-
随着我国经济的迅速发展,工业化和城市化的快速推进,大气污染已成为我国可持续发展所面临的主要环境问题之一[1]。大气污染问题在京津冀、长三角、珠三角、汾渭平原等城市群尤为突出,其对人体健康、大气环境和全球气候等方面具有重要影响[2-3]。研究表明,二次有机气溶胶(secondary organic aerosol,SOA)是灰霾的重要组成部分,2013年Huang等[1]通过北京、上海、西安、广州这四大城市重霾期间的外场观测,发现SOA分别占有机气溶胶(organic aerosol,OA)的44%—71%和PM2.5的30%—77%。已有的模型研究发现,SOA的模拟值远小于外场观测结果,其原因可能是由于模型中缺少对SOA贡献较大的前体物[4-5]。近年来,外场观测和实验室研究皆表明中等挥发性有机物(intermediate volatility organic compounds,IVOCs)对SOA具有重要贡献[5-6]。
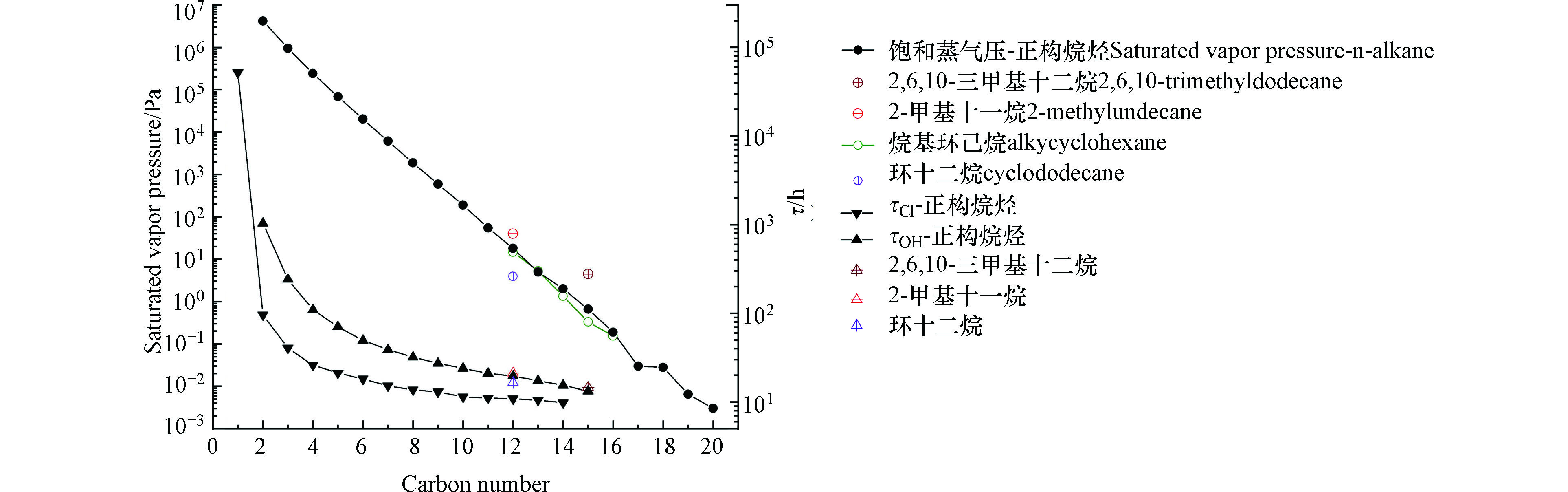
中等挥发性有机物的饱和蒸气压介于挥发性有机物(volatile organic compounds,VOCs)和难挥发性有机物之间。IVOCs主要存在于气相,少量分配在颗粒相。目前我国国标中还没有给出中等挥发性有机物的具体定义。北京大气污染物综合排放标准(DB 11/501-2007)采用欧标中对挥发性有机物VOCs的定义,将293 K下饱和蒸气压≥10 Pa的有机化合物称为VOCs。因此按照该标准,IVOCs的饱和蒸气压上限为10 Pa。国际上Donahue等[7]根据有效饱和蒸气压浓度对于IVOCs(103 μg·m−3≤C*≤106 μg·m−3)进行定义。以正构烷烃为例,正十二烷的饱和蒸气压为16 Pa,我国生态环境部(HJ 1010-2018)关于VOCs监测规范将正十二烷划入了57种VOCs中。因此,这里将中等挥发性有机物定义为298 K下饱和蒸气压在0.01 Pa—10 Pa的有机化合物,相当于C12—C20长链烷烃的饱和蒸气压范围。图1展示了C1—C20烷烃在298 K下的饱和蒸气压及其与OH自由基、Cl原子反应的大气寿命。除了长链烷烃以外,IVOCs还包括倍半萜烯,多环芳烃(polycyclic aromatic hydrocarbons,PAHs),单环芳烃,酚类化合物以及杂环化合物(表1)。
-
国内目前关于IVOCs的综述中,谭鑫等[51]和唐荣志等[52]都总结了大气中IVOCs的主要测量技术及研究进展,本章主要从离线测量技术和在线测量技术两个方面来介绍。
-
IVOCs的离线分析方法一般是基于毛细管电泳技术、气相色谱(gas chromatography,GC)、液相色谱、离子色谱以及色谱与质谱(mass spectra,MS)的联用技术。离线分析中,气相采样主要通过不锈钢罐、聚氟乙烯袋以及固体吸附管收集,颗粒采集主要通过膜采样器或者微孔均匀沉积式多级碰撞采样器等分级撞击采样器[52]。传统的预处理方法通常是使用溶剂萃取法(solvent extraction,SE)提取收集到的物质,然后通过气相/高效液相色谱与质谱联用的方法进行分离和检测。用溶剂萃取法进行预处理时,样品容易被污染、部分成分易丢失且耗时长,大量有机溶剂的使用还会对环境造成污染[53]。热脱附(thermal desorption,TD)是另一种预处理技术,它使用升温方法将有机物蒸发出来,以用于后续的GC-MS检测,操作方便且无需使用溶剂[54]。
然而,实际大气环境成分十分复杂,常规的一维GC无法实现对大气组分的完全分离和鉴别,尤其是痕量物种,会由于分离力不足而导致大量的峰共洗脱以及低色谱分辨率[55]。近年来,二维气相色谱(GC×GC)技术的出现为分析未识别复杂混合物提供了方向,它可以洗脱数千种可用质谱表征的化合物。GC×GC是一种色谱联用技术,通过将两根分离机制不同的色谱柱以串联的方式连接起来进而提高分离度[56]。Alam等[56]总结了GC×GC运用在外场观测时常用的检测器,最普遍的是飞行时间质谱(time of flight-mass spectrometer,tof-MS),其他常用的检测器还有氢火焰离子检测器(flame ionization detector,FID)、氮化学发光检测器和氮磷检测器等。传统质谱的电离源采用70 eV的电子轰击离子源(electron impact,EI)对有机物进行电离,但过高的能量会生成大量碎片。虽然EI有专门的质谱数据库可以比对,但由于缺少分子离子峰和有机物(如脂肪烃)的非特异性碎片,许多化合物仍然无法鉴别。为了解决这一问题,可以选择能量较低的软电离技术以保留分子离子峰来帮助化合物的识别:(1)电子捕获负化学电离法。该技术已被用于GC×GC-tof-MS的电离源来分析C8—C14的多氯代直链烷烃。(2)共振增强多光子离子化。使用强烈的紫外线激光脉冲进行电离,已被证明对于芳香族化合物具有高度的响应性与选择性,但不适合分析脂肪烃;(3)单光子电离。利用真空紫外光子进行电离,适合对环境样品进行全面分析,可以实现颗粒物中长链烷烃,链烷酸,芳烃,氧化的PAHs和硝基芳族化合物的无碎片电离,与共振增强多光子离子化技术可以互补使用。(4)GC与并联的电子轰击质谱(electron impact mass spectrometer,EI-MS)和场电离质谱(field ionization mass spectrometer,FI-MS)结合的方法。两台质谱会生成时间上同步的等效色谱图,从而可以将碎片离子(来自EI-MS)与分子离子(来自FI-MS)对应起来分析。(5)BenchTOF-Select-MS(Markes International, Llantrisant,UK)与GC×GC联用。它保持传统EI 70 ev的高电势差使电子从带负电的灯丝表面加速到带正电的离子室,但会在到达离子室之前降低加速电子的能量,这样可使电离能在10—70 eV的范围内变化来达到软电离的目的。
虽然离线测量对物种鉴别的能力非常高,但其能识别出的IVOCs物种有限,且前处理流程复杂,一般需要的样品量较大,样品在采样膜上停留时间长可能造成已采集的易挥发或不稳定物质消失,时间分辨率低,不能实时反映大气组分变化。
-
近年来,在线测量方法不断发展,具有时间分辨率高、自动化、节省人力和时间成本、实时反映大气组分等优点。在线测量方法主要有热解吸气相色谱(thermal desorption aerosol GC/MS-FID,TAG)、化学电离质谱(chemical ionization mass spectrometry,CIMS)和质子转移反应质谱(proton transfer reaction mass spectrometry,PTR-MS)等。
Williams等[57]搭建了热解吸气相色谱TAG用于对大气颗粒中的有机化合物进行自动、高时间分辨率的收集和GC-MS分析。随后他们搭建了基于GC×GC的二维TAG,提高了各种化合物的分离度,与一维TAG相比有了很大的改进[58]。之后又开发了可以准确定量蒸气压低于十四烷的有机物的新型SV-TAG[59]。化学电离质谱CIMS的特点有:(1)直接测量,响应时间短。(2)化学电离源是一种软电离技术,可以减少碎片峰,提供分子层面的信息。(3)通过切换合适的化学电离试剂,可以实现高选择性,适合复杂大气的监测[60]。Yatavelli等[61]研制了可以同时检测气相和颗粒相,并且能同时对所检测物质进行定性和定量分析的微米孔进样挥发撞击高分辨化学电离质谱(MOVI-HRToF-CIMS)。气体与气溶胶进样口耦合的FIGAERO-HRToF-CIMS也是一种可以同时测量气相与颗粒相化合物的在线仪器[62]。质子转移反应质谱PTR-MS具有高时间分辨率、高灵敏度、低检测限的特点,被广泛运用于外场观测[63]。Eichler等[64]将“气溶胶在线分析入口”(CHARON)与PTR-MS耦合,使原本只能在线检测气相有机物的PTR- MS也能检测气溶胶化学组分。
-
IVOCs中一部分是碳氢化合物,主要由一次源排放;另一部分是含杂原子(O、N等)的有机化合物,其中有来自于一次源排放的,也有来自于二次源光化学反应的。一次源排放的IVOCs是SOA的重要前体物[5],IVOCs的一次源主要包括化石燃料的燃烧和蒸发、生物质燃烧、挥发性化学产品(如农药、涂料、印刷油墨、清洁剂、个人护理产品等)等人为源排放,以及畜牧业、植物(如陆生高等植物、水生植物、浮游生物)和细菌等生物源排放。
与化石燃料有关的一次源排放,包括煤炭燃烧,汽油、柴油和煤油等燃油的燃烧和蒸发等,尤其是使用燃油的交通工具(道路和非道路车辆、轮船、飞机等)的尾气排放占了绝大部分[65-67]。燃油类型、行驶工况、尾气催化后处理装置、启动方式、发动机运转状态(如怠速工况)等都对交通工具排放IVOCs有重要影响[68-72]。Wang等[73]基于排放因子(emission factors,EFs)方法估算了长三角地区2017年车辆的IVOCs排放,卡车的IVOCs排放量占总IVOCs排放的70%以上,柴油车的IVOCs排放量远远高于汽油车。使用重质燃料油(heavy fuel oil,HFO)的大型轮船的IVOCs排放因子远大于汽油车,与柴油车相当;全球船舶的IVOCs排放量占陆地交通IVOCs排放量的四分之一,并且已超过汽油车的排放总量[74]。飞机使用的航空煤油几乎都是从原油的中间馏分中提取的,该馏分的碳原子数一般在C8-C16之间,低分子量多环芳烃和C12以上的长链烷烃是飞机尾气中的重要部分[75]。生物质燃烧包括木材燃烧、谷物秸秆燃烧、垃圾焚烧、家庭烹饪等,该过程不仅是含氧中等挥发性有机物(oxygenated-intermediate volatility organic compounds,O-IVOCs)如酚类化合物的重要来源,也是PAHs和长链烷烃的重要来源[76]。木材中的木质素热解会生成大量甲氧基苯酚,如2,6-二甲氧基苯酚和愈创木酚(2-甲氧基苯酚),2,6-二甲氧基苯酚普遍存在于落叶类植物的燃烧排放中,而愈创木酚则广泛存在于针叶类植物和落叶类植物的燃烧排放中[77]。建筑和工业涂料、印刷油墨、粘合剂、抛光剂、空气清新剂、香水、乳液等日常用品的使用过程中也会释放IVOCs,其中最常见有醇、酯、酮类等O-IVOCs。许多此类挥发性化学产品的VOCs排放因子比汽油车尾气高出1—2个数量级。Mcdonald等[78]根据外场观测和模型结果给出了这些挥发性化学产品中IVOCs的占比,其中直链烷烃占总挥发性有机物(total volatile organic compounds,TVOCs)的1.52%,支链烷烃占2.52%,环烷烃占1.53%,芳香烃占0.62%,O-IVOCs占4.75%。生物源中的畜牧业、植物和微生物等也会向大气中释放IVOCs,如倍半萜烯、酚类化合物、草酸、直链烷烃、吲哚等[79-82]。表2汇总了大气中常见的IVOCs的外场观测研究。包括国内外的城市、郊区、农村、森林、海洋和极地等地区。
IVOCs在气相和颗粒相的分配系数Kp是根据物理吸附和化学吸收原理定义的,与纯物质过冷液体状态下的饱和蒸气压有关[93]。Kp的理论值如下:
其中,Ns(site·cm−2)代表吸附位点(site)的表面积浓度,atsp(m2·g−1)代表颗粒物的比表面积,Q1(kJ·mol−1)代表从表面解吸的解吸焓,QV(kJ·mol−1)代表纯物质过冷液体的蒸发焓,
$ {P}_{L}^{0} $ (torr)代表纯物质过冷液体的饱和蒸气压,fom代表有机吸收相的质量分数,MWom代表有机吸收相的平均分子量,$ \xi $ 代表物种在有机吸收相的活度系数,R为理想气体常数,T为环境温度。该公式可简化为:Kp的实测值为:
当气粒分配平衡时,理论上mr会趋近于−1。目前已经许多文章测得了多环芳烃和正构烷烃的分配系数[94-96],将实测的Kp值代入理论方程拟合出的mr的绝对值大于1,则该地的气粒分配机理以物理吸附为主,若mr绝对值小于0.6,则以化学吸附为主[97]。若某物种的Kp×
$ {C}_{\mathrm{T}\mathrm{S}\mathrm{P}} $ >1,则物种主要分配在颗粒相,若Kp×$ {C}_{\mathrm{T}\mathrm{S}\mathrm{P}} $ <1,则物种主要分配在气相[95]。处于IVOCs蒸气压范围下限的菲在外场观测中的Kp×$ {C}_{\mathrm{T}\mathrm{S}\mathrm{P}} $ 在0.003—0.306之间($ {C}_{\mathrm{T}\mathrm{S}\mathrm{P}} $ =50 μg·m−3)[95],同样处于IVOCs蒸气压范围下限的十九烷的Kp×$ {C}_{\mathrm{T}\mathrm{S}\mathrm{P}} $ 在0.0150—0037之间($ {C}_{\mathrm{T}\mathrm{S}\mathrm{P}} $ ≈70 μg·m−3)[96],表2也给出了典型IVOCs分别在气相和颗粒相的浓度,结果表明大部分IVOCs都处于气相中。在研究气粒分配的外场观测中一般确保温度变化在10 ℃以内作为一个观测时间段,可以使用克劳修斯-克拉佩龙方程拟合气相浓度对于温度的依赖性[94, 98]:
其中,P是物质在大气中的分压,将测得的气相浓度通过理想气体状态方程转化为分压P,
$ {\Delta H}_{V} $ 是物质在过冷液体时的蒸发焓,R为理想气体常数,T为环境温度。一般将该式简化为:理论上m应为负值,但许多研究中的m为正值,此时温度对气粒分配的影响不大,斜率的高低与IVOCs排放源的远近有关,当排放源较近时斜率较低,当排放源较远时斜率较高。同时,温度升高会加速IVOCs在大气中的光化学反应,也会因此出现IVOCs气相浓度随着温度升高而降低的现象。
-
Fang等[85]于2019年秋季测量了珠三角地区大气环境中的萘与甲基萘。测得的萘,2-甲基萘和1-甲基萘的气相浓度分别为(94.0±23.1)、(25.3±4.5)、(11.8±2.1)ng·m−3。汽油车排放是萘和甲基萘的最大贡献者(39.1%),其次是生物质/煤燃烧(29.9%),柴油车排放(26.3%)和工业排放(4.7%)。Zhao等[6]在美国加州帕萨迪纳市的观测得出当地一次源IVOCs约占总IVOCs的60%。除公路柴油车外,其他与石油有关的污染源如非道路用车也是IVOCs排放的重要来源。Yang等[99]于2015年3月至2016年2月测得成都空气中气相和颗粒相总PAHs浓度的年度平均值为(300.35±176.6)ng·m−3。春季多环芳烃主要来源的贡献为煤炭(52%),机动车尾气和焦炭(48%);夏季为煤炭(52%),焦炭(21%)和机动车尾气(27%);秋季为煤炭(47%),汽车尾气(34%)和焦炭(19%);冬季则是煤炭(58%)和汽车尾气(42%)。Wu等[84]在武汉-鄂州地区的城乡结合部测量了当地16种PAHs的大气浓度,呈现明显的空间变化,与地理环境显著相关,并受到人为活动的影响。Wang等[63]利用高分辨NO+-PTR-MS分别对广州市区和华北平原农村区域的长链烷烃(C8—C21)进行了测量,其结果表明长链烷烃分别占广州市区和华北平原农村SOA贡献的(7.0±8.0)%和(7.1±9.5)%,与单环芳烃和萘的贡献相当甚至更高。Xu等[83]在伦敦市中心的3个观测点采用TD-GC×GC-tof-MS对气相和颗粒相中的C13—C36的长链烷烃和C10—C24的单环芳烃同时进行了鉴定和量化,城市空气和柴油机尾气中C12以上的脂肪族和芳香族组成的相似性表明了柴油车对城市空气质量的影响。Yan等[79]运用GC-FID和GC-MS研究了2012—2013年上海市区直链烷烃的潜在区域贡献源,冬春季以人为源(华北平原和华东地区)为主导;而夏秋季气温较高,逐渐向陆生植物蜡排放(安徽、浙江和江西)转变。佳乐麝香是空气、废水厂和地表水中含量最丰富的人工合成麝香,广泛应用于个人护理产品中,包括肥皂、洗发水、洗涤剂,以及除臭剂、空气清新剂和香水蜡烛等香水产品。Wong等[100]分别在加拿大安大略省20个家庭和办公室的室内、3个城乡样带室外、8个污水处理厂进行空气被动采样,在多伦多市和安大略湖附近农村进行主动采样,他们的结果发现,污水处理厂和室内环境可能是大气中佳乐麝香等人工合成麝香的主要来源。
-
Liu等[101]在江苏一工厂附近的农村采样发现当地的总多环芳烃主要来源于化石燃料和生物质的燃烧;二苯并呋喃类化合物主要来源于工业活动和燃烧过程。Chen等[102]测量了山西农村由于住宅燃料燃烧排放出的PAHs的室内浓度,该研究发现木材和泥炭的使用会导致最严重的PAHs污染和个人暴露。Chan等[82]对美国科罗拉多州曼尼托森林的生物排放进行了观测,观测到的生物源IVOCs包括长叶烯和丁香烯等倍半萜烯,人为源IVOCs包括C14—C19长链烷烃,以菲和联苯为主的多环芳烃以及香豆素和二苯并呋喃等O-IVOCs。Yee等[103]使用SV-TAG分别在亚马逊的雨季和旱季进行了观测,定性和定量了30种倍半萜烯,平均浓度在0.01—6.04 ng·m−3。
-
草酸也是一种中等挥发性有机物,是海洋气溶胶中含量最高的二羧酸。Miyazaki等[81]在北太平洋西部偏远地区研究了海洋生物对海洋气溶胶的贡献,发现在受海洋生物影响较大的气溶胶中,草酸的平均浓度是受海洋生物影响较小的气溶胶中的两倍。草酸的二次生成可能是通过海洋生物排放的长链二酸和乙醛酸在气溶胶水相中的氧化得到的。González-Gaya等[88]鉴定了大西洋、太平洋和印度洋对应的大气和海水样本中64种PAHs,并进行了PAHs大气-海洋通量的全球评估。PAHs在北大西洋的气相浓度最高,南大西洋、印度洋和太平洋的平均气相浓度相似,但在靠近大陆的海洋区域浓度更高。Singh等[104]研究了2—6月北极气溶胶中的PAHs(芴、菲等)、芳香酸(苯甲酸等)、芳香酮(二苯甲酮等)和芳杂环化合物(二苯并噻吩)等IVOCs的分布和来源。在极地日出时,北极上空运输的多环芳烃发生强烈的光化学降解。尽管春季芳香酮减少,但在极地日出期间由于芳香烃氧化而使某些芳香酸(例如苯甲酸和邻苯二甲酸)的浓度增加;同时,北极日出时还会生成大量的草酸。
-
汽油和柴油分别由C4—C10和C9—C25的复杂烃类混合物组成,因此车辆排放的尾气中也会存在这些烃类和它们热分解的产物[68]。Tang等[72]研究了中国道路汽油车中IVOCs的排放及其SOA生成潜势,其中B14—B16化合物(相当于C14—C16正构烷烃的挥发性分布)的贡献较大,正构烷烃的比例较高。Zhao等[12, 105]分别测试了道路和非道路用的柴油和汽油发动机排放IVOCs的特征。汽油车尾气中萘和C11—C14的取代萘占据已识别IVOCs的主导地位,伴随少量的长链烷烃;未识别IVOCs中主要是环状化合物,其次是支链化合物。C12—C20的烷烃占了柴油尾气中的主要部分,伴随着少量的多环芳烃、单环芳烃和烯烃,在未识别复杂混合物中大部分是环烷烃(75%),支链烷烃的比例小于20%。Alam等[106]运用软电离的GC×GC-tof-MS分别鉴定了柴油、润滑油和柴油车尾气,发现无环和单环烷烃不仅是柴油(76%)的主要成分,还是合成润滑油的主要成分(68%);柴油车尾气中的气相成分与柴油的组成极为相似,而颗粒相成分与润滑油的组成极为相似。非道路用车(如农业、林业、工程等非道路用机动机械和拖拉机等)的发动机与道路用车有一定区别,虽然其排放的IVOCs的挥发性分布和化学特性与道路用车类似,但IVOCs的排放因子会由于发动机类型不同而产生差异。非道路用车的发动机包括用于运输制冷单元(transportation refrigeration unit,TRU)的大型柴油发动机,使用汽油或柴油的二冲程和四冲程小型非道路发动机(small off-road engines,SORE)。TRU排放的IVOCs特征与老式卡车柴油发动机类似[12];使用二冲程发动机的非道路用车的IVOCs排放因子远高于其他非道路用车和道路用车发动机[105]。对于轮船和飞机的污染源排放观测,Lou等[71]的研究发现使用HFO的船舶主机排放的IVOCs远多于使用轻柴油(marine gas oil,MGO)的船用辅助发动机;轮船IVOCs的排放因子还与发动机负荷有关,在75%负荷时排放最少;与HFO的组成相比,排气中包含的PAHs比例更高,而烷基环己烷的比例则显著下降,排气中的IVOCs比HFO具有更高的挥发性。船用辅助发动机使用包括来自废食用油(waste cooking oil,WCO)的生物柴油,与MGO相比,可以将IVOC排放降低50%[107]。Agrawal等[75]首次研究了商用飞机的排放特征。研究发现,飞机涡轮发动机的排放率高度依赖于功率。萘类PAHs在总排放的PAHs中占据主要地位。在怠速工况下,长链烷烃的排放量最大。
工业锅炉是多环芳烃重要的排放源,Li等[108]研究了25个工业锅炉排放的PAHs。其中21台锅炉使用重油,2台使用柴油,1台使用重油/天然气的混合燃料,1台使用焦炉气/高炉煤气的混合燃料。烟囱排放的烟气中低分子量PAHs在总PAHs中占主导地位,萘是烟气中含量最丰富的PAHs。重油锅炉的PAHs排放因子是柴油锅炉和重油/天然气锅炉的4.6倍左右,是焦炉气/高炉煤气锅炉的64倍。化石燃料燃烧中的煤炭燃烧也是IVOCs的排放源之一。Cai等[109]使用PTR-MS研究了中国北方家庭家用低效炉灶中煤炭燃烧的烟气排放,其中含有大量IVOCs,约占排放的非甲烷烃的30%,远高于生物质燃烧排放的比例(约1.5%)。
对于生物质燃烧排放的研究中,Hatch等[110]运用GC×GC-Tof-MS测定了生物质燃烧烟气中的IVOCs,发现邻苯二酚是最丰富的苯二酚异构体,而不同的苯二酚异构体具有燃料依赖性。Dhammapala等[111]研究了秸秆燃烧释放的I/SVOCs的排放因子,这些污染物的EFs通常随着燃烧效率的提高而降低。在城市垃圾焚烧的观测中发现大量偶数碳的长链烷烃,可能主要来源于塑料制品的燃烧[112]。对于其他类型的源排放,Feilberg等[80]使用PTR-MS在丹麦农村猪圈的观测中检测到了对甲酚和吲哚等IVOCs,被认为是引起农村猪圈刺激性气味的关键物质之一。
-
大部分IVOCs都处于气相中,对一次有机气溶胶(primary organic aerosol,POA)的贡献不大,但其对SOA的贡献不容忽视。IVOCs光化学反应的实验室研究对于优化模式模拟有着重要作用。IVOCs一旦被释放到空气中,其主要通过与大气氧化剂发生氧化反应而被转化降解。到目前为止,部分IVOCs与对流层大气中常见的四种氧化剂OH自由基、O3、Cl原子和NO3自由基的反应速率常数及生成SOA的机理已经被报道过(表1)。本部分将按照不同类别IVOCs的反应机理、SOA产率和理化性质等实验室研究来介绍。Gentner等[113]评估了汽油车和柴油车尾气中鉴别出的物质的臭氧生成潜势,其中蒸气压处于IVOCs范围内的烷基苯、长链烷烃具有较高的臭氧生成潜势。Li等[114]研究了十几种IVOCs的臭氧生成潜势,其中含氧IVOCs的臭氧生成潜势普遍较高;此外,随着碳数的增加,正构烷烃的臭氧生成潜势也会随之增加。由于IVOCs的SOA产率普遍都很高,因此它们对SOA的贡献还需要看当地的浓度水平(表2)。
-
C12—C20左右的长链烷烃属于中等挥发性有机物,由于其化学结构中只有饱和共价键,在大气中与OH自由基和Cl原子反应的一级速率常数在10−11—10−10 cm3molecule−1s−1量级,与O3几乎不反应,与NO3自由基的反应慢到可以忽略不计[115]。从表1可以得知,长链烷烃与Cl原子反应的速率常数一般比OH自由基大了30倍左右。大气寿命的计算公式见式(1),
$ {\rm{\tau }}_{i} $ 是有机物种与氧化剂i反应的大气寿命,ki代表有机物种与氧化剂i反应的速率常数,[i]代表氧化剂i的浓度,见附录。如图1所示,在污染严重地区或海边地区,与Cl原子反应是长链烷烃主要的汇,在普通地区OH自由基占主导地位,但Cl原子的作用依旧重要[9]。由于OH自由基和Cl原子引发长链烷烃大气氧化反应的原理都是通过摘氢反应生成烷基自由基,因此Cl原子引发的长链烷烃生成SOA的机理与OH自由基类似。烷基自由基与O2反应生成烷基过氧自由基RO2后再进行后续反应。在低NOx条件下,长链烷烃的氧化是RO2+HO2主导的[116];在高NOx条件下,长链烷烃的氧化是RO2+NO主导的[117]。它们的气相产物一般是碎裂反应产生的小分子醇和醛酮[9],而生成的低挥发性产物会分配到颗粒相中继续反应。支链结构会促进碎裂反应,生成更多的挥发性产物,而环状结构的氧化速度更快,并导致较少的挥发性产物;数个多官能团的氢过氧化物形成过氧半缩醛的颗粒相反应是不同结构长链烷烃初始SOA生长的关键组成部分[116]。国际上普遍认为正十二烷是中等挥发性有机物,将其作为长链烷烃的代表做了许多研究。正十二烷(16 Pa)和己基环己烷(14.67 Pa)在298 K下的饱和蒸气压与我们定义的IVOCs在298 K下的饱和蒸气压的上限(10 Pa)差距不大,因此本章将正十二烷等C12长链烷烃的实验室研究也总结在内。在SOA产率方面,OH自由基引发的C12—C17的长链烷烃生成的SOA产率在20%—88%之间[4, 10, 117-121],Cl原子引发的正十二烷的SOA产率在110%—165%之间[8]。直链、支链和环烷烃等长链烷烃的同系物生成SOA的产率随C原子数的增加而增加[4, 8, 117-120],这是由前体烷烃及其反应产物的挥发性降低导致的[117];对于给定的C原子数,SOA产率遵循环烷烃>直链烷烃>支链烷烃的顺序[10, 117, 121],该顺序主要由烷氧自由基中间体的分解程度和所得产物的性质决定,前体烷烃的挥发性是次要的[117]。对于不同环境条件对SOA产率的影响,Lim等[117, 119]分别研究了有无种子条件下长链烷烃的SOA产率,发现有癸二酸二辛酯种子的情况下SOA产率增加了5%—15%左右。Loza等[10]发现高NOx条件下四种不同结构的C12烷烃的SOA产率略高于低NOx下的产率。虽然长链烷烃主要在交通繁忙的城市地区排放,但其与OH自由基或Cl原子的反应速率允许其向郊区输送,因此无论是NOx浓度高的城市地区还是NOx浓度低的郊区地区,长链烷烃都是SOA的重要贡献源。对于湿度的影响,Lim等[117]发现相对湿度47%的条件下,正十二烷的SOA产率比干燥条件下降低了11%—16%左右;Wang等[8]发现67%的相对湿度下,正十二烷的SOA产率比低湿下小了15%—55%。Lamkaddam等[122]研究了正十二烷SOA产率的温度依赖性,发现温度对SOA产率影响不大,可能是因为十二烷的SOA由足够低的低挥发性产物组成,温度变化不会显著影响产物的冷凝量。在长链烷烃SOA的光学性质方面,Li等[123]研究了高低NOx和有无种子的条件下十二烷、十五烷和十七烷光氧化产生的SOA的复折射率(Refractive Index,RI)。在532 nm波长下,它们生成SOA的RI在1.33—1.57之间;NOx水平引起的反应机理的改变和种子对气粒分配的作用都对SOA的化学组成有重要影响,进而影响到RI等光学性质。Li等[124]的另一项研究证明低温(278 K)下十二烷的氧化反应以低聚反应为主,低温生成的SOA的复折射率实部在波长532 nm和375 nm下明显增强。
高级烯烃也是重要的IVOCs,尤其是端烯烃(1-烯烃),它是污染边界层中重要的甲醛前体物[125]。OH自由基、O3和NO3自由基是烯烃在对流层主要的汇[115]。O3和NO3自由基主要通过对双键的加成反应来引发烯烃的大气氧化,大气寿命在几天左右[15];而OH自由基既可以通过加成通道也可以通过摘氢通道,不同的结构决定了两个通道的占比:与1-烯烃相比,内烯烃和2-甲基-1-烯烃中双键相邻烷基的给电子效应增加了加成通道的比例;随着链长的增加,摘氢通道变得越来越重要。高级烯烃与OH自由基反应的大气寿命大约在几个小时左右[126]。在NOx存在下OH自由基引发的C12—C17的端烯烃生成的SOA产率在46.8%—53.8%之间,C14—C17的内烯烃生成的SOA产率在10.5%—40.5%之间。SOA化学组成包括β-羟基硝酸酯和二羟基硝酸酯,以及由二羟基羰基化合物的颗粒相反应形成的环状半缩醛、二氢呋喃和二聚体[127]。生物源排放的倍半萜烯也是重要的烯烃类IVOCs,但目前实验室研究较少。
对于醛类化合物,OH自由基一般通过摘醛基氢来引发其光化学反应。蒎酮醛是α-蒎烯氧化生成的产率最高的一代产物,它的饱和蒸气压相当于正十五烷[128]。Chacon-Madrid等[128-129]在高低NOx条件下分别研究了OH自由基引发的蒎酮醛的光氧化。高NOx条件下蒎酮醛的SOA产率高于低NOx条件,是由于紫外光下低NOx条件生成的产物易被光解从而造成SOA产率的降低。他们的研究同时也给出了饱和蒸气压相似的十三醛的SOA产率,与蒎酮醛的SOA产率近似。对于酮类化合物,OH自由基一般优先摘取β-氢。Chacon-Madrid等的研究表明,2-十三酮比7-十三酮更易形成SOA,可能是由于7-十三酮的结构导致更多的碎裂通道而造成SOA产率较低[129]。
-
多环芳烃中约C10—C14的部分属于中等挥发性有机物,例如C10—C14的萘和烷基萘、联苯、苊烯、苊、芴、菲等。其中萘、C1-甲基萘和C2-甲基萘是城市大气中最丰富的多环芳烃[130]。因为多环芳烃具有不饱和键,所以OH自由基、Cl原子、O3和NO3自由基都能引发它们的大气氧化反应。苊烯是与四种大气氧化剂的反应速率都较快的一种多环芳烃,尤其是夜间与NO3自由基反应的大气寿命只有7 min左右[27]。
OH自由基、NO3自由基和O3引发萘、甲基萘、苊烯和苊等多环芳烃的反应机理都是它们在芳环上的加成反应,Cl原子引发萘的光氧化也是通过对不饱和键的加成开始的,但Cl原子引发烷基萘、苊等带有一定饱和碳的多环芳烃的光氧化主要通过摘氢通道[130-131]。在高NOx条件下,通过RO2+NO途径形成的烷氧自由基(RO)的解离或通过双环过氧机理生成的开环产物是主要的气相产物;由于低NOx条件下RO2+HO2途径的存在,环保留产物在气相产物中占主导地位[132]。萘环上烷基取代的位置是决定环裂解位点和异构产物分布的关键因素[130]。OH自由基引发的萘的氧化产物主要有萘酚、硝基萘、2-甲酰肉桂醛等。OH自由基、NO3自由基和O3这三种氧化剂引发的苊烯氧化都会生成氧杂萘-2-酮、萘-1,8-二甲醛等[27]。Cl原子与萘反应的产物主要为邻苯二甲酸酐和氯萘;苊、苊烯的氧化中环戊环类反应占主导,苊与Cl原子反应的主产物为苊酮,苊烯与Cl原子反应的主产物为苊酮、苊醌、1,8-萘二甲酸酐等。研究还发现多环芳烃与Cl原子反应生成SOA的产率很高,且大部分产物会分配到颗粒相[131]。这意味着在Cl原子丰富的地区,其对PAHs的氧化及SOA的生成有着很大的影响。萘和甲基萘的SOA产率在不同研究中跨度较大,在2%—181%之间;苊和苊烯的SOA产率分别在3%—11%,4%—13%之间。低NOx条件下萘与甲基萘的SOA产率高于高NOx条件,可能是由于生成的SOA中有大量的环保留产物,具有更低的挥发性。此外,OH自由基浓度、初始PAHs/NO比值、NO2/NO比值以及PAHs种类对SOA形成均有影响[23, 133-134]。Chen等[135]研究了间二甲苯对于OH自由基引发的萘和甲基萘生成SOA产率的影响,结果表明在低NOx条件下,间二甲苯的加入抑制了多环芳烃光氧化的SOA生长速率,但促进了早期粒子的成核。不同PAHs-间二甲苯混合物的SOA生长速率与初始[HO2]/[RO2]比值呈显著正相关,是由于HO2+RO2化学作用增强了SOA的形成;SOA生长速率与初始间二甲苯/NO的比值呈显著负相关,则归因于间二甲苯对OH自由基浓度的抑制。Riva等[136]研究了萘被OH自由基氧化和O3氧化的苊烯生成的SOA的光化学老化。在萘的SOA老化实验中,同时有OH自由基和光照的实验组检测到一些次级产物的形成。苊烯臭氧化生成的SOA的老化实验中,发现了明显的化学演化,H/C整体下降,O/C增加。老化过程中观察到的某些产物的耗尽可能是被臭氧光解生成的OH自由基二次氧化,不同类型的老化(OH自由基反应、光解或两者都有)会导致苊烯SOA组成的显著变化。
木质素是地球上第二丰富的聚合物,酚类是木质素预分解产生的,酚类和甲氧基酚类物质占木材烟雾中颗粒物的很大一部分。它们与NO3自由基、Cl原子和OH自由基反应的速率常数都很快。OH自由基、NO3自由基、O3与酚类化合物的反应都优先在芳环上加成[36, 39, 43, 137],甲氧基和羟基的存在会使OH自由基的加成位点倾向于取代基的邻位和对位;而Cl原子引发的反应是从摘苯环上的羟基H开始的[42]。愈创木酚和2,6-二甲氧基苯酚的SOA产率分别在9.46%—87%和10%—36%之间[43, 138-141]。SO2、种子、NOx都能促进愈创木酚SOA的生成,SO2与无机种子如(NH4)2SO4、NaCl种子对SOA的形成还具有协同作用[140-141]。随着SO2浓度的增加,诱导期变短,与通过酸催化的非均相反应生成低聚物相比,SO2在有机含硫化合物的形成和低分子量物种的形成或吸收中发挥了更重要的作用。对于其他酚类,Nakao等[142]研究了低NOx条件下多种酚类化合物生成的SOA,间甲酚、邻苯二酚、2,4-二甲基苯酚和3,5-二甲基苯酚的SOA产率分别为27%—31%、39%、67%—90%和17%—31%。Liu等[143]研究了OH自由基氧化丁香酚(4-烯丙基-2-甲氧基苯酚)的实验,SOA产量随OH暴露的增加而先升高后降低,并且也取决于丁香酚的浓度。另外,SO2和NO2的加入有利于提高SOA产率,分别能提高到38.6%和19.2%。
酚类化合物的云雾过程,特别是在生物质燃烧排放较多的地区,可能是形成低挥发性、高含氧有机物的重要途径,它们在云或雾蒸发后仍处于颗粒相,影响大气粒子的化学和光学性质。Sun等[144]使用高分辨率飞行时间气溶胶质谱仪、离子色谱和总有机碳分析仪来研究愈创木酚和2,6-二甲氧基苯酚通过液相反应生成SOA的化学性质及其形成机制,气溶胶质谱的谱图提供了通过苯氧自由基的C—C和C—O偶联形成二聚体以及聚合度更高的低聚物的证据。另一种途径是使芳香环羟基化,这是增加SOA氧化程度的一种机制。与酚类化合物的直接光化学反应相比,OH自由基引发的反应有利于生成较小的氧化产物,二聚体或低聚物较少。Kitanovski等[145]研究了愈创木酚的液相光硝化,得到的主要产物是4-硝基愈创木酚,6-硝基愈创木酚和4,6-二硝基愈创木酚。使用分离的化合物作为标准,用HPLC-ESI-MS×MS在来自卢布尔雅那市(斯洛文尼亚)的冬季PM10气溶胶中鉴定出4-硝基愈创木酚和4,6-二硝基愈创木酚,4,6-二硝基愈创木酚可能是大气中棕色碳的重要成分,尤其是在受生物质燃烧影响的地区[146]。有机化合物(如3,4-二甲氧基苯甲醛)的三重激发态(3C*)也是重要的水相氧化剂,在受生物质燃烧影响的地区,3C*是酚类化合物尤其是甲氧基苯酚类化合物在液相生成SOA的重要途径,有助于大气中棕色碳的生成[147]。
-
吲哚是IVOCs中常见的杂环化合物,与OH自由基、O3和NO3自由基的气相一级反应速率常数都很快。Montoya-Aguilera等[148]研究了OH自由基引发的低NOx条件下吲哚的光氧化,得到130%的SOA产率。由此产生的SOA具有很强的光吸收能力,其质量吸收截面(Mass absorption coefficients, MAC)值在可见光谱范围内为0.5 m2·g−1(λ=400 nm)和2 m2·g−1(λ=300 nm),接近于生物质燃烧中具有强烈吸收的棕色碳的MAC值。模拟结果表明,在OH自由基氧化吲哚的驱动下,吲哚SOA的形成具有很大的潜力;在光化学不活跃的深夜和清晨,吲哚与NO3的氧化反应可能也是重要的SOA生成途径。
杂环类化合物的液相反应在大气化学中的也非常重要,Feilberg等[149]研究了不同pH环境下喹啉与OH自由基液相反应的一级速率常数和OH-喹啉加成中间体与O2反应的速率常数。结果表明,喹啉在大气水滴中与OH自由基反应的大气寿命小于1 h。碱性条件下(pH=9)OH自由基加成的一级反应速率常数比酸性条件(pH=3)快了2倍左右;碱性条件下OH-喹啉加成中间体与O2反应的速率常数比酸性条件快了2个数量级,可能是由于在酸性条件下喹啉的液相降解占主导地位。Liu等[150]对二苯并噻吩液相氧化生成SOA进行了研究,结果表明,二苯并噻吩都可以被OH自由基和有机物三重激发态快速降解。在OH自由基光氧化下得到了32%的SOA产率,且生成的SOA具有相对较强的光吸收能力。
-
全球化学传输模型(GEOS-Chem)是目前世界上比较先进的三维全球对流层化学输送模型,已被广泛应用于大气组成成分的研究中。Hodzic等[151]更新了GEOS-Chem中SOA形成机制(半挥发性和中等挥发性有机化合物的壁校正产量和排放量)和清除机制(干湿沉降、光解和非均相氧化)的参数化方案。更新后的模型能更动态地刻画大气SOA的生命周期,其生成速率是更新前基准模型的3.9倍,清除速率是基准模型的3.6倍。与基准模型相比,更新后的模型更符合地面和飞机对有机气溶胶的观测结果。
Eluri等[152]使用基于挥发性分级方法的(volatility basis set,VBS)模型和统计氧化模型(statistical oxidation model,SOM),来模拟Jathar等[69]给出的氧化流动反应器中柴油机在两种不同发动机负载(怠速,负载)、两种燃料类型(柴油、生物柴油)和两种后处理配置(带或不带氧化催化剂和颗粒过滤器)下运行时SOA的形成和组成。利用基于最新实验室结果的参数化方案,基于VBS和SOM方法的两个模型都合理再现实验室得到的半挥发性和反应性一次有机气溶胶;SVOCs、IVOCs和VOCs反应生成的SOA;与氮氧化物相关的参数化方案;多级气相化学;以及气-粒动态分配。模型模拟表明,IVOCs平均占模型预测SOA前体物的70%,但其具体形成机制还需进一步深入研究。通过与CIMS的气相测量结果的比较,发现SOM模型能更好地再现气相有机物组分。Zhao等[153]用2D-VBS箱模型模拟了一系列的SOA形成的烟雾室实验,以确定最符合测量结果的2D-VBS参数,并将最优的参数化方案运用于区域多尺度空气质量模型(community multiscale air quality,CMAQ)中,结果表明,OA老化和IVOCs氧化机制的引入使中国东部地区OA和SOA浓度分别增加约40%和10倍,从而显著提高了模型模拟能力。POA和IVOCs都占OA浓度的40%以上;并且,与之前SOA来源解析结果不同,该研究发现IVOCs氧化对于SOA生成的贡献达到一半以上。Li等[154]使用区域空气质量模型系统(regional air quality modeling system,RAQMS)研究了2014年冬季京津冀地区的有机气溶胶分布,IVOCs对SOA的贡献占了9.1%。模型验证表明,该模型能够合理再现气象要素和主要的气溶胶组分,并且该模型通过考虑S/IVOC和相关的老化过程,可以显著改善SOA和OA的模拟。而Yang等[155]基于VBS的箱模型研究了2014年11月7日至8日在北京市区的SOA形成途径,其中IVOCs对SOA的贡献占了81%。Yao等[156]用扩展的综合空气质量模型(comprehensive air quality model with extensions,CAMx)模拟了珠三角地区SOA浓度,结果表明,基于传统SOA模块模拟的珠三角地区SOA年均浓度约为1—3 μg·m−3,远低于观测到的SOA浓度约为1—7 μg·m−3;而将1D-VBS模块中加入S/IVOCs的氧化机制后,模式模拟的珠三角地区SOA年均浓度约2—8 μg·m−3。珠三角地区的SOA主要来自该区域的机动车排放。生物质燃烧的贡献在17.4%—23.5%之内,远小于交通排放的贡献,但在夏季和秋季生物质燃烧的贡献增加。1D-VBS方案在模拟珠三角地区的SOA浓度上比1.5-D VBS方案表现出更好的性能。Wu等[157]将1D-VBS方法加入气象-化学在线全耦合模式(weather research and forecasting model coupled with chemistry,WRF-Chem),考虑到S/IVOCs排放量的不确定性,该模型可以解释约34%观测到的SOA平均浓度来源。他们的研究认为,所有人为源的S/IVOCs中,工业排放的S/IVOC对SOA形成的贡献最大,其次是道路交通、粉尘、生物质燃烧、住宅和非道路用车排放。
-
中等挥发性有机物已被证明是生成二次有机气溶胶的重要潜在源,但在复杂的实际大气条件下,现有的外场观测、实验室研究和模型模拟研究远远不足以评估IVOCs对有机气溶胶(OA)的贡献。目前,在中等挥发性有机物的研究方面还存在以下几个方向和挑战:
(1)检测技术的发展。实际环境中大量IVOCs还未能识别,需要分离度和分辨率更高的仪器,尤其是时间分辨率高的在线测量仪器,鉴别更多的IVOCs种类来更新排放清单,进而为实验室研究和模型模拟提供依据。
(2)对IVOCs反应机制更为全面、深入的研究。截至目前有关IVOCs反应机制的认识还十分匮乏,尤其是缺少IVOCs中脂肪醛、酮、羧酸、酯、醚类以及杂环化合物等物质的实验数据。目前已有的IVOCs的实验室研究更多的是气相反应研究,少有非均相反应、SOA老化和SOA光学性质的研究;IVOCs的壁效应对于实验室数据的准确性有一定的影响,需要更精确的壁损失校正方法;除了考查NOx水平、SO2、种子、氧化剂浓度、温度和湿度等环境条件的影响外,还需考虑VOCs的存在对于IVOCs氧化的影响,以使实验室结果逐步接近于实际大气,可以提高模型模拟的准确性和精确度。
(3)环境因素、区域性差异等条件的影响。在利用模型模拟时,考虑不同地区的差异。例如沿海地区的Cl原子浓度比其他地区高,而环境污染严重的内陆城市则是臭氧占主导地位。
附录
1. OH自由基的大气平均浓度为1.0×106 molecule·cm−3[158];
2. Cl原子在污染严重地区或海边的大气浓度为5.0×104 molecule·cm−3[159],普通地区大气Cl原子浓度为1.0×104 molecule·cm−3[160];
3. O3的大气浓度平均值为7×1011 molecule·cm−3[161];
4. NO3自由基夜间的典型对流层大气浓度为5×108 molecule·cm−3[162]。
中等挥发性有机物研究进展
Research progress of intermediate volatility organic compounds
-
摘要: 中等挥发性有机物是挥发性介于气态挥发性有机物和颗粒态难挥发性有机物的一类重要的有机物,其可以在气相和颗粒相进行配分,根据其结构可分为脂肪族及脂环族化合物、芳香族化合物以及杂环化合物等。近年来的研究发现其对大气氧化性及二次有机气溶胶的生成具有重要贡献,但由于测量技术及机理研究的局限,使得针对其研究和认识还处于起步阶段。本文从中等挥发性有机物的定义、测量技术、外场观测、实验室机制以及模型模拟等方面进行了综述。进一步针对已有关于中等挥发性有机物所面临的问题和挑战进行了展望。Abstract: Intermediate volatility organic compounds (IVOCs) are an important type of species that saturated vapor pressure between gaseous volatile organic compounds and particulate nonvolatile organic compounds. According to disparate structures, they can be divided into aliphatic and alicyclic compounds, aromatic compounds, and heterocyclic compounds. In recent years, it has been found that IVOCs have a significant contribution to the formation of atmospheric oxidization and secondary organic aerosol. However, due to the limitations of measurement technology and mechanism study, the research and understanding are still in the initial stage. This paper reviews the definition, measurement technologies, field observations, laboratory mechanisms, and model simulations of IVOCs. Furthermore, the problems and challenges faced have prospected.
-

-
图 1 C1—C20烷烃在298 K下的饱和蒸气压(圆形)及其与OH自由基(1.0×106 molecule·cm−3,上三角形)和Cl原子(5.0×104 molecule·cm−3,下三角形)反应的大气寿命。
Figure 1. Saturated vapor pressure (circle) of C1—C20 alkanes at 298 K and their atmospheric lifetimes for reactions with OH radicals (1.0×106 molecule·cm−3, up triangle) and Cl atoms (5.0×104 molecule·cm−3, down triangle).
表 1 大气中常见的IVOCs在298 K下的饱和蒸气压及其与大气活性物种的一级反应速率常数
Table 1. Saturated vapor pressures of common atmospheric IVOCs at 298 K and their first order reaction rate constants with atmospheric active species.
有机物
Organics饱和蒸气压a/Pa
Saturated vapor pressurekOH×(1011)/
(cm3·molecule−1·s−1)kCl×(1011)/
(cm3·molecule−1·s-1)$ {k}_{{\rm{O}}_{3}} $
(cm3·molecule−1·s−1)$ {k}_{{\rm{NO}}_{3}} $
(cm3·molecule−1·s−1)脂肪族及脂环族化合物 正十二烷(VOC) 16 1.32[8] 51.2[9] 环十二烷 3.93 1.47[10] 正己基环己烷(VOC) 14.67 1.74[10] 正十三烷 5 1.51[11] 53[9] 正十四烷 2 1.79(312 K)[11] 56.8[9] 正十五烷 0.66 2.07(312 K)[11] 正十六烷 0.19 2.32(312 K)[11] 正十七烷 0.03 2.1[12] 正十八烷 0.028 2.24[12] 正十九烷 0.01 2.38[12] 正庚基环己烷 5.33 1.91[13] 正辛基环己烷 1.33 2.05[13] 1-十四烯 2 4.96[14] 244[15] 0.287[15] 1-十四醇 0.02 3.56[16] 正癸酸 0.05 1.15[16] 草酸 0.03 0.064[16] 水杨酸 0.01 1.3[17] 2-十一烷酮 5.33 1.45[16] 丙位壬内酯 1.57 1.08[16] 三乙酸甘油酯 0.33 0.802[16] 二乙二醇丁醚 2.92 7.44[18] 芳香族化合物 萘 10.67 2.3[19] 0.42[20] <3[21] 200[22] 1-甲基萘 9.33 4.09[23] 12.1[24] <1.3[25] 2-甲基萘 8 4.86[23] 10.5[24] <4[26] 2,3-二甲基萘 0.15 7.68[26] 29.3[24] <4[26] 苊烯 0.64 10.9[27] 46.9[20] 3990[27] 44.2[27] 苊 0.29 5.8[19] 30.1[20] 1.79[28] 4.16[28] 芴 0.08 1.3[19] <2[19] 0.35[19] 菲 0.02 2.7[19] 4[29] 3.04[30] 五甲基苯 4 10.3[31] 肉桂醛 3.85 4.8[32] 22[32] 0.19[32] 5-甲基-2-硝基苯酚 2.67 0.672[33] 1.94[34] 对甲酚 5.3(293 K) 4.4[35] 4.71[21] 110[36] 邻苯二酚 4(293 K) 10.4[37] 64.6[38] 135[39] 980[40] 愈创木酚 13.3 7.5[41] 29.7[42] 4[39] 269[36] 2,6-二甲氧基苯酚 0.79 9.66[43] 27.1[42] 1584[36] 二苯胺 0.09 19.4[16] 水杨酸 0.01 1.3[17] 杂环化合物 二苯并噻吩 0.03 0.81[44] <6[44] <0.7[44] 喹啉 8 1.16[45] <1[45] 异喹啉 9.33 0.85[45] <1.1[45] 吲哚 1.62 15.4[45] 490[45] 1330[45] 佳乐麝香 0.07 2.71[46] 二苯并呋喃 0.33 0.35[19] <0.8[47] <0.02[47] 十二甲基环六硅氧烷 2.25 0.28[48] 0.25—0.32[48] a:数据来源:PubChem数据库(https://pubchem.ncbi.nlm.nih.gov/)[49]、文献[50]; 表 2 大气中常见IVOCs的外场观测浓度
Table 2. Concentrations of common IVOCs in the atmosphere from field observations
IVOCs 采样时间
Sampling date采样地点
Sampling location大气浓度Concentration/
(parts per trillion)分析仪器
Analysis
instrumentRef g p g+p 正十三烷及其异构体 2018年11—12月 华北农村 109±75 PTR-MS [63] 2018年9—11月 广州市区 66±60 PTR-MS [63] 正十三烷 2017年1—2月 伦敦市区 4.5 0.19 4.69 GC×GC-MS [83] 正十四烷及其异构体 2018年11—12月 华北农村 60±40 PTR-MS [63] 2018年9—11月 广州市区 50±47 PTR-MS [63] 正十四烷 2017年1—2月 伦敦市区 3.74 0.27 4 GC×GC-MS [83] 2011年8月 美国曼尼托森林 0.05—0.16 SV-TAG-AMS [82] 己基环己烷 2017年1—2月 伦敦市区 1.52 0.02 1.54 GC×GC-MS [83] 庚基环己烷 2017年1—2月 伦敦市区 1.91 0.01 1.92 GC×GC-MS [83] 辛基环己烷 2017年1—2月 伦敦市区 1.01 0.02 1.03 GC×GC-MS [83] 萘 2015年6—8月 武汉-鄂州 0.09—0.2 GC-MS [84] 2019年9—10月 广州 18 GC-MS [85] 2008年8月—2009年7月 哈尔滨 7.19 0.15 7.34 GC-MS [86] 2005年1月 北京海淀 729±521 11.33±15.18 740±521 GC-MS [87] 2010年12月—2011年7月 印度洋 0.03 0.03 GC-MS [88] C1-甲基萘 2017年1—2月 伦敦市区 3.06 0.24 3.30 GC×GC-MS [83] 1-甲基萘 2019年9—10月 广州 2.03 [85] 2-甲基萘 2019年9—10月 广州 4.35 [85] C2-甲基萘 2017年1—2月 伦敦市区 2.17 0.07 2.24 GC×GC-MS [83] 2010年12月—2011年7月 南太平洋 0.17 0.17 GC-MS [88] C3-甲基萘 2017年1—2月 伦敦市区 2.82 0.35 3.17 GC×GC-MS [83] 苊烯 2008年8月—2009年7月 哈尔滨 1.8 0.22 2.02 GC-MS [86] 2010年12月—2011年7月 北大西洋 0.03 0.03 GC-MS [88] 2005年1月 北京海淀 29.40±24.26 1.13±1.62 30.53±24.26 GC-MS [87] 苊 2010年12月—2011年7月 印度洋 0.02 0.02 GC-MS [88] 2008年8月—2009年7月 哈尔滨 0.38 0.02 0.4 GC-MS [86] 2005年1月 北京海淀 6.64±4.74 0.53±0.73 7.17±4.8 GC-MS [87] 芴 2015年6—8月 武汉-鄂州 0.06—0.37 GC-MS [84] 2008年8月—2009年7月 哈尔滨 1.32 0.22 1.54 GC-MS [86] 2006年8月—2007年6月 上海 0.22 GC [89] 2011年8月 美国曼尼托森林 0.02—0.16 SV-TAG-AMS [82] 2010年12月—2011年7月 北大西洋 0.17 0.17 GC-MS [88] 2005年1月 北京海淀 12.14±8.91 2.65±4.25 14.79±9.88 GC-MS [87] 菲 2015年6—8月 武汉-鄂州 0.30—7.91 GC-MS [84] 2008年8月—2009年7月 哈尔滨 2.43 2.03 4.46 GC-MS [86] 2006年8月—2007年6月 上海 2.28 GC [89] 2011年8月 美国曼尼托森林 0.06—0.33 SV-TAG-AMS [82] 2005年1月 北京海淀 10.74±6.86 14.95±31.69 25.69±32.38 GC-MS [87] 2010年12月—2011年7月 南太平洋 1.3 0.01 1.31 GC-MS [88] 对甲苯酚 2016年4月 济南 0.06 0.21 0.27 UHPLC-MS [90] 邻苯二酚 2016年4月 济南 0.42 0.24 0.66 UHPLC-MS [90] 愈创木酚 1988年11月—1989年2月 美国明尼阿波利斯市 3.15—88 GC-MS [91] 2,6-二甲氧基苯酚 1988年11月—1989年2月 美国明尼阿波利斯市 0.68—87 GC-MS [91] 佳乐麝香 2008年11月 土耳其,小学教室 25.29±5.3 0.24±0.09 25.53±5.3 GC-MS [92] 2009年6—7月 土耳其,妇女体育中心 13.6±5.72 0.09±0.05 13.69±5.72 GC-MS [92] 二苯并呋喃 2011年8月 美国曼尼托森林 0.07—0.68 SV-TAG-AMS [82] 二苯并噻吩 2010年12月—2011年7月 北大西洋 0.15 0.0004 0.15 GC-MS [88] -
[1] HUANG R J, ZHANG Y L, BOZZETTI C, et al. High secondary aerosol contribution to particulate pollution during haze events in China [J]. Nature, 2014, 514(7521): 218-222. doi: 10.1038/nature13774 [2] WANG Y, ZHANG R Y, SARAVANAN R. Asian pollution climatically modulates mid-latitude cyclones following hierarchical modelling and observational analysis [J]. Nature Communications, 2014, 5: 3098. doi: 10.1038/ncomms4098 [3] 曹军骥. 我国PM2.5污染现状与控制对策 [J]. 地球环境学报, 2012, 3(5): 1030-1036. CAO J J. Pollution status and control strategies of PM2.5 in China [J]. Journal of Earth Environment, 2012, 3(5): 1030-1036(in Chinese).
[4] PRESTO A A, MIRACOLO M A, DONAHUE N M, et al. Secondary organic aerosol formation from high-NOx photo-oxidation of low volatility precursors: N-alkanes [J]. Environmental Science & Technology, 2010, 44(6): 2029-2034. [5] ROBINSON A L, DONAHUE N M, SHRIVASTAVA M K, et al. Rethinking organic aerosols: Semivolatile emissions and photochemical aging [J]. Science , 2007, 315(5816): 1259-1262. doi: 10.1126/science.1133061 [6] ZHAO Y L, HENNIGAN C J, MAY A A, et al. Intermediate-volatility organic compounds: A large source of secondary organic aerosol [J]. Environmental Science & Technology, 2014, 48(23): 13743-13750. [7] DONAHUE N M, ROBINSON A L, PANDIS S N. Atmospheric organic particulate matter: From smoke to secondary organic aerosol [J]. Atmospheric Environment, 2009, 43(1): 94-106. doi: 10.1016/j.atmosenv.2008.09.055 [8] WANG D S, HILDEBRANDT RUIZ L. Chlorine-initiated oxidation of n-alkanes under high NOx conditions: Insights into secondary organic aerosol composition and volatility using a FIGAERO-CIMS [J]. Atmospheric Chemistry and Physics Discussions, 2018: 1-26. [9] SHI B, WANG W G, ZHOU L, et al. Atmospheric oxidation of C10~14 n-alkanes initiated by Cl atoms: Kinetics and mechanism [J]. Atmospheric Environment, 2020, 222: 117166. doi: 10.1016/j.atmosenv.2019.117166 [10] LOZA C L, CRAVEN J S, YEE L D, et al. Secondary organic aerosol yields of 12-carbon alkanes [J]. Atmospheric Chemistry and Physics, 2014, 14(3): 1423-1439. doi: 10.5194/acp-14-1423-2014 [11] ATKINSON R, AREY J. Atmospheric degradation of volatile organic compounds [J]. Chemical Reviews, 2003, 103(12): 4605-4638. doi: 10.1021/cr0206420 [12] ZHAO Y L, NGUYEN N T, PRESTO A A, et al. Intermediate volatility organic compound emissions from on-road diesel vehicles: Chemical composition, emission factors, and estimated secondary organic aerosol production [J]. Environmental Science & Technology, 2015, 49(19): 11516-11526. [13] CARTER W P L, LUO D M, MALKINA I L. Investigation of the ozone formation potentials of selected branched alkanes and mineral spirits samples[EB/OL]. [2021-03-29] [14] ASCHMANN S M, ATKINSON R. Rate constants for the gas-phase reactions of OH radicals with E-7-tetradecene, 2-methyl-1-tridecene and the C(7)-C(14) 1-alkenes at 295+/- 1 K [J]. Physical Chemistry Chemical Physics: PCCP, 2008, 10(28): 4159-4164. doi: 10.1039/b803527j [15] MASON S A, AREY J, ATKINSON R. Rate constants for the gas-phase reactions of NO3 radicals and O3 with C6−C14 1-alkenes and 2-methyl-1-alkenes at 296±2 K [J]. The Journal of Physical Chemistry A, 2009, 113(19): 5649-5656. doi: 10.1021/jp9014614 [16] DASHBOARD C C. CompTox Chemicals Dashboard [M]. U.S.; United States Environmental Protection Agency. 2020. [17] ATKINSON R. A structure-activity relationship for the estimation of rate constants for the gas-phase reactions of OH radicals with organic compounds [J]. International Journal of Chemical Kinetics, 1987, 19(9): 799-828. doi: 10.1002/kin.550190903 [18] ASCHMANN S M, MARTIN P, TUAZON E C, et al. Kinetic and product studies of the reactions of selected glycol ethers with OH radicals [J]. Environmental Science & Technology, 2001, 35(20): 4080-4088. [19] BRUBAKER W W, HITES R A. OH reaction kinetics of polycyclic aromatic hydrocarbons and polychlorinated dibenzo-p-dioxins and dibenzofurans [J]. The Journal of Physical Chemistry A, 1998, 102(6): 915-921. doi: 10.1021/jp9721199 [20] RIVA M, HEALY R M, FLAUD P M, et al. Kinetics of the gas-phase reactions of chlorine atoms with naphthalene, acenaphthene, and acenaphthylene [J]. The Journal of Physical Chemistry A, 2014, 118(20): 3535-3540. doi: 10.1021/jp5009434 [21] ATKINSON R, ASCHMANN S M, FITZ D R, et al. Rate constants for the gas-phase reactions of O3 with selected organics at 296 K [J]. International Journal of Chemical Kinetics, 1982, 14(1): 13-18. doi: 10.1002/kin.550140103 [22] ATKINSON R, AREY J. Mechanisms of the gas-phase reactions of aromatic hydrocarbons and pahs with oh and no 3 radicals [J]. Polycyclic Aromatic Compounds, 2007, 27(1): 15-40. doi: 10.1080/10406630601134243 [23] CHAN A W H, KAUTZMAN K, CHHABRA P, et al. Secondary organic aerosol formation from photooxidation of naphthalene and alkylnaphthalenes: implications for oxidation of intermediate volatility organic compounds (IVOCs) [J]. Atmospheric Chemistry and Physics, 2009, 9(9): 3049-3060. doi: 10.5194/acp-9-3049-2009 [24] WANG L, AREY J, ATKINSON R. Reactions of chlorine atoms with a series of aromatic hydrocarbons [J]. Environmental Science & Technology, 2005, 39(14): 5302-5310. [25] ATKINSON R, ASCHMANN S M. Kinetics of the gas-phase reactions of alkylnaphthalenes with O3, N2O5 and OH radicals at 298±2 K [J]. Atmospheric Environment (1967), 1987, 21(11): 2323-2326. doi: 10.1016/0004-6981(87)90367-2 [26] ATKINSON R, ASCHMANN S M. Kinetics of the reactions of naphthalene, 2-methylnaphthalene, and 2,3-dimethylnaphthalene with OH radicals and with O3 at (295±1) K [J]. International Journal of Chemical Kinetics, 1986, 18(5): 569-573. doi: 10.1002/kin.550180507 [27] ZHOU S M, WENGER J C. Kinetics and products of the gas-phase reactions of acenaphthylene with hydroxyl radicals, nitrate radicals and ozone [J]. Atmospheric Environment, 2013, 75: 103-112. doi: 10.1016/j.atmosenv.2013.04.049 [28] ZHOU S M, WENGER J C. Kinetics and products of the gas-phase reactions of acenaphthene with hydroxyl radicals, nitrate radicals and ozone [J]. Atmospheric Environment, 2013, 72: 97-104. doi: 10.1016/j.atmosenv.2013.02.044 [29] KEYTE I J, HARRISON R M, LAMMEL G. Chemical reactivity and long-range transport potential of polycyclic aromatic hydrocarbons – a review [J]. Chemical Society Reviews, 2013, 42(24): 9333. doi: 10.1039/c3cs60147a [30] ZHAO N, SHI X L, XU F, et al. Theoretical investigation on the mechanism of NO3 radical-initiated atmospheric reactions of phenanthrene [J]. Journal of Molecular Structure, 2017, 1139: 275-281. doi: 10.1016/j.molstruc.2017.03.063 [31] ASCHMANN S M, AREY J, ATKINSON R. Rate constants for the reactions of OH radicals with 1,2,4,5-tetramethylbenzene, pentamethylbenzene, 2,4,5-trimethylbenzaldehyde, 2,4,5-trimethylphenol, and 3-methyl-3-hexene-2,5-Dione and products of OH+1,2,4,5-tetramethylbenzene [J]. The Journal of Physical Chemistry A, 2013, 117(12): 2556-2568. doi: 10.1021/jp400323n [32] SMITH A M, RIGLER E, KWOK E S C, et al. Kinetics and products of the gas-phase reactions of 6-methyl-5-hepten-2-one andtrans-cinnamaldehyde with OH and NO3 Radicals and O3 at (296±2) K [J]. Environmental Science & Technology, 1996, 30(5): 1781-1785. [33] BEJAN I, BARNES I, OLARIU R, et al. Investigations on the gas-phase photolysis and OH radical kinetics of methyl-2-nitrophenols [J]. Physical Chemistry Chemical Physics, 2007, 9(42): 5686-5692. doi: 10.1039/b709464g [34] BEJAN I, DUNCIANU M, OLARIU R, et al. Kinetic study of the gas-phase reactions of chlorine atoms with 2-chlorophenol, 2-nitrophenol, and four methyl-2-nitrophenol isomers [J]. The Journal of Physical Chemistry A, 2015, 119(20): 4735-4745. doi: 10.1021/acs.jpca.5b02392 [35] ATKINSON R. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions [J]. Chemical Reviews, 1986, 86(1): 69-201. doi: 10.1021/cr00071a004 [36] LAURAGUAIS A, EL ZEIN A, COEUR C, et al. Kinetic study of the gas-phase reactions of nitrate radicals with methoxyphenol compounds: Experimental and theoretical approaches [J]. The Journal of Physical Chemistry. A, 2016, 120(17): 2691-2699. doi: 10.1021/acs.jpca.6b02729 [37] OLARIU R I, BARNES I, BECKER K H, et al. Rate coefficients for the gas-phase reaction of OH radicals with selected dihydroxybenzenes and benzoquinones [J]. International Journal of Chemical Kinetics, 2000, 32(11): 696-702. doi: 10.1002/1097-4601(2000)32:11<696::AID-KIN5>3.0.CO;2-N [38] BEJAN I B I, OLARIU R, WENGER J C. A kinetic study of gas phase reactions of chlorine atoms with 1, 2-benzenediols and benzoquinones. [J]. 21st International Symposium on Gas Kinetics , 2010, 6: 12. [39] ZEIN A E, COEUR C, OBEID E, et al. Reaction kinetics of catechol (1,2-benzenediol) and guaiacol (2-methoxyphenol) with ozone [J]. The Journal of Physical Chemistry A, 2015, 119(26): 6759-6765. doi: 10.1021/acs.jpca.5b00174 [40] OLARIU R I, BEJAN I, BARNES I, et al. Rate coefficients for the gas-phase reaction of NO3 radicals with selected dihydroxybenzenes [J]. International Journal of Chemical Kinetics, 2004, 36(11): 577-583. doi: 10.1002/kin.20029 [41] COEUR-TOURNEUR C, CASSEZ A, WENGER J C. Rate coefficients for the gas-phase reaction of hydroxyl radicals with 2-methoxyphenol (guaiacol) and related compounds [J]. The Journal of Physical Chemistry A, 2010, 114(43): 11645-11650. doi: 10.1021/jp1071023 [42] LAURAGUAIS A, BEJAN I, BARNES I, et al. Rate coefficients for the gas-phase reaction of chlorine atoms with a series of methoxylated aromatic compounds [J]. The Journal of Physical Chemistry A, 2014, 118(10): 1777-1784. doi: 10.1021/jp4114877 [43] LAURAGUAIS A, COEUR-TOURNEUR C, CASSEZ A, et al. Rate constant and secondary organic aerosol yields for the gas-phase reaction of hydroxyl radicals with syringol (2,6-dimethoxyphenol) [J]. Atmospheric Environment, 2012, 55: 43-48. doi: 10.1016/j.atmosenv.2012.02.027 [44] KWOK E S C, ATKINSON R, AREY J. Kinetics of the gas-phase reactions of dibenzothiophene with OH radicals, NO3 radicals, and O3 [J]. Polycyclic Aromatic Compounds, 1999, 13(3): 175-189. doi: 10.1080/10406639908020562 [45] ATKINSON R, TUAZON E C, AREY J, et al. Atmospheric and indoor chemistry of gas-phase indole, quinoline, and isoquinoline [J]. Atmospheric Environment, 1995, 29(23): 3423-3432. doi: 10.1016/1352-2310(95)00103-6 [46] LI Y F, SUN Y H, ZHANG Q Z. Theoretical and kinetic properties of OH radical-initiated oxidation of galaxolide in the atmosphere [J]. The Journal of Physical Chemistry A, 2018, 122(47): 9151-9159. doi: 10.1021/acs.jpca.8b07456 [47] KWOK E S C, AREY J, ATKINSON R. Gas-phase atmospheric chemistry of dibenzo-p-dioxin and dibenzofuran [J]. Environmental Science & Technology, 1994, 28(3): 528-533. [48] SAFRON A, STRANDELL M, KIERKEGAARD A, et al. Rate constants and activation energies for gas-phase reactions of three cyclic volatile methyl siloxanes with the hydroxyl radical [J]. International Journal of Chemical Kinetics, 2015, 47(7): 420-428. doi: 10.1002/kin.20919 [49] PUBCHEM. PubChem [M]. U.S.; National Library of Medicine National Center for Biotechnology Information. 2020. [50] GOSS K U, SCHWARZENBACH R P. Empirical prediction of heats of vaporization and heats of adsorption of organic compounds [J]. Environmental Science & Technology, 1999, 33(19): 3390-3393. [51] TAN X, YUAN B, WANG C, et al. Progress in measurements of semi-/intermediate-volatile organic compounds in ambient air [J]. China Environmental Science, 2020, 40(10): 4224-36. [52] 唐荣志, 王辉, 刘莹, 等. 大气半/中等挥发性有机物的组成及其对有机气溶胶贡献 [J]. 化学进展, 2019, 31(1): 180-190. TANG R Z, WANG H, LIU Y, et al. Constituents of atmospheric semi-volatile and intermediate volatility organic compounds and their contribution to organic aerosol [J]. Progress in Chemistry, 2019, 31(1): 180-190(in Chinese).
[53] NOZIÈRE B, KALBERER M, CLAEYS M, et al. The molecular identification of organic compounds in the atmosphere: State of the art and challenges [J]. Chemical Reviews, 2015, 115(10): 3919-3983. doi: 10.1021/cr5003485 [54] KIM J W, KIM Y M, MOON H M, et al. Comparative study of thermal desorption and solvent extraction-gas chromatography-mass spectrometric analysis for the quantification of phthalates in polymers [J]. Journal of Chromatography A, 2016, 1451: 33-40. doi: 10.1016/j.chroma.2016.05.014 [55] ALAM M S, WEST C E, SCARLETT A G, et al. Application of 2D-GCMS reveals many industrial chemicals in airborne particulate matter [J]. Atmospheric Environment, 2013, 65: 101-111. doi: 10.1016/j.atmosenv.2012.10.014 [56] ALAM M S, HARRISON R M. Recent advances in the application of 2-dimensional gas chromatography with soft and hard ionisation time-of-flight mass spectrometry in environmental analysis [J]. Chemical Science, 2016, 7(7): 3968-3977. doi: 10.1039/C6SC00465B [57] WILLIAMS B J, GOLDSTEIN A H, KREISBERG N M, et al. An in situ instrument for speciated organic composition of atmospheric aerosols: Thermal desorption aerosol GC/MS-FID (TAG) [J]. Aerosol Science and Technology, 2006, 40(8): 627-638. doi: 10.1080/02786820600754631 [58] WORTON D R, KREISBERG N M, ISAACMAN G, et al. Thermal desorption comprehensive two-dimensional gas chromatography: An improved instrument for in situ speciated measurements of organic aerosols [J]. Aerosol Science and Technology, 2012, 46(4): 380-393. doi: 10.1080/02786826.2011.634452 [59] ZHAO Y L, KREISBERG N M, WORTON D R, et al. Development of an in situ thermal desorption gas chromatography instrument for quantifying atmospheric semi-volatile organic compounds [J]. Aerosol Science and Technology, 2013, 47(3): 258-266. doi: 10.1080/02786826.2012.747673 [60] ZHAO R. The recent development and application of chemical ionization mass spectrometry in atmospheric chemistry[EB/OL]. 2018 [61] YATAVELLI R L N, LOPEZ-HILFIKER F, WARGO J D, et al. A chemical ionization high-resolution time-of-flight mass spectrometer coupled to a micro orifice volatilization impactor (MOVI-HRToF-CIMS) for analysis of gas and particle-phase organic species [J]. Aerosol Science and Technology, 2012, 46(12): 1313-1327. doi: 10.1080/02786826.2012.712236 [62] LOPEZ-HILFIKER F D, MOHR C, EHN M, et al. A novel method for online analysis of gas and particle composition: Description and evaluation of a Filter Inlet for Gases and AEROsols (FIGAERO) [J]. Atmospheric Measurement Techniques, 2014, 7(4): 983-1001. doi: 10.5194/amt-7-983-2014 [63] WANG C M, WU C H, WANG S H, et al. Measurements of higher alkanes using NO+PTR-ToF-MS: Significant contributions of higher alkanes to secondary organic aerosols in China[EB/OL]. 2020 [64] EICHLER P, MÜLLER M, D'ANNA B, et al. A novel inlet system for online chemical analysis of semi-volatile submicron particulate matter [J]. Atmospheric Measurement Techniques, 2015, 8(3): 1353-1360. doi: 10.5194/amt-8-1353-2015 [65] CHEN Y C, LEE W J, UANG S N, et al. Characteristics of polycyclic aromatic hydrocarbon (PAH) emissions from a UH-1H helicopter engine and its impact on the ambient environment [J]. Atmospheric Environment, 2006, 40(39): 7589-7597. doi: 10.1016/j.atmosenv.2006.06.054 [66] COOPER D A. Exhaust emissions from ships at berth [J]. Atmospheric Environment, 2003, 37(27): 3817-3830. doi: 10.1016/S1352-2310(03)00446-1 [67] KANG M J, REN L J, REN H, et al. Primary biogenic and anthropogenic sources of organic aerosols in Beijing, China: Insights from saccharides and n-alkanes [J]. Environmental Pollution, 2018, 243: 1579-1587. doi: 10.1016/j.envpol.2018.09.118 [68] GENTNER D R, JATHAR S H, GORDON T D, et al. Review of urban secondary organic aerosol formation from gasoline and diesel motor vehicle emissions [J]. Environmental Science & Technology, 2017, 51(3): 1074-1093. [69] JATHAR S H, FRIEDMAN B, GALANG A A, et al. Linking load, fuel, and emission controls to photochemical production of secondary organic aerosol from a diesel engine [J]. Environmental Science & Technology, 2017, 51(3): 1377-1386. [70] CROSS E S, HUNTER J F, CARRASQUILLO A J, et al. Online measurements of the emissions of intermediate-volatility and semi-volatile organic compounds from aircraft [J]. Atmospheric Chemistry and Physics, 2013, 13(3): 8065-8100. [71] LOU H J, HAO Y J, ZHANG W W, et al. Emission of intermediate volatility organic compounds from a ship main engine burning heavy fuel oil [J]. Journal of Environmental Sciences, 2019, 84: 197-204. doi: 10.1016/j.jes.2019.04.029 [72] TANG R, LU Q, GUO S, et al. Measurement report: Distinct emissions and volatility distribution of intermediate-volatility organic compounds from on-road Chinese gasoline vehicles: Implication of high secondary organic aerosol formation potential [J]. Atmos Chem Phys, 2021, 21(4): 2569-2583. doi: 10.5194/acp-21-2569-2021 [73] 王倩, 黄凌, 王杨君, 等. 长江三角洲2017年机动车IVOCs排放清单构建及其对SOA的生成影响 [J]. 环境科学, 2020, 41(1): 125-132. WANG Q, HUANG L, WANG Y J, et al. Emission inventory of intermediate volatility organic compounds from vehicles in the Yangtze River Delta in 2017 and the impact on the formation potential of secondary organic aerosols [J]. Environmental Science, 2020, 41(1): 125-132(in Chinese).
[74] HUANG C, HU Q Y, LI Y J, et al. Intermediate volatility organic compound emissions from a large cargo vessel operated under real-world conditions [J]. Environmental Science & Technology, 2018, 52(21): 12934-12942. [75] AGRAWAL H, SAWANT A A, JANSEN K, et al. Characterization of chemical and particulate emissions from aircraft engines [J]. Atmospheric Environment, 2008, 42(18): 4380-4392. doi: 10.1016/j.atmosenv.2008.01.069 [76] SIMONEIT B R T. Biomass burning—a review of organic tracers for smoke from incomplete combustion [J]. Applied Geochemistry, 2002, 17(3): 129-162. doi: 10.1016/S0883-2927(01)00061-0 [77] MAZZOLENI L R, ZIELINSKA B, MOOSMÜLLER H. Emissions of levoglucosan, methoxy phenols, and organic acids from prescribed burns, laboratory combustion of wildland fuels, and residential wood combustion [J]. Environmental Science & Technology, 2007, 41(7): 2115-2122. [78] MCDONALD B C, de GOUW J A, GILMAN J B, et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions [J]. Science, 2018, 359(6377): 760-764. doi: 10.1126/science.aaq0524 [79] LYU Y, XU T T, YANG X, et al. Seasonal contributions to size-resolved n-alkanes (C8—C40) in the Shanghai atmosphere from regional anthropogenic activities and terrestrial plant waxes [J]. Science of the Total Environment, 2017, 579: 1918-1928. doi: 10.1016/j.scitotenv.2016.11.201 [80] FEILBERG A, LIU D Z, ADAMSEN A P S, et al. Odorant emissions from intensive pig production measured by online proton-transfer-reaction mass spectrometry [J]. Environmental Science & Technology, 2010, 44(15): 5894-5900. [81] MIYAZAKI Y, KAWAMURA K, SAWANO M. Size distributions and chemical characterization of water-soluble organic aerosols over the western North Pacific in summer [J]. Journal of Geophysical Research Atmospheres, 2010, 115(D23): D23210. doi: 10.1029/2010JD014439 [82] CHAN A W H, KREISBERG N M, HOHAUS T, et al. Speciated measurements of semivolatile and intermediate volatility organic compounds (S/IVOCs) in a pine forest during BEACHON-RoMBAS 2011 [J]. Atmospheric Chemistry and Physics, 2016, 16(2): 1187-1205. doi: 10.5194/acp-16-1187-2016 [83] XU R X, ALAM M S, STARK C, et al. Composition and emission factors of traffic- emitted intermediate volatility and semi-volatile hydrocarbons (C10-C36) at a street canyon and urban background sites in central London, UK [J]. Atmospheric Environment, 2020, 231: 117448. doi: 10.1016/j.atmosenv.2020.117448 [84] WU D H, LIU H X, WANG Z G, et al. Atmospheric concentrations and air-soil exchange of polycyclic aromatic hydrocarbons (PAHs) in typical urban-rural fringe of Wuhan-Ezhou region, central China [J]. Bulletin of Environmental Contamination and Toxicology, 2020, 104(1): 96-106. doi: 10.1007/s00128-019-02743-6 [85] FANG H, LUO S L, HUANG X Q, et al. Ambient naphthalene and methylnaphthalenes observed at an urban site in the Pearl River Delta region: Sources and contributions to secondary organic aerosol [J]. Atmospheric Environment, 2021, 252: 118295. doi: 10.1016/j.atmosenv.2021.118295 [86] MA W L, LI Y F, QI H, et al. Seasonal variations of sources of polycyclic aromatic hydrocarbons (PAHs) to a northeastern urban city, China [J]. Chemosphere, 2010, 79(4): 441-447. doi: 10.1016/j.chemosphere.2010.01.048 [87] LIU Y N, TAO S, YANG Y F, et al. Inhalation exposure of traffic police officers to polycyclic aromatic hydrocarbons (PAHs) during the winter in Beijing, China [J]. Science of the Total Environment, 2007, 383(1/2/3): 98-105. [88] GONZÁLEZ-GAYA B, FERNÁNDEZ-PINOS M C, MORALES L, et al. High atmosphere–ocean exchange of semivolatile aromatic hydrocarbons [J]. Nature Geoscience, 2016, 9(6): 438-442. doi: 10.1038/ngeo2714 [89] WANG X Y, LI Q B, LUO Y M, et al. Characteristics and sources of atmospheric polycyclic aromatic hydrocarbons (PAHs) in Shanghai, China [J]. Environmental Monitoring and Assessment, 2010, 165(1/2/3/4): 295-305. [90] LI M, WANG X F, LU C Y, et al. Nitrated phenols and the phenolic precursors in the atmosphere in urban Jinan, China [J]. Science of the Total Environment, 2020, 714: 136760. doi: 10.1016/j.scitotenv.2020.136760 [91] HAWTHORNE S B, MILLER D J, LANGENFELD J J, et al. PM-10 high-volume collection and quantitation of semi- and nonvolatile phenols, methoxylated phenols, alkanes, and polycyclic aromatic hydrocarbons from winter urban air and their relationship to wood smoke emissions [J]. Environmental Science & Technology, 1992, 26(11): 2251-2262. [92] SOFUOGLU A, KIYMET N, KAVCAR P, et al. Polycyclic and nitro musks in indoor air: A primary school classroom and a women's sport center [J]. Indoor Air, 2010, 20(6): 515-522. doi: 10.1111/j.1600-0668.2010.00674.x [93] PANKOW J F. An absorption model of gas/particle partitioning of organic compounds in the atmosphere [J]. Atmospheric Environment, 1994, 28(2): 185-188. doi: 10.1016/1352-2310(94)90093-0 [94] ESEN F, TASDEMIR Y, BOZKURT Y M. Assessments of seasonal trend, gas-particle partitioning and deposition flux of polycyclic aromatic hydrocarbons at a semi-rural site [J]. Journal of Environmental Science and Health, Part A, 2019, 54(6): 582-591. doi: 10.1080/10934529.2019.1574158 [95] AKYÜZ M, ÇABUK H. Gas-particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak, Turkey [J]. Science of the Total Environment, 2010, 408(22): 5550-5558. doi: 10.1016/j.scitotenv.2010.07.063 [96] MANDALAKIS M, TSAPAKIS M, TSOGA A, et al. Gas-particle concentrations and distribution of aliphatic hydrocarbons, PAHs, PCBs and PCDD/Fs in the atmosphere of Athens (Greece) [J]. Atmospheric Environment, 2002, 36(25): 4023-4035. doi: 10.1016/S1352-2310(02)00362-X [97] GOSS K U, SCHWARZENBACH R P. Gas/solid and gas/liquid partitioning of organic compounds: Critical evaluation of the interpretation of equilibrium constants [J]. Environmental Science & Technology, 1998, 32(14): 2025-2032. [98] LOHMANN R, HARNER T, THOMAS G O, et al. A comparative study of the gas-particle partitioning of PCDD/fs, PCBs, and PAHs [J]. Environmental Science & Technology, 2000, 34(23): 4943-4951. [99] YANG J, XU W L, CHENG H Y. Seasonal variations and sources of airborne polycyclic aromatic hydrocarbons (PAHs) in Chengdu, China [J]. Atmosphere, 2018, 9(2): 63. doi: 10.3390/atmos9020063 [100] WONG F, ROBSON M, MELYMUK L, et al. Urban sources of synthetic musk compounds to the environment [J]. Environmental Science. Processes & Impacts, 2019, 21(1): 74-88. [101] LIU W, ZHAO J, XU S, et al. Concentrations, sources, and potential human health risks of PCDD/fs, dl-PCBs, and PAHs in rural atmosphere around chemical plants in Jiangsu Province, China [J]. Bulletin of Environmental Contamination and Toxicology, 2020, 104(6): 846-851. doi: 10.1007/s00128-020-02864-3 [102] CHEN Y, SHEN G, HUANG Y, et al. Household air pollution and personal exposure risk of polycyclic aromatic hydrocarbons among rural residents in Shanxi, China [J]. Indoor Air, 2016, 26(2): 246-258. doi: 10.1111/ina.12204 [103] YEE L D, ISAACMAN-VANWERTZ G, WERNIS R A, et al. Observations of sesquiterpenes and their oxidation products in central Amazonia during the wet and dry seasons [J]. Atmospheric Chemistry and Physics, 2018, 18(14): 10433-10457. doi: 10.5194/acp-18-10433-2018 [104] SINGH D K, KAWAMURA K, YANASE A, et al. Distributions of polycyclic aromatic hydrocarbons, aromatic ketones, carboxylic acids, and trace metals in arctic aerosols: Long-range atmospheric transport, photochemical degradation/production at polar sunrise [J]. Environmental Science & Technology, 2017, 51(16): 8992-9004. [105] ZHAO Y L, NGUYEN N T, PRESTO A A, et al. Intermediate volatility organic compound emissions from on-road gasoline vehicles and small off-road gasoline engines [J]. Environmental Science & Technology, 2016, 50(8): 4554-4563. [106] ALAM M S, ZERAATI-REZAEI S, LIANG Z R, et al. Mapping and quantifying isomer sets of hydrocarbons (≥C12) in diesel exhaust, lubricating oil and diesel fuel samples using GC×GC-ToF-MS [J]. Atmospheric Measurement Techniques, 2018, 11(5): 3047-3058. doi: 10.5194/amt-11-3047-2018 [107] SU P H, HAO Y J, QIAN Z, et al. Emissions of intermediate volatility organic compound from waste cooking oil biodiesel and marine gas oil on a ship auxiliary engine [J]. Journal of Environmental Sciences, 2020, 91: 262-270. doi: 10.1016/j.jes.2020.01.008 [108] LI C T, MI H H, LEE W J, et al. PAH emission from the industrial boilers [J]. Journal of Hazardous Materials, 1999, 69(1): 1-11. doi: 10.1016/S0304-3894(99)00097-7 [109] CAI S Y, ZHU L, WANG S X, et al. Time-resolved intermediate-volatility and semivolatile organic compound emissions from household coal combustion in Northern China [J]. Environmental Science & Technology, 2019, 53(15): 9269-9278. [110] HATCH L E, RIVAS-UBACH A, JEN C N, et al. Measurements of I/SVOCs in biomass-burning smoke using solid-phase extraction disks and two-dimensional gas chromatography [J]. Atmospheric Chemistry and Physics, 2018, 18(24): 17801-17817. doi: 10.5194/acp-18-17801-2018 [111] DHAMMAPALA R, CLAIBORN C, SIMPSON C, et al. Emission factors from wheat and Kentucky bluegrass stubble burning: Comparison of field and simulated burn experiments [J]. Atmospheric Environment, 2007, 41(7): 1512-1520. doi: 10.1016/j.atmosenv.2006.10.008 [112] AGARWAL R, SHUKLA K, KUMAR S, et al. Chemical composition of waste burning organic aerosols at landfill and urban sites in Delhi [J]. Atmospheric Pollution Research, 2020, 11(3): 554-565. doi: 10.1016/j.apr.2019.12.004 [113] GENTNER D R, WORTON D R, ISAACMAN G, et al. Chemical composition of gas-phase organic carbon emissions from motor vehicles and implications for ozone production [J]. Environmental Science & Technology, 2013, 47(20): 11837-11848. [114] LI W H, LI L J, CHEN C L, et al. Potential of select intermediate-volatility organic compounds and consumer products for secondary organic aerosol and ozone formation under relevant urban conditions [J]. Atmospheric Environment, 2018, 178: 109-117. doi: 10.1016/j.atmosenv.2017.12.019 [115] ATKINSON R. Gas-phase tropospheric chemistry of volatile organic compounds: 1. alkanes and alkenes [J]. Journal of Physical and Chemical Reference Data, 1997, 26(2): 215-290. doi: 10.1063/1.556012 [116] YEE L D, CRAVEN J S, LOZA C L, et al. Effect of chemical structure on secondary organic aerosol formation from C12 alkanes [J]. Atmospheric Chemistry and Physics, 2013, 13(21): 11121-11140. doi: 10.5194/acp-13-11121-2013 [117] LIM Y B, ZIEMANN P J. Effects of molecular structure on aerosol yields from OH radical-initiated reactions of linear, branched, and cyclic alkanes in the presence of NOx [J]. Environmental Science & Technology, 2009, 43(7): 2328-2334. [118] LAMBE A T, ONASCH T B, CROASDALE D R, et al. Transitions from functionalization to fragmentation reactions of laboratory secondary organic aerosol (SOA) generated from the OH oxidation of alkane precursors [J]. Environmental Science & Technology, 2012, 46(10): 5430-5437. [119] LIM Y B, ZIEMANN P J. Products and mechanism of secondary organic aerosol formation from reactions of n-alkanes with OH radicals in the presence of NOx [J]. Environmental Science & Technology, 2005, 39(23): 9229-9236. [120] JORDAN C E, ZIEMANN P J, GRIFFIN R J, et al. Modeling SOA formation from OH reactions with C8—C17 n-alkanes [J]. Atmospheric Environment, 2008, 42(34): 8015-8026. doi: 10.1016/j.atmosenv.2008.06.017 [121] TKACIK D S, PRESTO A A, DONAHUE N M, et al. Secondary organic aerosol formation from intermediate-volatility organic compounds: Cyclic, linear, and branched alkanes [J]. Environmental Science & Technology, 2012, 46(16): 8773-8781. [122] LAMKADDAM H, GRATIEN A, PANGUI E, et al. High-NOx photooxidation of n-dodecane: Temperature dependence of SOA formation [J]. Environmental Science & Technology, 2017, 51(1): 192-201. [123] LI J L, LI K, WANG W G, et al. Optical properties of secondary organic aerosols derived from long-chain alkanes under various NOx and seed conditions [J]. Science of the Total Environment, 2017, 579: 1699-1705. doi: 10.1016/j.scitotenv.2016.11.189 [124] LI J L, WANG W G, LI K, et al. Temperature effects on optical properties and chemical composition of secondary organic aerosol derived from n-dodecane [J]. Atmospheric Chemistry and Physics, 2020, 20(13): 8123-8137. doi: 10.5194/acp-20-8123-2020 [125] WERT B P, TRAINER M, FRIED A, et al. Signatures of terminal alkene oxidation in airborne formaldehyde measurements during TexAQS 2000 [J]. Journal of Geophysical Research: Atmospheres, 2003, 108(D3): 4014. doi: 10.1029/2002jd002502 [126] ZIEMANN P J. Effects of molecular structure on the chemistry of aerosol formation from the OH-radical-initiated oxidation of alkanes and alkenes [J]. International Reviews in Physical Chemistry, 2011, 30(2): 161-195. doi: 10.1080/0144235X.2010.550728 [127] MATSUNAGA A, DOCHERTY K S, LIM Y B, et al. Composition and yields of secondary organic aerosol formed from OH radical-initiated reactions of linear alkenes in the presence of NOx: Modeling and measurements [J]. Atmospheric Environment, 2009, 43(6): 1349-1357. doi: 10.1016/j.atmosenv.2008.12.004 [128] CHACON-MADRID H J, HENRY K M, DONAHUE N M. Photo-oxidation of pinonaldehyde at low NOx: From chemistry to organic aerosol formation [J]. Atmospheric Chemistry and Physics, 2013, 13(6): 3227-3236. doi: 10.5194/acp-13-3227-2013 [129] CHACON-MADRID H J, DONAHUE N M. Fragmentation vs. functionalization: Chemical aging and organic aerosol formation [J]. Atmospheric Chemistry and Physics, 2011, 11(20): 10553-10563. doi: 10.5194/acp-11-10553-2011 [130] WANG L, ATKINSON R, AREY J. Dicarbonyl products of the OH radical-initiated reactions of naphthalene and the C1- and C2-alkylnaphthalenes [J]. Environmental Science & Technology, 2007, 41(8): 2803-2810. [131] RIVA M, HEALY R M, FLAUD P M, et al. Gas- and particle-phase products from the chlorine-initiated oxidation of polycyclic aromatic hydrocarbons [J]. The Journal of Physical Chemistry A, 2015, 119(45): 11170-11181. doi: 10.1021/acs.jpca.5b04610 [132] KAUTZMAN K E, SURRATT J D, CHAN M N, et al. Chemical composition of gas- and aerosol-phase products from the photooxidation of naphthalene [J]. The Journal of Physical Chemistry A, 2010, 114(2): 913-934. doi: 10.1021/jp908530s [133] SHAKYA K M, GRIFFIN R J. Secondary organic aerosol from photooxidation of polycyclic aromatic hydrocarbons [J]. Environmental Science & Technology, 2010, 44(21): 8134-8139. [134] CHEN C L, KACARAB M, TANG P, et al. SOA formation from naphthalene, 1-methylnaphthalene, and 2-methylnaphthalene photooxidation [J]. Atmospheric Environment, 2016, 131: 424-433. doi: 10.1016/j.atmosenv.2016.02.007 [135] CHEN C L, LI L J, TANG P, et al. SOA formation from photooxidation of naphthalene and methylnaphthalenes with m-xylene and surrogate mixtures [J]. Atmospheric Environment, 2018, 180: 256-264. doi: 10.1016/j.atmosenv.2018.02.051 [136] RIVA M, ROBINSON E S, PERRAUDIN E, et al. Photochemical aging of secondary organic aerosols generated from the photooxidation of polycyclic aromatic hydrocarbons in the gas-phase [J]. Environmental Science & Technology, 2015, 49(9): 5407-5416. [137] XU C, WANG L. Atmospheric oxidation mechanism of phenol initiated by OH radical [J]. The Journal of Physical Chemistry A, 2013, 117(11): 2358-2364. doi: 10.1021/jp308856b [138] LAURAGUAIS A, COEUR-TOURNEUR C, CASSEZ A, et al. Atmospheric reactivity of hydroxyl radicals with guaiacol (2-methoxyphenol), a biomass burning emitted compound: Secondary organic aerosol formation and gas-phase oxidation products [J]. Atmospheric Environment, 2014, 86: 155-163. doi: 10.1016/j.atmosenv.2013.11.074 [139] YEE L D, KAUTZMAN K E, LOZA C L, et al. Secondary organic aerosol formation from biomass burning intermediates: Phenol and methoxyphenols [J]. Atmospheric Chemistry and Physics, 2013, 13(16): 8019-8043. doi: 10.5194/acp-13-8019-2013 [140] LIU C G, CHEN T Z, LIU Y C, et al. Enhancement of secondary organic aerosol formation and its oxidation state by SO2 during photooxidation of 2-methoxyphenol [J]. Atmospheric Chemistry and Physics, 2019, 19(4): 2687-2700. doi: 10.5194/acp-19-2687-2019 [141] LIU C G, LIU J, LIU Y C, et al. Secondary organic aerosol formation from the OH-initiated oxidation of guaiacol under different experimental conditions [J]. Atmospheric Environment, 2019, 207: 30-37. doi: 10.1016/j.atmosenv.2019.03.021 [142] NAKAO S, CLARK C, TANG P, et al. Secondary organic aerosol formation from phenolic compounds in the absence of NOx [J]. Atmospheric Chemistry and Physics, 2011, 11(20): 10649-10660. doi: 10.5194/acp-11-10649-2011 [143] LIU C G, LIU Y C, CHEN T Z, et al. Rate constant and secondary organic aerosol formation from the gas-phase reaction of eugenol with hydroxyl radicals [J]. Atmospheric Chemistry and Physics, 2019, 19(3): 2001-2013. doi: 10.5194/acp-19-2001-2019 [144] SUN Y L, ZHANG Q, ANASTASIO C, et al. Insights into secondary organic aerosol formed via aqueous-phase reactions of phenolic compounds based on high resolution mass spectrometry [J]. Atmospheric Chemistry and Physics, 2010, 10(10): 4809-4822. doi: 10.5194/acp-10-4809-2010 [145] KITANOVSKI Z, ČUSAK A, GRGIĆ I, et al. Chemical characterization of the main products formed through aqueous-phase photonitration of guaiacol [J]. Atmospheric Measurement Techniques, 2014, 7(8): 2457-2470. doi: 10.5194/amt-7-2457-2014 [146] KITANOVSKI Z, GRGIĆ I, de VERMEYLEN R, et al. Liquid chromatography tandem mass spectrometry method for characterization of monoaromatic nitro-compounds in atmospheric particulate matter [J]. Journal of Chromatography A, 2012, 1268: 35-43. doi: 10.1016/j.chroma.2012.10.021 [147] YU L, SMITH J, LASKIN A, et al. Chemical characterization of SOA formed from aqueous-phase reactions of phenols with the triplet excited state of carbonyl and hydroxyl radical [J]. Atmospheric Chemistry and Physics, 2014, 14(24): 13801-13816. doi: 10.5194/acp-14-13801-2014 [148] MONTOYA-AGUILERA J, HORNE J R, HINKS M L, et al. Secondary organic aerosol from atmospheric photooxidation of indole [J]. Atmospheric Chemistry and Physics, 2017, 17(18): 11605-11621. doi: 10.5194/acp-17-11605-2017 [149] FEILBERG A, HOLCMAN J, NIELSEN T, et al. Atmospheric oxidation of N-PAC and nitro substituted N-PAC in water droplets [J]. Polycyclic Aromatic Compounds, 1999, 14(1/2/3/4): 137-150. [150] LIU Y, LU J C, CHEN Y F, et al. Aqueous-phase production of secondary organic aerosols from oxidation of dibenzothiophene (DBT) [J]. Atmosphere, 2020, 11(2): 151. doi: 10.3390/atmos11020151 [151] HODZIC A, KASIBHATLA P S, JO D S, et al. Rethinking the global secondary organic aerosol (SOA) budget: Stronger production, faster removal, shorter lifetime [J]. Atmospheric Chemistry and Physics, 2016, 16(12): 7917-7941. doi: 10.5194/acp-16-7917-2016 [152] ELURI S, CAPPA C D, FRIEDMAN B, et al. Modeling the formation and composition of secondary organic aerosol from diesel exhaust using parameterized and semi-explicit chemistry and thermodynamic models [J]. Atmospheric Chemistry and Physics, 2018, 18(19): 13813-13838. doi: 10.5194/acp-18-13813-2018 [153] ZHAO B, WANG S X, DONAHUE N M, et al. Quantifying the effect of organic aerosol aging and intermediate-volatility emissions on regional-scale aerosol pollution in China [J]. Scientific Reports, 2016, 6(1): 1-10. doi: 10.1038/s41598-016-0001-8 [154] LI J, HAN Z W, LI J W, et al. The formation and evolution of secondary organic aerosol during haze events in Beijing in wintertime [J]. Science of the Total Environment, 2020, 703: 134937. doi: 10.1016/j.scitotenv.2019.134937 [155] YANG W Y, LI J, WANG M, et al. A case study of investigating secondary organic aerosol formation pathways in Beijing using an observation-based SOA box model [J]. Aerosol and Air Quality Research, 2018, 18(7): 1606-1616. doi: 10.4209/aaqr.2017.10.0415 [156] YAO T, LI Y, GAO J H, et al. Source apportionment of secondary organic aerosols in the Pearl River Delta region: Contribution from the oxidation of semi-volatile and intermediate volatility primary organic aerosols [J]. Atmospheric Environment, 2020, 222: 117111. doi: 10.1016/j.atmosenv.2019.117111 [157] WU L Q, WANG X M, LU S H, et al. Emission inventory of semi-volatile and intermediate-volatility organic compounds and their effects on secondary organic aerosol over the Pearl River Delta region [J]. Atmospheric Chemistry and Physics, 2019, 19(12): 8141-8161. doi: 10.5194/acp-19-8141-2019 [158] PRINN R G, WEISS R F, MILLER B R, et al. Atmospheric trends and lifetime of CH3CCI3 and global OH concentrations [J]. Science, 1995, 269(5221): 187-192. doi: 10.1126/science.269.5221.187 [159] HOSSAINI R, CHIPPERFIELD M P, SAIZ-LOPEZ A, et al. A global model of tropospheric chlorine chemistry: Organic versus inorganic sources and impact on methane oxidation [J]. Journal of Geophysical Research: Atmospheres, 2016, 121(23): 14271-14297. doi: 10.1002/2016JD025756 [160] WINGENTER O W, KUBO M K, BLAKE N J, et al. Hydrocarbon and halocarbon measurements as photochemical and dynamical indicators of atmospheric hydroxyl, atomic chlorine, and vertical mixing obtained during Lagrangian flights [J]. Journal of Geophysical Research: Atmospheres, 1996, 101(D2): 4331-4340. doi: 10.1029/95JD02457 [161] LIN C Y C, JACOB D J, FIORE A M. Trends in exceedances of the ozone air quality standard in the continental United States, 1980-1998 [J]. Atmospheric Environment, 2001, 35(19): 3217-3228. doi: 10.1016/S1352-2310(01)00152-2 [162] SHU Y H, ATKINSON R. Atmospheric lifetimes and fates of a series of sesquiterpenes [J]. Journal of Geophysical Research Atmospheres, 1995, 100(D4): 7275-7281. doi: 10.1029/95JD00368 -



 下载:
下载:

















