-
药品及个人护理产品(pharmaceutical and personal care products,简称PPCPs)污染正受到越来越多的关注[1]。因其组分具有较强的生物活性、极性和旋光性,PPCPs在环境介质中的迁移转化行为会给环境和人类健康带来潜在风险[2-3]。作为一类重要的PPCPs,β受体阻断剂主要用于人类高血压、心肌梗塞、心律不齐、心力衰竭、婴儿血管瘤和其他心血管疾病的治疗[4-5]。人体对该类药物的不完全吸收以及动物体对该类药物的不完全代谢,使其随着生物体排泄等方式进入到废水中,最终进入城市污水处理厂。但是,目前污水处理厂不能完全去除这些污染物[6-7]。因此,β受体阻断剂就不可避免地进入到自然水体和土壤环境中[8]。研究表明,β受体阻断剂会干扰藻类的光合作用,并影响一些脊椎动物或无脊椎动物的心率[9-11]。因此,研究β受体阻断剂在自然环境中的迁移转化行为十分重要。
针铁矿(goethite,简称Goe)是自然界中广泛存在的一种铁氧化物,常见于土壤、海洋、河流湖泊及沉积物中。之前研究表明,针铁矿对环境中的重金属[12-14]和药物及个人护理产品[15-16]有较强的吸附能力。目前,关于针铁矿对β受体阻断剂吸附的研究鲜有报道。微塑料(microplastics,简称Mps)是环境中常见的外来颗粒污染物,因其具有难降解、粒径小、比表面积大和分布广的特性,可以将环境中污染物吸附并富集在其表面上,对环境中污染物的迁移行为产生影响[17]。目前已有很多学者研究了微塑料对重金属及抗生素类药物的吸附[18-20],同时也有一些文献报道了微塑料对β受体阻断剂的吸附。例如, Razanajatovo等[21]在24 ℃避光条件下研究了聚乙烯微塑料对包括普萘洛尔在内的3种药物的吸附和解吸,实验结果表明,普萘洛尔的吸附率为21.61%。Puckowski等[22]在21 ℃黑暗的玻璃管中水平旋转搅拌24 h研究了4种不同类型的微塑料对9种药物的吸附,实验结果证明微塑料对普萘洛尔的吸附系数最大为2.4 L·kg−1。微塑料对β受体阻断剂的吸附作用很有可能会影响β受体阻断剂在针铁矿上的吸附行为。然而,目前关于微塑料对β受体阻断剂在针铁矿上吸附行为的影响研究尚鲜见报道。
本文以针铁矿、微塑料(聚氯乙烯微塑料)为吸附剂,以β受体阻断剂(普萘洛尔)为吸附质,通过批量吸附实验,考察了微塑料、针铁矿及微塑料共存时针铁矿对普萘洛尔的吸附动力学、吸附等温线,探讨了影响吸附的主要因素如pH、离子强度及腐殖酸,以确定微塑料对β受体阻断剂在针铁矿上吸附的影响。
-
盐酸普萘洛尔(98%,上海九鼎化学科技有限公司),针铁矿(湖北万得化工有限公司)过100目筛,聚氯乙烯微塑料(中新塑胶原料有限公司)1000目,无进一步处理。腐殖酸钠购自上海麦克林生化科技有限公司。甲醇、甲酸和乙腈均为色谱纯,氯化钠、氯化钙、氢氧化钠和盐酸均为分析纯,以上药品均购自天津市科密欧化学试剂有限公司。实验用水为超纯水。ZD-85恒温振荡器(国华企业),pH计FE28(梅特勒托利多仪器(上海)有限公司),1260型液相色谱仪(美国安捷伦科技有限公司)。
-
采用批量吸附法,实验在40 mL玻璃管中进行。在玻璃管中加入1 mL的普萘洛尔母液,使得玻璃管中普萘洛尔浓度为25 mg·L−1,随后加入一定量的吸附剂(3种体系的吸附剂加入量分别为:针铁矿0.2 g、微塑料0.2 g、针铁矿与微塑料各0.2 g)。在25 ℃下,于200 r·min−1的水平摇床中振荡反应。分别在5、10、20、30、60、360、720、1440 min时,用1 mL的注射器抽取样品,并用0.22 μm滤膜过滤,以保证固液分离。分离后的液相保存在2 mL棕色液相色谱瓶中,用高效液相色谱仪进行分析。为保证所得数据可靠,每个点均做两个平行样。
-
先在玻璃管中加入不同量的普萘洛尔母液,使得40 mL玻璃管中普萘洛尔的初始浓度呈一定梯度(5、10、15、20、25、50、100、150、200、250 mg·L−1)。随后再加入一定量的吸附剂,3种体系的吸附剂加入量与吸附动力学实验一致。在25 ℃下,于200 r·min−1的水平摇床中振荡反应,24 h后用1 mL的注射器抽取样品,并用0.22 μm滤膜过滤,保存与测样方法与吸附动力学一致。为保证所得数据可靠,每个点均做两个平行样。
-
考虑实际环境情况,本文分别在酸性、中性、碱性条件下,测定了普萘洛尔的吸附率;研究了不同腐殖酸浓度(0、5、10、15、20、25、50、100、150、200 mg·L−1)对普萘洛尔吸附行为的影响。此外,还用不同浓度的NaCl、CaCl2溶液(0、5、10、20、25、50、100、150、200、250 mg·L−1)检验离子强度的影响。
-
高效液相色谱法测定上清液,色谱柱规格:美国安捷伦,4.6 mm×150 mm,SB-C18柱。在290 nm下进行等度洗脱:0.1%甲酸∶乙腈(67∶33,V∶V)。普萘洛尔吸附量和吸附率分别采用公式(1)和公式(2)进行计算:
式中,q(mg·g−1)为普萘洛尔在吸附剂上的吸附量,C0和Ce(mg·L−1)为普萘洛尔的初始浓度和平衡浓度,V(L)表示溶液的体积,m(g)为加入的吸附剂的质量,E为吸附率,%。
-
吸附速度和吸附动态平衡涉及到物质的传质现象和扩散速度。因此,本研究采用伪一级动力学方程和伪二级动力学方程对吸附进行拟合分析,拟合方程式如下所示[23]:
式中,qe为吸附平衡后的吸附量(mg·g−1);t为达到吸附平衡所需时间(min);qt为在t时刻普萘洛尔的吸附量(mg·g−1);K1为伪一阶吸附速率常数(L·min−1);K2为伪二阶吸附速率常数(g·(g·min)−1)。
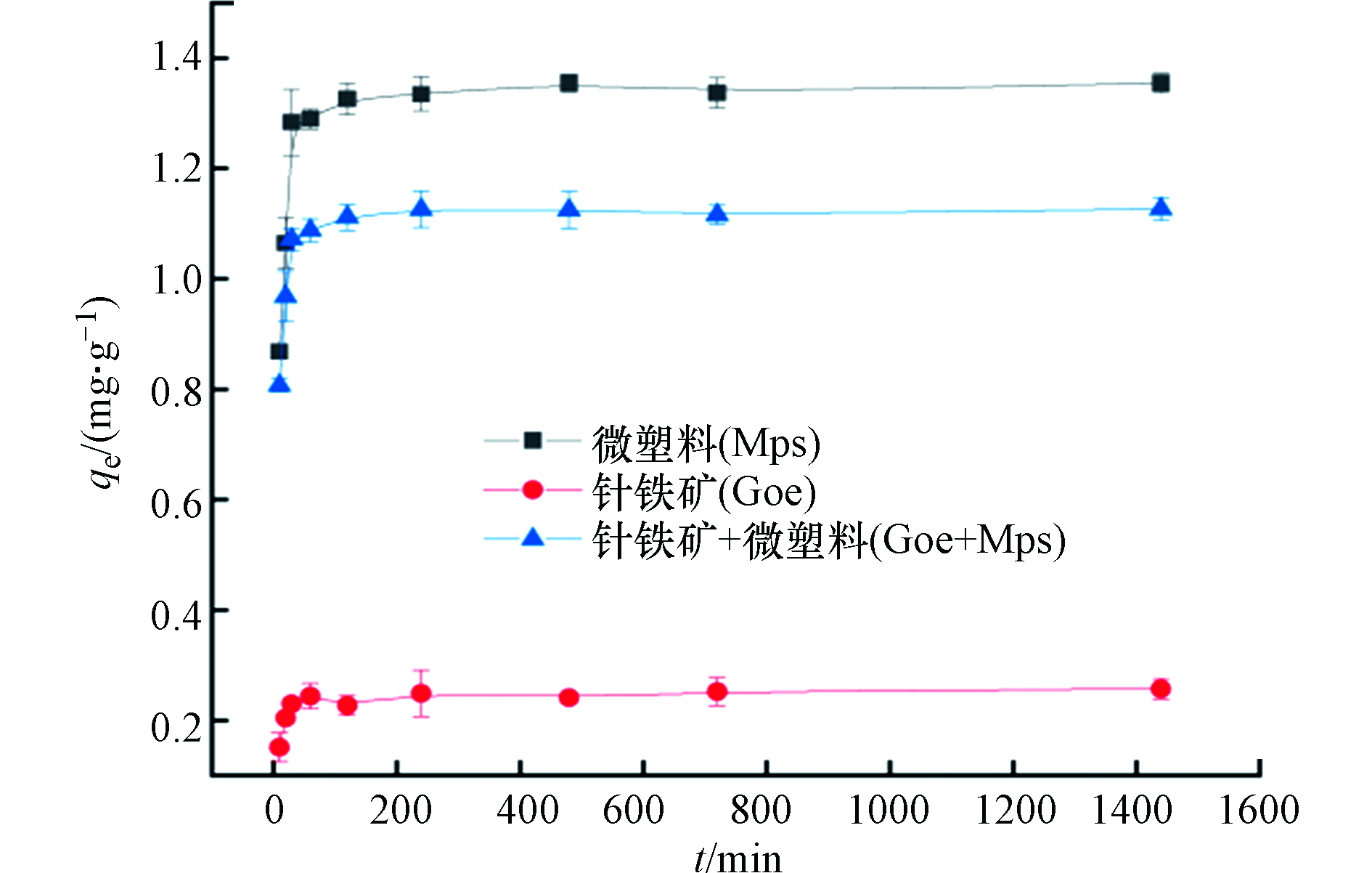
图1显示了普萘洛尔在针铁矿、微塑料以及针铁矿和微塑料共存时的吸附动力学。从图1可以看出,在前60 min,普萘洛尔的吸附量随时间的增加而急剧上升,这是因为针铁矿与微塑料的粒径小,比表面积大,表面的吸附位点多。随着吸附的进行,吸附量逐渐达到动态平衡,吸附剂表面的活性位点逐渐被占据[24]。普萘洛尔在共存体系中的吸附平衡时间早于单一体系,30 min时,普萘洛尔在针铁矿上的吸附量为0.23 mg·g−1,超过平衡吸附量的2/3;在微塑料上的吸附量为1.28 mg·g−1,低于平衡吸附量;在针铁矿和微塑料共存体系上的吸附量与平衡吸附量基本一致,说明两种吸附剂共存时能更早地达到吸附平衡。分别用伪一阶动力学模型和伪二阶动力学模型对所得数据进行拟合,结果如表1所示。
从表1可看出,伪二阶动力学模型相关性系数均比伪一阶动力学模型相关性系数高,且伪二阶动力学模型平衡浓度qe接近实验所得吸附量值,说明普萘洛尔在3种体系上的吸附均符合伪二阶动力学模型。进一步推测出普萘洛尔在3种体系上的吸附行为包含多种吸附共同作用,其中在吸附剂表面上活性位点的吸附可能是最主要的吸附过程[21-23]。
-
本研究用Langmuir和Freundlich等温线模型对普萘洛尔在3种体系上的吸附行为进行了描述,模型公式如下所示[24]:
式中,Ce为普萘洛尔的平衡浓度(mg·L−1),qe为平衡时的吸附量(mg·g−1),qm为最大平衡吸附量(mg·g−1),KL为Langmuir吸附常数,KF为Freundlich吸附常数,1/n为非均质参数。
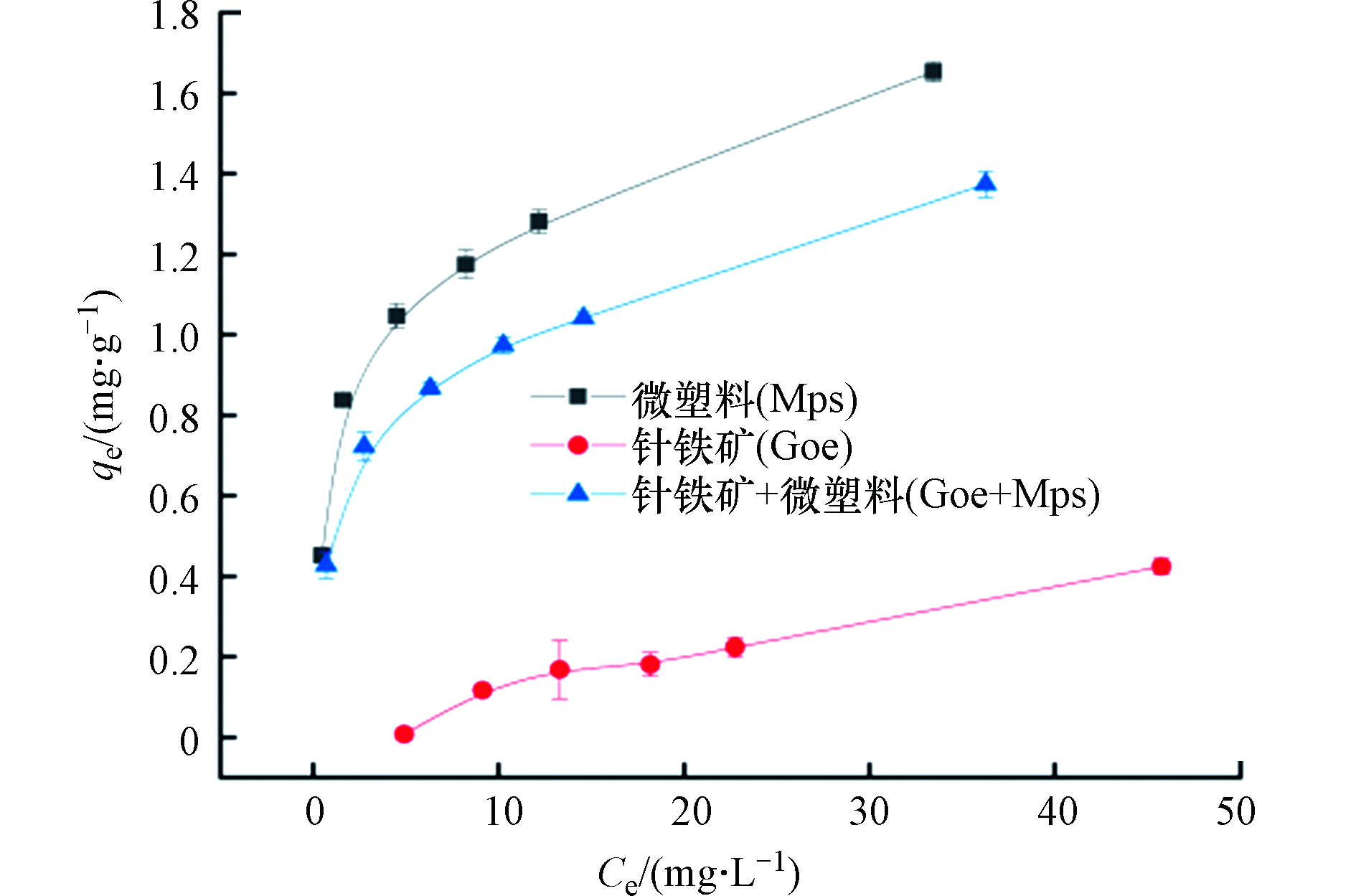
图2显示了普萘洛尔在不同体系上的吸附量随普萘洛尔浓度变化的情况。从图2看出,不同体系中普萘洛尔的吸附量均随平衡浓度的增加而增加。在针铁矿体系中普萘洛尔的吸附量仅为0.42 mg·g−1,这说明针铁矿对普萘洛尔的吸附力较弱,相反微塑料对普萘洛尔的吸附量最高。在微塑料与针铁矿共存时,吸附量却有所下降,说明微塑料与针铁矿两者之间也存在一定的界面作用。可能是因为微塑料表面带负电荷,而针铁矿带正电荷,两者可通过静电相互作用而产生吸附,从而导致吸附剂表面的部分活性位点被占据,使得普萘洛尔吸附量降低,这与Luo等[25]的研究结果一致。
Langmuir和Freundlich吸附方程参数见表2,在针铁矿体系中,Langmuir模型和Freundlich模型的的线性相关系数分别为0.9970和0.9469,说明普萘洛尔在针铁矿上的吸附行为是非线性的。这可能是因为针铁矿表面含有多种活性位点,在吸附开始时,普萘洛尔首先占据针铁矿表面的高能活性位点,但随着吸附的进行,高能吸附位点基本被占据,使得普萘洛尔只能占据针铁矿表面的低能活性位点,而且针铁矿表面含有较多的羟基并带负电荷,因此针铁矿的吸附机理也包括表面络合作用[23]和静电相互作用。这进一步表明普萘洛尔在针铁矿上的吸附行为是在多种作用力下协同进行的。在微塑料体系和针铁矿与微塑料共存体系中,两种模型相关性系数均大于0.9,说明普萘洛尔在微塑料和针铁矿与微塑料共存体系上的吸附情况与针铁矿相似,也是非线性的,既包含了单层吸附又包含了多层吸附[25]。
-
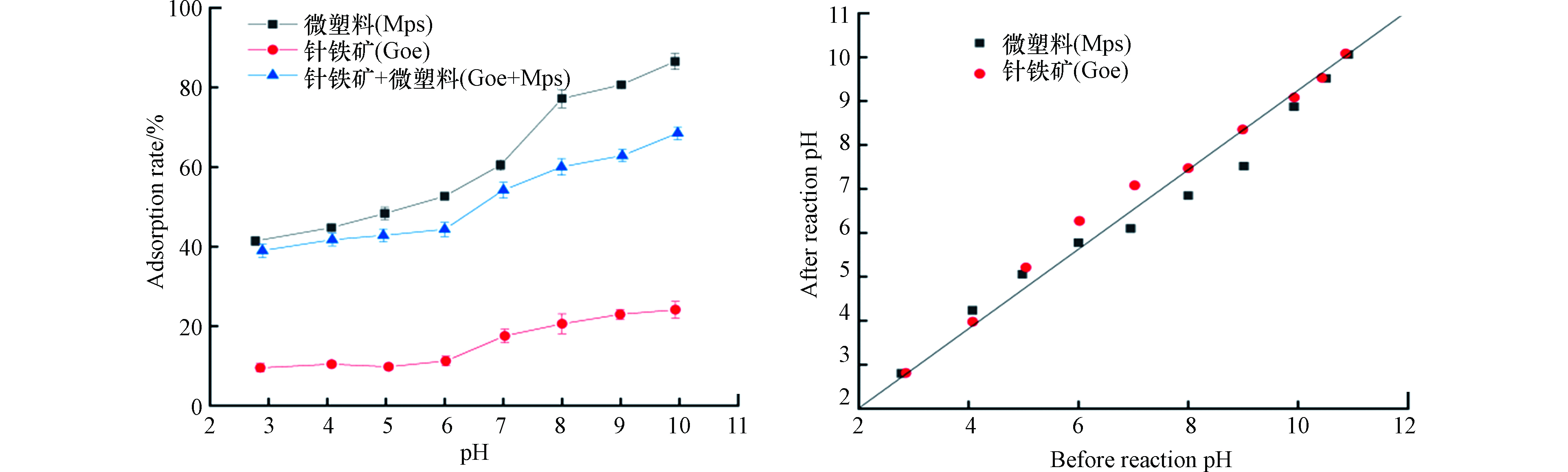
普萘洛尔pKa为9.5,当pH值小于9.5,主要以阳离子形式存在;当pH值大于9.5,主要以分子形式存在,且pH越高中性分子数越多,这会导致普萘洛尔与吸附剂之间的静电引力作用减弱[26]。因此,本文研究了不同pH对普萘洛尔吸附行为的影响,见图3。从图3可看出,以针铁矿为吸附剂时,普萘洛尔在碱性条件下的吸附率有所升高,从9.55 %上升到24.08 %。在pH值 2—10范围内,针铁矿对普萘洛尔的吸附率整体较低,这可能与普萘洛尔在不同pH条件下呈现的形态以及在不同pH条件下针铁矿表面所带的电荷不同有关[27]。针铁矿的等电点是8.5,所以在pH值小于8.5时表面是带正电的,在pH值大于8.5时表面带负电荷。这进一步说明,当pH在酸性范围时,针铁矿表面带正电荷,而普萘洛尔是以阳离子形式存在的,使得这两者间存在静电斥力,导致了吸附率较低。从图3可知,反应后pH有一定的升高,这可能是由于溶液中的H+在针铁矿的表面发生了吸附,导致溶液中H+浓度降低,pH上升。当pH在碱性范围内时(pH>8.5),针铁矿表面带负电荷,其与普萘洛尔之间的静电斥力减小,两者间静电引力增强,进而导致吸附率增加。
当微塑料为吸附剂时,酸性环境中的H+浓度较高,会与普萘洛尔竞争微塑料表面的活性位点,导致吸附率较低。随着pH的升高,H+浓度降低,竞争作用减弱,使吸附率上升。溶液pH值在4.5—8范围内增加时,吸附率急剧增加。当pH值大于8时,吸附率增加减缓,表明H+浓度降低有利于普萘洛尔吸附,即微塑料在吸附普萘洛尔的过程中静电引力起着重要作用。从图3可知,pH值在3—9范围内,反应后H+浓度高于反应前H+浓度,推测出在吸附过程中可能发生了阳离子交换作用。
当微塑料和针铁矿共存时,普萘洛尔的吸附率并不等于两种吸附剂单独对普萘洛尔吸附率的加和。这说明微塑料与针铁矿两者之间发生了一定的界面作用,导致吸附率有所下降[28]。由图3可知,在pH值为2—4.5范围内,普萘洛尔在两种吸附剂共存时的吸附率与微塑料单独存在时的吸附率十分相近。这说明在pH值小于4.5时,微塑料对普萘洛尔的吸附效果较好,并且微塑料与针铁矿的界面作用也较弱。共存体系中随pH的继续增加,普萘洛尔的吸附率有所上升,这可能是因为H+浓度降低,竞争作用减弱,使得吸附率增加[26]。
-
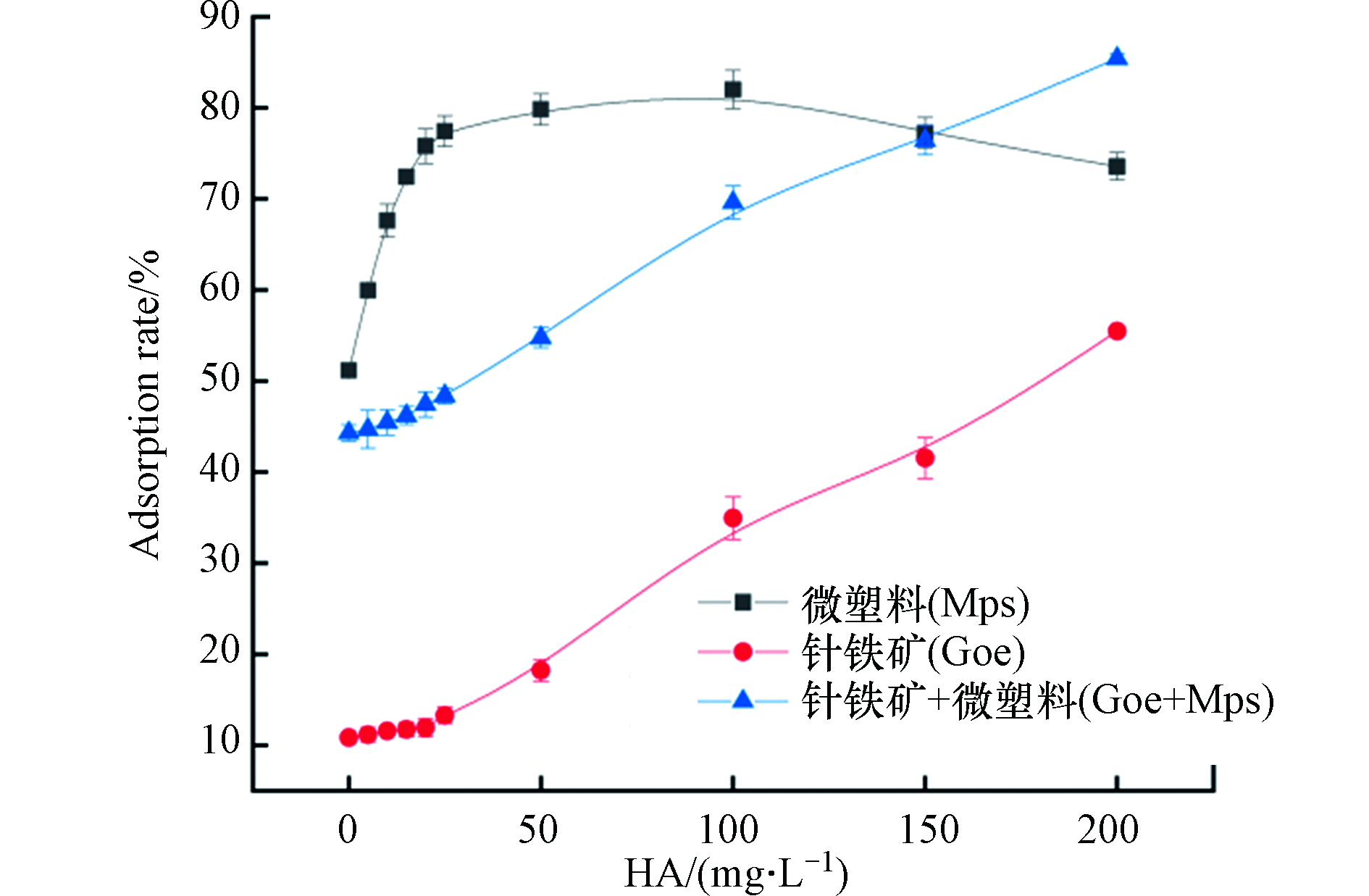
近年来,自然水体富营养化程度不断加强,水生植物及浮游植物的大量生长,导致水体环境中腐殖酸的含量增加。据相关研究表明,腐殖酸会吸附在针铁矿上[29]。此外,腐殖酸可通过疏水性作用、静电相互作用、氢键作用、离子交换及表面络合等作用与有机污染物进行反应[30]。因此,本文将腐殖酸作为一项重要影响因素进行了研究。由图4可看出,腐殖酸的加入促进了普萘洛尔的吸附,且随着腐殖酸浓度的增加,普萘洛尔的吸附率均有一定程度的增加。这可能是因为腐殖酸自身对普萘洛尔具有一定的吸附能力,且腐殖酸会吸附在吸附剂上,促进普萘洛尔在吸附剂表面的吸附,从而降低了普萘洛尔的浓度[31]。由4图可知,当腐殖酸浓度达到200 mg·L−1时,普萘洛尔在针铁矿上的吸附率提高了46%,在针铁矿与微塑料共存体系中的吸附率提高了42%,但在微塑料上的吸附率只提高了22%。当腐殖酸浓度超过150 mg·L−1时,针铁矿与微塑料共存体对普萘洛尔的吸附率超过了微塑料体系,说明高浓度的腐殖酸抑制普萘洛尔在微塑料体系上的吸附。此外,在腐殖酸浓度低于150 mg·L−1时,微塑料单独体系对普萘洛尔的吸附率仍为最高,针铁矿与微塑料混合体系中,普萘洛尔的吸附率低于微塑料体系的吸附率。这说明腐殖酸的加入并不能抑制针铁矿和微塑料之间的界面作用。
-
在自然环境中普萘洛尔以阳离子形式存在,可能会与自环境中的阳离子产生竞争吸附。从图5可知,Na+对普萘洛尔吸附的影响不大,但随Ca2+浓度的增加,普萘洛尔的吸附率均有所降低,是因为Ca2+结合阳离子交换位点的能力较强,表明Ca2+与普萘洛尔之间产生了较强的竞争作用。
-
普萘洛尔在针铁矿、微塑料以及针铁矿和微塑料共存时的吸附动力学均符合伪二阶吸附动力学模型,吸附等温模型均符合Langmuir吸附模型,说明普萘洛尔在3种情况下的吸附行为主要以单层吸附为主。pH值在2—10范围内变化时,普萘洛尔在针铁矿上的吸附率随着pH的增高先降低后增加,其可能的吸附机理主要为静电引力;而普萘洛尔在微塑料上的吸附率随pH的升高逐渐增加,推测主要吸附机理为疏水相互作用和阳离子交换作用;在针铁矿和微塑料共存体系中,普萘洛尔的吸附率随pH的增高逐渐增加,但整体吸附率偏低,这可能是针铁矿和微塑料之间存在的静电作用抑制了普萘洛尔的吸附。当腐殖酸存在时,普萘洛尔在不同体系中的吸附率均有明显升高。当体系中加入Ca2+时对普萘洛尔的吸附表现出较高的抑制性。
微塑料对普萘洛尔在针铁矿上吸附的影响
Effect of microplastics on the adsorption of propranolol on goethite
-
摘要: 本实验研究了微塑料对新兴污染物普萘洛尔在针铁矿上吸附行为的影响,探讨了针铁矿、微塑料及微塑料共存时针铁矿对普萘洛尔的吸附动力学、吸附等温线,考察了pH、腐殖酸浓度、离子强度对普萘洛尔吸附行为的影响。结果表明,普萘洛尔在3种吸附剂上的吸附动力学均符合伪二阶动力学模型,吸附等温线均符合Langmuir等温吸附模型。对比发现,普萘洛尔的吸附效率顺序为微塑料>微塑料+针铁矿>针铁矿。溶液pH值在2—6时,微塑料对普萘洛尔在针铁矿上吸附的影响较弱,当溶液pH值大于6时,微塑料能显著影响普萘洛尔在针铁矿上吸附。普萘洛尔在3种吸附剂上的吸附量随着腐殖酸浓度的增加而增加,且对普萘洛尔在针铁矿上吸附的促进作用最强。同时,Ca2+的加入对普萘洛尔的吸附抑制较强。本文的研究结果可为全面认识微塑料共存时污染物在环境中的迁移行为提供基础数据。Abstract: This study focused on investigating the influence of microplastics on the adsorption behavior of the emerging pollutant propranolol on goethite. The adsorption kinetics and isotherms of propranolol on goethite, microplastics, and their mixed adsorbents were studied respectively. Besides, the effects of pH, humic acid concentration, and ionic strength on the adsorption efficiency of propranolol were also investigated. Results indicated that the pseudo-second-order model and Langmuir isotherm model fitted the adsorption process well. Moreover, the adsorption efficiency order of propranolol follows microplastics > microplastics + goethite > goethite. Furthermore, compared with the pH range of 2—6, a pH higher than six was more helpful for the adsorption of propranolol on goethite by microplastics. In addition, the adsorption efficiency of propranolol in the above three adsorbents was all promoted with the addition of humic acid, and the goethite exhibited the best. Meanwhile, the adsorption efficiency of propranolol was hampered in the presence of cation, especially for the addition of Ca2+. This study provided basic data for a comprehensively understanding of the migration behavior of propranolol in the environment when microplastics coexist.
-
Key words:
- propranolol /
- goethite /
- microplastics /
- adsorption /
- interaction
-

-
表 1 普萘洛尔在3种吸附剂上的吸附动力学参数
Table 1. Adsorption kinetic parameters of propranolol on three adsorbents
吸附剂
Sorbent伪一阶动力学模型
Pseudo-first-order equation伪二阶动力学模型
Pseudo-second-order equationK1/(L·min−1) qe/(mg·g−1) R2 K2/(g·(g·min)−1) qe/(mg·g−1) R2 针铁矿 0.0872 0.2504 0.7371 0.8981 0.2532 0.8524 微塑料 0.0720 1.3564 0.7675 0.1262 1.3754 0.9924 针铁矿+微塑料 0.0936 1.1325 0.7246 0.2211 1.1440 0.9686 表 2 普萘洛尔在3种吸附剂上的吸附等温线参数
Table 2. Adsorption isotherm parameters of propranolol on three adsorbents
吸附剂
SorbentLangmuir Freundlich KL/(L·mg−1) qm/(mg·g−1) R2 KF/ (mg·g−1) n R2 针铁矿 0.1817 0.5124 0.9970 0.1746 3.9432 0.9469 微塑料 0.2325 2.6838 0.9933 0.7480 2.9700 0.9659 针铁矿+微塑料 0.3813 0.9203 0.9835 0.3770 4.3159 0.9164 -
[1] ZHAO X, ZHENG Y, HU S, et al. Improving urban drainage systems to mitigate PPCPs pollution in surface water: A watershed perspective [J]. Journal of Hazardous Materials, 2021, 411: 125047. doi: 10.1016/j.jhazmat.2021.125047 [2] LI L, ZHAO X L, LIU D, et al. Occurrence and ecological risk assessment of PPCPs in typical inflow rivers of Taihu lake, China [J]. Journal of Environmental Management, 2021, 285: 112176. doi: 10.1016/j.jenvman.2021.112176 [3] HAMID N, JUNAID M, WANG Y, et al. Chronic exposure to PPCPs mixture at environmentally relevant concentrations (ERCs) altered carbohydrate and lipid metabolism through gut and liver toxicity in zebrafish [J]. Environmental Pollution, 2021, 273: 116494. doi: 10.1016/j.envpol.2021.116494 [4] 邓月华. 改性凹凸棒土吸附去除水中铅离子、心得安及溶解性有机物[D]. 南京: 南京大学, 2012. DENG Y H. Adsorption and removal of lead ion, propranolol and dissolved organic matter in water by modified attapulgite [D]. Nanjing: Nanjing University, 2012(in Chinese).
[5] NIE W J, LI Y N, CHEN L Y, et al. Interaction between multi-walled carbon nanotubes and propranolol [J]. Scientific Reports, 2020, 10(1): 1025-1029. doi: 10.1038/s41598-020-57894-y [6] KUMAR R, SARMAH A K, PADHYE L P. Fate of pharmaceuticals and personal care products in a wastewater treatment plant with parallel secondary wastewater treatment train [J]. Journal of Environmental Management, 2019, 233: 649-659. [7] VALDES M E, AME M V, BISTONI M D L A, et al. Occurrence and bioaccumulation of pharmaceuticals in a fish species inhabiting the Suquía River basin (Córdoba, Argentina) [J]. Science of The Total Environment, 2014, 472: 389-396. doi: 10.1016/j.scitotenv.2013.10.124 [8] BARBIERI M, LICHA T, NOEDLER K, et al. Fate of β-blockers in aquifer material under nitrate reducing conditions: batch experiments [J]. Chemosphere, 2012, 89(11): 1272-1277. doi: 10.1016/j.chemosphere.2012.05.019 [9] MADUREIRA T V, ROCHA M J, CRUZEIRO C, et al. The toxicity potential of pharmaceuticals found in the Douro River estuary (Portugal): Evaluation of impacts on fish liver, by histopathology, stereology, vitellogenin and CYP1A immunohistochemistry, after sub-acute exposures of the zebrafish model [J]. Environmental Toxicology & Pharmacology, 2012, 34(1): 34-45. [10] VIENO N M, TUHKANEN T, KRONBERG L. Analysis of neutral and basic pharmaceuticals in sewage treatment plants and in recipient rivers using solid phase extraction and liquid chromatography-tandem mass spectrometry detection [J]. Journal of Chromatography A, 2006, 1134(12): 101-111. [11] STANLEY J K, RAMIREZ A J, MOTTALEB M, et al. Enantiospecific toxicity of the beta-blocker propranolol to Daphnia magna and Pimephales promelas [J]. Environmental Toxicology & Chemistry, 2006, 25(7): 1780-1786. [12] DASH B, DASH B, RATH S S. A thorough understanding of the adsorption of Ni (Ⅱ), Cd (Ⅱ) and Zn (Ⅱ) on goethite using experiments and molecular dynamics simulation [J]. Separation and Purification Technology, 2020, 240: 1-14. [13] 尹雪斐, 杨蕊嘉, 刘玉玲, 等. Cd(Ⅱ)与As(Ⅴ)在土壤铁氧化物和细菌表面上的共吸附研究 [J]. 生态环境学报, 2021, 30(03): 614-620. YIN X F, YANG R J, LIU Y L, et al. CO adsorption of CD (Ⅱ) and as (Ⅴ) on soil iron oxides and bacterial surfaces [J]. Journal of ecological environment, 2021, 30(03): 614-620(in Chinese).
[14] ZHAO Y, LIU F, QIN X P. Adsorption of diclofenac onto goethite: Adsorption kinetics and effects of pH [J]. Chemosphere, 2017, 180: 373-378. doi: 10.1016/j.chemosphere.2017.04.007 [15] FILEP T, SZABOÓ L, KONDOR A C, et al. Evaluation of the effect of the intrinsic chemical properties of pharmaceutically active compounds (PhACs) on sorption behaviour in soils and goethite [J]. Ecotoxicology and Environmental Safety, 2021, 215: 112120. doi: 10.1016/j.ecoenv.2021.112120 [16] LI J C, ZHAO L, ZHANG R C, et al. Transformation of tetracycline antibiotics with goethite: Mechanism, kinetic modeling and toxicity evaluation [J]. Water Research, 2021, 199: 117196. doi: 10.1016/j.watres.2021.117196 [17] ALIMI O S, BUARZ J F, HERNANDEZ L M, et al. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport [J]. Environmental Science & Technology, 2018, 52(4): 1704-1724. [18] GUO X T, HU G L, FAN X Y, et al. Sorption properties of cadmium on microplastics: The common practice experiment and A two-dimensional correlation spectroscopic study [J]. Ecotoxicology and Environmental Safety, 2020, 190: 110118. doi: 10.1016/j.ecoenv.2019.110118 [19] YU F, YANG C F, HUANG G Q, et al. Interfacial interaction between diverse microplastics and tetracycline by adsorption in an aqueous solution [J]. Science of the Total Environment, 2020, 721: 13772-13779. [20] HUANG D F, XU Y B, YU X Q, et al. Effect of cadmium on the sorption of tylosin by polystyrene microplastics [J]. Ecotoxicology and Environmental Safety, 2021, 207: 111255. doi: 10.1016/j.ecoenv.2020.111255 [21] RAZANAJATOVO R M, DING J N, ZHANG S S, et al. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics [J]. Marine Pollution Bulletin, 2018, 136: 516-523. doi: 10.1016/j.marpolbul.2018.09.048 [22] PUCKOWSKI A, CWIĘK W, MIODUSZEWSKA K, et al. Sorption of pharmaceuticals on the surface of microplastics [J]. Chemosphere, 2021, 263: 127976. doi: 10.1016/j.chemosphere.2020.127976 [23] 郭学涛. 针铁矿/腐殖酸对典型抗生素的吸附及光解机理研究[D]. 广州: 华南理工大学, 2014. GUO X T. Study on adsorption and photolysis mechanism of goethite / humic acid on Typical Antibiotics [D]. Guangzhou: South China University of Technology, 2014(in Chinese).
[24] DENGG Y H, LI Y N, NIE W J, et al. Fast removal of propranolol from water by attapulgite/graphene oxide magnetic ternary composites [J]. Materials, 2019, 12(6): 924. doi: 10.3390/ma12060924 [25] LUO Y Y, ZHANG Y Y, XU Y B, et al. Distribution characteristics and mechanism of microplastics mediated by soil physicochemical properties [J]. Science of the Total Environment, 2020, 726: 138389. doi: 10.1016/j.scitotenv.2020.138389 [26] 邓月华, 王文姬, 贺艳, 等. 普萘洛尔在太湖沉积物上的吸附特征 [J]. 环境化学, 2021, 40(1): 263-271. doi: 10.7524/j.issn.0254-6108.2020060104 DENG Y H, WANG W J, HE Y, et al. Adsorption of propranolol on Taihu Lake sediments [J]. Environmental Chemistry, 2021, 40(1): 263-271(in Chinese). doi: 10.7524/j.issn.0254-6108.2020060104
[27] LEUNG K, CRISCENTI L J. Lead and selenite adsorption at water–goethite interfaces from first principles [J]. Journal of Physics:Condensed Matter, 2017, 29(36): 365101. doi: 10.1088/1361-648X/aa7e4f [28] ZHANG Y, LUO Y, GUO X, et al. Charge mediated interaction of polystyrene nanoplastic (PSNP) with minerals in aqueous phase [J]. Water Research, 2020, 178: 115861. doi: 10.1016/j.watres.2020.115861 [29] WANG L, LI Y T, WENG L P, et al. Using chromatographic and spectroscopic parameters to characterize preference and kinetics in the adsorption of humic and fulvic acid to goethite [J]. Science of the Total Environment, 2019, 666: 766-777. doi: 10.1016/j.scitotenv.2019.02.235 [30] CHIANESE S, FENTI A, IOVINO P, et al. Sorption of organic pollutants by humic acids: A review [J]. Molecules, 2020, 25(4): 918. doi: 10.3390/molecules25040918 [31] 许佳瑶, 孙红文, 汪磊. β-受体阻断剂在粘土上的吸附行为 [J]. 环境化学, 2013, 32(11): 2109-2114. doi: 10.7524/j.issn.0254-6108.2013.11.013 XU J Y, SUN H W, WANG L. Adsorption behavior of β-receptor blocker on clay [J]. Environmental Chemistry, 2013, 32(11): 2109-2114(in Chinese). doi: 10.7524/j.issn.0254-6108.2013.11.013
-



 下载:
下载: