-
内分泌干扰物(endocrine disrupting chemicals, EDCs)是继“温室效应”和“臭氧层破坏”之后被世界公认的第三大环境问题[1-2],探究此类物质的去除方法有着重要的科学和现实意义。目前,去除EDCs的方法主要有以下几种:微生物降解法,活性炭吸附法,膜过滤法和光催化降解法[3-4]。与其他几种方法相比,光催化降解法具有成本更低,降解速度更快,降解程度更高等明显优势[5]。作为光催化技术的核心环节,催化剂材料的理化性质直接制约着光催化技术的适用范围和应用效果。在众多被研究的半导体催化剂材料中,TiO2因其廉价、易得、环境友好等优势受到了人们的广泛关注[6-7]。然而,由于TiO2固有的宽带隙特征,其在光催化过程中只能依靠紫外光激发而无法利用可见光,严重限制了其实际应用发展[8]。为了克服这一问题,多种制备和调控策略被尝试,如形貌调控、晶面取向控制、掺杂等[9]。在这其中,构建复合材料的方法被广泛使用。不久前,Dong课题组将CdS引入TiO2体系,成功将催化剂的光响应范围从紫外光区拓展至可见光区,并在可见光照射下实现了对4-氯芬的高效降解[10];Du等将AgInS2引入TiO2体系,构建了AgInS2/TiO2复合型催化剂,其在可见光照射下展现了良好的催化杀菌效果[11]。一系列研究结果表明,构建复合材料是拓宽TiO2光响应范围、获得基于可见光驱动的TiO2基催化材料的有效方法。
相比于常见的过渡金属氧化物和硫化物等无机材料,不含金属的石墨相氮化碳(g-C3N4)因拥有更为低廉的价格、良好的催化活性以及优异的结构稳定性得到了科研工作者更多青睐[12]。近年来,一些TiO2与g-C3N4的复合材料被相继报道,如Li等通过控制退火温度,构筑了一系列g-C3N4-TiO2复合材料,证实其能够在可见光照射下高效分解水中丙烯[13];Huang等将锐钛矿型TiO2沉积在g-C3N4纳米片表面,制备了能够在可见光驱动下快速降解恩诺沙星的复合型催化材料[14]。然而,当前复合材料的制备过程大多使用化学复合方法,如水热、溶剂热、煅烧等[15-17],尽管能够满足复合材料的制备需求,但高能耗以及难以精确控制各组分间的比例的缺点始终存在,这些问题往往会造成能源与原材料的大量浪费,这在注重绿色合成的今天是需要改善的。
本研究采用简便的机械混合处理工艺,将TiO2纳米颗粒与不同质量的g-C3N4进行复合,成功制备了一系列TiO2/g-C3N4复合型材料。利用扫描电子显微镜(SEM)、透射电子显微镜(TEM)、X射线粉末衍射(XRD)以及X射线光电子能谱(XPS)等技术对所合成材料进行了表征。以典型EDCs双酚A(BPA)为污染物分子模型,在最优条件下研究了TiO2/g-C3N4对BPA的可见光降解活性,并分析了催化降解机制。本项研究不仅实现了复合材料制备的低能耗与组分比例的精准合成,而且证实了机械混合法可以得到结构均匀且具有良好稳定性的复合材料。
-
三聚氰胺、六次甲基四胺、聚乙烯醇、钛酸四丁酯、无水乙醇(分析纯)均购置于国药集团化学试剂有限公司;双酚A(BPA,分析纯),购置于天津市光复精细化工研究所。
-
分别称取3.0 g三聚氰胺和1.8 g六次甲基四胺,至于玛瑙研钵中,研磨20 min,使两种材料混合均匀,将混合物置于坩埚中,在550 ℃下煅烧4 h,升温速率为2.5 ℃·min−1,降温速率为5.5 ℃·min−1,当降温至室温时,得到石墨相氮化碳(后用g-C3N4表示)。
-
称取5 mL钛酸四丁酯和0.20 g聚乙烯醇置于25 mL去离子水中,超声分散1 h,得到钛酸四丁酯分散液;将溶液转移至30 mL高温反应釜中,160 ℃下加热10 h,自然冷却至室温,得到固体粉末样品,分别用蒸馏水和无水乙醇离心洗涤粉末样品各3次,随后将样品在60 ℃下干燥2 h,得到TiO2纳米颗粒。
-
分别称取TiO2纳米颗粒1.0 g和不同质量的g-C3N4粉末(0.05、0.08、0.12、0.20 g)置于玛瑙研钵中,研磨2 h,即可得到TiO2/g-C3N4复合材料。根据g-C3N4投料量的不同,将得到的复合材料分别命名为:TiO2/g-C3N4-A(g-C3N4投料0.05 g);TiO2/g-C3N4-B(g-C3N4投料0.08 g);TiO2/g-C3N4-C(g-C3N4投料0.12 g);TiO2/g-C3N4-D(g-C3N4投料0.20 g)。
-
催化剂的化学组成和结构信息通过X射线粉末衍射(XRD,XRD-6100,日本Shimadzu)、X射线光电子能谱(XPS,PHI-5700 ESCA,美国PerkinElmer)和拉曼光谱(Raman,XploRA PLUS,美国HORIBA Scientific)确定;形貌特征通过扫描电子显微镜(SEM,Nova Nano SEM 460,美国FEI)和透射电子显微镜(TEM,Talos F200X,美国FEI)确定;比表面积和孔结构通过N2吸附仪(NOVA 4000e,美国Quantachrome)测定;光催化反应前后的结构特征和表面官能团通过红外光谱(IR,Frontier Mid-IR FTIR)确定;催化剂的光响应特征和带隙结构通过紫外可见吸收光谱(UV-Vis,Lambda 750 UV/VIS/NIR,美国PerkinElmer)确定;光生载流子分离活性和动力学行为通过荧光光谱(PL,fluorolog-3-tau,美国Jobin Yvon)确定。
-
所使用反应装置为光催化反应仪(南京胥江机电厂,中国),光源为800 W氙灯(波长≥ 420 nm)。取50 mg催化剂样品置于50 mL含有BPA(浓度为2.28 mg·L−1)溶液中,磁力搅拌,在黑暗条件下暗处理1 h,达到吸附平衡。随后打开光源,开始光催化降解反应,每间隔1 h取5 mL反应溶液,离心过滤催化剂,再将溶液滤过0.45 μm滤膜,测试过滤后溶液中的BPA浓度。通过比较不同反应时间的BPA浓度变化,可以由下面公式计算催化剂的光催化降解效率(η):
其中,C0为BPA的初始浓度,C为降解后的BPA浓度。中间产物浓度通过高效液相色谱-质谱(HPLC-MS,Xevo TQ-S,美国Waters)联用仪确定。
-
将光催化降解BPA反应后的催化剂样品收集,通过蒸馏水和无水乙醇洗涤,将洗涤后的样品在60 ℃下干燥2 h,再次得到TiO2/g-C3N4复合材料。将得到的催化剂再次进行与“1.5节”中相同条件下的光催化降解过程,如此反复3次,考察催化剂的循环催化稳定性。
-
为探究光催化降解BPA过程中不同活性物种的贡献,分别向反应体系中加入1 mmol·L−1的空穴自由基(h+)捕获剂草酸铵、羟基自由基(•OH)捕获剂异丙醇和超氧自由基(
${\rm{O}}_2^{\cdot -} $ )捕获剂超氧化物歧化酶(SOD),考察加入活性物种捕获剂后催化剂对BPA的降解效率变化,从而理清活性物种贡献,阐明催化降解机理。 -
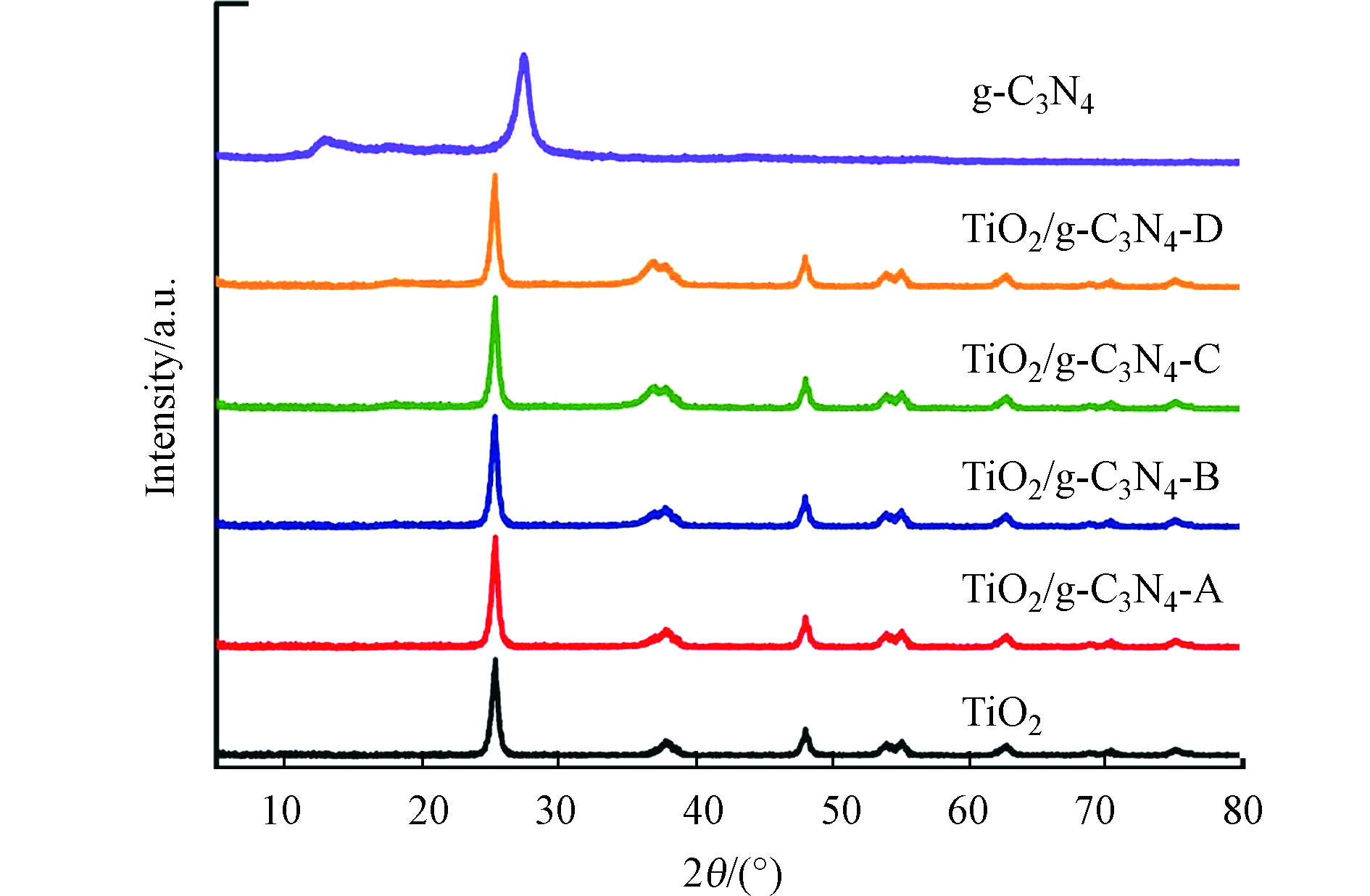
材料的结构组成首先通过XRD测定。如图1所示,一系列TiO2/g-C3N4复合材料保持了与锐钛矿型TiO2相同的衍射峰形状[18],这说明g-C3N4与TiO2的复合过程没有引起TiO2自身结构的变化。值得注意的是,在TiO2/g-C3N4复合材料的XRD谱图中未观察到属于g-C3N4的特征衍射峰,这是由于g-C3N4含量低导致的。
-
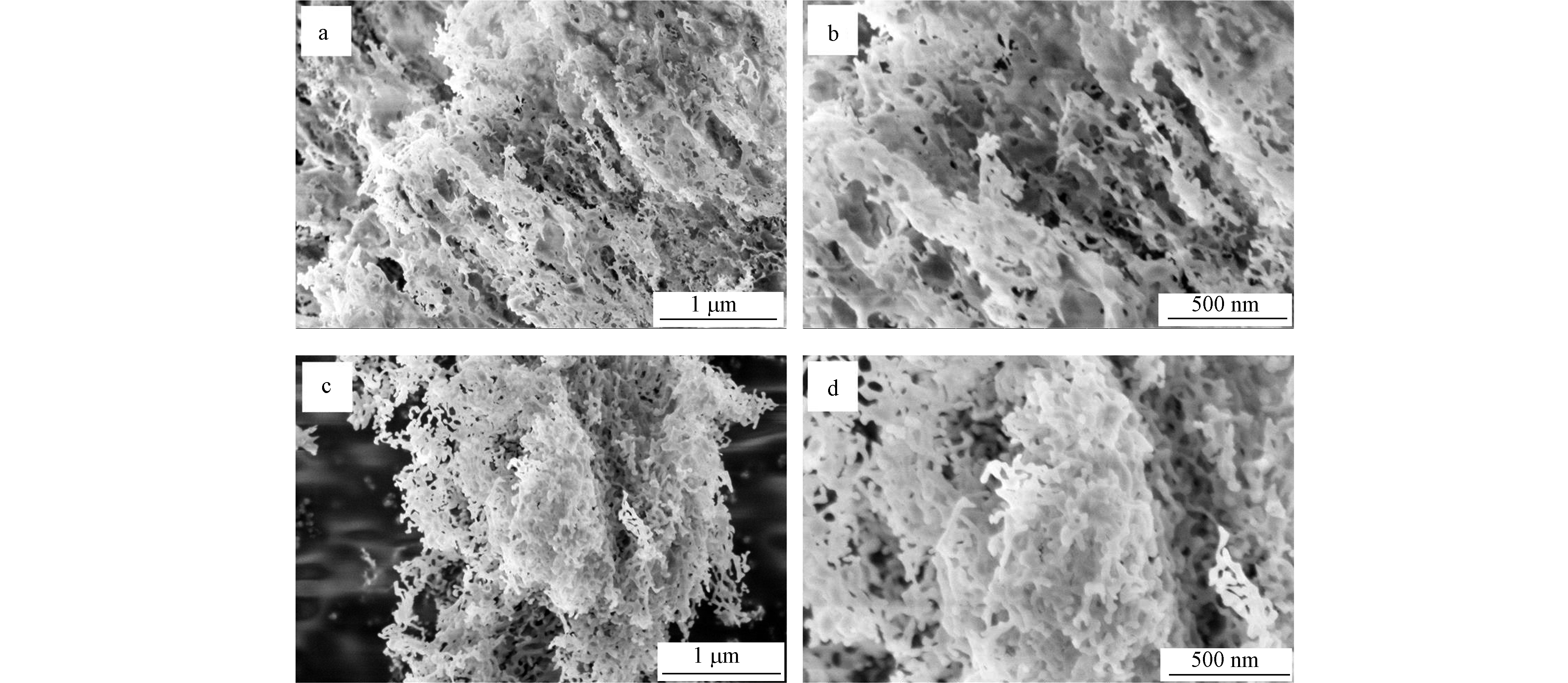
图2为g-C3N4和TiO2/g-C3N4复合材料(以TiO2/g-C3N4-B为例)的SEM图,可以看出,与TiO2的复合没有改变g-C3N4的形貌特征,这说明TiO2仅复合在g-C3N4表面;图3为g-C3N4和TiO2/g-C3N4复合材料的TEM图。图3a显示g-C3N4为常见的片状结构。图3b证实TiO2/g-C3N4保持了与g-C3N4相同的结构;图3c为TiO2/g-C3N4的高分辨TEM图,可以观察到属于TiO2(101)晶面的晶格条纹(0.353 nm)和归属于g-C3N4的石墨相无定形结构。图3d—g为TiO2/g-C3N4表面的元素分布情况,证实N和Ti元素共存于复合材料表面,且Ti元素分布高度均匀,再次印证TiO2/g-C3N4复合材料的形成。
-
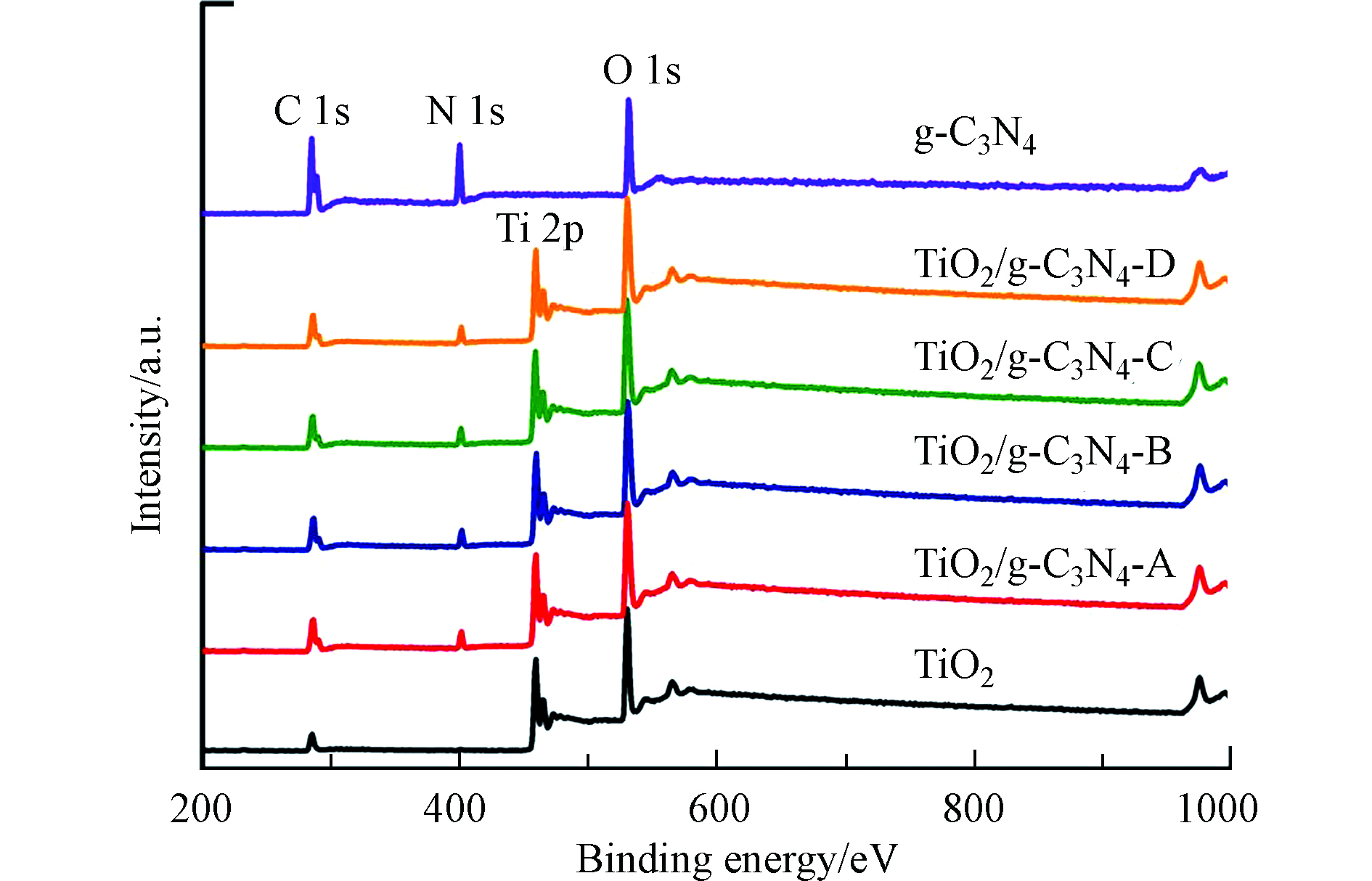
为了进一步观察复合材料表面特征,测试了TiO2/g-C3N4的XPS谱。从图4中可以看出,复合材料表面的元素分布为C、N、O和Ti,其中N元素来源于g-C3N4,Ti元素来自TiO2,再次证实复合材料为g-C3N4与TiO2的共同体,且TiO2附着在g-C3N4表面。综合表征结果,可确定TiO2/g-C3N4复合材料被成功制备。
-
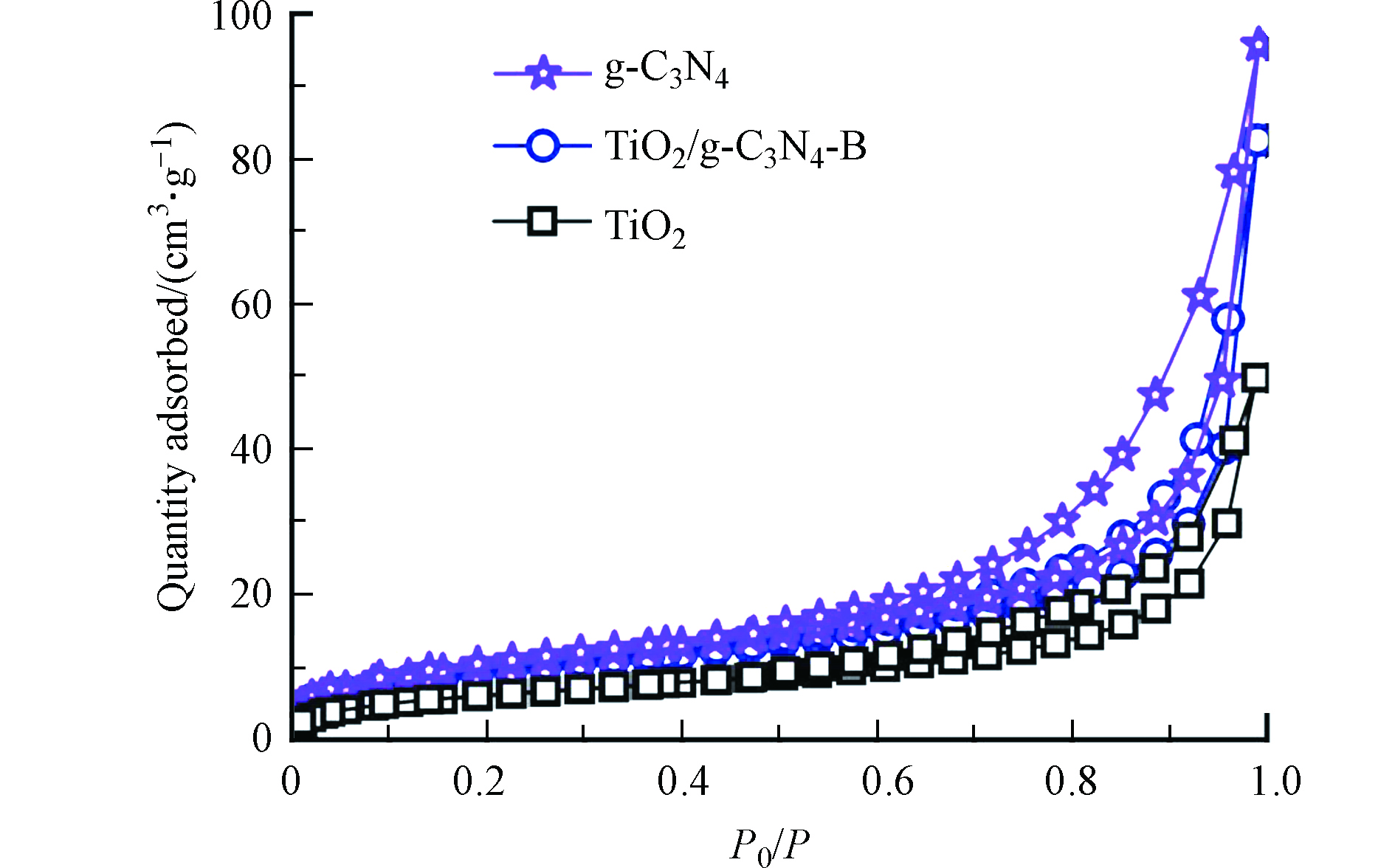
图5为材料的N2吸附-脱附等温线。几种材料的吸附-脱附等温线均可归属于Ⅳ型滞后回环,表明g-C3N4、TiO2和TiO2/g-C3N4-B均为介孔材料[19],这与TEM观察到的丰富孔结构相符。表1列出几种材料的BET比表面积和孔容量,可以看出,随着g-C3N4复合量的增加,材料的比表面积和孔容量均呈现逐渐增大趋势,导致这一现象的原因可归因于g-C3N4相对较高的比表面积和较大的孔容量,同时,这也再次证实了TiO2成功与g-C3N4复合。
-
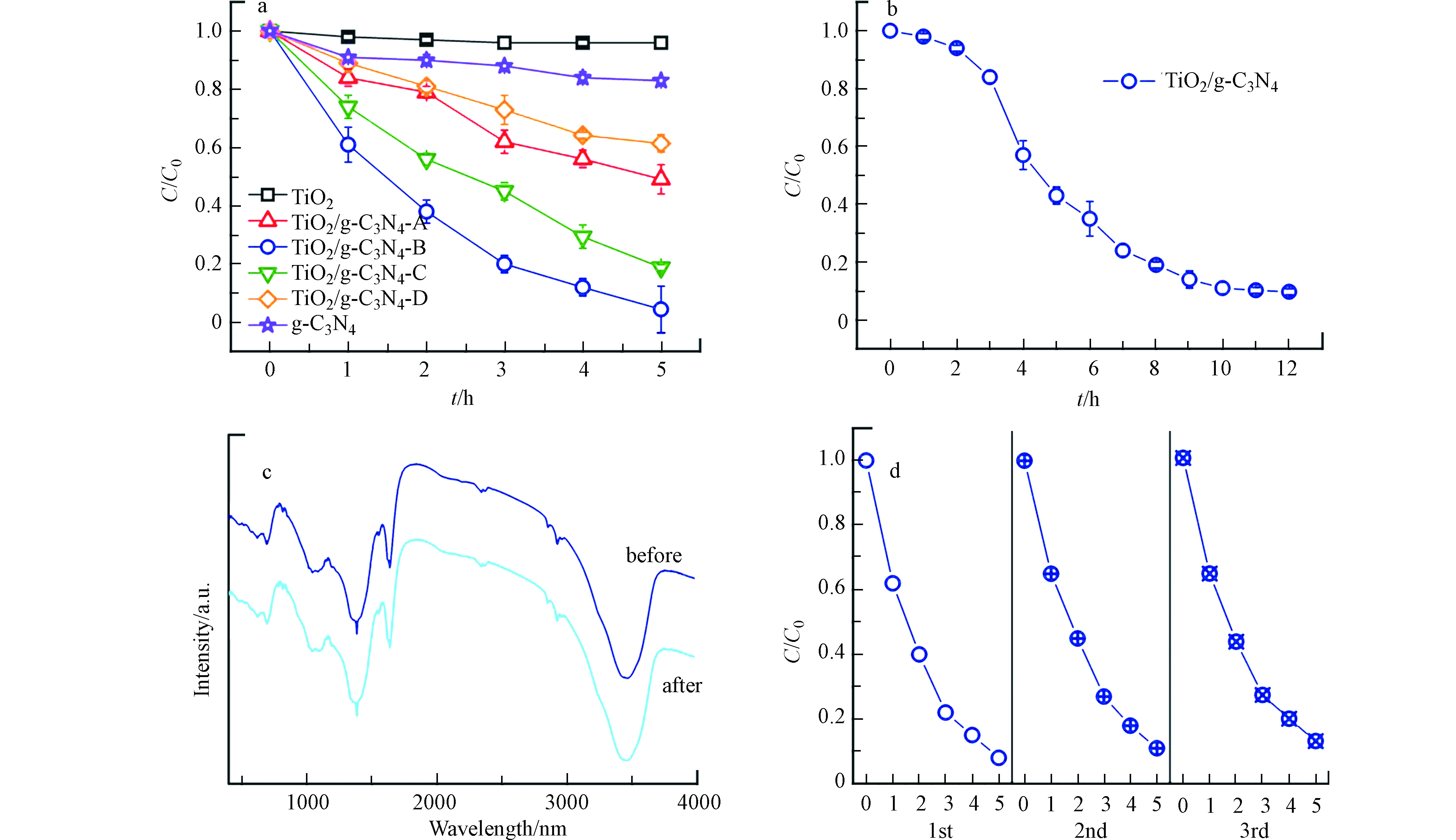
图6a为所制备材料在可见光照射下对BPA的降解效率。与g-C3N4和TiO2相比,全部TiO2/g-C3N4样品呈现出更高的降解效率。
在一系列TiO2/g-C3N4复合材料中,TiO2/g-C3N4-B展现了最高的光催化降解效率,当可见光照射5 h后,其对BPA的去除率达到95.6%。此时g-C3N4对BPA的去除率只有17.2%,而TiO2几乎不能对BPA实现降解,其它几种TiO2/g-C3N4复合材料对BPA的去除率分别为50.9%(TiO2/g-C3N4-A);81.2%(TiO2/g-C3N4-C);38.6%(TiO2/g-C3N4-D)。由于TiO2/g-C3N4-B展现了最佳的光催化降解活性,因此本文深入探究了其对BPA的矿化能力。如图6b所示,在可见光照射12 h后,TiO2/g-C3N4-B对BPA的矿化率达到90.3%,再次证实g-C3N4能够有效提升TiO2在可见光驱动下的催化活性。
催化剂的稳定性是衡量其能否推广应用的重要指标,本文探究了TiO2/g-C3N4-B在水溶液中的结构稳定性和光催化循环稳定性。将光催化反应后的样品进行收集,其IR谱的特征峰与反应前样品的IR谱相同(图6c),表明光催化反应后的TiO2/g-C3N4-B拥有与反应前样品相同的结构特征与表面官能团,初步证实其能够稳定存在于水环境中。为了进一步理解TiO2/g-C3N4-B的结构稳定性,利用TEM光电子能谱(TEM-EDS)对反应后样品表面的Ti/N原子比值进行测定,实验结果显示,反应后样品的Ti、N原子比为7∶2,这与投料时的原子比相近(25∶7),可以认为TiO2/g-C3N4-B在光催化过程中保持良好的结构稳定性。图6d显示在经过3次循环反应后,光催化降解效率仅降低了4.7%,说明TiO2/g-C3N4-B具有良好的光催化循环稳定性。
-
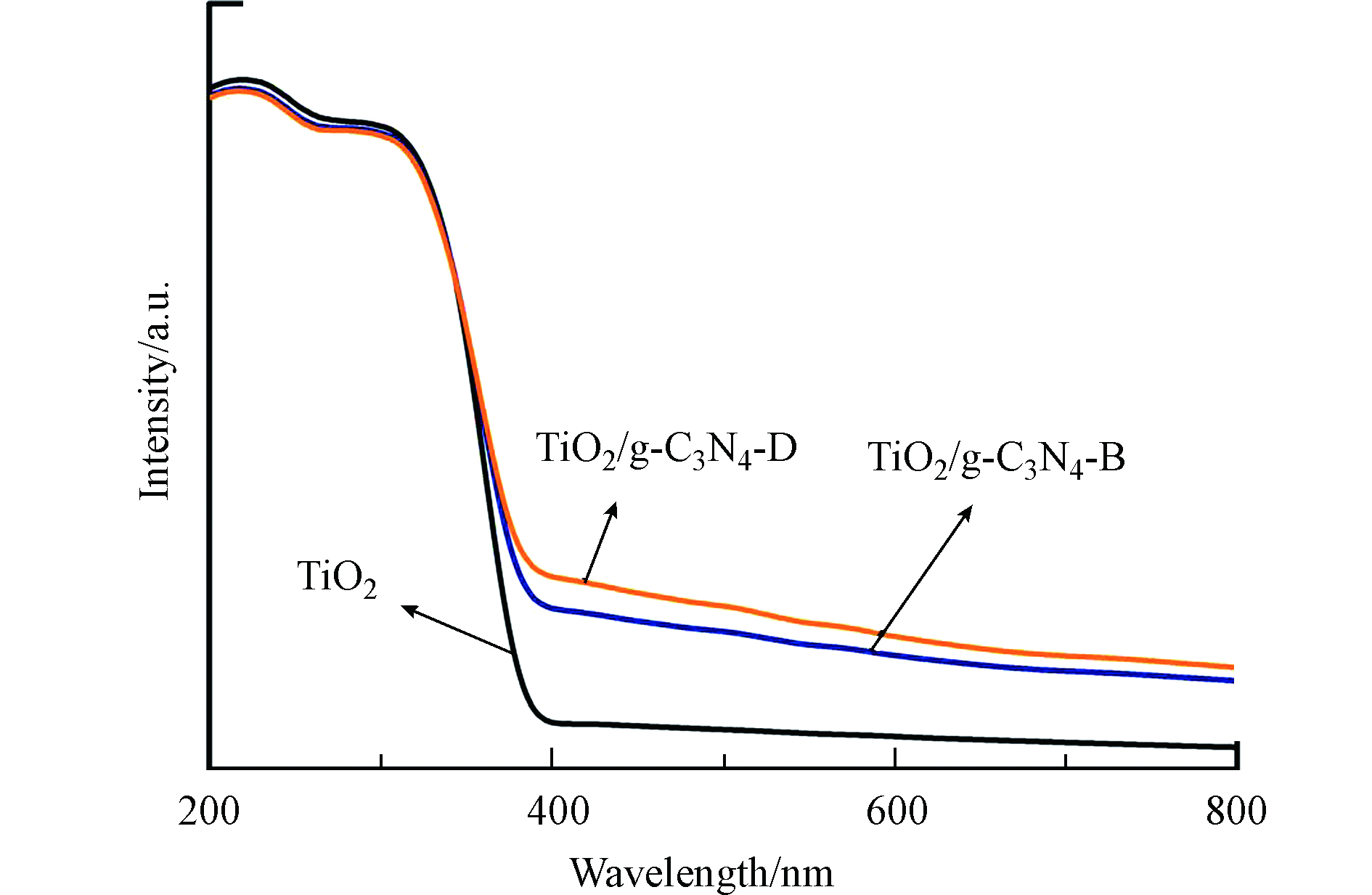
为了研究TiO2/g-C3N4-B拥有优异光催化活性的原因,首先通过UV-Vis考察了催化剂对光的响应能力(这里选择TiO2、具有最佳催化活性的TiO2/g-C3N4-B 和复合材料中催化活性最低的TiO2/g-C3N4-D为研究对象)。由图7可见,相比TiO2,复合材料整体呈现出更高的可见光吸收能力,说明机械混合法向TiO2体系引入g-C3N4能够有效增强其对可见光的吸收,这一结论与TiO2/g-C3N4-B更好的光催化活性并不相符。为了揭示TiO2/g-C3N4-B具有更高效光催化活性的原因,样品载流子分离特性的差异有待得到进一步研究。
-
催化剂表面光生载流子的分离能力直接决定其光催化活性[20]。首先运用稳态PL光谱对TiO2和TiO2/g-C3N4进行了考察。图8a显示,TiO2/g-C3N4的PL强度明显弱于TiO2,表明TiO2/g-C3N4表面的光生载流子具有更高的分离效率,预示TiO2/g-C3N4具有更高的光催化活性。通过对比TiO2/g-C3N4-B和TiO2/g-C3N4-D的PL强度可知,g-C3N4的引入有助于降低复合材料的PL强度,促进其光生载流子的分离;然而,当g-C3N4的引入量达到一定时(TiO2/g-C3N4-B),继续增加g-C3N4的引入量会导致复合材料光生载流子分离能力降低,导致这一现象的原因可能在于过量的g-C3N4形成了堆积,此时TiO2与g-C3N4所形成的复合材料在整体材料中占有比例缩小,整体材料更多的显现出g-C3N4自身光催化活性,因而载流子分离效率降低。值得注意的是,3种催化剂光生载流子分离能力与其对BPA的光催化降解效率相符,可以认为良好的载流子分离能力是使TiO2/g-C3N4-B拥有最高BPA降解效率的主要原因。
为了进一步理解光催化性能差异的由来,采用瞬态PL光谱测量了3种催化剂的光生载流子寿命。如图8b所示,光生载流子的存活寿命顺序为TiO2/g-C3N4-B>TiO2/g-C3N4-D>TiO2,这一顺序同样与催化剂对BPA的光催化降解效率相符,再一次证实TiO2/g-C3N4-B表面的光生载流子分离效率最高。综合对催化剂光生载流子特性的考察,可以认为尽管g-C3N4的引入有助于提升TiO2对可见光的吸收,但良好的光生载流子分离能力以及更长的载流子存活寿命是导致TiO2/g-C3N4-B具有最佳光催化活性的关键因素。此外,良好的光生载流子分离能力可以归因于在TiO2和g-C3N4之间会形成传统的异质结构,当可见光照射TiO2/g-C3N4表面,光生电子和光生空穴分别位于g-C3N4和TiO2上,增加了二者的复合难度,从而延长了光生载流子寿命[21]。
-
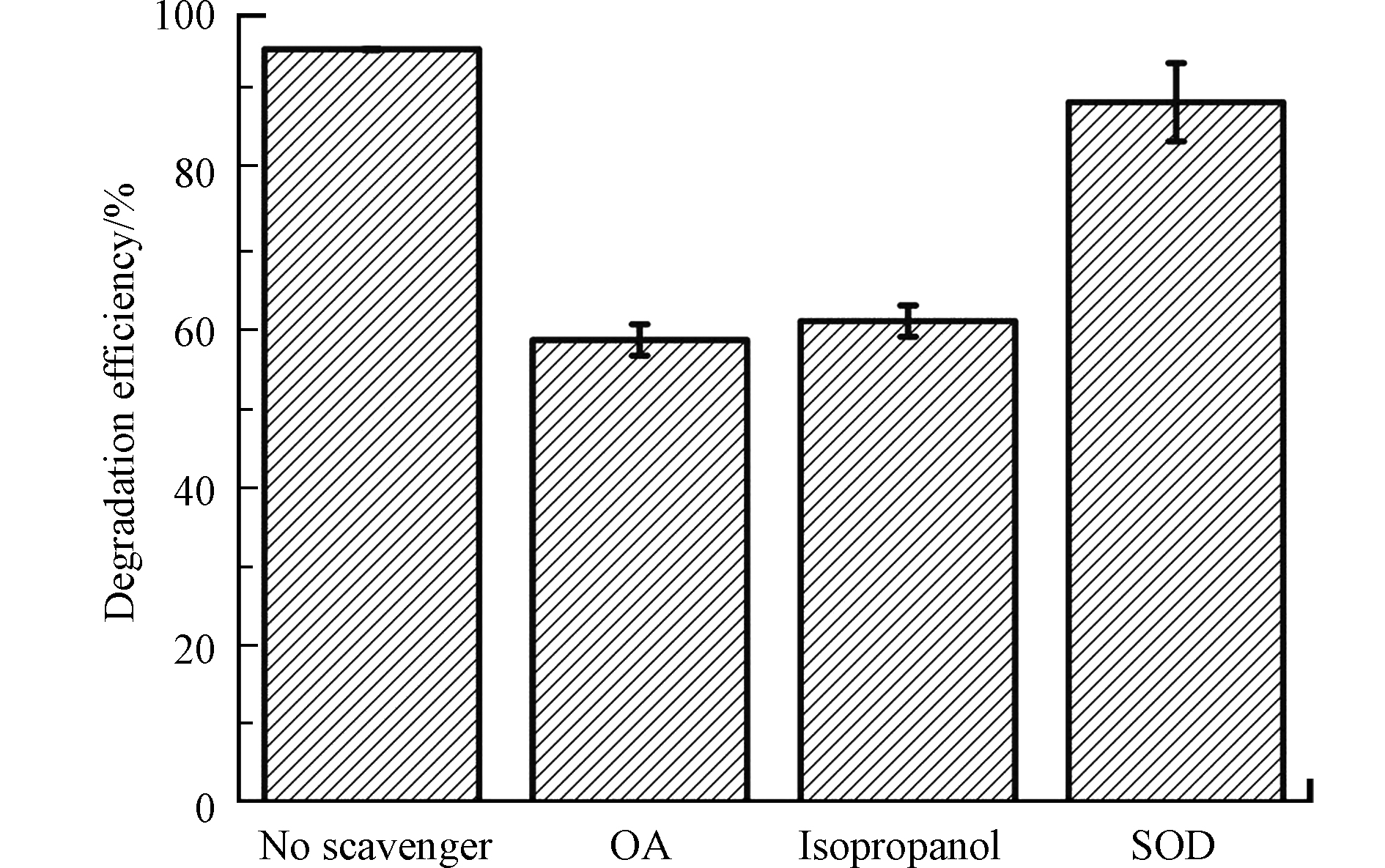
为了深入理解TiO2/g-C3N4-B对BPA的光催化降解机制,通过向催化反应体系中添加自由基捕获剂的方式探究了不同光生自由基在降解过程中的贡献(图9)。当向反应体系中分别加入草酸铵(oxamic acid,(OA),h+捕获剂)、异丙醇(isopropanol,•OH捕获剂)和SOD(
${\rm{O}}_2^{\cdot -}$ 捕获剂)[22],发现TiO2/g-C3N4-B对BPA的光催化降解效率分别降低了36.9%、34.5%和6.7%,这意味着在降解过程中起最主要作用的自由基为h+和•OH,而${\rm{O}}_2^{\cdot -} $ 的贡献相对较少。进一步的,根据式1—5可知,•OH主要通过${\rm{O}}_2^{\cdot -} $ 的再还原过程(式1— 4)和h+氧化水分子(式5)等两条途径形成[23],因此在加入SOD的反应体系中,催化剂对BPA不明显的降解效率变化还说明反应体系中的•OH并不来自${\rm{O}}_2^{\cdot -} $ 的再还原过程,而主要来自h+对水分子的氧化,这表示TiO2/g-C3N4-B光催化体系中起主导催化作用的h+,其贡献不仅在于直接氧化降解BPA分子,还在于氧化水分子,促进•OH形成。 -
为了进一步明确BPA的降解过程,运用HPLC-MS联用技术检测了催化过程中不同时间点的中间产物。图10a展示了各种中间产物随催化反应进行的浓度变化,可以看出在可见光照射5 h后,溶液中的BPA分子几乎被完全去除,这与图6a的检测结果相符;各中间产物在反应12 h左右几乎完全消失,这与之前的矿化结果(图6b)相符,证实TiO2/g-C3N4-B在可见光照射下对BPA具有良好的降解活性和矿化能力。此外,基于HPLC-MS对中间产物质荷比(离子质量/离子电荷)的检测结果,BPA的降解路径被提出(图10b)。
-
本文采用简单的机械混合方法将TiO2纳米颗粒与g-C3N4复合,制备一系列g-C3N4含量不同的TiO2/g-C3N4复合材料。通过对比在可见光作用下对水中BPA的光催化降解效率发现,TiO2/g-C3N4的光催化活性明显优于TiO2和g-C3N4,其中尤其以TiO2/g-C3N4-B的光催化活性最高。值得注意的是,机械混合方法构建的复合材料能够在水中保持良好的结构稳定和光催化循环稳定性。通过对复合材料光催化性能的机理研究发现,机械混合方法引入的g-C3N4不仅能够将TiO2的吸光范围拓展至可见光区,而且可以有效阻碍光生空穴电子对的复合。更为重要的是,机械混合法能够促使两相材料混合均匀,这有利于载流子在两相界面间的迁移与扩散。根据本实验结果推测,高度均匀的混合结构组成是TiO2/g-C3N4具有良好光催化性能的关键,后续将进行更多的研究需要进行。本项工作在丰富了可见光驱动的TiO2基光催化剂的设计思路的同时,也为精准构建复合材料提供了新方法。
机械混合法制备TiO2/g-C3N4复合材料及其光催化降解双酚A的性能
Preparation of TiO2/g-C3N4 composite material by mechanical mixing method and study on its photocatalytic degradation performance of bisphenol A
-
摘要: 本工作采用机械混合方法,将TiO2纳米颗粒与不同质量的g-C3N4复合,制备了一系列具有不同g-C3N4含量的TiO2/g-C3N4复合材料。运用X射线粉末衍射(XRD)、X射线光电子能谱(XPS)、拉曼光谱(Raman)、比表面积分析仪(BET)、扫描电镜(SEM)和透射电镜(TEM)对材料的化学组成和形貌特征进行了表征。比较了g-C3N4、TiO2和一系列TiO2/g-C3N4在可见光驱动下对水中双酚A(BPA)的降解效率和矿化能力差异,发现TiO2/g-C3N4的光催化活性显著高于g-C3N4和TiO2,其中以TiO2/g-C3N4-B的光催化活性最高(g-C3N4的投料量为0.08 g)。为了揭示TiO2/g-C3N4良好光催化活性的产生机理,采用紫外可见光谱、稳态和瞬态荧光光谱对催化剂(以TiO2/g-C3N4-B为研究模型)可见光响应能力、带隙结构以及光生载流子分离能力进行了分析。结果证实,通过机械混合方法向TiO2体系引入适量的g-C3N4不仅能够将TiO2的光响应范围拓宽,而且可以提升光生载流子的分离能力并延长载流子存活寿命。此方法构建的复合材料能够在水环境中保持结构和性能的稳定。对降解过程中自由基分布特征的监测表明,降解过程中的主要活性物种为空穴自由基和羟基自由基。Abstract: In this work, a series of TiO2/g-C3N4 composites with different g-C3N4 content were prepared by combining TiO2 nanoparticles with g-C3N4 of different masses through using a mechanical mixing method. The chemical composition and morphological structure of the composites were analyzed by X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy (Raman), Brunauer-Emmett-Teller (BET), scanning electron microscope (SEM) and transmission electron microscope (TEM). Driven by visible light, the differences in degradation efficiency and mineralization ability of bisphenol A (BPA) in water with g-C3N4, TiO2 and a series of TiO2/g-C3N4 were compared. Among them, TiO2/g-C3N4-B has the highest photocatalytic activity (the content of g-C3N4 is 0.08 g). To reveal the generation mechanism of the good photocatalytic activity of TiO2/g-C3N4, UV-visible spectroscopy, steady-state and transient fluorescence spectroscopy were used to analyze the visible light response capability, band gap structure, and photogenerated carrier separation ability of the catalyst (using TiO2/g-C3N4-B as the research model). The results confirmed that the introduction of an appropriate amount of g-C3N4 into the TiO2 system through the mechanical mixing method can not only broaden the photoresponse range of TiO2, but also enhance the separation ability of photogenerated carriers and extend the carrier lifetime. The composite material constructed by this method can maintain the stability of structure and performance in the water environment. The monitoring of the distribution characteristics of radicals shows that the main active species in the degradation process are hole and hydroxyl radicals.
-
Key words:
- mechanical mixing /
- composite materials /
- photocatalytic /
- bisphenol A
-

-
图 6 (a)不同催化剂对BPA的降解效率比较;(b)TiO2/g-C3N4-B对BPA的矿化效果;(c)TiO2/g-C3N4-B光催化反应前后的IR图;(d)TiO2/g-C3N4-B对BPA的循环催化效果
Figure 6. (a) comparison of different catalyst degradation efficiency of BPA; (b) the mineralization effect of TiO2/g-C3N4-B on BPA; (c)IR spectrum of the sample before and after photocatalytic reaction; (d)cyclic photocatalytic effect of TiO2/g-C3N4-B on BPA
表 1 不同样品的比表面积和孔容量
Table 1. Specific surface area and pore volume of different samples
样品 Samples 比表面积/(m2·g−1) BET 孔容量/(cm3·g−1) Pore volume g-C3N4 105 0.71 TiO2 26 0.06 TiO2/g-C3N4-A 43 0.29 TiO2/g-C3N4-B 64 0.34 TiO2/g-C3N4-C 69 0.37 TiO2/g-C3N4-D 73 0.39 -
[1] DAI R, GUO H, TANG C Y, et al. Hydrophilic selective nanochannels created by metal organic frameworks in nanofiltration membranes enhance rejection of hydrophobic endocrine-disrupting compounds [J]. Environmental Science & Technology, 2019, 53(23): 13776-13783. [2] GRAJALES D M, BERNARDES G J L, VERBEL J O. Urban endocrine disruptors targeting breast cancer proteins [J]. Chemical Research in Toxicology, 2016, 29(2): 150-161. doi: 10.1021/acs.chemrestox.5b00342 [3] WANG Z, SUN P, LI Y, et al. Reactive nitrogen species mediated degradation of estrogenic disrupting chemicals by biochar/monochloramine in buffered water and synthetic hydrolyzed urine [J]. Environmental Science & Technology, 2019, 53(21): 12688-12696. [4] BASILE T, PETRELLA A, PETRELLA M, et al. Review of endocrine-disrupting-compound removal technologies in water and wastewater treatment plants: An EU perspective [J]. Industrial & Engineering Chemistry Research, 2011, 50(14): 8389-8401. [5] 陈紫盈, 孙洁, 罗雪文, 等. BiVO4晶面生长调控及其光催化氧化罗丹明B和还原Cr(Ⅵ)的性能 [J]. 环境化学, 2020, 39(8): 2129-2136. doi: 10.7524/j.issn.0254-6108.2019061101 CHEN Z, SUN J, LUO X, et al. Growth regulation of BiVO4 crystal plane and photocatalytic oxidation of Rhodamine B and reduction of Cr(Ⅵ) [J]. Environmental Chemistry, 2020, 39(8): 2129-2136(in Chinese). doi: 10.7524/j.issn.0254-6108.2019061101
[6] 刘子薇, 胡丽君, 孙振亚, 等. TiO2-FeOOH/Mmt纳米复合材料的表面酸碱性质及光催化性能 [J]. 环境化学, 2020, 39(3): 745-754. doi: 10.7524/j.issn.0254-6108.2019092707 LIU Z, HU L J, SUN Z Y, et al. The surface acidity and basicity and photocatalytic activity of TiO2-FeOOH/Mmt nanocomposites [J]. Environmental Chemistry, 2020, 39(3): 745-754(in Chinese). doi: 10.7524/j.issn.0254-6108.2019092707
[7] ZHOU X, LIU N, SCHMUKI P. Photocatalysis with TiO2 nanotubes: “Colorful” reactivity and designing site-specific photocatalytic centers into TiO2 nanotubes [J]. ACS Catalysis, 2017, 7(5): 3210-3235. doi: 10.1021/acscatal.6b03709 [8] ZHAO Z, SHEN B, HU Z, et al. Recycling of spent alkaline Zn-Mn batteries directly: combination with TiO2 to construct a novel Z-scheme photocatalytic system [J]. Journal of Hazardous Materials, 2020, 400: 123236. doi: 10.1016/j.jhazmat.2020.123236 [9] SCHNEIDER J, MATSUOKA M, TAKEUCHI M, et al. Understanding TiO2 photocatalysis: Mechanisms and materials [J]. Chemical Reviews, 2014, 114(19): 9919-9986. doi: 10.1021/cr5001892 [10] ZHANG J, ZHOU D, DONG S, et al. Respective construction of Type-Ⅱ and direct Z-scheme heterostructure by selectively depositing CdS on {001} and {101} facets of TiO2 nanosheet with C-Dots modification: a comprehensive comparison [J]. Journal of Hazardous Materials, 2019, 366: 311-320. doi: 10.1016/j.jhazmat.2018.12.013 [11] DU J, MA S, LIU H, et al. Uncovering the mechanism of novel AgInS2 nanosheets/TiO2 nanobelts composites for photocatalytic remediation of combined pollution [J]. Applied Catalysis B:Environmental, 2019, 259: 118062. doi: 10.1016/j.apcatb.2019.118062 [12] WANG H, SU Y, ZHAO H, et al. Photocatalytic oxidation of aqueous ammonia using atomic single layer graphitic-C3N4 [J]. Environmental Science & Technology, 2014, 48(20): 11984-11990. [13] LI J, ZHANG M, LI X, et al. Effect of the calcination temperature on the visible light photocatalytic activity of direct contact Z-scheme g-C3N4-TiO2 heterojunction [J]. Applied Catalysis B:Environmental, 2017, 212: 106-114. doi: 10.1016/j.apcatb.2017.04.061 [14] HUANG J, LI D, LI R, et al. One-step synthesis of phosphorus/oxygen co-doped g-C3N4/anatase TiO2 Z-scheme photocatalyst for significantly enhanced visible-light photocatalysis degradation of enrofloxacin [J]. Journal of Hazardous Materials, 2020, 386: 121634. doi: 10.1016/j.jhazmat.2019.121634 [15] XIAO J, HAN Q, XIE Y, et al. Is C3N4 chemically stable toward reactive oxygen species in sunlight-driven water treatment? [J]. Environmental Science & Technology, 2017, 51(22): 13380-13387. [16] CHAN M, CHEN C, LEE I, et al. Near-infrared light-mediated photodynamic therapy nanoplatform by the electrostatic assembly of upconversion nanoparticles with graphitic carbon nitride quantum dots [J]. Inorganic Chemistry, 2016, 55(20): 10267-10227. doi: 10.1021/acs.inorgchem.6b01522 [17] WANG W, NIU Q, ZENG G, et al. 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction [J]. Applied Catalysis B:Environmental, 2020, 273(15): 119051. [18] GUO S, ZHEN M, LIU L, et al. Facile preparation and lithium storage properties of TiO2@graphene composite electrodes with low carbon content [J]. Chemistry-A European Journal, 2016, 22(34): 11943-11948. doi: 10.1002/chem.201602532 [19] GUO S, ZHANG X, ZHOU Z, et al. Facile preparation of hierarchical Nb2O5 microspheres with photocatalytic activities and electrochemical properties [J]. Journal of Materials Chemistry A, 2014, 2(24): 9236-9243. doi: 10.1039/C4TA01567C [20] WANG L, GUO S, CHEN Y, et al. A mechanism investigation of how the alloying effect improves the photocatalytic nitrate reduction activity of bismuth oxyhalide nanosheets [J]. ChemPhotoChem, 2020, 4(2): 110-119. doi: 10.1002/cptc.201900217 [21] QIU B, ZHU Q, DU M, et al. Efficient solar light harvesting CdS/Co9S8 hollow cubes for Z-scheme photocatalytic water splitting [J]. Angewandte Chemie-International Edition, 2017, 56(10): 2684-2688. doi: 10.1002/anie.201612551 [22] LI J, ZHANG Z, CUI W, et al. The spatially oriented charge flow and photocatalysis mechanism on internal van der Waals heterostructures enhanced g-C3N4 [J]. ACS Catalysis, 2018, 8(9): 8376-8385. doi: 10.1021/acscatal.8b02459 [23] ZHANG L, YANG C, LV K, et al. SPR effect of bismuth enhanced visible photoreactivity of Bi2WO6 for NO abatement [J]. Chinese Journal of Catalysis, 2019, 40(5): 755-764. doi: 10.1016/S1872-2067(19)63320-6 -



 下载:
下载: