-
持久性有毒有机污染物(persistent toxic organic substances, PTOS),作为持久性有毒化学品(persistent toxic substances , PTS)中最重要的组成部分,主要包括原油和精炼油泄漏,化石燃料、木材和煤灰的不完全燃烧,以及金属冶炼有关过程产生的多环芳烃[1];广泛用于农业和工业生产的有机氯农药(organochlorine pesticides, OCPs)和多氯联苯(polychlorinated biphenyls, PCBs);用于各种消费品和工业过程的全氟和多氟烷基化合物(perfluoroalkyl and polyfluoroalkyl substances, PFASs)[2],作为阻燃剂出现在电子产品、纺织品、建筑材料或家具等组成中的多溴二苯醚(poly brominated diphenyl ethers, PBDEs)[3]以及一些损害机体正常内分泌功能的环境内分泌干扰物(environmental endocrine disruptors, EED)等。常见的主要PTOS种类及性质见表1,这些高毒性和高脂溶性污染物[4]来源多样,环境行为复杂,且难以降解,极大的威胁着生态环境与人体健康[5]。由于PTOS在全球普遍存在,且具有生物累积性、难降解性及内分泌干扰等特性,探索和采用有效的方法来快速地去除PTOS,开发建立环境介质中快速痕量检测PTOS的方法,成为了生态环境领域研究的重要方面[6]。
近几十年来,研究者针对各类PTOS的去除方法已有大量报道,如高级氧化法[7]、生物降解法[8]、膜分离法[9]、吸附法[10]等。其中,吸附法由于操作简便、可扩展性强、成本效益高,以及高达95%以上的污染物去除效率[11],一直以来备受青睐,而吸附剂则是影响吸附性能的重要因素[12-13]。
目前,商业中一般采用沸石[14]、活性炭[15]、黏土[16]等微孔材料[17]作为吸附剂。然而,这些传统吸附剂往往因吸附速率慢,吸附能力低且再生困难等问题在实际应用中受到限制[18-19]。因此,设计合成新型高效可再生的吸附材料成为了亟待解决的关键问题[20-21]。
金属-有机骨架(metal organic frameworks, MOFs)作为一类由金属离子或金属簇与有机配体通过配位作用自组装而成的一种新型的多孔材料[22],受到了广泛关注和重视[23-28]。根据金属离子与有机配体种类及连接方式的不同[29],MOFs大致可分为网状金属-有机骨架材料(isoreticular metal-organic frameworks, IRMOF)、来瓦希尔骨架材料 (material institute lavoisier frameworks, MIL)、类沸石咪唑酯骨架材料(zeoliticim idazolate framework, ZIF)和UiO (University of Oslo)系列[30]。由于MOFs具有高的孔隙率和比表面积,易调控的孔径和孔结构以及良好的结晶度,使其广泛应用于吸附去除、传感检测[31]、催化[32]、药物传递[33]、气体储存[34]、质子传导[35]、分离[36]等方面。如表2所示,相比于传统吸附剂,MOFs因为其独特的形貌、规则均匀的气孔、丰富的配位不饱和中心,以及表面可功能化,展示了其高效吸附去除环境中抗生素[37-40]、止疼药 [41-42]、芳香类化合物[43-45]、农药[46-48]、染料[49-51]及重金属[52-54]等各类污染物的良好性能。另外,由于有些MOFs自身具有丰富的发光中心,主要可通过金属离子发光、配体发光、电荷转移发光以及客体分子发光等方式释放荧光[55],且由于MOFs的多孔结构及优异的吸附性能,可将分析物分子快速吸附富集在孔道中,使其成为了一类性能良好的传感器材料。
-
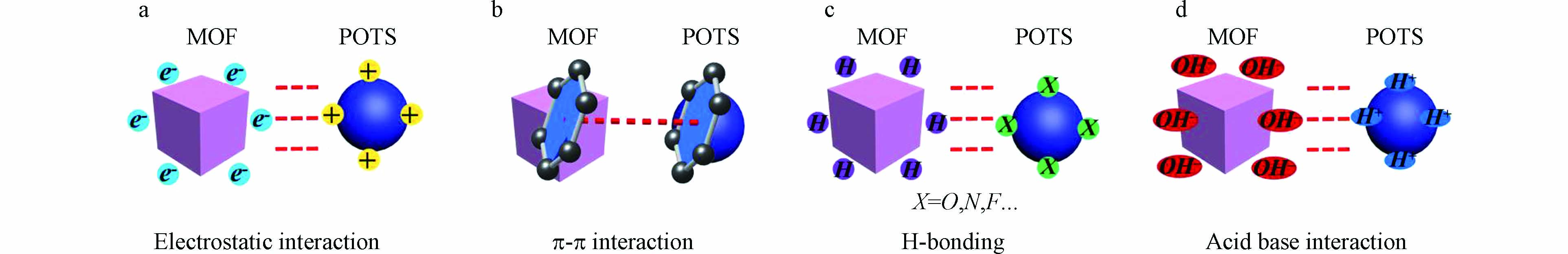
由于不同PTOS分子中含有不同的官能团,且MOFs配体具有多样性,配体中的苯环、羧基、氨基和羟基可以选择性地与有机污染物之间通过相互作用实现高效吸附。就目前研究看来,MOFs材料对PTOS分子的吸附机理主要包括氢键、π-π堆叠、静电作用及酸碱作用等(图1)。值得注意的是,这些相互作用可单独存在,也可能同时存在于同一吸附过程中。
-
MOFs中心金属离子和有机配体上的不饱和位点会导致部分金属位点暴露,由于空间位阻降低,不饱和金属位点可以通过主-客体络合作用与富含N和O的有机分子结合[56],从而形成氢键,有利于吸附的发生。2017年,Ahmed等[57]在1000℃下碳化了ZIF-8,合成了一种新型多孔碳材料MDC-1000,用来吸附去除水中的一种抗生素磺胺甲恶唑(sulfamethoxazole, SMX)。同年,该课题组[58]通过高温碳化含离子液体的ZIF-8进一步合成了多孔碳材料IMDC,并研究了其对水中敌草隆(diuron, DUR)和2,4-二氯苯氧乙酸(2,4-dichlorophenoxyacetic acid, 2,4-D)的吸附。实验结果表明,IMDC对水中DUR和2,4-D的最大吸附量分别可达284 mg·g−1和448 mg·g−1。研究证明,不论是MDC-1000吸附去除水中SMX,还是IMDC吸附去除水中DUR和2,4-D,一方面归因于材料表面存在大量的活性位点可用来吸附污染物分子;另一方面,MOFs材料作为H-供体和污染物分子作为H-受体结合形成氢键的过程在吸附中发挥更重要的作用。
-
π-π堆积是芳香化合物的一种特殊空间排布,常常发生在芳香环之间的弱相互作用,通常存在于相对富电子和缺电子的两个分子之间,是一种与氢键同样重要的非共价键相互作用。由于MOFs材料的配体和PTOS分子中一般存在芳香环结构,因此π-π作用也可能是吸附过程的主要机理之一。Wang等[59]通过扩散法,利用Zn2+和均苯三甲酸(1,3,5-benzenetricarboxylic acid, H3BTC)合成了一种具有菊花形状的MOF(Zn-BTC),并将这一富含芳香环的材料应用于吸附水中杂环类农药。实验发现,农药的吸附量随着共轭双键和缩聚环的增加而增加,说明π-π堆叠相互作用在吸附该类污染物中发挥了重要作用。Duo等[60]合成了类梭状磁性金属有机骨架材料Fe3O4-NH2@MOF-235,MOF-235中有许多芳香环,有利于其他具有π结构的化合物通过,可通过π-π作用,实现对甲酰脲类杀虫剂的固相萃取。
-
研究报道发现在吸附实验中,材料对污染物的吸附能力依赖于体系的pH[61],这种现象通常是由于吸附过程以静电吸附为主导致的。在不同pH条件下,分析物可能以不同离子形式(阴离子、阳离子、两性离子)存在,MOFs材料表面也可能带正电或负电,这就导致材料与分析物之间可能存在静电吸引作用或静电排斥作用,从而促进或阻碍吸附过程。基于此,研究人员通常设计表面带电荷的MOFs,通过静电作用从液相中吸附去除带电液体污染物。Jia等[61]设计了一种新型三维阴离子MOF(NKU-101),该阴离子MOF在乙醇中对以阳离子形式存在的甲基紫精(Methylviologen, MV)和敌草快(Diquat, DQ)有较强的静电亲和力,使其对MV和DQ分子的吸附容量达到160—200 mg·g−1,这是MOFs材料用于捕获有毒阳离子除草剂的首次研究。
-
酸-碱作用是化学反应中最常见的作用之一。在MOFs的研究中,可通过引入酸性基团等方式,构造酸性位点,从而增强对碱性有机毒物的吸附。同样,也可以通过引入碱性基团,从而增强对酸性有机毒物的吸附。2013年,Hasan等[62]分别以氨基甲磺酸(aminomethylsulfonic acid, AMSA)和乙二胺(ethylenediamine, EDA)为―SO3H和―NH2源合成了酸性的AMSA-MIL-101和碱性的ED-MIL-101,研究发现,通过EDA-MIL-101中的碱性―NH2与两种污染物中―COOH间的酸-碱相互作用,其对萘普生和氯菲酸的吸附量分别为MIL-101的1.17倍和1.10倍,而酸性的AMSA-MIL-101则表现出较差的吸附能力。
-
除上文提到的几种主要吸附机理外,MOFs材料对PTOS的吸附过程还存在几种其他的作用,如官能团间的相互作用、极性作用、疏水作用等。Abdelhameed等[63]以Cu为中心金属原子,以H3BTC为配体合成了一种Cu-BTC,并通过Cu与棉织物中纤维素官能团的相互作用,成功得到了产物Cu-BTC@棉布,并发现其对有机磷杀虫剂乙硫磷具有良好的吸附性能。如图2所示,Cu–BTC@棉布不仅可通过材料表面的孔结构实现对乙硫磷的物理吸附,同时也可通过材料与杀虫剂间形成Cu―S键以及O―纤维素官能团之间形成的氢键进行化学吸附,两种吸附过程同时存在使其对乙硫磷的吸附量高达182 mg·g−1。另外,由于Zr―OH和缺陷的存在,Zr-MOF常被用作有机磷农药的良好吸附剂。Jamali等[64]研究发现,由于较大的空腔和Zr―OH的存在,UiO-67对敌敌畏和敌百虫有较强的吸附能力,吸附量分别为571.43 mg·g−1和378.78 mg·g−1。另外,Zhu等[48]2020年通过探究UiO-67对草甘膦和草铵膦的吸附能力再次提出,由于UiO-67形成了大量的Zr―OH,其对有机磷农药中的磷酸基团具有较高的亲和力,因此UiO-67成为了捕获草甘膦和草铵膦的“天然锚定物”。2015年,Bhadra等[65]比较了典型MOF(MIL-101)与活性炭(Active Carbon, AC)在水相和非水相中对芳香族化合物的吸附能力,发现在低极性溶剂,如正辛烷中,MIL-101能更有效的吸附污染物,这种吸附归因于极性作用;而在极性溶剂中,AC对芳香族化合物的吸附性能更佳,这归因于AC的疏水作用。
-
吸附过程往往不是单独某种吸附机理的作用,而是多种机理协同作用导致的。在MOFs材料对PTOS进行吸附时,这种协同作用可有效提高材料的吸附能力。2013年,Jiang等[66]将ZIF-8用于新兴污染物苯并三氮唑(1H-benzotriazole, BTri)和5-甲苯基三唑(5-tolyltriazole, 5-TTri)的吸附去除。研究发现,ZIF-8的疏水作用、BTri和5-TTri的芳环与ZIF-8的芳族咪唑环之间的π-π相互作用以及BTri和5-TTri分子中的氮原子与ZIF-8中的Zn2+离子的配位共同促进吸附过程的进行。Azhar等[67]首次进行了MOF材料用于吸附去除废水中的磺胺类抗生素磺胺氯哒嗪(Sulfachloropyridazine, SCP)的研究。由于HKUST-1与SCP之间存在π-π作用、氢键作用和静电作用,使其对SCP有相对较大的吸附能力。此外,Wang等[68]合成了Fe3O4@SiO2-GO-MOFs,通过氢键作用、π-π作用和疏水作用实现了对含苯类杀虫剂的萃取和预富集。
除以上总结的相关研究以外,其他经典MOFs对PTOS的吸附机理见表3。
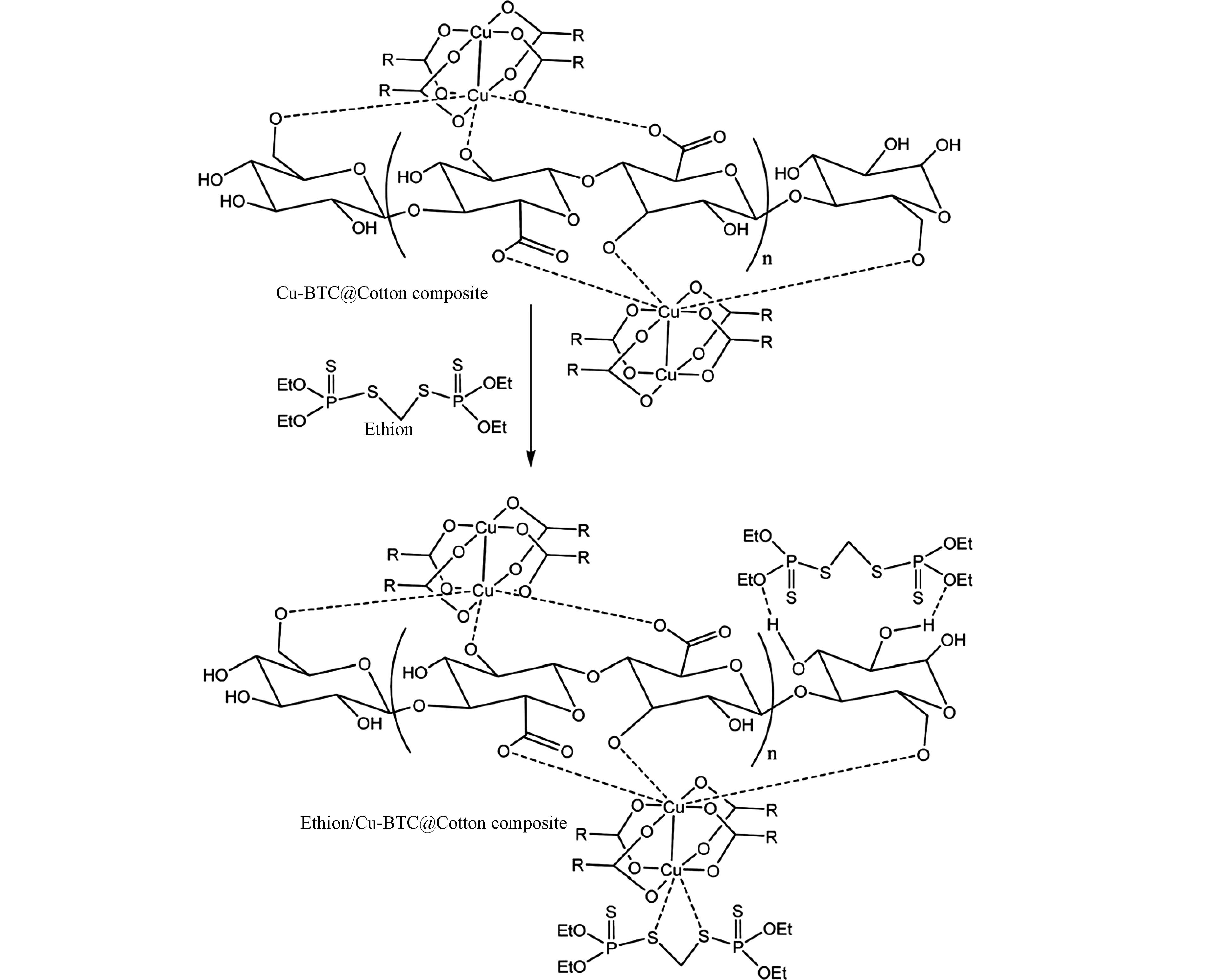
由上可见,MOFs材料对于PTOS的吸附主要是基于配体与PTOS官能团之间的氢键、π-π作用及静电作用等,也有一些是由于MOFs金属中心与有机配体的不饱和配位使得材料可暴露一定的金属中心活性位点,而该类活性位点通过络合或螯合作用与PTOS分子进行结合,实现对PTOS的吸附,如Abdelhameed等[63]合成的Cu-BTC@棉布可通过材料的金属中心Cu2+与杀虫剂S原子间形成Cu―S键而实现对乙硫磷的吸附。
由于PTOS的多样性、在环境中存在形态的多样性和环境介质的复杂性,需进一步研究探索MOFs对于水环境中多种PTOS共存、多种形态共存及多重介质下的协同吸附去除PTOS的机理。另外,可以根据MOFs的可设计性,设计需要的MOFs材料,实现其在复杂环境介质中,对多种PTOS以及同一PTOS不同形态的同时吸附或高选择性吸附,提高吸附去除性能,解决水环境复合型污染问题。
-
尽管有些MOFs对PTOS的饱和吸附量很大,但对污染物的亲和力却很低,导致吸附速率较低。大量研究证明,MOFs的吸附性能(例如吸附动力学,吸附容量,吸附热力学,选择性,稳定性和可回收性)由其高度有序的骨架结构,亲水性,比表面积,功能性以及孔径分布来控制[82]。而随着对MOFs研究的深入,人们发现MOFs的表面可以很容易地进行功能化并转化为各种功能组件,例如后修饰―SO3H、―NH2、―OH、―COCH3和其他活性物质或基质,而不改变材料的拓扑结构[83]。因此利用MOFs结构灵活可控的优点,通过物理或化学改性,可对MOFs结构和性质进行一定的调整,使其吸附性能得到提高。
-
Shemirani与Gu等分别于2019年和2020年研究了UiO-67对敌敌畏和敌百虫以及草甘膦和草铵膦的去除效果,进一步证明了由于Zr―OH基团的存在,UiO-67中形成大量的活性位点,可有效实现对有机磷农药的吸附[64, 48]。因此,材料的表面积和活性位点在吸附过程中发挥着重要作用。然而,虽然MOFs材料可直接作为吸附剂使用,但由于MOFs具有大量的空隙空间和有限的不饱和金属中心,导致吸附亲和力较弱,降低了PTOS的去除效率。因此,越来越多的研究集中在了MOFs基材料的制备上[84]. 目前有研究证明,在MOFs的基础上,通过碳化等方式[85],在维持MOFs原有形貌的基础上,直接构建介孔材料,从而增加材料的表面积或暴露更多的活性位点,可以有效提高其吸附性能。
Ahmed等[57]通过碳化ZIF-8,合成了一种新型多孔碳材料MDC。BET表征和N2吸附曲线显示,相较于ZIF-8,该材料的表面积和孔体积分别从1073 m2·g−1和0.51 cm3·g−1提高到1635 m2·g−1和1.32 cm3·g−1,其孔隙率几乎是ZIF-8的两倍。研究表明,利用MDC吸附去除水中的抗生素SMX,其吸附量是原ZIF-8材料的20倍左右。Pang等[86]通过溶剂热法,用NH2-MIL-125和g-C3N4原位合成了g-C3N4@NH2-MIL-125,然后在1000℃下碳化,得到了一种新型多孔碳材料C-(C3N4@MOF)。BET表征证明,在与C3N4复合后,材料的比表面积从268 m2·g−1提高到了544 m2·g−1。将该材料作涂料制成C-(C3N4@MOF)涂层纤维后,成功对蔬菜和水果中有机磷农药(Organophosphorus Pesticides, OPPs)进行了固相萃取。Liu等[87]以MOFs为模板,用糠醇浸泡ZIF-8并在900℃下高温碳化,糠醇在ZIF-8空隙内发生聚合和碳化,同时ZIF-8骨架也发生碳化和热分解,最终得到了MOF-C。表征发现MOF-C比表面积和总孔体积达到1320 m2·g−1和1.48 m2·g−1,具有较高的比表面积和较大的孔隙体积,可作为分散固相吸附剂,实现对水和柑橘中苯并脲类杀虫剂二氟脲、三氟脲、六氟脲和四氟脲的高效预富集。Abdelillah等[88]以Ce-BTC金属有机骨架为原料,通过原子力显微镜(Atomic Force Microscopy, AFM)分析表明,在650℃下煅烧3 h后,Ce-BTC表面形成了微米/纳米级结构,得到了高比表面积的CeO2纳米纤维。以间歇式吸附方式探究了CeO2纳米纤维对水中2,4-D的吸附能力。研究证明,CeO2纳米纤维可通过π-π作用和静电作用对2,4-D的进行高效吸附,其容量可达86.16 mg·g−1,这主要归因于微米/纳米级结构形成的高比表面积与暴露的活性中心。2016年Wang等[71]以MIL-125(Ti)为核心,原位合成了In2S3@MIL-125(Ti)核-壳微粒(MLS)。该复合材料虽表面积较MIL-125(Ti)较小,但表面In2S3暴露的In3+成为了该材料吸附水中四环素(Tetracycline, TC)的良好的活性位点。最终实验结果证明,MLS对水中TC的吸附量从14.2 mg·g−1提高到119.2 mg·g−1,吸附去除效率可达99.9%。如图3所示,该吸附过程主要依赖于材料表面In3+与TC极性官能团间的表面络合作用、π-π相互作用、氢键以及静电作用。
除直接构建多孔材料外,将MOFs与其他活性材料复合[89],通过不同组分间的协同效应,与单个材料相比,可显著增加材料的比表面积或活性位点数目,也是提高材料吸附和分离性能的一种有效手段。
氧化石墨烯(Graphene Oxide, GO)是单层石墨片,其较大的比表面积使其成为了良好的吸附材料,而与MOFs复合后,吸附性能可进一步提高。Yang等[90]通过溶剂热合成法,让UiO-67颗粒致密有序的排列在GO表面,最终制成UiO-67/GO。利用GO较大的表面积和丰富的Zr-OH基团作为活性位点,增强了复合材料与OPPs之间的螯合作用,极大的增强了其对草甘膦的吸附能力,吸附容量可达到482.69 mg·g−1。Wang等[68]以Cu-MOFs、氧化石墨烯(GO)和Fe3O4纳米颗粒(NPs)为基础,将Cu-MOFs和Fe3O4纳米颗粒加载到GO上,通过化学键合的方法制备了磁性纳米复合材料Fe3O4@SiO2-GO-MOFs。生长在GO表面的MOFs会形成微孔结构,形成较大的表面积,有利于对芳香族杀虫剂的萃取。最终实验结果证明,该材料对吡虫啉等含苯类杀虫剂表现出良好的萃取效果,检测限为0.38 μg·L−1,是一种高效的磁性固相萃取(MSPE)吸附剂。2018年,Liu等[91]通过对MOFs的进一步改性,利用Fe3O4/GO的稳定结构以及其结构中的值值―NH2和―N═CH―NH―基团,合成了一种新型氨基功能化的Zn-MOF材料(M-IRMOF)。扫描电子显微镜(Scanning Electron Microscope, SEM)和透射电子显微镜(Transmission Electron Microscope, TEM)表征显示,未进行Fe3O4/GO处理的IRMOF是表面光滑的三维花瓣状结构,而处理后M-IRMOF则是高度粗糙、多孔的块状结构。其良好的多孔结构,加强了含氮杂环性杀菌剂中的氧基与―N═C―N基团与GO中的―OH和―COOH基团间的氢键和π-π作用,使M-IRMOF对含氮杂环性杀菌剂的吸附量较IRMOF大幅度提高,吸附容量达到2.34—5.62 mg·g−1。2019年,Liu等[92]成功在Fe3O4-GO表面生长MOF,并将其与β-环糊精(β-Cyclodextrin, β-CD)结合,不仅提高了材料的水稳定性和机械强度,还形成了超顺磁性和高比表面积。由于该材料表面存在大量的―OH以及六元碳环的平面结构,增强了氢键和π-π作用,成功提高了材料对丙氯佐和三唑杀菌剂的分离性能。Yang等[93]合成了一种新型榴莲形磁性多孔复合材料Fe3O4@SiO2@UiO-66 (MSU(Zr)),通过MSU(Zr)中Zr带正电的Zr中心原子与硝基间苯二酚(Nitroresorcinol, NRC)中―OH结合,Zr―OH变为了Zr―NRC,实现了MSU(Zr)对NRC分子的良好吸附。
另外,MOFs也经常与其他材料,如纤维素纸、多壁碳纳米管等材料复合,通过不同组分间的协同作用,提高其吸附性能。Jiang等[94]将MIL-101(Cr)包覆于壳聚糖基质中,利用纤维素纸,制备成薄膜,用于薄膜微萃取(Thin-Film Microextraction, TFME),由于萃取相表面积与吸附薄膜体积的比值较高,使其对三嗪类除草剂有良好萃取分离效果。多壁碳纳米管(Multiwalled Carbon Nanotube, MWCNT)是一种由石墨片组成的三维碳材料[95],由于其较大的表面积,中空多孔的结构以及优异的稳定性,使其与MOFs的复合材料可具有较好的吸附性能。Liu等[96]2018年运用MWCNT和ZIF-8合成了磁性材料M-M-ZIF-8,如图4。从TEM和SEM表征可以看出,ZIF-8均匀生长在MWCNT表面,形成了较大的比表面积和多孔结构,通过有机磷农药分子与M-M-ZIF-8的空位活性位点之间存在的共享或交换电子,通过静电作用促进材料对有机磷农药的吸附过程,吸附容量可达203.0 mg·g−1。另外,也有研究者曾用凹凸棒(Attapulgite, ATP)改性MOFs材料,使其具有良好的吸附分离性能。2020年,Niu等[97]通过ATP的―OH与磁性ZIF-8的金属离子特异性结合,合成了磁性材料ATP@Fe3O4@ZIF-8。傅里叶红外光谱仪(Fourier transform infrared spectroscopy, FT-IR)光谱证明,ZIF-8成功固定在了ATP@Fe3O4纳米杂化材料表面。实验证明,通过将功能化凹凸棒加入到ZIF-8中,使其具有良好的稳定性的同时提供更多的吸附位点,成为一种良好的固相萃取剂可对甲酰脲类杀虫剂的检测限为0.7—3.2 μg·g−1。
不论是通过处理MOFs直接构筑多孔材料的方式,还是将MOFs与其他活性物质复合形成复合材料的方式,究其根本,都是使材料暴露更多的活性位点,从而促进吸附过程的进行。但是,在对MOFs进行处理的过程中,应关注MOFs的热稳定性及化学稳定性,避免MOFs结构的坍塌与破坏。
-
除通过以上物理改性增加材料的表面积和活性位点外,利用羟基、氨基、磺酸基等特定官能团官能化MOFs也可以提供额外的吸附位点,实现对材料的化学改性,进而增强材料与分析物之间的π-π络合、氢键作用以及静电作用等相互作用强度,不仅是实现材料吸附性能提高的一个重要手段,还可以提高MOFs对污染物的选择性[82]。
当前,随着化合物和化学药品用于生产和生活中的快速增加,越来越多的药物及个人护理用品(Pharmaceuticals and personal care products, PPCP)以各种方式直接或间接排放到生态系统中,从而对不同的环境区域造成潜在威胁[24]。近十年来,官能团(例如―NH2,―OH)修饰的MOFs材料成为了吸附去除水中PPCPs良好材料。如前所述,Guo等[39]将极性―SO3H基团引入MIL-101中,合成了磺酸化的MIL-101-SO3H。研究发现,在pH值从1到6的上升过程中,由于―SO3H基团的电离,MIL-101-SO3H的电负性急剧升高,从而与抗生素中质子化的哌嗪基间形成强烈的静电作用,使其对三种氟喹诺酮类抗生素的吸附量可达到408.2—450.4 mg·g−1。也有研究[51]分别用―NH2和―SO3H对UiO-66进行了修饰,发现除UiO-66本身与双氯芬酸钠(Diclofenac Sodium, DCF)之间的静电作用外,SO3H-UiO-66对DFC的吸附性能由于酸-碱作用的存在较UiO-66提高了39.1%,而NH2-UiO-66则由于碱基互斥效应的存在导致吸附量降低了43.9%。对于某些H-供体和H-受体的数量较多的PPCP,该课题组[76]研究了羟基官能团化的MOFs对水中5种PPCP的吸附研究,并对吸附中的氢键进行了定量分析。实验结果表明,合成的MIL-101、MIL-101-OH和MIL-101-(OH)3材料对5种PPCP的吸附量随PPCP中的作为H-受体的O的数量的增加而增加,且随MOF中―OH的数量增加,其吸附量增大。原因归结为PPCP分子作为H-受体,MOFs中的―OH作为H-供体,二者之间形成氢键,从而实现了对PPCP的高效吸附。Zhang等[98]制备了磁性复合材料(MNP@UiO-66-NH2),材料既拥有MOFs的特殊结构,又增加了磁性材料的磁分离性能,从而使MNP@UiO-66-NH2对水杨酸(SA)和阿司匹林(ASA)的吸附能力分别达到87.57 mg·g−1和90.74 mg·g−1。该材料对水杨酸和阿司匹林的高吸附能力是由氢键、羧基与Zr―O团簇的亲和力以及静电作用等共同作用的结果。
除PPCPs外,化石燃料燃烧排放出的SOx和NOx容易转化为酸雨,对环境,森林和人造结构产生负面影响,因此,从燃料中去除含硫和含氮化合物也成为了当今研究的热点之一。Jhung及其课题组对燃料中的吲哚(Indole, IND)和喹啉(Quinoline, QUI)的吸附去除进行了一系列研究。2016年,他们[99]利用游离的羧酸(―COOH)修饰UiO-66合成了UiO-66-COOH,虽然―COOH修饰后的UiO-66的孔隙率降低,但对燃料中IND的吸附量有一定的提高,这可能是因为―COOH的引入有利于与IND形成氢键作用,进而提高其吸附性能。2017年,将氨基(―NH2)和磺酸基(―SO3H)修饰在MOF中,合成了UiO-66-NH-SO3H,实现了对IND去除效率的提高[100]。探究其吸附机理发现,IND中的H原子可与―COOH中的O原子形成氢键,从而提高了材料的吸附能力。2019年,该课题组[101]又合成了MIL-125和MIL-125-NH3,并用―NH―、―C(O) ―和―C(O)OH―对后者进行进一步修饰(记为MIL-125-VFG),实验结果表明,MIL-125-VFG在从燃料中吸附IND和QUI方面表现出非常出色的性能。这些研究发现,MOFs材料在吸附IND时,IND常常作为H-供体,而通过将含可作为H-受体的N、O等位点的官能团修饰到MOF材料上的方式,可促进IND和材料之间形成较稳定的氢键,进而提高材料的吸附去除性能。综上,我们发现氢键是官能化MOFs吸附吲哚的关键作用力。在对吸附吲哚进行研究的同时,他们对喹啉的吸附也进行了探究[100-101]。研究结果显示,由于喹啉是一种碱性物质,UiO-66-COOH、和MIL-125-VFG主要通过酸-碱作用,在与氢键的协同作用下,很好地实现对喹啉的吸附,而UiO-66-NH-SO3H则由于空间位阻的存在,使得在高浓度喹啉溶液中,部分喹啉分子不能接触到酸性位点(―SO3H),导致吸附量下降。
随着MOFS材料吸附去除PTOS研究的深入,官能团改性的MOFs材料逐渐被用于各类化学品的吸附,如将―NH2基团引入MIL-68(In)中[102],增强π-π作用和氢键作用,有效的提高了MOFs材料对有机砷化合物4-氨基苯胂酸(p-Arsanilic Acid, p-ASA)的吸附;用―NH2基团修饰MIL-101(Al)[103],通过增强氢键作用,可表现出对对硝基苯酚(p-nitrophenol, PNP)较高的吸附能力;将含π电子的配体引入MOFs中[104],可实现对阿特拉津除草剂的有效吸附,并进一步证明了化学官能团在吸附中的作用。
大量研究证明,与未官能化的MOFs相比,带有一个或多个基团的官能化MOFs各种性能得到了提高,具有更好的吸附能力,被看做是简单易用的材料[105-107]。
-
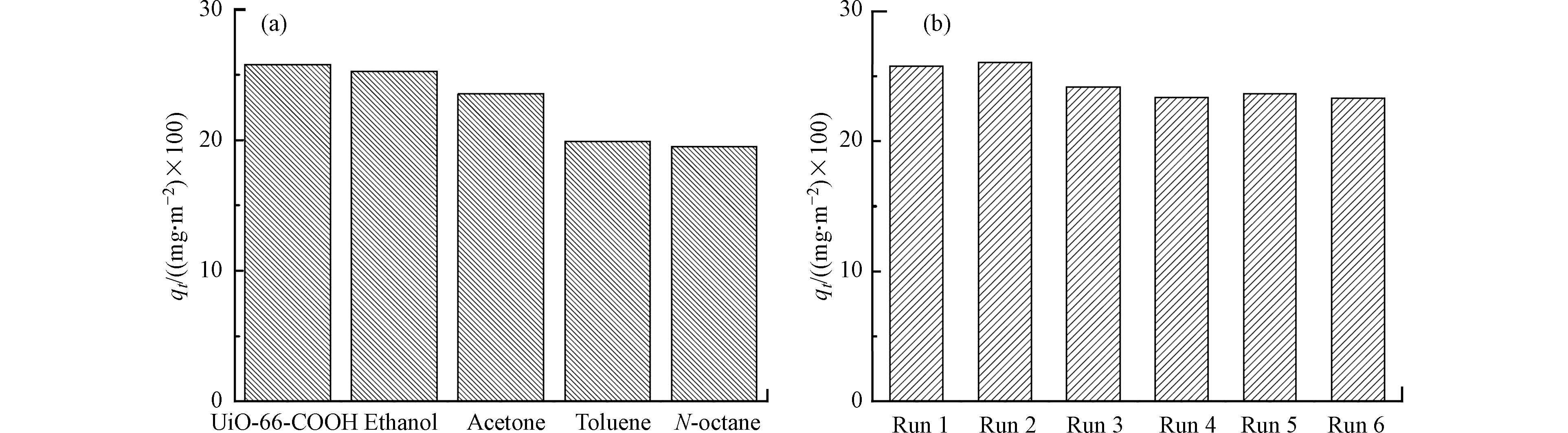
对于吸附剂的研究与开发,除了其吸附性能的提高外,提高吸附剂的解析和再生性能对于资源节约、高效利用具有重要意义。因此,对于开发研究的MOFs吸附材料,其再生性能的评估是不可缺失的部分。在MOFs材料对PTOS进行吸附后,可以采用对PTOS有较强溶解能力的溶剂(如甲醇、乙醇等有机溶剂)进行再生,利用溶剂与PTOS之间较强的亲和力,实现PTOS的脱附和MOFs的再生。2017年,Jhung等[57-58]在分别利用MDC-1000和IMDC成功实现对DUR和SMX的吸附去除后,利用乙醇溶剂对DUR和SMX进行了脱附并探究了材料的再生性能。XRD和FTIR光谱证明,通过乙醇洗涤后,回收后的材料依旧保持了结构的完整性,且4次循环后,依旧保持良好的吸附能力。Abdelhameed等[63]探究了Cu–BTC@棉布复合材料吸附乙硫磷后的再生性能,为使乙硫磷分子从Cu–BTC@棉布复合材料上脱附,吸附完成后将其浸泡在乙腈溶液中,结果表明,经过5个重复再生、再利用的循环后,该材料对乙硫磷的去除效率仍能达到85%以上。2016年,Seo等[99]在合成了UiO-66-COOH并将其应用于燃料中吲哚的吸附去除后,还比较了乙醇,丙酮,甲苯和正辛烷等各种溶剂对吲哚的脱附效果,其中,乙醇是回收UiO-66-COOH的最佳溶剂(图5(a))。6个吸附解析循环后,吸附性能虽略有下降,但仍能保持较高的吸附性能(图5(b))。Guo等[39]探究了MIL-101(Cr)-SO3H的再生性能。在将MIL-101(Cr)-SO3H样品在水溶液中对诺氟沙星进行了饱和吸附后,用丙酮洗涤,在100℃真空下干燥数小时。再生的MIL-101(Cr)-SO3H再次用于对诺氟沙星的吸附,循环7次后,该材料对诺氟沙星的吸附量仅略有下降,表明MIL-101(Cr)-SO3H具有良好的再生性能。综上所述,MOFs材料不仅具有良好的吸附性能,且具有良好的再生性,因此,该类材料在未来PTOS的去除方面具有潜在的实际应用前景。
-
化学传感器旨在通过其性质(例如,电导率,颜色,发光度或电容)的变化来识别分析物[108],而通过荧光传感的方式,利用荧光淬灭或增强的现象,可以实现溶液中分析物的荧光检测,且由于该方法具有灵敏性高、易操作、响应时间短、实时监测等特点而使得基于荧光淬灭对分析物进行识别检测成为了一种重要的化学传感方式。由于MOFs中金属离子/团簇和有机配体的存在,使其具有良好的发光特性,而且还可以通过它们之间的相互作用调节其荧光性能[109],使其可以检测不同类型的分析物,如金属离子、硝基芳族化合物、挥发性有机化合物等。大量研究充分利用MOFs材料的发光特性,设计出荧光MOFs,通过荧光共振能量转移(Fluorescence Resonance Energy Transfer, FRET)、光诱导电子转移(Photoinduced Electron Transfer, PET)以及动态/静态淬灭机制等,实现了MOFs对各类污染物的分析检测(表4)。而MOFs材料天然的多孔结构,使得荧光MOFs传感器较其他类型传感器更具有响应时间短、灵敏度高、检出限低且选择性好等优势。首先,孔隙度限制了分析物与MOFs的距离,从而确保MOFs与分析物之间发生紧密相互作用。其次,孔的尺寸和化学环境(如亲疏水性)的变化可用于控制传感器与传感分子之间相互作用的选择性[111]。最重要的是,MOFs的多孔结构和优异的吸附性能可将分析物快速吸附到MOFs的孔道中,使分析物在传感器处预先富集[110],从而大大提高传感灵敏度,能够实现水环境中痕量PTOS的快速检测。
Wang等[122]筛选发现,带有孤立笼子的MOFs不能很好地识别多氯代二恶英(Polychlorinated Dibenzo-p-dioxins, PCDD),这是因为他们的孔空间不匹配以及缺少π-π堆叠的相互作用。由此,该课题组用较小的有机连接基H4CPTTA合成了BUT-17,用来识别PCDD中毒性最强的2,3-二氯二苯并-对-二恶英(2,3-dichlorodibenzo-p-dioxin, BCDD)和2,3,7,8-四氯二苯并-对二英 (2,3,7,8-tetrachlorodibenzo-p-dioxin, TCDD)。实验发现,BUT-17对BCDD和TCDD的检出限分别低至27和57 μg·L−1。
单晶X射线分析和密度泛函理论(density functional theory, DFT)计算表明,开放的一维通道具有合适的孔径用于孔限制,芳香族空隙表面与客体分子具有π-π堆叠和氢键相互作用共同增强了宿主BUT-17的特异性识别和检测BCDD和TCDD分子的能力。Xing等[123]设计了一种氨基功能化的Zn-MOF,[NH2(CH3)2] [Zn4O(bpt)2(bdc-NH2)0.5] 5DMF,并将此MOF应用于硝基爆炸物(trinitrophenol, TNP)的荧光检测。TNP羟基上的质子与MOF上的氨基结合,MOF的质子化氨基与TNP分子的酚性氧原子之间有较强的氢键作用,有利于TNP分子吸附到MOF孔道内,实现MOF的荧光淬灭,从而实现对TNP分子的传感。传感TNP后的MOF材料几乎不吸附N2分子,进一步证明了该检测过程是基于将TNP分子吸附到孔道中进行的。Zhu等[112]合成了一种Zn-MOF(FCS-1),并将其作为一种高灵敏度、快速响应的化学传感器应用于模拟废水中磺胺类抗生素的检测。实验结果表明,FCS-1能够通过光诱导电子转移引起荧光猝灭而有效地检测到一系列磺胺类抗生素。值得注意的是,磺胺类抗生素的荧光猝灭作用归因于π-π相互作用,该相互作用促进了抗生素和FCS-1之间通过表面吸附的相互作用,从而促进了荧光淬灭过程的发生。该荧光猝灭过程不受重金属离子和溶液pH的影响,且检测限较低,意味着该材料在检测实际水样中磺胺类抗生素方面有很好的应用。
虽然MOFs在基于吸附的PTOS荧光检测中的应用已经得到了广泛的探索,但是MOFs的水稳定性和光稳定性较差依旧限制了MOFs在PTOS荧光检测中的应用。因此,研究出具有高水稳定性和光稳定性的MOFs成为了必然。2018年,Yang等[124]开发出一种新型的基于MOFs的磁性纳米复合材料Fe3O4@SiO2@UiO-67,可以实现选择性识别、检测和去除水环境中有机磷农药的智能吸附。因为吸附剂中含有对磷酸基团有高亲和力的Zr-OH基团,从而对草甘膦具有很强的选择性识别及吸附能力;而且草甘膦分子与Fe3O4@SiO2@UiO-67分子结合时会发生荧光强度的变化,在加入SiO2后,阻碍了UiO-67/GO与磁芯之间的电子转移,又增强了材料检测草甘膦分子的灵敏度,达到了较低的检测限(0.093 mg·L−1),实现了同时检测和去除水中的草甘膦分子的目的。Zhou等[114]合成了一个高度稳定的发光双功能Zr-MOF (PCN-128Y),通过荧光猝灭的方式,用来检测和去除水中的四环素(TC)。理论和实验研究表明,TC在激发波长上的强吸收和PCN-128Y上配体向TC的光诱导电子转移是荧光猝灭的主要原因。然而,PCN-128Y孔道中对TC的预富集使TC与骨架之间的接触更加充分,显著提高了TC的传感效率。2019年,He等[116]基于Zr簇合成了一种高水稳定性和发光性的荧光MOF(Zr-LMOF),可快速灵敏的吸附和传感甲基对硫磷分子。研究发现,Zr-LMOF卓越的吸附能力和甲基对硫磷分子在框架结构中的扩散,是传感甲基对硫磷分子的基础。材料的吸附能力实现了对痕量甲基对硫磷的富集,骨架中的孔使甲基对硫磷分子容易扩散至荧光团,使材料与甲基对硫磷分子之间发生光激发电子的转移,引起荧光淬灭,实现了对甲基对硫磷的分析检测。
-
综上所述,MOFs材料具有自身可调控的孔径和孔道结构、较大的表面积和大量的活性中心等诸多优点,可作为一种新型多孔材料用于水中有机污染物的吸附去除。我们还可通过前期设计或后期修饰来对它的组成和结构进行一定的调控,使其更易通过氢键作用、π-π络合、静电作用等实现对水中PTOS的吸附去除。尽管如此,目前研究者对于MOFs基材料的研究仍处于初级阶段,仍然存在合成成本高、水稳定性差、材料不易从分析物溶液中分离以及重复利用性差等亟待解决的问题,因此设计新型稳定且高效的MOFs基材料是目前研究者的关注重点。未来的研究中,(1)应从改进材料的合成过程入手,合成的MOFs基材料应具有良好的热稳定性、化学稳定性以及机械稳定性,以来维持其吸附前后的结构,从而确保去除水中PTOS的有效性及可重复利用性。在合成的过程中,应通过对MOFs的进一步改性,在提高其水稳定性的同时,还应该充分利用分析物的结构特征,有目的的设计或改良MOFs,以提高其吸附性能。(2)充分考虑各种PTOS在不同环境条件下具有独特的物理和化学性质(如电离,溶解度的不同),这是导致不同的反应途径的重要原因。因此,必须进一步考虑MOFs基材料对PTOS吸附去除的可能机制,除本文提到的几种机理外还包括呼吸作用、不饱和化合物的配位及其他未知的吸附机理,以开发更有效的吸附分离材料。(3)为了大规模生产MOFs并提高MOFs作为工业吸附剂的适用性,MOFs基材料的回收、再生技术也应该是接下来研究的重点之一,只有研究出可重用性高,副产物毒性低,且具有优异的机械强度和成本效益的MOFs基材料,才能让MOFs基材料的工业化应用成为可能。
金属有机骨架材料吸附与荧光检测水中持久性有毒有机污染物的研究与应用
Highly efficient adsorption and fluorescence detection of persistent toxic organic pollutants in water with MOFs: A review
-
摘要: 近年来,环境中的持久性有毒有机污染物(persistent toxic organic substances, PTOS)因具有毒性高、生物累积性、致癌致突变性和内分泌干扰等特性引起了广泛关注,但是其在水环境介质中含量低、可与其他物质发生相互作用、迁移转化能力强等特点为其高效分离去除和快速的痕量分析检测带来了挑战。因此,开发新材料、新技术、新方法用于水中PTOS的高效分离去除、建立其快速的痕量分析检测方法对于水环境保护、再生水安全利用以及PTOS的环境风险评价具有重要意义。相对于传统的吸附材料,金属有机骨架材料(metal organic frameworks, MOFs)由于其较高的孔隙率和比表面积,易调控的孔径,大量的活性位点、可功能化以及具有良好的发光特性等优点,在吸附去除和分析检测环境中PTOS表现出优异性能。本文综述了MOFs、功能化MOFs以及MOFs衍生物材料在水溶液中吸附去除PTOS的研究与应用进展,归纳总结相应的吸附机理,特别对如何通过功能化手段来提高MOFs材料对PTOS的吸附性能进行了讨论。同时,本文还对基于吸附的荧光传感方法检测PTOS进行了归纳总结,对未来MOFs材料在吸附去除和检测水环境中PTOS方面的应用进行了展望。
-
关键词:
- 金属有机骨架材料 /
- 功能化 /
- 持久性有毒有机污染物 /
- 吸附 /
- 荧光传感
Abstract: Persistent Toxic Organic Substances (PTOS) have aroused extensive concerns due to their bioaccumulation, long-distance transmission, carcinogenicity, mutagenicity and endocrine disturbance. However, owning to their low content, co-existing with other substances and easy migration and transformation in environmental media, highly efficient removing them from water and their rapid and trace detection techniques are becoming emergence for water environment protection, safe utilization of recycled-water and their environmental risk assessment. Compared to traditional adsorption materials, MOFs synthesized via the self-assembling combination of metals and organic ligands are considered as potential highly efficient adsorbents to removal and detection of PTOS from water due to their high specific surface areas, good adsorption performance, tunable porosity, modifiable structures, and great fluorescence property. In this paper, we comprehensively summarized the development of MOFs, functional MOFs and MOFs derivative materials applied in adsorptive removal of PTOS from aqueous solution. We discussed the adsorptive purifications of contaminated water with MOFs materials and the mechanism of adsorption of various PTOS by MOFs materials. In particularly, in order to improve the adsorption capacity of MOFs materials to PTOS, the strategies of functionalizing MOFs were comprehensively summarized. In addition, the fluorescence sensing methods for detecting PTOS based on the adsorption of PTOS with MOFs are summarized. Finally, the challenges of further application of MOFs materials in adsorption and removal of PTOS from water are briefly commented, and the future research prospects in this field are prospected.-
Key words:
- MOFs /
- functional /
- persistent toxic organic substances /
- adsorption /
- fluorescence sensing
-

-
图 5 (a)解析溶剂种类对再生UiO-66-COOH吸附吲哚的影响 (b)乙醇洗涤次数对再生UiO-66-COOH吸附吲哚的影响
Figure 5. (a) Effect of solvents applied in the regeneration of UiO-66-COOH for the adsorption of IND; (b) effect of regeneration cycles on the performances of adsorptive removal of IND over UiO-66-COOH regenerated by washing with ethanol.
表 1 环境中存在的主要PTOSTable 1 Major PTOS in the environment
PTOS 主要类别
Main
categories用途
Application主要代表物质
Principal
representative
substance毒性
Virulence农药 有机氯农药 防治植物病、虫、草害 六六六、滴滴涕 刺激神经中枢,产生小脑失调、造血器官障碍 有机磷农药 敌敌畏、敌百虫、马拉硫磷 神经中毒症状,如出汗、震颤严重者会出现呼吸麻痹,甚至死亡 有机氮农药 敌草隆、灭草隆 对人、畜的急性毒性都不大,不易发生药害 药物及个人
护理产品抗生素 治疗生物体内被微小的病原体所引发的感染现象 磺胺甲恶唑、氧氟沙星、氯霉素 破坏肠道微生物环境,导致肝、肾功能受损 激素 对机体的代谢、生长、发育、繁殖、性别、性欲和性活动等起调节作用 雌酮、雌二醇、炔雌醇 引起内分泌紊乱失调,引起肥胖 止痛剂和消炎药 抗炎、止痛、抗风湿、退热和抗凝血等作用 双氯酚酸、布洛芬、
对乙酰氨基酚刺激肠胃,导致肝、肾功能受损 芳香剂 改善或增强香味特征 佳乐麝香、吐纳麝香 刺激皮肤黏膜,损害神经系统 工业化学品 多氯联苯 可作绝缘油、热载体和润滑油等,还可作为许多工业产品的添加剂 三氯联苯、四氯联苯、 易累积在脂肪组织,造成脑部、皮肤及内脏的疾病,并影响神经、生殖及免疫系统 六氯苯 用于生产花炮,作焰火色剂 — 影响肝脏、中枢神经系统和心血管系统,导致皮肤溃疡 生产中的副产品 二噁英 — 2,3,7,8-四氯二苯并-对-二噁英(2,3,7,8-TCDD) 剧毒,可能导致染色体损伤、心力衰竭、癌症等 呋喃 用于制取吡咯、噻吩、
四氢呋喃等— 吸入后可引起头痛、头晕、恶心、呼吸衰竭。 表 2 不同吸附剂对水环境中污染物的吸附量
Table 2. The adsorption capacity of pollutants with different adsorbents
污染物
Pollutant吸附剂
Adsorbent吸附量/(mg·g-1)
Amount adsorption参考文献
References氧氟沙星 氮化硼纳米片 72.5 [37] 膨润土CVL黏土 116.72 [38] MIL-101(Cr)-SO3H 433.7 [39] ZIF-8 194.1 [40] 双氯芬酸钠 碳纳米管 27 [41] 商业活性炭 76 [42] UiO-66 189 [42] 18%SO3H-UiO-66 263 [42] 吲哚 活性炭 118 [43] MIL-101 410 [44] 喹啉 活性炭 145 [43] MIL-101 446 [44] 草甘膦 MgAl-LDH 184.6 [45] 蒙脱石 49.9 [46] 明矾 85.9-113.6 [47] UiO-67 537 [48] Pb2+ PBC@SiO2-NH2 120 [49] g-C3N4/Mt 182.7 [50] ZIF-8@GO 356 [51] 甲基蓝 坡缕石 57.47 [52] 沸石/壳聚糖复合材料 199 [53] Amine-MOF-Fe 312.5 [54] 表 3 部分MOFs材料对PTOS分子的吸附机理
Table 3. Adsorption mechanism of PTOS in MOFs
PTOS分子
PTOSMOFs材料
MOFs material吸附量/(mg·g−1)
Amount adsorption机理
Mechanism参考文献
ReferencesTNR UiO-66-NH2 24 氢键 [69] PA UiO-66-NH2 22.5 氢键 [69] 2,4-DNP UiO-66-NH2 29.6 氢键 [69] 对氯苯氧异丁酸 MIL-101 312 静电作用 [70] 四环素 MLS 119.2 表面络合、π-π相互作用、氢键以及静电作用 [71] ZIF-8 303.0 π-π作用 [72] 邻苯二甲酸 ZIF-8 654 静电作用、酸碱作用 [73] UiO-66Zr) 187 静电作用、酸碱作用 [73] 丙硫膦 ZIF-8 210.8 氢键 [74] ZIF-67 261.1 氢键 [74] 呋喃西林 BUT-12 — 疏水作用 [75] 对氯间二甲苯酚 (OH)3-MIL 101 (Cr) 79 氢键 [76] 苯酚 ZIF-67 378 静电作用 [77] 全氟辛酸 MIL-101(Cr) 459.6 静电作用 [78] 苯并三唑 MOF-5(Co) 389 氢键、π-π作用 [79] 苯并咪唑 MOF-5(Co) 175 氢键、π-π作用 [79] 洛克沙胂 MIL-100(Fe) 387 配位不饱和位点 [80] MCPP UiO-66 370 静电作用 [81] 表 4 MOFs材料对污染物的荧光检测
Table 4. Fluorescence detection of pollutants by MOFs
MOFs 污染物
Pollutant机理
Mechanism检出限
LOD参考文献
ReferencesFCS-1 磺胺类抗生素 电子由FCS-1的导带转移到磺胺类抗生素的最低空轨道上 — [112] CTGU-7 奥硝唑 分析物附着在MOF表面,减小配体到金属中心的能量转移,实现荧光淬灭 0.8 μmol·L−1 [113] 硝基苯酚 0.3—1.5 mg·L−1 PCN-128Y 四环素 光诱导电子转移过程 30 nmol·L−1 [114] Eu-BAC 呋喃妥因 荧光共振能量转移 0.21 μmol·L−1 [115] 呋喃西林 0.16 μmol·L−1 BUT-172/BUT-173 诺氟沙星等喹
诺酮类抗生素MOF和抗生素分子之间对激发光的竞争吸收及荧光共振能量转移过程 0.18—0.22 μmol·L−1 [31] Zr-LMOF 甲基对硫磷 甲基对硫磷中强吸电子基团-NO2的存在导致Zr-LMOF光激电子向对硫磷甲基转移,引起荧光淬灭 0.43 nmol·L−1 [116] [Y1.8Eu0.1Tb0.1(PDA)3(H2O)1]2H2O 谷硫磷 静态猝灭,激发光的竞争吸收以及荧光共振能量转移 212 μg·L−1 [117] Zn2(bpdc)2(bpee) DNT 光诱导电子转移 — [118] FJI-C8 2,4-DNP 荧光共振能量转移和12,4-DNP与MOF之间对于紫外光的竞争吸收 0.0028 mmol·L−1 [119] Tb3+@Cd-MOF Fe3+ Fe3+与Tb3+之间的离子交换引起的荧光淬灭 0.01 mmol·L−1 [120] Cr2O72- Cr2O72−与Tb3+@Cd-MOF配体之间对于紫外光的竞争吸收 0.012 mmol·L−1 Tb-MOF Pb2+ Pb2+的电子结构和Tb-MOF中酚氧的Lewis碱性位点之间的相互作用显著的增强了配体向Tb3+之间能量转移的效率 — [121] -
[1] YOON S J, HONG S, KIM T, et al. Occurrence and bioaccumulation of persistent toxic substances in sediments and biota from intertidal zone of Abu Ali Island, Arabian Gulf [J]. Marine Pollution Bulletin, 2019, 144: 243-252. doi: 10.1016/j.marpolbul.2019.05.008 [2] SCHULTZ M M, BAROFSKY D F, FIELD J A. Fluorinated alkyl surfactants [J]. Environ Eng, 2003, 20(5): 487-501. [3] FROMME H, BWCHER G, HILGER B, et al. Brominated flame retardants-Exposure and risk assessment for the general population [J]. International Journal of Hygiene and Environmental Health, 2016, 219(1): 1-23. doi: 10.1016/j.ijheh.2015.08.004 [4] XIE F, YANG M, JIANG M, et al. Carbon-based nanomaterials-A promising electrochemical sensor toward persistent toxic substance [J]. TrAC Trends in Analytical Chemistry, 2019, 119: 115624. doi: 10.1016/j.trac.2019.115624 [5] BARAKAT A O. Assessment of persistent toxic substances in the environment of Egypt [J]. Environment International, 2004, 30(3): 309-322. doi: 10.1016/S0160-4120(03)00181-8 [6] XU J, LI K, ZHANG S, et al. Removal of endocrine-disrupting chemicals from environment using a robust platform based on metal-organic framework nanoparticles [J]. ACS Applied Nano Materials, 2020, 3(4): 3646-3651. doi: 10.1021/acsanm.0c00347 [7] HE L, ZHANG Y, ZHENG Y, et al. Degradation of tetracycline by a novel MIL-101(Fe)/TiO2 composite with persulfate [J]. Journal of Porous Materials, 2019, 26(6): 1839-1850. doi: 10.1007/s10934-019-00778-y [8] BENSON J J, SAKKOS J K, RADIAN A, et al. Enhanced biodegradation of atrazine by bacteria encapsulated in organically modified silica gels [J]. Journal of Colloid and Interface Science, 2018, 510: 57-68. doi: 10.1016/j.jcis.2017.09.044 [9] WANG J, ZHANG Y, WANG Y, et al. Bimetallic Ce-UiO-66-NH2/diatomite (CUD) self-assembled membrane simultaneously with synergetic effect of phase equilibrium and rate separation [J]. Journal of Membrane Science, 2020, 598: 117730. doi: 10.1016/j.memsci.2019.117730 [10] NEHRA M, DILBAGHI N, SINGHAL N K, et al. Metal organic frameworks MIL-100(Fe) as an efficient adsorptive material for phosphate management [J]. Environmental Research, 2019, 169: 229-236. doi: 10.1016/j.envres.2018.11.013 [11] WANG D, JIA F, WANG H, et al. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs [J]. Journal of Colloid and Interface Science, 2018, 519: 273-284. doi: 10.1016/j.jcis.2018.02.067 [12] ZHANG N, ISHAG A, LI Y, et al. Recent investigations and progress in environmental remediation by using covalent organic framework-based adsorption method: A review [J]. Journal of Cleaner Production, 2020, 277: 123360. doi: 10.1016/j.jclepro.2020.123360 [13] BHATNAGAR A, SILLANPAA M. Removal of natural organic matter (NOM) and its constituents from water by adsorption - A review [J]. Chemosphere, 2017, 166: 497-510. doi: 10.1016/j.chemosphere.2016.09.098 [14] JIANG N, SHANG R, HEIJMAN S G J, et al. Adsorption of triclosan, trichlorophenol and phenol by high-silica zeolites: Adsorption efficiencies and mechanisms [J]. Separation and Purification Technology, 2019, 235: 116152. [15] LYU Y, LIU X, LIU W, et al. Adsorption/oxidation of ethyl mercaptan on Fe-N-modified active carbon catalyst [J]. Chemical Engineering Journal, 2020, 393: 124680. doi: 10.1016/j.cej.2020.124680 [16] KUMAR P, BANSAK V, KIM K, et al. Metal-organic frameworks (MOFs) as futuristic options for wastewater treatment [J]. Journal of Industrial and Engineering Chemistry, 2018, 62: 130-145. doi: 10.1016/j.jiec.2017.12.051 [17] KUMAR P, KIM K, LEE J, et al. Metal-organic framework for sorptive/catalytic removal and sensing applications against nitroaromatic compounds [J]. Journal of Industrial and Engineering Chemistry, 2020, 84: 87-95. doi: 10.1016/j.jiec.2019.12.024 [18] XU W, HUSSAIN A, LIU Y. A review on modification methods of adsorbents for elemental mercury from flue gas [J]. Chemical Engineering Journal (Lausanne, Switzerland:1996), 2018, 346: 692-711. [19] ZHAO R, MA T, ZHAO S, et al. Uniform and stable immobilization of metal-organic frameworks into chitosan matrix for enhanced tetracycline removal from water [J]. Chemical Engineering Journal (Lausanne, Switzerland:1996), 2020, 382: 122893. [20] MU R, LIU B, CHEN X, et al. Hydrogel adsorbent in industrial wastewater treatment and ecological environment protection [J]. Environmental Technology & Innovation, 2020, 20: 101107. [21] LU T, ZHU Y, QI Y, et al. Tunable superporous magnetic adsorbent prepared via eco-friendly Pickering MIPEs for high-efficiency adsorption of Rb+ and Sr2+ [J]. Chemical Engineering Journal (Lausanne, Switzerland:1996), 2019, 368: 988-998. [22] ROCIO-BAUTISTA P, MARTINEZ-BENITO C, PINO V, et al. The metal-organic framework HKUST-1 as efficient sorbent in a vortex-assisted dispersive micro solid-phase extraction of parabens from environmental waters, cosmetic creams, and human urine [J]. Talanta, 2015, 139: 13-20. doi: 10.1016/j.talanta.2015.02.032 [23] ISLAMOGLU T, CHEN Z, WASSON M C, et al. Metal–Organic Frameworks against toxic chemicals [J]. Chemical Reviews, 2020, 120(16): 8130-8160. doi: 10.1021/acs.chemrev.9b00828 [24] RASHEEDT, BILAL M, HASSAN A A, et al. Environmental threatening concern and efficient removal of pharmaceutically active compounds using metal-organic frameworks as adsorbents [J]. Environmental Research, 2020, 185: 109436. doi: 10.1016/j.envres.2020.109436 [25] JOSEPH L, JUN B, JANG M, et al. Removal of contaminants of emerging concern by metal-organic framework nanoadsorbents: A review [J]. Chemical Engineering Journal, 2019, 369: 928-946. doi: 10.1016/j.cej.2019.03.173 [26] KITAO T, ZHANG Y, KITAGAWA S, et al. Hybridization of MOFs and polymers [J]. Chemical Society Reviews, 2017, 46(11): 3108-3133. doi: 10.1039/C7CS00041C [27] KARMAKAR A, PRABAKARAN V, ZHAO D, et al. A review of metal-organic frameworks (MOFs) as energy-efficient desiccants for adsorption driven heat-transformation applications [J]. Applied Energy, 2020, 269: 115070. doi: 10.1016/j.apenergy.2020.115070 [28] JERME C, FATEEVA A, GUO Y, et al. Water adsorption in MOFs: Fundamentals and applications [J]. Chemical Society Reviews, 2014, 43(16): 5594-5617. doi: 10.1039/C4CS00078A [29] 崔继方, 崔文权. 金属有机骨架材料在光催化领域的应用研究进展 [J]. 陶瓷, 2019, 11: 65-70. doi: 10.3969/j.issn.1002-2872.2019.11.008 CUI J F, CUI W Q. Advance of metal organic framework material in catalysis application [J]. Ceramic, 2019, 11: 65-70(in Chinese). doi: 10.3969/j.issn.1002-2872.2019.11.008
[30] 张贺. 金属有机骨架材料在吸附分离研究中的应用进展 [J]. 化学学报, 2017, 75: 841-859. doi: 10.6023/A17040168 ZHANG H, LI G, ZHANG K. Advances of metal-organic frameworks in adsorption and separation applications [J]. Acta Chimica. Sinica, 2017, 75: 841-859(in Chinese). doi: 10.6023/A17040168
[31] ZHONG W, LI R, LV J, et al. Two isomeric In(Ⅲ)-MOFs: unexpected stability difference and selective fluorescence detection of fluoroquinolone antibiotics in water [J]. Inorganic Chemistry Frontiers, 2020, 7(5): 1161-1171. doi: 10.1039/C9QI01490J [32] 赵玲, 刘恒恒, 胡晴, 等. 金属有机骨架材料MOF-5催化吸附SO2 [J]. 环境化学, 2017, 36(9): 1914-1922. doi: 10.7524/j.issn.0254-6108.2016110901 ZHAO L, LIU H H, HU Q, et al. Synthesis of MOF-5 catalysts and their catalytic oxidation of sulfur dioxide [J]. Environmental Chemistry, 2017, 36(9): 1914-1922(in Chinese). doi: 10.7524/j.issn.0254-6108.2016110901
[33] HORCAJADA P, CHALATI T, SERRE C, et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging [J]. Nature Materials, 2009, 9(2): 172-178. [34] YANG X, XU Q. Bimetallic metal-organic frameworks for gas storage and separation [J]. Crystal Growth & Design, 2017, 17(4): 1450-1455. [35] RAMASWAMY P, WONG N E, SHIMIZU G K. MOFs as proton conductors-challenges and opportunities [J]. Chem Soc Rev, 2014, 43(16): 5913-5932. doi: 10.1039/C4CS00093E [36] LI J, SCULLEY J, ZHOU H. Metal-organic frameworks for separations [J]. Chemical Reviews, 2011, 112(2): 869-932. [37] BANGARI R S, SINHA N. Adsorption of tetracycline, ofloxacin and cephalexin antibiotics on boron nitride nanosheets from aqueous solution [J]. Journal of Molecular Liquids, 2019, 293: 111376. doi: 10.1016/j.molliq.2019.111376 [38] ANTONELLI R, MARTINS F R, MALPASS G R P, et al. Ofloxacin adsorption by calcined Verde-lodo bentonite clay: Batch and fixed bed system evaluation [J]. Journal of Molecular Liquids, 2020, 315: 113718. doi: 10.1016/j.molliq.2020.113718 [39] GUO X, KANG C, HUANG H, et al. Exploration of functional MOFs for efficient removal of fluoroquinolone antibiotics from water [J]. Microporous and Mesoporous Materials, 2019, 286: 84-91. doi: 10.1016/j.micromeso.2019.05.025 [40] YU R, WU Z. High adsorption for ofloxacin and reusability by the use of ZIF-8 for wastewater treatment [J]. Microporous and Mesoporous Materials, 2020, 308: 110494. doi: 10.1016/j.micromeso.2020.110494 [41] WEI H, DENG S, HUANG Q, et al. Regenerable granular carbon nanotubes/alumina hybrid adsorbents for diclofenac sodium and carbamazepine removal from aqueous solution [J]. Water Res, 2013, 47(12): 4139-4147. doi: 10.1016/j.watres.2012.11.062 [42] HASAN Z, KHAN N A, JHUNG S H. Adsorptive removal of diclofenac sodium from water with Zr-based metal-organic frameworks [J]. Chemical Engineering Journal, 2016, 284: 1406-1413. doi: 10.1016/j.cej.2015.08.087 [43] WEN J, HAN X, LIN H, et al. A critical study on the adsorption of heterocyclic sulfur and nitrogen compounds by activated carbon: Equilibrium, kinetics and thermodynamics [J]. Chemical Engineering Journal (Lausanne, Switzerland:1996), 2010, 164(1): 29-36. [44] AHMED I, JHUNG S H. Remarkable adsorptive removal of nitrogen-containing compounds from a model fuel by a graphene oxide/MIL-101 composite through a combined effect of improved porosity and hydrogen bonding [J]. J Hazard Mater, 2016, 314: 318-325. doi: 10.1016/j.jhazmat.2016.04.041 [45] LI F, WANG Y, YANG Q, et al. Study on adsorption of glyphosate (N-phosphonomethyl glycine) pesticide on MgAl-layered double hydroxides in aqueous solution [J]. J. Hazard Mater, 2005, 125: 89-95. doi: 10.1016/j.jhazmat.2005.04.037 [46] KHOURY G A, GEHRIS T C,TRIBE L, et al. Glyphosate adsorption on montmorillonite: an experimental and theoretical study of surface complexes [J]. Appl Clay Sci, 2010, 50: 167-175. doi: 10.1016/j.clay.2010.07.018 [47] HU Y S, ZHAO Y Q, SOROHAN B. Removal of glyphosate from aqueous environment by adsorption using water industrial residual [J]. Desalination, 2011, 271: 150-156. doi: 10.1016/j.desal.2010.12.014 [48] ZHU X, LI B, YANG J, et al. Effective adsorption and enhanced removal of organophosphorus pesticides from aqueous solution by Zr-Based MOFs of UiO-67 [J]. ACS Applied Materials & Interfaces, 2014, 7(1): 223-231. [49] LIU Y, XU J, CAO Z, et al. Adsorption behavior and mechanism of Pb(Ⅱ) and complex Cu(Ⅱ) species by biowaste-derived char with amino functionalization [J]. Journal of Colloid and Interface Science, 2020, 559: 215-225. doi: 10.1016/j.jcis.2019.10.035 [50] WAN X, KHAN M A, WANG F, et al. Facile synthesis of protonated g-C3N4 and acid-activated montmorillonite composite with efficient adsorption capacity for PO43- and Pb(Ⅱ) [J]. Chemical Engineering Research & Design, 2019, 152: 95-105. [51] WANG J, LI Y, LV Z, et al. Exploration of the adsorption performance and mechanism of zeolitic imidazolate framework-8@graphene oxide for Pb(Ⅱ) and 1-naphthylamine from aqueous solution [J]. J Colloid Interface Sci, 2019, 542: 410-420. doi: 10.1016/j.jcis.2019.02.039 [52] DALI Y L, BELAROUI L S, LOPEZ-GALINDO A. Adsorption of a cationic methylene blue dye on an Algerian palygorskite [J]. Applied Clay Science, 2019, 179: 105145. doi: 10.1016/j.clay.2019.105145 [53] KHANDAY W A, ASIF M, HAMEED B H. Cross-linked beads of activated oil palm ash zeolite/chitosan composite as a bio-adsorbent for the removal of methylene blue and acid blue 29 dyes [J]. Int J Biol Macromol, 2017, 95: 895-902. doi: 10.1016/j.ijbiomac.2016.10.075 [54] PAIMAN S H, RAHMAN M A, UCHIKOSHI T, et al. Functionalization effect of Fe-type MOF for methylene blue adsorption [J]. Journal of Saudi Chemical Society, 2020, 24(11): 896-905. doi: 10.1016/j.jscs.2020.09.006 [55] 张爱琴, 郭斌, 柳利龙. 荧光金属有机骨架材料在离子检测中的应用[J]. 广州化工, 2020, 48(24): 20-23. ZHANG A Q, GUO B, LIU L L. Application of luminescent metal-organic frameworks in ions detection[J] Guangzhou Chemical Industry, 2020, 48(24): 20-23(in Chinese).
[56] GAO Y, LIU G, GAO M, et al. Recent advances and applications of magnetic metal-organic frameworks in adsorption and enrichment removal of food and environmental pollutants [J]. Critical Reviews in Analytical Chemistry, 2019, 50(5): 1-13. [57] AHMED I, BHADRA B N, LEE H J, et al. Metal-organic framework-derived carbons: Preparation from ZIF-8 and application in the adsorptive removal of sulfamethoxazole from water [J]. Catalysis Today, 2018, 301: 90-97. doi: 10.1016/j.cattod.2017.02.011 [58] SARKER M, AHMED I, JHUNG S H. Adsorptive removal of herbicides from water over nitrogen-doped carbon obtained from ionic liquid@ZIF-8 [J]. Chemical Engineering Journal, 2017, 323: 203-211. doi: 10.1016/j.cej.2017.04.103 [59] WANG X, MA X, WANG H, et al. A zinc(II) benzene tricarboxylate metal organic framework with unusual adsorption properties, and its application to the preconcentration of pesticides [J]. Microchimica Acta, 2017, 184(10): 3681-3687. doi: 10.1007/s00604-017-2382-1 [60] DUO H, LU X, WANG S, et al. Synthesis of magnetic metal–organic framework composites, Fe3O4-NH2@MOF-235, for the magnetic solid-phase extraction of benzoylurea insecticides from honey, fruit juice and tap water samples [J]. New Journal of Chemistry, 2019, 43(32): 12563-12569. doi: 10.1039/C9NJ01988J [61] JIA Y, ZHANG Y, XU J, et al. A high-performance “sweeper” for toxic cationic herbicides: An anionic metal–organic framework with a tetrapodal cage [J]. Chemical Communications, 2015, 51(98): 17439-17442. doi: 10.1039/C5CC07249B [62] HASAN Z, CHOI E, JHUNG S H. Adsorption of naproxen and clofibric acid over a metal–organic framework MIL-101 functionalized with acidic and basic groups [J]. Chemical Engineering Journal, 2013, 219: 537-544. doi: 10.1016/j.cej.2013.01.002 [63] ABDELHAMEED R M, ABDEL-GAWAD H, ELSHAHAT M, et al. Cu-BTC@cotton composite: design and removal of ethion insecticide from water [J]. RSC Advances, 2016, 6(48): 42324-42333. doi: 10.1039/C6RA04719J [64] JAMALI A, SHEMIRANI F, MORSALI A. A comparative study of adsorption and removal of organophosphorus insecticides from aqueous solution by Zr-based MOFs [J]. Journal of Industrial and Engineering Chemistry, 2019, 80: 83-92. doi: 10.1016/j.jiec.2019.07.034 [65] BHADEA B N, CHO K H, KHAN N A, et al. Liquid-Phase adsorption of aromatics over a metal-organic framework and activated carbon: effects of hydrophobicity/hydrophilicity of adsorbents and solvent polarity [J]. The Journal of Physical Chemistry C, 2015, 119(47): 26620-26627. doi: 10.1021/acs.jpcc.5b09298 [66] JIANG J, YANG C, YAN X. Zeolitic Imidazolate Framework-8 for Fast Adsorption and Removal of Benzotriazoles from Aqueous Solution [J]. ACS Applied Materials & Interfaces, 2013, 5(19): 9837-9842. [67] AZHAR M R, ABID H R, SUN H, et al. Excellent performance of copper-based metal organic framework in adsorptive removal of toxic sulfonamide antibiotics from wastewater [J]. Journal of Colloid and Interface Science, 2016, 478: 344-352. doi: 10.1016/j.jcis.2016.06.032 [68] WANG X, MA X, HUANG P, et al. Magnetic Cu-MOFs embedded within graphene oxide nanocomposites for enhanced preconcentration of benzenoid-containing insecticides [J]. Talanta, 2018, 181: 112-117. doi: 10.1016/j.talanta.2018.01.004 [69] XU Z, WEN Y, TIAN L, et al. Efficient and selective adsorption of nitroaromatic explosives by Zr-MOF [J]. Inorganic Chemistry Communications, 2017, 77: 11-13. doi: 10.1016/j.inoche.2017.01.025 [70] HASAN Z, JEON J, JHUNG S H. Adsorptive removal of naproxen and clofibric acid from water using metal-organic frameworks [J]. Journal of Hazardous Materials, 2012, 209-210: 151-157. doi: 10.1016/j.jhazmat.2012.01.005 [71] WANG H, YUAN X, WU Y, et al. In situ synthesis of In2S3@MIL-125(Ti) core-shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis [J]. Applied Catalysis B:Environmental, 2016, 186: 19-29. doi: 10.1016/j.apcatb.2015.12.041 [72] LI N, ZHOU L, JIN X, et al. Simultaneous removal of tetracycline and oxytetracycline antibiotics from wastewater using a ZIF-8 metal organic-framework [J]. Journal of Hazardous Materials, 2019, 366: 563-572. doi: 10.1016/j.jhazmat.2018.12.047 [73] KHAN N A, JUNG B K, HASAN Z, et al. Adsorption and removal of phthalic acid and diethyl phthalate from water with zeolitic imidazolate and metal-organic frameworks [J]. Journal of Hazardous Materials, 2015, 282: 194-200. doi: 10.1016/j.jhazmat.2014.03.047 [74] ABDWLHAMEED R M, TAHA M, ABDEL-GAWAD H, et al. Zeolitic imidazolate frameworks: Experimental and molecular simulation studies for efficient capture of pesticides from wastewater [J]. Journal of Environmental Chemical Engineering, 2019, 7(6): 103499. doi: 10.1016/j.jece.2019.103499 [75] WANG B, LV X, FENG D, et al. Highly stable Zr(IV)-based metal-organic frameworks for the detection and removal of antibiotics and organic explosives in water [J]. Journal of the American Chemical Society, 2016, 138(19): 6204-6216. doi: 10.1021/jacs.6b01663 [76] SONG J Y, JHUNG S H. Adsorption of pharmaceuticals and personal care products over metal-organic frameworks functionalized with hydroxyl groups: Quantitative analyses of H-bonding in adsorption [J]. Chemical Engineering Journal, 2017, 322: 366-374. doi: 10.1016/j.cej.2017.04.036 [77] PAN Y, LI Z, ZHANG Z, et al. Adsorptive removal of phenol from aqueous solution with zeolitic imidazolate framework-67 [J]. Journal of Environmental Management, 2016, 169: 167-173. [78] LIU K, ZHANG S, HU X, et al. Understanding the adsorption of PFOA on MIL-101(Cr)-Based Anionic-Exchange Metal–Organic Frameworks: Comparing DFT Calculations with Aqueous Sorption Experiments [J]. Environmental Science & Technology, 2015, 49(14): 8657-8665. [79] SARKER M, BHADRA B N, SEO P W, et al. Adsorption of benzotriazole and benzimidazole from water over a Co-based metal azolate framework MAF-5(Co) [J]. Journal of Hazardous Materials, 2017, 324: 131-138. doi: 10.1016/j.jhazmat.2016.10.042 [80] JUN J W, TONG M, JUNG B K, et al. Effect of central metal ions of analogous metal-organic frameworks on adsorption of organoarsenic compounds from water: Plausible mechanism of adsorption and water purification [J]. Chemistry - A European Journal, 2015, 21(1): 347-354. doi: 10.1002/chem.201404658 [81] SEO Y S, KHAN N A, JHUNG S H. Adsorptive removal of methylchlorophenoxypropionic acid from water with a metal-organic framework [J]. Chemical Engineering Journal, 2015, 270: 22-27. doi: 10.1016/j.cej.2015.02.007 [82] ARIS A Z, MOHD HIR A, RAZAK M R. Metal-organic frameworks (MOFs) for the adsorptive removal of selected endocrine disrupting compounds (EDCs) from aqueous solution: A review [J]. Applied Materials Today, 2020, 21: 100796. doi: 10.1016/j.apmt.2020.100796 [83] LI J, WANG X, ZHAO G, et al. Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions [J]. Chemical Society Reviews, 2018, 47(7): 2322-2356. doi: 10.1039/C7CS00543A [84] WANG Y, ZHAO W, QI Z, et al. Designing ZIF-8/hydroxylated MWCNT nanocomposites for phosphate adsorption from water: Capability and mechanism [J]. Chemical Engineering Journal, 2020, 394: 124992. doi: 10.1016/j.cej.2020.124992 [85] GUO Y, JIN H, QI Z, et al. Carbonized-MOF as a sulfur host for aluminums Sulfur batteries with enhanced capacity and cycling life [J]. Advanced Functional Materials, 2019, 29(7): 1807676. [86] PANG Y, ZANG X, LI H, et al. Solid-phase microextraction of organophosphorus pesticides from food samples with a nitrogen-doped porous carbon derived from g-C3N4 templated MOF as the fiber coating [J]. Journal of Hazardous Materials, 2020, 384: 121430. doi: 10.1016/j.jhazmat.2019.121430 [87] LIU X, WANG C, WANG Z, et al. Nanoporous carbon derived from a metal organic framework as a new kind of adsorbent for dispersive solid phase extraction of benzoylurea insecticides [J]. Microchemical Acta, 2015, 182(11-12): 1903-1910. doi: 10.1007/s00604-015-1530-8 [88] ABDELILLAH A E E, ŞAHIN S, BAYAZIT Ş S. Preparation of CeO2 nanofibers derived from Ce-BTC metal-organic frameworks and its application on pesticide adsorption [J]. Journal of Molecular Liquids, 2018, 255: 10-17. doi: 10.1016/j.molliq.2018.01.165 [89] YU J, MU C, YAN B, et al. Nanoparticle/MOF composites: Preparations and applications [J]. Materials Horizons, 2017, 4(4): 557-569. doi: 10.1039/C6MH00586A [90] YANG Q, WANG J, ZHANG W, et al. Interface engineering of metal organic framework on graphene oxide with enhanced adsorption capacity for organophosphorus pesticide [J]. Chemical Engineering Journal, 2017, 313: 19-26. doi: 10.1016/j.cej.2016.12.041 [91] LIU G, HUANG X, LU M, et al. Facile synthesis of magnetic zinc metal-organic framework for extraction of N-containing heterocyclic fungicides from lettuce vegetable samples [J]. Journal of Separation Science, 2019, 42(7): 1451-1458. doi: 10.1002/jssc.201801169 [92] LIU G, LI L, GAO Y, et al. A beta-cyclodextrin-functionalized magnetic metal organic framework for efficient extraction and determination of prochloraz and triazole fungicides in vegetables samples [J]. Ecotoxicology and Environmental Safety, 2019, 183: 109546. doi: 10.1016/j.ecoenv.2019.109546 [93] YANG Q, ZHAO Q, REN S, et al. Assembly of Zr-MOF crystals onto magnetic beads as a highly adsorbent for recycling nitrophenol [J]. Chemical Engineering Journal, 2017, 323: 74-83. doi: 10.1016/j.cej.2017.04.091 [94] JIANG Y, MA P, PIAO H, et al. Solid-phase microextraction of triazine herbicides via cellulose paper coated with a metal-organic framework of type MIL-101(Cr), and their quantitation by HPLC-MS [J]. Microchimica Acta, 2019, 186(11): 1-8. [95] GANGU K K, MADDILA S, MUKKAMALA S B, et al. Characteristics of MOF, MWCNT and graphene containing materials for hydrogen storage: A review [J]. Journal of Energy Chemistry, 2019, 30: 132-144. doi: 10.1016/j.jechem.2018.04.012 [96] LIU G, LI L, HUANG X, et al. Adsorption and removal of organophosphorus pesticides from environmental water and soil samples by using magnetic multi-walled carbon nanotubes@organic framework ZIF-8 [J]. Journal of Materials Science, 2018, 53(15): 10772-10783. doi: 10.1007/s10853-018-2352-y [97] NIU M, LI Z, HE W, et al. Attapulgite modified magnetic metal-organic frameworks for magnetic solid phase extraction and determinations of benzoylurea insecticides in tea infusions [J]. Food Chemistry, 2020, 317: 126425-126425. doi: 10.1016/j.foodchem.2020.126425 [98] ZHANG R, WANG Z, ZHOU Z, et al. Highly effective removal of pharmaceutical compounds from aqueous solution by magnetic Zr-Based MOFs composites [J]. Industrial & Engineering Chemistry Research, 2019, 58(9): 3876-3884. [99] SEO P W, AHMED I, JHUNG S H. Adsorptive removal of nitrogen-containing compounds from a model fuel using a metal–organic framework having a free carboxylic acid group [J]. Chemical Engineering Journal, 2016, 299: 236-243. doi: 10.1016/j.cej.2016.04.060 [100] AHMED I, KHAN N A, JHUNG S H. Adsorptive denitrogenation of model fuel by functionalized UiO-66 with acidic and basic moieties [J]. Chemical Engineering Journal, 2017, 321: 40-47. doi: 10.1016/j.cej.2017.03.093 [101] SHIN S, SARKER M, LEE H, et al. Metal-organic framework with various functional groups: Remarkable adsorbent for removal of both neutral indole and basic quinoline from liquid fuel [J]. Chemical Engineering Journal, 2019, 370: 1467-1473. doi: 10.1016/j.cej.2019.03.290 [102] LV Y, ZHANG R, ZENG S, et al. Removal of p-arsanilic acid by an amino-functionalized indium-based metal–organic framework: Adsorption behavior and synergetic mechanism [J]. Chemical Engineering Journal, 2018, 339: 359-368. doi: 10.1016/j.cej.2018.01.139 [103] LIU B, YANG F, ZOU Y, et al. Adsorption of phenol and p-nitrophenol from aqueous solutions on metal-organic frameworks: Effect of hydrogen bonding [J]. Journal of Chemical & Engineering Data, 2014, 59(5): 1476-1482. [104] AKPINAR I, DROUT R J, ISLAMOGLU T, et al. Exploiting π–π interactions to design an efficient sorbent for atrazine removal from water [J]. ACS Applied Materials & Interfaces, 2019, 11(6): 6097-6103. [105] SILVA B C E , IRIKURA K , REGINA C G F, et al. Effect of Cu(BDC-NH2) MOF deposited on Cu/Cu2O electrode and its better performance in photoelectrocatalytic reduction of CO2 [J]. Journal of Electroanalytical Chemistry, 2020, 880: 114856. doi: 10.1016/j.jelechem.2020.114856 [106] CAI Z, BIEN C E, LIU Q, et al. Insights into CO2 adsorption in M-OH functionalized MOFs [J]. Chemistry of materials, 2020, 32(10): 4257-4264. doi: 10.1021/acs.chemmater.0c00746 [107] ZHANG X, WANG J, DONG X X, et al. Functionalized metal-organic frameworks for photocatalytic degradation of organic pollutants in environment [J]. Chemosphere, 2020, 242(Mar.): 125144.1-125144.15. [108] MALLICK A, EL-ZOHRY A M, SHEKHAH O, et al. Unprecedented ultralow detection limit of amines using a Thiadiazole-Functionalized Zr(Ⅳ)-Based metal-organic framework [J]. Journal of the American Chemical Society, 2019, 141(18): 7245-7249. doi: 10.1021/jacs.9b01839 [109] CUI Y, ZHU F, CHEN B, et al. Metal-organic frameworks for luminescence thermometry [J]. Chem Commun (Camb), 2015, 51(35): 7420-7431. doi: 10.1039/C5CC00718F [110] LUSTUG W, MUKHERJEE S, RUDD N, et al. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications [J]. Chemical Society Reviews, 2017, 46(11): 3242-3285. doi: 10.1039/C6CS00930A [111] HU Z, TAN K, LUSTIG W P, et al. Effective sensing of RDX via instant and selective detection of ketone vapors [J]. Chemical Science (Cambridge), 2014, 5(12): 4873-4877. [112] ZHU X, ZHANG K, WANG Y, et al. Fluorescent metal-organic framework (MOF) as a highly sensitive and quickly responsive chemical sensor for the detection of antibiotics in simulated wastewater [J]. Inorganic Chemistry, 2018, 57(3): 1060-1065. doi: 10.1021/acs.inorgchem.7b02471 [113] HAN M, WEN G, DONG W, et al. A heterometallic sodium–europium-cluster-based metal–organic framework as a versatile and water-stable chemosensor for antibiotics and explosives [J]. Journal of Materials Chemistry C, Materials for Optical and Electronic Devices, 2017, 5(33): 8469-8474. doi: 10.1039/C7TC02885G [114] ZHOU Y, YANG Q, ZHANG D, et al. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF [J]. Sensors and Actuators B:Chemical, 2018, 262: 137-143. doi: 10.1016/j.snb.2018.01.218 [115] ZHANG F, YAO H, CHU T, et al. A lanthanide MOF thin-film fixed with Co3O4 nano-anchors as a highly efficient luminescent sensor for nitrofuran antibiotics [J]. Chemistry, 2017, 23(43): 10293-10300. doi: 10.1002/chem.201701852 [116] HE K, LI Z, WANG L, et al. A water-stable luminescent metal-organic framework for rapid and visible sensing of organophosphorus pesticides [J]. ACS Applied Materials & Interfaces, 2019, 11(29): 26250-26260. [117] SINGHA D K, MAJEE P, MONDAL S K, et al. Detection of pesticide using the large stokes shift of luminescence of a mixed lanthanide co-doped metal–organic framework [J]. Polyhedron, 2019, 158: 277-282. doi: 10.1016/j.poly.2018.10.066 [118] LAN A, LI K, WU H, et al. A luminescent microporous metal–organic framework for the fast and reversible detection of high explosives [J]. Angewandte Chemie International Edition, 2009, 48(13): 2334-2338. doi: 10.1002/anie.200804853 [119] WANG X S, LI L, YUAN D Q, et al. Fast highly selective and sensitive anionic metal-organic framework with nitrogen-rich sites fluorescent chemosensor for nitro explosives detection [J]. Journal of Hazardous Materials, 2018, 344: 283-290. doi: 10.1016/j.jhazmat.2017.10.027 [120] WENG H, Yan B. A flexible Tb (Ⅲ) functionalized cadmium metal organic framework as fluorescent probe for highly selectively sensing ions and organic small molecules [J]. Sensors and Actuators B:Chemical, 2016, 228: 702-708. doi: 10.1016/j.snb.2016.01.101 [121] JI G, LIU J, GAO X, et al. A luminescent lanthanide MOF for selectively and ultra-high sensitively detecting Pb2+ ions in aqueous solution [J]. Journal of Materials Chemistry A, 2017, 5(21): 10200-10205. doi: 10.1039/C7TA02439H [122] WANG B, WANG P, XIE L H, et al. A stable zirconium-based metal-organic framework for specific recognition of representative polychlorinated dibenzo-p-dioxin molecules [J]. Nature Communications, 2019, 10(1): 1-8. doi: 10.1038/s41467-018-07882-8 [123] XING S, BING Q, QI H, et al. Rational design and functionalization of a zinc metal-organic framework for highly selective detection of 2, 4, 6-Trinitrophenol [J]. ACS Appl Mater Interfaces, 2017, 9(28): 23828-23835. doi: 10.1021/acsami.7b06482 [124] YANG Q, WANG J, CHEN X, et al. The simultaneous detection and removal of organophosphorus pesticides by a novel Zr-MOF based smart adsorbent [J]. Journal of Materials Chemistry A, 2018, 6(5): 2184-2192. doi: 10.1039/C7TA08399H -



 下载:
下载: