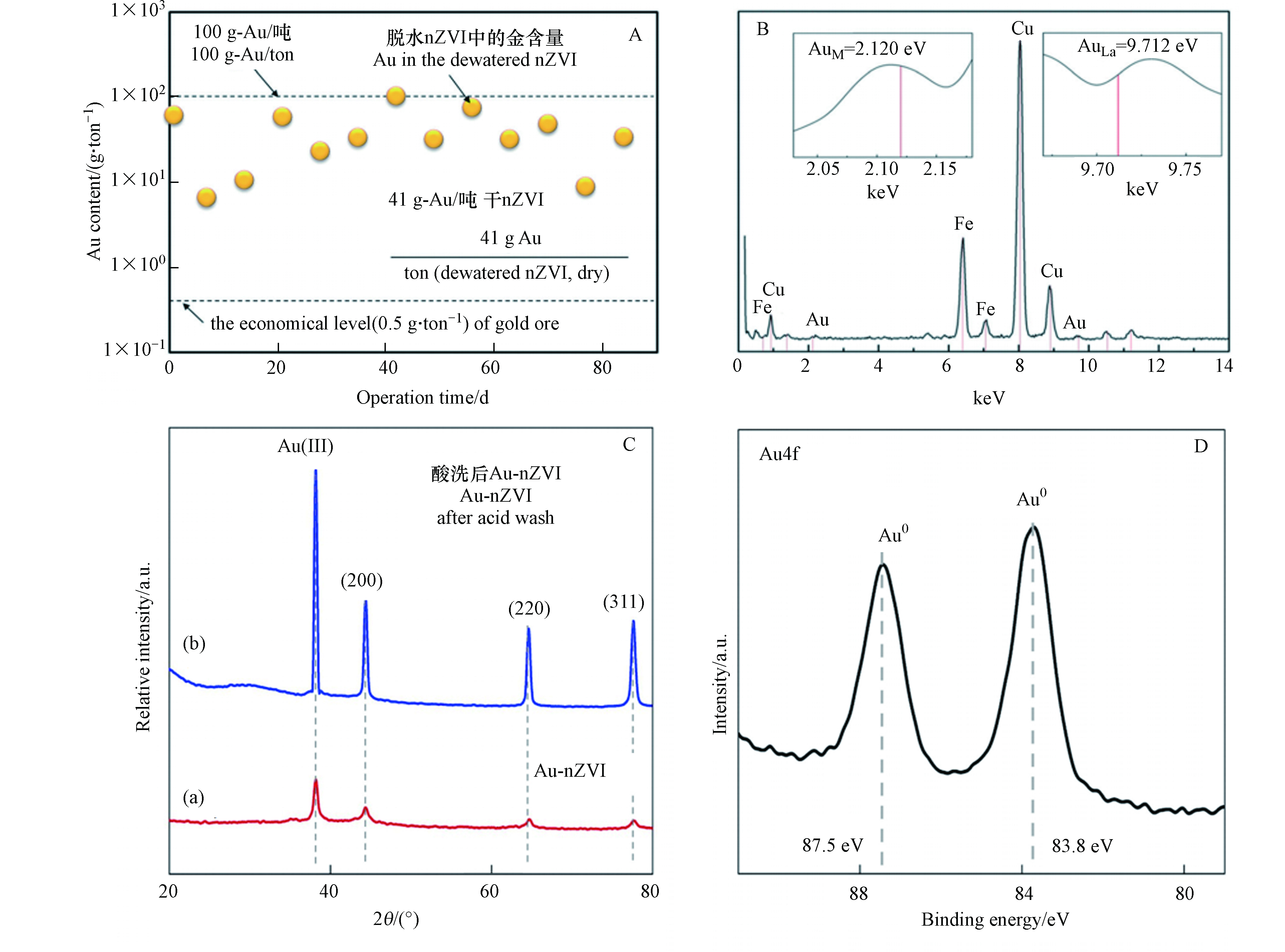
在nZVI (150 kg·d−1)处理大规模重金属冶金废水(350 m3·d−1)工程中时,废水中痕量的金(平均为37 μg·L−1)能够被分离、富集和回收,每吨水中大约回收41 g金. 在nZVI反应器处理重金属冶炼废水12个月的运行周期中,处理了大约120000 m3的工业废水,共回收约5000 g金. 该废水(300—400 m3·d−1)主要来自电化学方法精炼铜阳极泥的化学浸出溶液(使用NaClO3、HCl和 H2SO4),泥中的金的形态为Au(Ⅲ).
废水处理厂每月产生近25 t脱水nZVI铁泥(水含量:58%,W/W),在3个月运行时间内,取13个污泥样品用于成分分析(图9). 结果表明样品中金的含量为41 g-Au·(t-nZVI)−1,即是金矿石的80倍(0.5 g-Au·t−1);每吨脱水后的nZVI可回收90 kg的铜, nZVI 泥样中的Cu含量比在传统的铜矿石中(每吨约6 kg)高15倍. 脱水的nZVI铁泥中所含的Au和Cu的价值达到近每吨2600 美元. 干燥后的nZVI用扫描表征透射电子显微镜能量色散 X射线(STEM-EDX)进行了表征. EDX元素谱图显示(图9B),在 Mα=2.12 keV处存在峰值,证明了脱水nZVI中存在贵金属单质金. 在较低浓度下(~0.1‰,每吨103 克)仍然能观察到金原子,这是由于金原子序数较大,其在对应的区域相对较高的缘故. 从EDX光谱中还可以看出铜的含量较高,0.93、8.05、8.91 keV处为Cu的能量散射谱的吸收峰. 通过XRD和XPS分析表明,在nZVI泥样中的Au是以单质形式存在的(图9C、D).
该废水处理工程,每年能够从废水中回收5 kg Au、1100 kg Cu,以及一些其他有毒但是有价值的金属. 回收的金、铜等贵金属,部分抵消了消耗的成本nZVI. 本文中含重金属废水处理中试及水处理工程案例中应用的nZVI材料为本研究团队生产,成本相对较低,而且回收过程不会产生有害金属. 传统的石灰沉淀法不但不能有效回收重金属,而且生成固体废物,产生额外的处理成本.
-
综上所示,nZVI可有效同步去除实际废水中铜、砷、铅、锌等多种重金属,表现出较高去除负荷. 通过“反应—分离—回用”及两级间回流使nZVI在反应器内充分循环,有效提高了nZVI材料利用率;同时,利用Eh在线反馈调控系统,有效提高了反应器稳态控制能力. 本研究提供的基于纳米零价铁的重金属废水处理新方法,拓展了纳米零价铁应用领域,也为其他纳米材料水处理应用及重金属废水处理提供理论及技术借鉴.
笔者所在的研究团队已经在实验室小试、现场中试、工程应用规模上验证nZVI处理含重金属工业废水的可行性及效能,nZVI技术可有效弥补传统石灰法废水处理缺陷. 但nZVI技术处理含重金属工业废水,仍有一些实际问题有待深入研究. 主要存在的问题有:(1)含重金属的工业废水中共存的有小分子有机污染物可能与重金属离子发生络合反应,从而对纳米零价铁去除重金属产生影响;(2)废水中与重金属离子共存的较高浓度的碳酸根和磷酸根,使零价铁表面形成钝化层,影响其对污染物的去除,需评估碳酸根和磷酸根等对重金属去除效能的影响程度;(3)工业废水中可能存在的硝酸根等氧化性物质,影响体系的氧化还原电位,不仅会影响对重金属的去除效果,也会导致干扰Eh反馈调控而使系统不稳定.后续的研究需要考虑的问题包括:如何排除小分子有机污染物、碳酸根、磷酸根和硝酸根等的干扰,建立最佳的nZVI技术体系;同时,重金属离子被nZVI分离固定后,具有一定的经济价值,可探索建立污泥中有价金属回收再利用技术体系.
纳米零价铁处理含重金属工业废水研究进展
Advance of heavy metal-loading industrial wastewater treatment with nanoscale zero-valent iron
-
摘要: 纳米零价铁材料(nanoscale zero-valent iron, nZVI)是环境领域应用最广泛的纳米材料之一, 因其原材料来源丰富、反应产物环境友好,在分离/固定水中重金属方面得到了广泛的研究. 实验室研究表明,nZVI能够有效去除复杂实际废水中铜、砷、铅、锌、金等多种重金属,表现出较高的去除负荷. 本研究团队在国内首先研究以nZVI技术为核心,开发分离、固定重金属工业废水中重金属的针对性废水处理工艺. 构建了废水处理“反应-分离-回用”式纳米零价铁反应器(nano iron reactor, NIR)装置,通过“小试—中试—工程应用”逐级科学放大,将其应用于多种重金属工业废水的处理当中. 本文总结了纳米零价铁废水处理工艺,综述了NIR反应器技术处理典型重金属废水的中试和工程应用案例,为nZVI的实际环境应用以及重金属废水处理提供了理论及技术借鉴.Abstract: Nanoscale zero-valent iron (nZVI) is one of the most widely used nanomaterials for environmental remediation. The iron-based materials are easy to obtain, as well as the final products are environmental friendly, so nZVI has been widely studied in the field of separation and stabilization of heavy metal from wastewater. Our research group firstly designed the nano iron reactor (NIR) for heavy metals containing wastewater treatment focused on “reaction, separation and reuse” process. Key factors influencing the removal capacity has been investigated for the treatment of heavy metals containing wastewater using NIR. The technology of nZVI reactor for wastewater treatment has been invented after it has been studied in the scale of laboratory, pilot and large scale engineering application. It was proved that nZVI could effectively and simultaneously remove multiple heavy metal such as Cu, As, Pb, Zn from industrial wastewater, showing a high removal capacity. In general, this paper summarized the advance of wastewater treatment with nZVI, and focus on the pilot- and full- scale application of the treatment of wastewater containing multiple heavy metal. The article can provide the theoretical fundamentals and engineering experience for the environmental application of nZVI and the treatment of heavy metal wastewater.
-
Key words:
- nanoscale zerovalent iron /
- heavy metals /
- wastewater treatment /
- reactor /
- pilot study /
- full-scale application
-
重金属在环境中是一般不能被降解的,采矿、冶金、机械制造、化工、电子等行业的重金属废水,若不经处理直接排入环境中,会对生态环境和人体健康造成重大危害,重金属污染已成为全球性重大环境问题之一[1-4]. 锌、铜、锰和镍等重金属虽是人体必需微量元素,但此类重金属在体内长时间累积也可能引起心血管、肺、神经和内分泌等多方面问题 [4-6]. 汞(Hg)、镉(Cd)、铅(Pb)、铬(Cr)和砷(As)等非生命活动所必需的“五毒”重金属,对人、动植物和微生物更是具有显著毒性[4-7]. 人们长期饮用含砷(As)地下水,会引发皮肤癌、黑脚病及其他神经系统疾病(如孟加拉国砷污染事件)[4-6];饮用铅(Pb)离子超标的水,会导致人身器官损害、痴呆、骨萎缩等疾病(如血铅事件)[7]等;镉在体内蓄积,会造成肾损伤,进而导致骨软化症(日本著名的公害病──痛痛病)[8-12];汞金属进入人体内,会导致汞中毒,如20世纪50年代,日本九州水俣湾发生的“水俣病”事件 [11-15]. 据不完全估计,全世界平均每年向环境排放重金属达千万吨:Cu约340万吨,Pb约500万吨,Ni约100万吨等[16]. 重金属污染的水体和土壤环境修复已成为当前环境保护领域的重点、热点和难点问题.
纳米零价铁(nano scale zero valent iron,nZVI)是最早用于环境治理的纳米材料之一,其原材料来源丰富、反应产物环境友好,适用于水体中重金属的治理与修复. nZVI去除水中重金属的研究开始于2000年,科学家们以聚合树脂、硅胶等作为载体,制备了粒径在10—30 nm的负载型铁纳米材料,并应用于水溶液中Cr(Ⅵ)和Pb(Ⅱ)的固定和修复,实验证明具有良好的修复效果[17]. 此后,韩国科学家在2005年报道了nZVI异位处理地下水中的As污染,结果表明nZVI对水体中砷的去除率可达95%以上 [18]. 2006年用nZVI去除水溶液中镍离子的研究表明,每克铁对于镍的去除容量达到0.13 g Ni·g−1 Fe,吸附容量比所有的吸附材料都高[19]. 此后,有关nZVI去除重金属的研究层出不穷 [20-30]. 至今,已有多篇文献报道了nZVI用于土壤及地下水卤代有机污染的原位修复研究 [31-38],但尚未有关于重金属污染土壤、地下水的实际场地修复的报道.
2014年,本研究团队首次进行了nZVI技术处理含重金属工业废水的中试研究[39],针对我国水体重金属污染控制现实需求,在nZVI处理工业废水(特别是重金属工业废水)工艺、过程及机理上进行创新型拓展,为纳米材料对含重金属工业废水的有效处置提供了解决方案. 本文重点总结了nZVI技术在工业含重金属废水处理中的原理、技术和最新进展,为本领域的研究者提供参考.
1. 纳米零价铁用于重金属废水的基本原理(Mechanism of heavy metal wastewater treatment with nZVI)
已有研究表明,不论是采用硼氢化钠化学还原氯化铁(硫酸亚铁)、氢气还原氧化铁等化学还原方法制备的nZVI,还是化学气相沉积、微米铁机械球磨方法等制备的nZVI,均具有核-壳结构[40-42],即内核为零价铁,壳层由多种铁氧化物组成,厚度为几个nm,因此nZVI兼具纳米材料和零价铁材料的双重特性. 在纳米零价铁去除重金属的过程中,壳层氧化铁不但能够作为污染物的活性位点,同时由于具有缺陷及导电性等特质,可提供电子转移通道[43-46],使部分重金属污染物被还原.
在水处理过程中,nZVI在水中发生系列反应,生成Fe2+、Fe(OH)2、FeOOH、Fe2O3 、Fe3O4 等一系列腐蚀产物[47-51],如式(1—9). 水中的重金属离子与nZVI可发生以下反应:(1)在nZVI表面快速形成表面络合物;(2)与铁氧化物共沉淀;(3)金属离子通过nZVI壳层的缺陷到达核-壳结构零价铁的界面,继而还原为零价金属[46,49].
Fe0+2H2O⟶Fe2++2OH−+H2 (1) Fe0+O2+2H2O⟶2Fe2++4OH− (2) Fe0+2H+⟶Fe2++H2 (3) Fe2++2OH−⟶Fe(OH)2 (4) 6Fe2++O2+6H2O⟶2Fe3O4+12H+ (5) 4Fe2++O2+10H2O⟶4Fe(OH)3+8H+ (6) 4Fe(OH)2+2H2O+O2⟶4Fe(OH)3 (7) 6Fe(OH)2+O2⟶2Fe3O4+6H2O (8) 4Fe3O4+6H2O+O2⟶12FeOOH (9) 研究表明,纳米零价铁去除水中重金属的主要作用机理包括:吸附、还原、沉淀及共沉淀(图1). nZVI的双纳米相结构为水相中的重金属离子的分离和转化提供有效且独特的解决方案[50-53]. 研究团队基于实验室小试中nZVI对重金属废水良好的处理效果,进一步构建“反应-分离-回用”式纳米零价铁反应器,用于实际废水的中试和工程应用研究,实验室小试、中试、工程应用中nZVI对于重金属的去除机理相同[39,54-58].
2. 纳米零价铁处理废水工艺( Wastewater treatment process with nZVI )
纳米零价铁进行重金属工业废水处理时,反应需在适宜的反应器内完成. 为此,笔者所在的研究团队设计了纳米零价铁反应器(nano iron reactor, NIR),将其用于实际工业废水处理的中试和工程应用[39,54-58],开拓了nZVI环境应用新领域. NIR反应器由反应区、分离区和污泥回流系统组成,采用全混合搅拌釜式反应器(continuous stirred tank reactor, CSTR)作为反应区,竖流式沉淀池作为分离区,沉降于分离区底部的污泥通过蠕动泵或离心泵进行回流. 在中试试验中,另配有纳米零价铁投药泵和在线pH 、氧化还原电位计检测记录仪等(图2).
纳米零价铁与水经预分散后由蠕动泵泵入反应区. 待处理废水由离心泵从现场废水调节池抽提至纳米零价铁反应器内,管阀及流量计控制进水流量[57-58]. 在纳米零价铁反应器进行含重金属工业废水去除时,需要考虑水力停留时间、系统运行稳定性、反应后产物固液分离等对于反应效率的影响. 在处理含有强氧化性的重金属且浓度波动剧烈的工业废水时,需要预先设定反应区Eh控制范围并在线监测反应区其数值实时变化. 当Eh升高并突破控制范围时及时补充新鲜nZVI,使出水水质控制在排放标准范围以内 [39,54-58]. 在分离区中,纳米零价铁能够团聚成微米级尺寸的团聚体,通过重力沉降进行固液分离. 实验室研究证明,5 g·L−1的纳米零价铁,10 min的静态沉降效率可达96%,1 h的静态沉降效率达99%(图3A). 且纳米零价铁的浓度越高,静态沉降速度就越快;动态沉降的效果与表面水力负荷有关,由图3B可见,沉淀池表面负荷越低,纳米零价铁的沉降效果越好.
3. 纳米零价铁反应器处理重金属工业废水实际工程应用案例(Practical application of NIR for treatment of industry wastewater containing heavy metals )
研究团队选取含铜线路板废水(江苏昆山)、含砷冶炼废水(湖北大冶)、含铅含锌制酸废水(湖南水口山)及含铜含砷冶炼废水(江西贵溪)作为处理对象,用纳米零价铁技术进行了中试及工程应用研究[39,54-58]. 重金属工业实际废水具有成分复杂、重金属浓度高、波动大等缺点(主要原水水质特征见表1),各废水中均同时存在多种重金属,主要为铜、砷、锌、铅、镍. 该类废水多为强酸性,其中部分废水中同时存在较高的盐分及氨氮等杂质,因此采用传统的石灰、硫化钠、吸附剂等材料无法同步去除废水中多种重金属阳离子和含氧阴离子。但纳米零价铁处理工艺在此类废水的中试及工程应用中均表现出了优异的性能.
指标Index 线路板废水中试Pilot study of printed circulate board wastewater 冶炼废水中试Pilot study of smelting wastewaterwastewater 制酸废水中试Pilot study of acid-making wasterwater 冶炼废水工程Full-scale study of smelting wasterwater pH 2.0—6.5 4.5—6.1 1.8—3 5.9—7.3 Eh /mV 240—610 450—590 380—440 310—530 主要重金属及其浓度范围/(mg·L−1) Cu(8—234)Ni(0.1—16.9) As(400—1020)Cu(12—115)Zn(3.3—22.7)Ni(5.6—20) Pb(44—2580)Zn(60—320)Cd(17—280)Cu(1—175) As(14—415)Cu(11—488)Ni(<6.5)Zn(<4.3)Pb(<3.8) 其他特征 磷,SS 高盐度(8%) 强酸性 高盐度(15%) 高氨氮(0.6%) COD(3900 mg·L-1) 3.1 纳米零价技术处理高浓度含铜线路板废水中试
2014年,研究团队首先报导了纳米零价铁反应器(NIR)技术处理江苏昆山某高浓度含铜线路板废水的中试结果,证明了该技术处理含铜废水的可行性[38]. 整个中试共使用55 kg纳米零价铁,处理了250 t含铜废水([Cu(Ⅱ)]~70 mg·L−1). 在中试规模上,首次研究了纳米零价铁处理高浓度含铜废水效果及关键影响因素,通过改变进水容量,重点考察了该反应器水力停留时间(HRT)对装置长期运行的影响;此外,还考察了进出水铜浓度变化、反应区pH、Eh变化、去除负荷变化等因素(图4). 结果证明在流量为1.0 m3·h−1(HRT=1.6 h),nZVI投量为0.23 kg·m−3时,铜的去除率最高,可达97%. 废水中平均铜浓度从进水的52.5 mg·L−1降至出水的1.7 mg·L−1,且出水水质稳定(图4D). 该研究表明通过“反应—分离—回用”处理流程,nZVI在反应器内充分循环反应,除铜负荷高达343 mg Cu·g−1 nZVI,反应区nZVI容积负荷高达 1876 g-Cu·(m3·d)−1,且污泥中铜含量高达25%,具有回收价值.
nZVI对废水中铜(重金属)的去除负荷(removal capacity, mg Cu·g−1 nZVI,)可通过式10计算(以铜为例):
去除负荷(R)=CuinT−CuoutTFeT (10) 其中,
CuinT CuoutT 中试结果发现,混合液氧化还原电位(Eh)和相应出水中Cu(Ⅱ)浓度具有较强的相关性:当出水中Cu(Ⅱ)浓度提高时(呈数量级升高时),对应时刻反应区内混合液Eh同样出现升高趋势;而当Eh降低并低于−0.2 V时, 对应出水中Cu(Ⅱ)浓度低于0.1 mg·L−1(图4E). 因此,通过监测反应区Eh变化,来判断反应器内的运行情况,尤其适用于含强氧化性重金属且浓度波动较大的工业废水.
采用 XRD对中试纳米零价铁反应产物进行了成分分析,如图4F所示. 新鲜纳米零价铁仅出现α-Fe的3个特征衍射峰,而含铜废水中试处理后获得泥样的XRD峰谱中出现新的特征衍射峰,经 PDF卡片对比确认为Cu2O和Cu0两种物质,溶液化学小试研究也出现同样情况. (图4F). 上述结果表明,水中Cu(Ⅱ)被纳米零价铁还原生成了零价铜和赤铜矿(Cu2O). 与采用加碱沉淀或硫化物沉淀形成的氢氧化铜或硫化铜相比,金属或氧化亚铜形式的铜具有更低的生物可利用性[59]. 换言之,采用纳米零价铁进行含铜废水处理,其固体产物或污泥更加稳定.
此外,对中试过程中产生的污泥进行了铜含量分析,以评估其回收价值. 研究发现,当进水流量为1.5 m3·h−1或更低时,产生的污泥中铜含量占污泥干重的10%(W/W)以上,当进水流量为1.0 m3·h−1时,污泥中铜含量高达25%. 一般而言,自然界铜矿工业品位(Cu,质量分数)达到0.5%及以上即具有开采价值. 这些铜矿石再经过一系列复杂的选矿过程后,铜含量富集,成为铜精矿. 根据国家标准[60],铜精矿按铜品位分为5个等级,其中一级品铜含量不低于32%,五级品铜含量不低于13%. 按此标准,本次中试获得的污泥属于铜精矿中的二级品,具有相当高的回收价值. 而采用传统加碱沉淀法处理产生的含铜污泥,通常因为污泥量大、铜含量低(一般低于5%)而成为危险废物,由专业危险废物处置中心进行安全处置,从而又产生了一笔不小的处置费用[20]. 本研究还对中试获得的含铜污泥进行酸洗,以浓缩提纯铜还原产物. 如图3F所示,经过盐酸加热酸洗后,污泥中残余零价铁被完全溶解,剩余固体主要成分为CuO和Cu2O. 可见,通过简单酸洗操作,即可将纳米零价铁处理后含铜污泥进一步富集提纯.
3.2 NIR中试规模处理含砷复杂废水
对湖北某地含砷冶炼废水,采用了两级纳米零价铁反应器在中试规模上研究了nZVI工艺处理含砷复杂多金属冶炼废水的可行性[54,58](图5A). 两级NIR是在一级反应器的基础上,增加了二级反应区,将单级循环与两级间回流相结合,同时联合曝气混凝沉淀处理工艺,提高废水中重金属的去除率和nZVI的利用率. 中试进水流量为400 L·h−1(各级 HRT=4 h),共使用75 kg的nZVI,处理35 m3冶炼废水. 研究表明,两级纳米零价铁反应器可同步稳定去除废水中砷、铜、锌、镍等7种重金属污染物,平均砷、铜去除率皆可达到99.9%,总体出水中各重金属浓度均低于0.1 mg·L−1,达到当地排放标准. 从而证明,采用两级纳米零价铁反应器联合“曝气混凝沉淀”处理工艺能够进一步加强了废水中重金属去除能力.
本中试研究中,纳米零价铁总投量为75 kg,两级纳米零价铁反应器去除/截留砷的质量为17.9 kg,去除负荷为 239 mg-As·(g-nZVI)−1. 通过文献研究比较发现,本次中试获得的除砷负荷较其他吸附材料平均高出几十甚至上百倍[61-69],如活性氧化铝(~15 mg·g−1)[62-63]、活性炭(3—30 mg·g−1)[64-65]、 二氧化钛(~30 mg·g−1)[66-67]等. 废水中大部分砷在第一级反应器内被去除(砷去除率为94%),若按一级反应器纳米零价铁浓度占总投量的 60%—80%计(第二级反应器中部分纳米零价铁被回流至第一级),则第一级反应器中纳米零价铁的除砷负荷可以高达 300—400 mg-As·(g-nZVI)−1. 假设纳米零价铁的比表面积为 30 m2·g−1,砷在氧化铁外壳(FeOOH)上的吸附密度为 2.6 位点·(nm2)−1[68], 则砷在纳米零价铁表面的吸附容量约为几十 mg-As·(g-nZVI)−1,仅为本次中试获得的除砷负荷的10%. 可见,纳米零价铁较高的除砷负荷并非仅由吸附作用达到.
对中试过程中获得的纳米零价铁污泥进行了XPS表征(图5C). 第一级、第二级纳米零价铁反应器中获得的泥样分别简称nZVI1和nZVI2,新鲜纳米零价铁简称nZVIF. 新鲜纳米零价铁表面元素主要为铁、氧和碳等. 经与冶炼废水反应后,在nZVI1表面出现了As(As3d,44 eV)、Cu(Cu2p,932 eV)、Cr(Cr2p,576 eV)、Pb(Pb4f,138 eV)等重金属元素特征峰,其相对含量高低与进水中含量高低一致:As>Cu>Cr>Pb. 此外,在nZVI2表面同样检出了As,但其余重金属元素(Cu、Cr、Pb)特征峰并不明显,可能是因为其在 nZVI2 中的相对含量较低.
一级反应器污泥中砷含量高达10%,主要是通过单级反应器内循环和两级反应器之间纳米零价铁回流实现了重金属的富集. 污泥产物的XPS固相表征显示,在一级反应器污泥中同时存在3种价态的砷,即As(Ⅴ)以及其两种还原产物As(Ⅲ)、As(0),而在二级反应器污泥中As(0)较少. 进一步证明二级反应器中纳米零价铁回流至一级反应器当中. 对污泥的XRD表征表明,纳米零价铁与水中As(Ⅴ)反应后产生了Fe3(AsO4)2沉淀,进一步证实纳米零价铁较高的除砷负荷是多种去除机理协同作用的结果(即吸附、还原、沉淀及共沉淀作用)[65].
3.3 NIR技术处理含铅含锌制酸废水研究
对湖南水口山的制酸废水,研究团队进行了中试试验,比较了传统的石灰中和沉淀法和纳米零价铁“反应-分离-回用”处理系统对铅、锌等重金属离子的去除效率差异[56]. 制酸废水具有较强的酸性 (pH=2.5)及高浓度的铅、锌等重金属离子(平均铅浓度高达610 mg·L−1,锌的浓度高达195 mg·L-1),经石灰中和处理后出水中仍残留铅19 mg·L−1、锌18 mg·L−1,远超排放标准(Pb:0.1 mg·L−1,Zn:1.0 mg·L−1). 研究采用“石灰沉淀+NIR+混凝沉淀”工艺对该废水进行了处理研究(图6A). 其中石灰沉淀法采用该厂原有处理设备,包括一个中和反应池和一个辐流式沉淀池(Unit Ⅰ). 经过石灰沉淀处理后,大部分废水储于调节池(Unit Ⅱ)并经泵抽提至该厂综合废水处理站(生物处理),少部分废水经pH调节后(pH调至6.0)进入纳米零价铁反应器. 采用一级纳米零价铁反应器对废水进一步处理,控制进水流量为400 L·h−1,每天向反应区投加两次纳米零价铁(每次2—3 kg),出水中铅锌浓度均低于0.1 mg·L−1,且出水水质未受进水波动影响. 研究表明,采用纳米零价铁处理技术,可弥补传统石灰法重金属废水处理工艺不足,提高出水水质以达到日趋严格的排放标准.
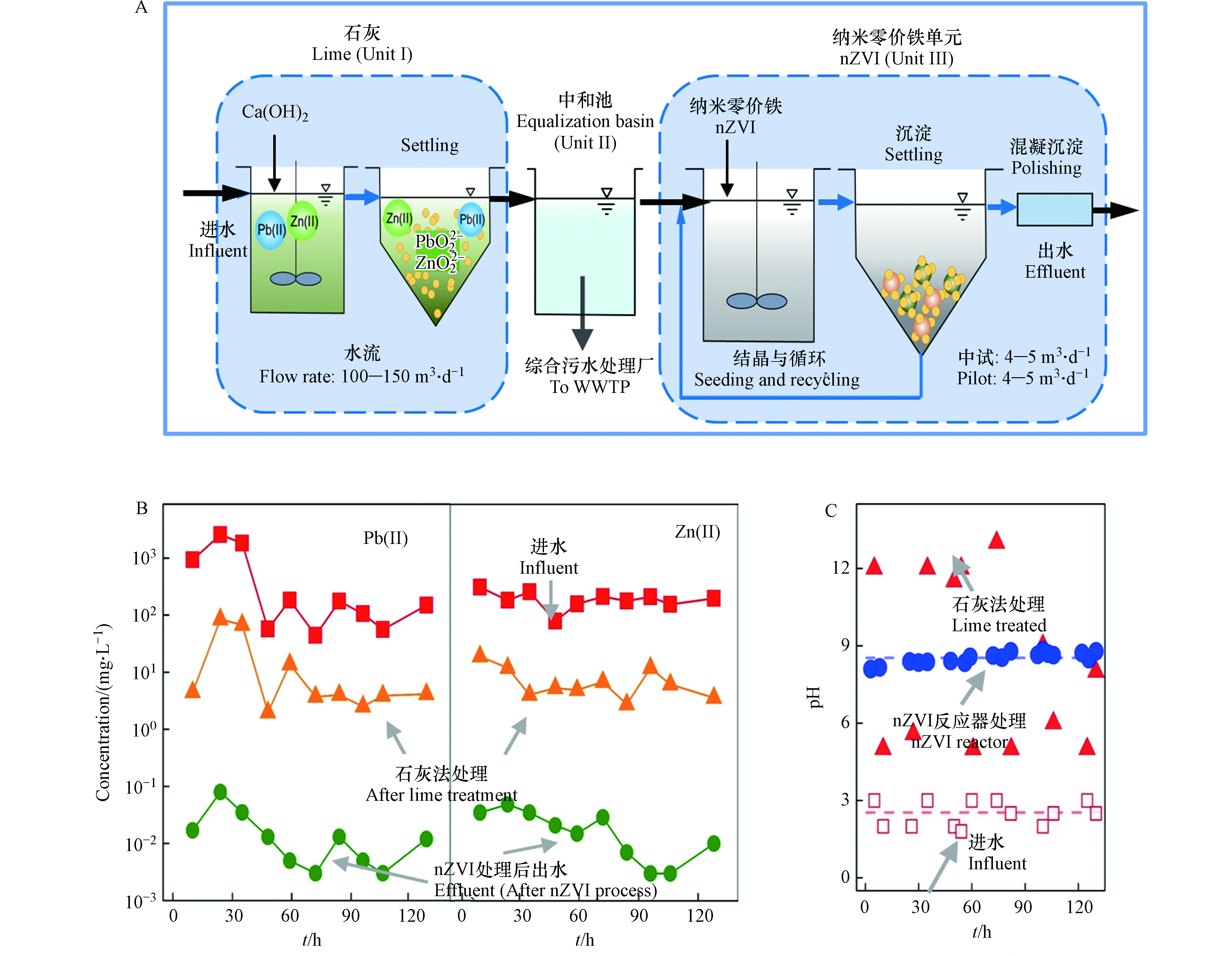 图 6 (A)石灰与纳米零价铁处理含铅含锌废水中试规模装置,(B) 纳米零价铁处理含铅含锌废水中试运行情况:Pb(Ⅱ)、Zn(Ⅱ)含量变化,(C)pH 变化[66].
图 6 (A)石灰与纳米零价铁处理含铅含锌废水中试规模装置,(B) 纳米零价铁处理含铅含锌废水中试运行情况:Pb(Ⅱ)、Zn(Ⅱ)含量变化,(C)pH 变化[66].本次中试共采用33.5 kg零价铁处理了27.6 m3的废水,纳米零价铁平均投加量为1.2 g·L−1,每天连续运行10—12 h,进行了连续6 d的实验. 研究表明,在流量为0.4 m3·h−1,纳米零价铁投量为 1.2 kg·m−3(纳米零价铁用量为33.5 kg,废水处理量为27.6 m3)条件下,出水中铅、锌浓度均低于 0.1 mg·L−1,且出水水质未受进水波动影响,表现出较强抗冲击负荷能力,且反应产物粒径大(是石灰处理产物的5倍)、易沉降分离. 这是由于纳米零价铁-水体系同时具有相对温和的pH(8.6)环境、OH−缓释特性及晶种效应,从而可实现废水中Pb(Ⅱ)、Zn(Ⅱ)等多种重金属离子的同步去除. 综上所述,NIR技术适用于高浓度铅、锌废水深度处理,有效弥补现行传统重金属废水处理工艺(如石灰沉淀法)不足,出水可稳定达标排放[27].
图6B可见,废水经石灰中和沉淀处理后Pb(Ⅱ)、Zn(Ⅱ)去除率均大于90%,出水中Pb(Ⅱ)、Zn(Ⅱ)平均浓度分别为19 mg·L−1和18 mg·L−1,仍无法达到相应排放标准(Pb:0.1 mg·L−1,Zn:1.0 mg·L−1). 当废水水质出现较大波动时,石灰法出水中Pb(Ⅱ)、Zn(Ⅱ)浓度也随之波动. 例如,当原水中Pb(Ⅱ)浓度超过1500 mg·L−1时,相应石灰法出水中Pb(Ⅱ)浓度增加至100 mg·L−1(图6B). 结果表明,石灰法可去除废水中大部分Pb(Ⅱ)、Zn(Ⅱ)等重金属离子,但无法保证出水水质稳定达标. 图6B可见,石灰法出水中残余的Pb(Ⅱ)、Zn(Ⅱ)、Cu(Ⅱ)、Cd(Ⅱ)等重金属离子在纳米零价铁反应器内得到有效去除,处理后出水中4种重金属浓度均降至0.1 mg·L−1以下,去除率大于99%,且整个中试运行过程中出水水质稳定达标. 后续混凝沉淀过程主要去除了纳米零价铁反应器出水中残余的悬浮物和铁离子. 此外,石灰法出水直接进行混凝沉淀处理时,出水中重金属离子浓度并未显著降低(如处理后Zn(Ⅱ)浓度仍有近5 mg·L−1),表明纳米零价铁反应器在去除Pb(Ⅱ)、Zn(Ⅱ)等重金属污染物时起到了关键作用(而非混凝沉淀过程).
纳米零价铁-水体系具有pH自我调节功能(图6C). 在纳米零价铁反应器中纳米零价铁浓度为2—5 g·L−1时,反应区pH保持在8—9范围内. 因此,利用纳米零价铁的自我pH调节功能,保持反应区pH稳定在弱碱性环境,从而有效减缓因进水水质波动而可能造成的不利影响. 其次,纳米零价铁可有效去除水中低浓度重金属离子. 纳米零价铁对水中Pb(Ⅱ)、Zn(Ⅱ)的去除机理包括吸附、还原、沉淀及共沉淀作用. 在几种作用协同作用下,纳米零价铁可有效同步去除水中多种低浓度(10—100 mg·L−1)重金属离子,而石灰或NaOH则无法达到类似效果. 此外,利用反应器不断“反应—分离—回用”纳米零价铁,使废水中低浓度的重金属在反应器污泥上得到富集,其中铅和锌含量分别占1.9%和1.1%. 最后,纳米零价铁反应产物具有优良沉降性能. 与石灰法沉淀池出水相比,纳米零价铁反应器沉淀区上清液浊度较低,相应重金属总浓度低于0.5 mg·L−1. 这是由于纳米零价铁在去除 Pb(Ⅱ)、Zn(Ⅱ)过程中扮演了晶种的角色,与重金属的反应、沉淀及生长提供了巨大的表面,从而形成尺寸大、密实、易沉降 的反应产物,并通过重力沉降方式实现快速固液分离.
综上,在现有石灰法预处理基础上,通过增加纳米零价铁深度处理单元,实现了强酸性高浓度铅、锌废水高效处理并稳定达标排放.
3.4 工程规模上NIR技术处理含铜含砷废水
纳米零价铁技术也已经应用于江西贵溪某铜矿含铜、含砷冶炼生产废水处理工程[57-58]. 该废水酸性强(pH=1)、盐度高(~15%)、重金属浓度高,其中Cu(Ⅱ)浓度高达8000 mg·L−1,As(Ⅴ)浓度高达2000 mg·L−1. 废水首先经石灰中和沉淀预处理,再经pH调节(pH调至6—7)后作为本次工程进水. 本次工程中(图7),进水流量平均为30 m3·h−1,各级反应区HRT为2 h,纳米零价铁平均投量为 0.4—0.5 kg·m−3(单月累计使用4400 kg纳米零价铁处理9520 m3废水). 该系统长期平稳运行(3 年以上),废水中砷、铜平均浓度分别从预处理后的110 mg·L−1、103 mg·L−1降至0.29 mg·L−1、0.16 mg·L−1;纳米零价铁除砷、除铜负荷分别达245 mg·g−1 (As) nZVI和226 mg·g−1 (Cu)nZVI,总体重金属去除负荷超过 500 mg·g−1(重金属) nZVI.
提取该工程中的剩余污泥进行了分析,检测到As、Cu、Fe、Na、O及C含量较多,并检查到少量的Pb的存在.其中铜的含量达到10%、砷为8%,值得进一步回收. 最终产物的 形态主要为单质铜(Cu)、氧化亚铜(Cu2O)、Fe3(AsO4)2沉淀及铁的腐蚀产物(Fe3O4). 工程规模引用nZVI技术去除废水工业中的重金属,原理与实验室小试、中试相同[58].
3.5 NIR技术处理含金冶炼废水
对于含金冶炼废水,也在工程应用规模上证明了纳米零价铁工艺的可行性,研究证明,nZVI可以从重金属冶炼废水中进行贵金属金的回收[69]. 这是有关用nZVI进行水中痕量黄金回收的工程应用案例报道. 废水中金的回收分为3个步骤,从废水溶液中去除、在纳米铁颗粒表面富集和铁泥中金的回收,步骤如示意图8所示:在反应器中,废水中的金离子首先被纳米铁颗粒去除,在反应器和分离装置中进一步团聚,产生的铁泥经过脱水后得到金. nZVI主要通过以下3种原理对金进行回收:(1)nZVI是一种有效的还原剂,5.5—25000 μg·L−1浓度范围内的溶解性的金都可以被有效回收;(2)nZVI对金的富集能力主要取决于nZVI的持久反应活性、适宜的粒径和独特的铁-水化学作用等因素;(3)nZVI晶种效应,铁纳米颗粒作为晶种促进金纳米颗粒的生成. 晶种效应有效增大反应产物粒径并促进金-nZVI的沉淀分离.
在nZVI (150 kg·d−1)处理大规模重金属冶金废水(350 m3·d−1)工程中时,废水中痕量的金(平均为37 μg·L−1)能够被分离、富集和回收,每吨水中大约回收41 g金. 在nZVI反应器处理重金属冶炼废水12个月的运行周期中,处理了大约120000 m3的工业废水,共回收约5000 g金. 该废水(300—400 m3·d−1)主要来自电化学方法精炼铜阳极泥的化学浸出溶液(使用NaClO3、HCl和 H2SO4),泥中的金的形态为Au(Ⅲ).
废水处理厂每月产生近25 t脱水nZVI铁泥(水含量:58%,W/W),在3个月运行时间内,取13个污泥样品用于成分分析(图9). 结果表明样品中金的含量为41 g-Au·(t-nZVI)−1,即是金矿石的80倍(0.5 g-Au·t−1);每吨脱水后的nZVI可回收90 kg的铜, nZVI 泥样中的Cu含量比在传统的铜矿石中(每吨约6 kg)高15倍. 脱水的nZVI铁泥中所含的Au和Cu的价值达到近每吨2600 美元. 干燥后的nZVI用扫描表征透射电子显微镜能量色散 X射线(STEM-EDX)进行了表征. EDX元素谱图显示(图9B),在 Mα=2.12 keV处存在峰值,证明了脱水nZVI中存在贵金属单质金. 在较低浓度下(~0.1‰,每吨103 克)仍然能观察到金原子,这是由于金原子序数较大,其在对应的区域相对较高的缘故. 从EDX光谱中还可以看出铜的含量较高,0.93、8.05、8.91 keV处为Cu的能量散射谱的吸收峰. 通过XRD和XPS分析表明,在nZVI泥样中的Au是以单质形式存在的(图9C、D).
该废水处理工程,每年能够从废水中回收5 kg Au、1100 kg Cu,以及一些其他有毒但是有价值的金属. 回收的金、铜等贵金属,部分抵消了消耗的成本nZVI. 本文中含重金属废水处理中试及水处理工程案例中应用的nZVI材料为本研究团队生产,成本相对较低,而且回收过程不会产生有害金属. 传统的石灰沉淀法不但不能有效回收重金属,而且生成固体废物,产生额外的处理成本.
4. 总结与展望(Summary and outlook)
综上所示,nZVI可有效同步去除实际废水中铜、砷、铅、锌等多种重金属,表现出较高去除负荷. 通过“反应—分离—回用”及两级间回流使nZVI在反应器内充分循环,有效提高了nZVI材料利用率;同时,利用Eh在线反馈调控系统,有效提高了反应器稳态控制能力. 本研究提供的基于纳米零价铁的重金属废水处理新方法,拓展了纳米零价铁应用领域,也为其他纳米材料水处理应用及重金属废水处理提供理论及技术借鉴.
笔者所在的研究团队已经在实验室小试、现场中试、工程应用规模上验证nZVI处理含重金属工业废水的可行性及效能,nZVI技术可有效弥补传统石灰法废水处理缺陷. 但nZVI技术处理含重金属工业废水,仍有一些实际问题有待深入研究. 主要存在的问题有:(1)含重金属的工业废水中共存的有小分子有机污染物可能与重金属离子发生络合反应,从而对纳米零价铁去除重金属产生影响;(2)废水中与重金属离子共存的较高浓度的碳酸根和磷酸根,使零价铁表面形成钝化层,影响其对污染物的去除,需评估碳酸根和磷酸根等对重金属去除效能的影响程度;(3)工业废水中可能存在的硝酸根等氧化性物质,影响体系的氧化还原电位,不仅会影响对重金属的去除效果,也会导致干扰Eh反馈调控而使系统不稳定.后续的研究需要考虑的问题包括:如何排除小分子有机污染物、碳酸根、磷酸根和硝酸根等的干扰,建立最佳的nZVI技术体系;同时,重金属离子被nZVI分离固定后,具有一定的经济价值,可探索建立污泥中有价金属回收再利用技术体系.
-
图 6 (A)石灰与纳米零价铁处理含铅含锌废水中试规模装置,(B) 纳米零价铁处理含铅含锌废水中试运行情况:Pb(Ⅱ)、Zn(Ⅱ)含量变化,(C)pH 变化[66].
Figure 6. (A) Pilot-scale NIR for treatment of wastewater containing lead and zinc with nZVI and lime [51],(B)Pilot performance of nZVI reactor for Pb(Ⅱ) and Zn(Ⅱ) wastewater treatment : changes of Pb(Ⅱ) and Zn(Ⅱ) concentrations,(C) Changes in solution pH[66]
指标Index 线路板废水中试Pilot study of printed circulate board wastewater 冶炼废水中试Pilot study of smelting wastewaterwastewater 制酸废水中试Pilot study of acid-making wasterwater 冶炼废水工程Full-scale study of smelting wasterwater pH 2.0—6.5 4.5—6.1 1.8—3 5.9—7.3 Eh /mV 240—610 450—590 380—440 310—530 主要重金属及其浓度范围/(mg·L−1) Cu(8—234)Ni(0.1—16.9) As(400—1020)Cu(12—115)Zn(3.3—22.7)Ni(5.6—20) Pb(44—2580)Zn(60—320)Cd(17—280)Cu(1—175) As(14—415)Cu(11—488)Ni(<6.5)Zn(<4.3)Pb(<3.8) 其他特征 磷,SS 高盐度(8%) 强酸性 高盐度(15%) 高氨氮(0.6%) COD(3900 mg·L-1) -
[1] HARARI Y N. Sapiens: A Brief History of Humankind[J]. New York: Harper Perennial, 2014. [2] BANG S, CHOI J W, CHO K, et al. Simultaneous reduction of copper and toxicity in semiconductor wastewater using protonated alginate beads [J]. Chemical Engineering Journal, 2016, 288: 525-531. doi: 10.1016/j.cej.2015.12.025 [3] VIKRANT K, KUMAR V, VELLINGIRI K, et al. Nanomaterials for the abatement of cadmium (II) ions from water/wastewater [J]. Nano Research, 2019, 12(7): 1489-1507. doi: 10.1007/s12274-019-2309-8 [4] HUGHES M F. Arsenic toxicity and potential mechanisms of action [J]. Toxicology Letters, 2002, 133(1): 1-16. doi: 10.1016/S0378-4274(02)00084-X [5] FENDORF S, MICHAEL H A, van GEEN A. Spatial and temporal variations of groundwater arsenic in South and Southeast Asia [J]. Science, 2010, 328(5982): 1123-1127. doi: 10.1126/science.1172974 [6] ZHENG Y. Global solutions to a silent poison [J]. Science, 2020, 368(6493): 818-819. doi: 10.1126/science.abb9746 [7] LARSON C. China gets serious about its pollutant-laden soil [J]. Science, 2014, 343(6178): 1415-1416. doi: 10.1126/science.343.6178.1415 [8] FU F L, WANG Q. Removal of heavy metal ions from wastewaters: A review [J]. Journal of Environmental Management, 2011, 92(3): 407-418. doi: 10.1016/j.jenvman.2010.11.011 [9] KURNIAWAN T A, CHAN G Y S, LO W H, et al. Physico-chemical treatment techniques for wastewater laden with heavy metals [J]. Chemical Engineering Journal, 2006, 118(1/2): 83-98. [10] SATARUG S. Long-term exposure to cadmium in food and cigarette smoke, liver effects and hepatocellular carcinoma [J]. Current Drug Metabolism, 2012, 13(3): 257-271. doi: 10.2174/138920012799320446 [11] KUMAR R, CHAWLA J. Removal of cadmium ion from water/wastewater by nano-metal oxides: A review [J]. Water Quality, Exposure and Health, 2014, 5(4): 215-226. doi: 10.1007/s12403-013-0100-8 [12] KUMAR R, CHAWLA J, KAUR I. Removal of cadmium ion from wastewater by carbon-based nanosorbents: A review [J]. Journal of Water and Health, 2015, 13(1): 18-33. doi: 10.2166/wh.2014.024 [13] van GESTEL C A M, KOOLHAAS J E. Water-extractability, free ion activity, and pH explain cadmium sorption and toxicity to Folsomia candida (Collembola) in seven soil-pH combinations [J]. Environmental Toxicology and Chemistry, 2004, 23(8): 1822-1833. doi: 10.1897/03-393 [14] HARADA M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution [J]. Critical Reviews in Toxicology, 1995, 25(1): 1-24. doi: 10.3109/10408449509089885 [15] SINGH O V, LABANA S, PANDEY G, et al. Phytoremediation: an overview of metallic ion decontamination from soil [J]. Applied Microbiology and Biotechnology, 2003, 61(5/6): 405-412. [16] 刘金燕, 刘立华, 薛建荣, 等. 重金属废水吸附处理的研究进展 [J]. 环境化学, 2018, 37(9): 2016-2024. doi: 10.7524/j.issn.0254-6108.2017110105 LIU J Y, LIU L H, XUE J R, et al. Research progress on treatment of heavy metal wastewater by adsorption [J]. Environmental Chemistry, 2018, 37(9): 2016-2024(in Chinese). doi: 10.7524/j.issn.0254-6108.2017110105
[17] KURNIAWAN T A, CHAN G Y S, LO W H, et al. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals [J]. Science of the Total Environment, 2006, 366(2/3): 409-426. [18] PONDER S M, DARAB J G, MALLOUK T E. Remediation of Cr(Ⅵ) and Pb(Ⅱ) aqueous solutions using supported, nanoscale zero-valent iron [J]. Environmental Science & Technology, 2000, 34(12): 2564-2569. [19] KANEL S R, MANNING B, CHARLET L, et al. Removal of arsenic (Ⅲ) from groundwater by nanoscale zero-valent iron [J]. Environmental Science & Technology, 2005, 39(5): 1291-1298. [20] LI X Q, ZHANG W X. Iron nanoparticles: The core-shell structure and unique properties for Ni(Ⅱ) sequestration [J]. Langmuir, 2006, 22(10): 4638-4642. doi: 10.1021/la060057k [21] LIU A R, WANG W, LIU J, et al. Nanoencapsulation of arsenate with nanoscale zero-valent iron (nZVI): A 3D perspective [J]. Science Bulletin, 2018, 63(24): 1641-1648. doi: 10.1016/j.scib.2018.12.002 [22] LING L, ZHANG W X. Visualizing arsenate reactions and encapsulation in a single zero-valent iron nanoparticle [J]. Environmental Science & Technology, 2017, 51(4): 2288-2294. [23] TANG L, FENG H P, TANG J, et al. Treatment of arsenic in acid wastewater and river sediment by Fe@Fe2O3 nanobunches: The effect of environmental conditions and reaction mechanism [J]. Water Research, 2017, 117: 175-186. doi: 10.1016/j.watres.2017.03.059 [24] LING L, ZHANG W X. Enrichment and encapsulation of uranium with iron nanoparticle [J]. Journal of the American Chemical Society, 2015, 137(8): 2788-2791. doi: 10.1021/ja510488r [25] MU Y, AI Z H, ZHANG L Z, et al. Insight into core–shell dependent anoxic Cr(Ⅵ) removal with Fe@Fe2O3 nanowires: Indispensable role of surface bound Fe(Ⅱ) [J]. ACS Applied Materials & Interfaces, 2015, 7(3): 1997-2005. [26] SHI L N, ZHANG X, CHEN Z L. Removal of Chromium (Ⅵ) from wastewater using bentonite-supported nanoscale zero-valent iron [J]. Water Research, 2011, 45(2): 886-892. doi: 10.1016/j.watres.2010.09.025 [27] ZHANG Y L, SU Y M, ZHOU X F, et al. A new insight on the core-shell structure of zerovalent iron nanoparticles and its application for Pb(II) sequestration [J]. Journal of Hazardous Materials, 2013, 263: 685-693. doi: 10.1016/j.jhazmat.2013.10.031 [28] PONDER S M, DARAB J G, BUCHER J, et al. Surface chemistry and electrochemistry of supported zerovalent iron nanoparticles in the remediation of aqueous metal contaminants [J]. Chemistry of Materials, 2001, 13(2): 479-486. doi: 10.1021/cm000288r [29] HUANG Q, GU T H, LIU A R, et al. Probing pollutant reactions at the iron surface: A case study on selenite reactions with nanoscale zero-valent iron [J]. Environmental Science:Nano, 2021, 8(9): 2650-2659. doi: 10.1039/D1EN00458A [30] YAN W L, RAMOS M A V, KOEL B E, et al. Multi-tiered distributions of arsenic in iron nanoparticles: Observation of dual redox functionality enabled by a core–shell structure [J]. Chemical Communications, 2010, 46(37): 6995. doi: 10.1039/c0cc02311f [31] FAN D M, ANITORI R P, TEBO B M, et al. Reductive sequestration of pertechnetate ( 99TcO−4 ) by nano zerovalent iron (nZVI) transformed by abiotic sulfide [J]. Environmental Science & Technology, 2013, 47(10): 5302-5310.[32] ELLIOTT D W, ZHANG W X. Field assessment of nanoscale bimetallic particles for groundwater treatment [J]. Environmental Science & Technology, 2001, 35(24): 4922-4926. [33] YAN W L, LIEN H L, KOEL B E, et al. Iron nanoparticles for environmental clean-up: Recent developments and future outlook [J]. Environmental Science. Processes & Impacts, 2013, 15(1): 63-77. [34] WANG C B, ZHANG W X. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs [J]. Environmental Science & Technology, 1997, 31(7): 2154-2156. [35] 邱心泓, 方战强. 修饰型纳米零价铁降解有机卤化物的研究 [J]. 化学进展, 2021, 22(增刊1): 291-297. QIU X H, FANG Z Q. Degradation of halogenated organic compounds by modified nano zero-valent iron [J]. Progress in Chemistry, 2021, 22(增刊1): 291-297(in Chinese).
[36] ZHANG W X, WANG C B, LIEN H L. Treatment of chlorinated organic contaminants with nanoscale bimetallic particles [J]. Catalysis Today, 1998, 40(4): 387-395. doi: 10.1016/S0920-5861(98)00067-4 [37] XU Y, ZHANG W X. Subcolloidal Fe/Ag particles for reductive dehalogenation of chlorinated benzenes [J]. Industrial & Engineering Chemistry Research, 2000, 39(7): 2238-2244. [38] LIEN H L, ZHANG W X. Nanoscale iron particles for complete reduction of chlorinated ethenes [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2001, 191(1/2): 97-105. [39] LI S L, WANG W, YAN W L, et al. Nanoscale zero-valent iron (nZVI) for the treatment of concentrated Cu(II) wastewater: A field demonstration [J]. Environmental Science. Processes & Impacts, 2014, 16(3): 524-533. [40] LI S L, YAN W L, ZHANG W X. Solvent-free production of nanoscale zero-valent iron (nZVI) with precision milling [J]. Green Chemistry, 2009, 11(10): 1618. doi: 10.1039/b913056j [41] LIU A R, ZHANG W X. Fine structural features of nanoscale zero-valent iron characterized by spherical aberration corrected scanning transmission electron microscopy (Cs-STEM) [J]. The Analyst, 2014, 139(18): 4512-4518. doi: 10.1039/C4AN00679H [42] YAN W L, HERZING A A, KIELY C J, et al. Nanoscale zero-valent iron (nZVI): Aspects of the core-shell structure and reactions with inorganic species in water [J]. Journal of Contaminant Hydrology, 2010, 118(3/4): 96-104. [43] WANG C M, BAER D R, AMONETTE J E, et al. Morphology and oxide shell structure of iron nanoparticles grown by sputter-gas-aggregation[J]. 2007, 18(25): 255603. [44] ANTONY J, QIANG Y, BAER D R, et al. Synthesis and characterization of stable iron–iron oxide core–shell nanoclusters for environmental applications [J]. Journal of Nanoscience and Nanotechnology, 2006, 6(2): 568-572. doi: 10.1166/jnn.2006.925 [45] WANG C M, BAER D R, AMONETTE J E, et al. Morphology and electronic structure of the oxide shell on the surface of iron nanoparticles [J]. Journal of the American Chemical Society, 2009, 131(25): 8824-8832. doi: 10.1021/ja900353f [46] LIU A R, LIU J, ZHANG W X. Transformation and composition evolution of nanoscale zero valent iron (nZVI) synthesized by borohydride reduction in static water [J]. Chemosphere, 2015, 119: 1068-1074. doi: 10.1016/j.chemosphere.2014.09.026 [47] LIU A R, LIU J, PAN B C, et al. Formation of lepidocrocite (γ-FeOOH) from oxidation of nanoscale zero-valent iron (nZVI) in oxygenated water [J]. RSC Adv, 2014, 4(101): 57377-57382. doi: 10.1039/C4RA08988J [48] DONG H R, JIANG Z, DENG J M, et al. Physicochemical transformation of Fe/Ni bimetallic nanoparticles during aging in simulated groundwater and the consequent effect on contaminant removal [J]. Water Research, 2018, 129: 51-57. doi: 10.1016/j.watres.2017.11.002 [49] LIU A R, LIU J, HAN J H, et al. Evolution of nanoscale zero-valent iron (nZVI) in water: Microscopic and spectroscopic evidence on the formation of nano- and micro-structured iron oxides [J]. Journal of Hazardous Materials, 2017, 322: 129-135. doi: 10.1016/j.jhazmat.2015.12.070 [50] MAGALHÃES J M, SILVA J E, CASTRO F P, et al. Physical and chemical characterisation of metal finishing industrial wastes [J]. Journal of Environmental Management, 2005, 75(2): 157-166. [51] 刘静, 刘爱荣, 张伟贤. 纳米零价铁及其在环境介质中氧化后性质演变研究进展 [J]. 环境化学, 2014, 33(4): 576-583. doi: 10.7524/j.issn.0254-6108.2014.04.009 LIU J, LIU A R, ZHANG W X. Review on transformation of oxidized nanoscale zero valent iron in environment media [J]. Environmental Chemistry, 2014, 33(4): 576-583(in Chinese). doi: 10.7524/j.issn.0254-6108.2014.04.009
[52] 刘静, 顾天航, 王伟, 等. 纳米零价铁在水相反应中的表面化学和晶相转化 [J]. 化学学报, 2019, 77(2): 121-129. doi: 10.6023/A18100412 LIU J, GU T H, WANG W, et al. Surface chemistry and phase transformation of nanoscale zero-valent iron(nZVI) in aquatic media [J]. Acta Chimica Sinica, 2019, 77(2): 121-129(in Chinese). doi: 10.6023/A18100412
[53] 黄潇月, 王伟, 凌岚, 等. 纳米零价铁与重金属的反应: “核-壳”结构在重金属去除中的作用 [J]. 化学学报, 2017, 75(6): 529-537. doi: 10.6023/A17020051 HUANG X Y, WANG W, LING L, et al. Heavy metal-nZVI reactions: The core-shell structure and applications for heavy metal treatment [J]. Acta Chimica Sinica, 2017, 75(6): 529-537(in Chinese). doi: 10.6023/A17020051
[54] LI S L, WANG W, LIU Y Y, et al. Zero-valent iron nanoparticles (nZVI) for the treatment of smelting wastewater: A pilot-scale demonstration [J]. Chemical Engineering Journal, 2014, 254: 115-123. doi: 10.1016/j.cej.2014.05.111 [55] WANG W, LI S L, LEI H, et al. Enhanced separation of nanoscale zero-valent iron (nZVI) using polyacrylamide: Performance, characterization and implication [J]. Chemical Engineering Journal, 2015, 260: 616-622. doi: 10.1016/j.cej.2014.09.042 [56] WANG W, HUA Y L, LI S L, et al. Removal of Pb(Ⅱ) and Zn(Ⅱ) using lime and nanoscale zero-valent iron (nZVI): A comparative study [J]. Chemical Engineering Journal, 2016, 304: 79-88. doi: 10.1016/j.cej.2016.06.069 [57] LI S L, WANG W, LIANG F P, et al. Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application [J]. Journal of Hazardous Materials, 2017, 322: 163-171. doi: 10.1016/j.jhazmat.2016.01.032 [58] 王伟. 纳米零价铁处理重金属废水应用研究[D]. 上海: 同济大学, 2016. WANG W. Research on the Application of Nanoscale Zero-valent Iron[D]. Shanghai: Tongji University, 2016.
[59] BOULAY N, EDWARDS M. Copper in the urban water cycle [J]. Critical Reviews in Environmental Science and Technology, 2000, 30(3): 297-326. doi: 10.1080/10643380091184192 [60] 国家发展和改革委员会. 中华人民共和国有色金属行业标准: 铜精矿 YS/T 318—2007[S]. 北京: 中国标准出版社, 2007. National Development and Reform Commission of the People's Republic of China. Non-ferrous MetallurgyStandard of the People's Republic of China: Copper concentrate. YS/T 318—2007[S]. Beijing: Standards Press of China, 2007(in Chinese).
[61] MOHAN D, PITTMAN C U Jr. Arsenic removal from water/wastewater using adsorbents—A critical review [J]. Journal of Hazardous Materials, 2007, 142(1/2): 1-53. [62] LIN T F, WU J K. Adsorption of arsenite and arsenate within activated alumina grains: Equilibrium and kinetics [J]. Water Research, 2001, 35(8): 2049-2057. doi: 10.1016/S0043-1354(00)00467-X [63] TAKANASHI H, TANAKA A, NAKAJIMA T, et al. Arsenic removal from groundwater by a newly developed adsorbent [J]. Water Science and Technology, 2004, 50(8): 23-32. doi: 10.2166/wst.2004.0479 [64] PATTANAYAK J, MONDAL K, MATHEW S, et al. A parametric evaluation of the removal of As(Ⅴ) and As(Ⅲ) by carbon-based adsorbents [J]. Carbon, 2000, 38(4): 589-596. doi: 10.1016/S0008-6223(99)00144-X [65] CHUANG C L, FAN M, XU M, et al. Adsorption of arsenic(Ⅴ) by activated carbon prepared from oat hulls [J]. Chemosphere, 2005, 61(4): 478-483. doi: 10.1016/j.chemosphere.2005.03.012 [66] DUTTA P K, RAY A K, SHARMA V K, et al. Adsorption of arsenate and arsenite on titanium dioxide suspensions [J]. Journal of Colloid and Interface Science, 2004, 278(2): 270-275. doi: 10.1016/j.jcis.2004.06.015 [67] PENA M E, KORFIATIS G P, PATEL M, et al. Adsorption of As(Ⅴ) and As(Ⅲ) by nanocrystalline titanium dioxide [J]. Water Research, 2005, 39(11): 2327-2337. doi: 10.1016/j.watres.2005.04.006 [68] DIXIT S, HERING J G. Comparison of arsenic(Ⅴ) and arsenic(Ⅲ) sorption onto iron oxide minerals: Implications for arsenic mobility [J]. Environmental Science & Technology, 2003, 37(18): 4182-4189. [69] LI S L, LI J H, WANG W, et al. Recovery of gold from wastewater using nanoscale zero-valent iron [J]. Environmental Science:Nano, 2019, 6(2): 519-527. doi: 10.1039/C8EN01018H [70] JANA N R, SAU T K, PAL T. Growing small silver particle as redox catalyst [J]. The Journal of Physical Chemistry B, 1999, 103(1): 115-121. doi: 10.1021/jp982731f -






 下载:
下载: