-
有机氯农药(organochlorine pesticides,OCPs)、多氯联苯(polychlorinated biphenyls,PCBs)和多环芳烃(polycyclic aromatic hydrocarbons,PAHs)等持久性有机污染物(persistent organic pollutants,POPs),具有难降解性、生物蓄积性、高毒性和长距离迁移性[1],是全球重点关注的一类有机污染物。POPs经一次源排放后,由于其难降解性和长距离迁移性,在全球各环境介质中广泛分布[2];土壤因其较高的吸附容量,成为多数POPs的重要储存库[3]。
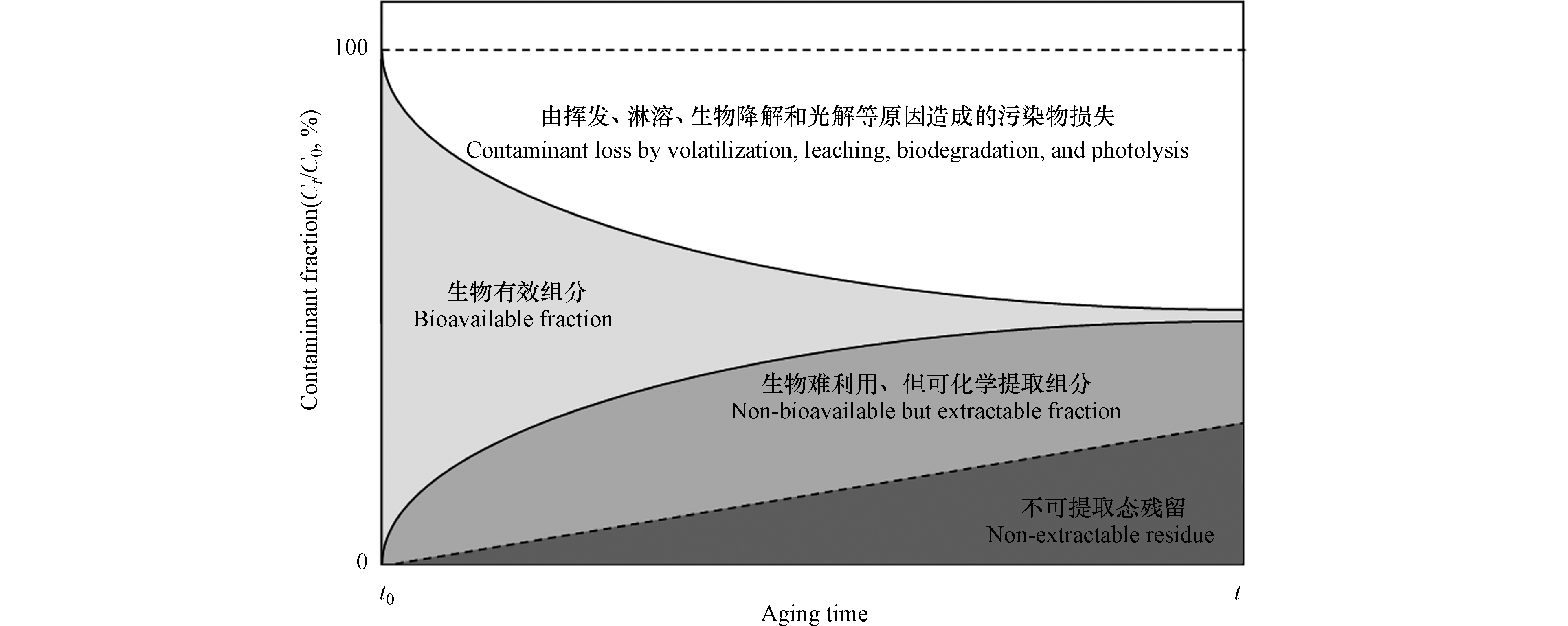
有机污染物(包括POPs)进入土壤后,一部分组分是可移动的,可经历各种迁移(比如挥发[4]和淋溶[5])和转化(比如生物降解[6])过程。随着污染物与土壤基质间的相互作用逐渐增强,一部分污染物与土壤基质紧密结合,其生物有效性(bioavailability)下降,发生老化(aging)现象[7-8](图1)。与基质紧密结合的污染物中,有部分组分(包括母体化合物和降解产物)在经过不明显改变化合物本身和基质结构的化学萃取后,仍存留于土壤基质中,这部分组分被称为不可提取态残留(non-extractable residue,NER)[11-13](又称结合态残留(bound residue)[14-15])。
土壤中有机污染物的环境归趋以前被认为仅受挥发、淋溶和生物降解等过程控制[16],但研究发现有机污染物在土壤的实际残留时间会超过基于污染物理化性质预测的理论残留时间[17]。典型案例是,Steinberg等[18]发现,尽管1,2-二溴乙烷(一种农业熏蒸剂)具有较高的挥发性、水溶性和较短的生物降解半衰期(预测值为18 d[19]),被禁用后仍在土壤中持续残留19年之久,其中一部分组分变成NER。近年来,同位素标记技术的广泛应用使NER的精确定量成为可能,并揭示了NER生成是土壤中有机污染物一个至关重要的环境过程。据估算[20],农业土壤中平均高达1/3的农药在施放后最终变成了NER[21]。但当周围环境条件发生改变时,NER可能会重新释放[20, 22],造成潜在的环境和健康风险。
作为被《关于持久性有机污染物的斯德哥尔摩公约》[23]和《远距离越境空气污染公约》[24]等多个国际公约管控的污染物,土壤中POPs-NER的生成及释放与POPs的控制和削减成效密切相关。本文对土壤中POPs-NER的测试方法、生成机理与动力学特征以及环境风险等方面进行了总结,并对未来的研究趋势进行展望。
-
同位素标记(比如14C和13C)是有机污染物NER(包括POPs-NER)的常用研究手段。该方法通过人为添加同位素标记的有机污染物,将污染物的NER组分与土壤基质中的背景值(比如碳)区分开来[25],从而识别和研究有机污染物NER组分的生成和迁移转化行为。一些研究[26]尝试用破坏基质结构的处理方法来提取某一特定类型的NER组分。
-
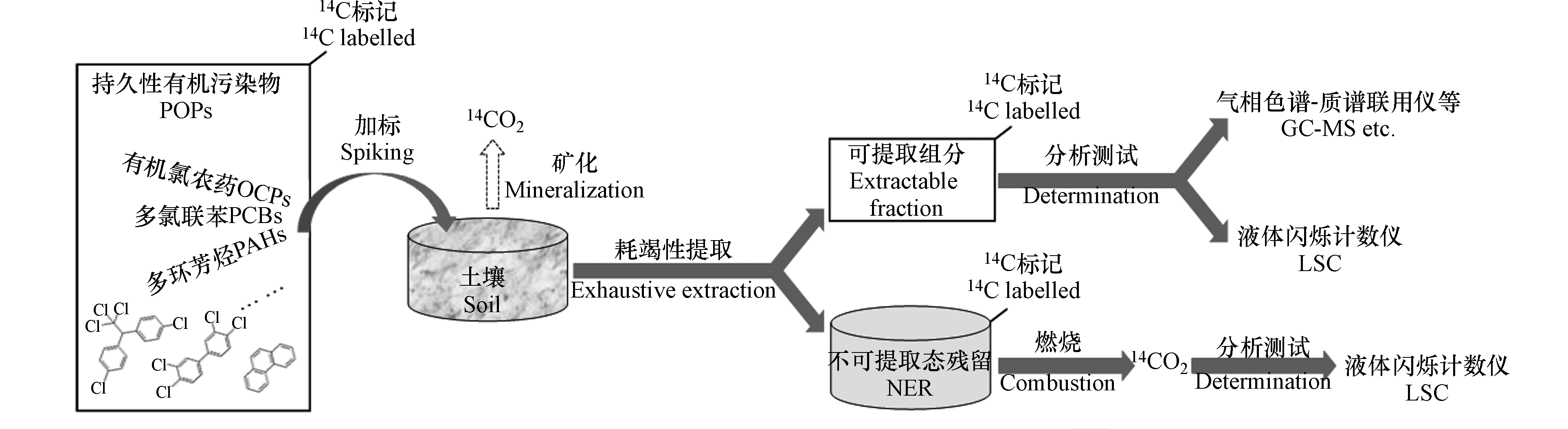
利用具放射性的14C标记化合物是研究土壤中有机污染物迁移转化(包括NER生成)的最常用方法。在14C-POPs加入供试土壤后,随时间增长,土壤中的14C-POPs发生老化并生成NER(14C标记)。土壤中14C-POPs的母体化合物及其降解产物的一部分可被各种常规萃取技术所提取,即可提取组分;土壤中剩余组分即NER。萃取后包含NER的土壤经燃烧生成14CO2,14CO2气体经收集后加入闪烁体,利用液体闪烁计数仪(liquid scintillation counter,LSC)测定14C活度,即获得NER的含量(图2)。
比如,Burauel和Führ[28]利用蒸渗仪,基于14C-苯并[a]芘、14C-荧蒽、14C-PCB 28和14C-PCB 52研究了PAHs和PCBs的迁移转化与NER生成。结果表明,在第4月—第28月的场地试验中,有约20%的PAHs和约16%的PCBs最终变成了NER。黄焕芳[29]利用室内微宇宙实验,基于14C-γ-六氯环己烷和14C-六氯苯研究了土壤中OCPs的NER生成特征及不同浓度黑碳对NER生成的影响。结果显示,在未添加黑碳的情况下,γ-六氯环己烷和六氯苯的NER生成比例(第103 d)分别为5%和0.6%;在添加10%的黑碳组分下,γ-六氯环己烷和六氯苯的NER生成比例(第103 d)分别变为4%和30%,反映了黑碳的添加不能促进γ-六氯环己烷的NER生成但能显著提高六氯苯的NER生成比例。
然而,在测定NER的萃取方法上缺乏统一标准,导致不同来源报道的NER数据缺乏可比性[27]。基于此,Loeffler[27]评估了加标土壤中42种有机化合物在不同萃取技术下的萃取效率,结果显示加压液体萃取(pressurized liquid extraction,PLE)(又称加速溶剂萃取(ASE))的萃取效率最高,优于基于氯化钙-甲醇/水-丙酮/甲醇的三步批萃取(3SBE)、超声溶剂萃取(USE)和微波辅助溶剂萃取(MASE)。PLE可作为耗竭性(exhaustive)提取的标准方法[27]。
-
除常见的放射性14C标记手段外,使用单体稳定同位素标记研究土壤中有机污染物的NER生成特征的报道也逐渐增多[30],比如13C标记[31]和15N标记[32](与12C和14N(相对丰度分别为98.9%和99.6%)相比,13C和15N(相对丰度分别为1.1%和0.4%[33])均为自然环境中低丰度的稳定碳同位素和稳定氮同位素)。对于POPs-NER,常见的稳定同位素标记手段一般为13C标记,其他标记手段较少报道。
Richnow等[34]以蒽作为PAHs模式化合物,利用稳定同位素比质谱仪(IRMS)研究了土壤中13C-蒽的矿化与NER生成,并利用LSC对比了14C-蒽的NER生成特征。研究发现,在室内实验第291 d,13C-蒽中有11%转化为NER,86%矿化为CO2,且NER组分在第188 d后几乎保持恒定;14C-蒽实验中,NER呈现出与13C标记实验相似的长期稳定性,但NER生成率(20%)稍高于13C标记实验,CO2的矿化率(78%)稍低。此研究揭示了利用稳定同位素研究NER生成的可行性以及与放射性同位素标记实验结果的可比性。
在NER转化研究中,与13C标记相比,14C标记表现出的优点[34]包括:(1)比13C标记有更高的准确度和灵敏度(尤其是低浓度下);(2)土壤中14C同位素的自然本底极低(痕量),不需要背景校正;而13C标记实验结果需要背景校正。但使用稳定同位素标记的示踪剂(如13C)比放射性物质(如14C)更加环境友好,实验中不需要额外的放射性安全防护措施,可直接应用到开放体系的场地试验中[34]。
-
通过高温燃烧含14C标记化合物的土壤,并对生成的14CO2进行测试,可精确定量NER,但该方法无法得知NER的化学组成[35](比如,无法得知NER是否为母体化合物或降解产物)。不破坏土壤结构的传统耗竭性和温和萃取手段均无法提取出与土壤有机质(SOM)紧密结合的污染物(即NER)[36]。为了表征NER的分子结构,许多学者尝试用破坏土壤结构的处理方法来提取NER,再使用色谱/质谱等技术进行分析。比如将土壤硅烷化(silylation)[37-38]或水解(hydrolysis)[39-41]后结合气相色谱(GC)分析,或者利用热裂解气相色谱-质谱联用(Pyrolysis-GC-MS)[42]和核磁共振(NMR)[43]技术来分析固体基质样品。硅烷化的作用是使SOM裂解,从而释放出NER[37];碱性水解[39-40]可用于断裂有机污染物与SOM间的酯键;高温水解[41]可断裂有机污染物与SOM间的醚键。热裂解气相色谱-质谱手段的局限在于无法区分热解产物的来源(NER源或SOM源);核磁共振手段的局限在于灵敏度较低,土壤中的稳定同位素(13C和15N)背景值通常较高[35]。
Kästner等[35]和欧洲化学品管理局(ECHA)[44]按照生成机制将NER分为3类:Ⅰ型、Ⅱ型和Ⅲ型NER。Ⅰ型NER与SOM的结合模式为非共价键结合;Ⅱ型NER与SOM的结合模式为共价键结合;Ⅲ型NER为生物同化产物,结合在生物残体(比如蛋白质)内(详细介绍见2.1小节)。因此,可根据不同类型NER的结合方式,将NER与SOM分离,从而完成对特定类型NER的表征和定量。表1所列方法为土壤中3种类型NER的常见定量方法。土壤硅烷化和乙二胺四乙酸(EDTA)萃取可提取Ⅰ型NER;化学降解(chemical degradation)[42]和热化学降解(thermochemolysis)[50]等破坏共价键可提取出Ⅱ型NER;对SOM中的蛋白质进行酸性水解(acidic hydrolysis),可对其水解产物氨基酸中的Ⅲ型NER进行分析测试。
在化学降解处理中,为更好区分不同处理方法下测得的(Ⅱ型)NER组分,可将土壤按表1中的3种化学降解方法分开(separate)处理或逐级(stepwise)处理[52]。Schäffer等[25]推荐了区分Ⅰ型和Ⅱ型NER组分的标准测试流程,即先利用硅烷化释放出Ⅰ型NER(可通过GC-MS或LSC等方式测定萃取液中的浓度或活度),土壤中余下组分即为Ⅱ型NER(可通过燃烧法经LSC测定活度);或者在得知总NER的含量后,分别减去Ⅰ型NER和Ⅲ型NER的含量,即为Ⅱ型NER含量。
然而,特定类型NER的测试方法仍存在较多不确定性[53-54]。比如,化学降解和热化学降解在破坏NER和SOM间共价键的同时,也会使SOM结构破坏,从而使与SOM结合更松散的Ⅰ型NER释放出来。因此,可对土壤依次进行破坏程度较轻的硅烷化或EDTA萃取→破坏程度较高的化学降解或热化学降解,分别提取Ⅰ型NER和Ⅱ型NER。但是,在提取Ⅰ型或Ⅱ型的土壤处理过程中(包括Schäffer等[25]的推荐方法),也可能会有部分Ⅲ型NER释放,导致Ⅰ型或Ⅱ型NER比例的高估[55-56]。此外,基于蛋白质水解产物氨基酸的分析测试结果能否较好代表Ⅲ型NER也需要进一步研究[55]。
-
NER过去被认为是仅由有机污染物(母体化合物或降解产物)与SOM或黏土间的相互作用而产生[57],即生成Ⅰ型NER或Ⅱ型NER(两者又称xenobiotic NER或xeno-NER)。近年来同位素标记实验揭示了易生物降解化合物的NER也可在生物同化过程中产生,即生成Ⅲ型NER(又称biogenic NER或bio-NER),NER的定义和分类因此得到拓展[35]。
有机污染物与土壤基质经非共价键结合生成Ⅰ型NER,主要结合模式为吸附(adsorption)和包埋(entrapment),也称锁定(sequestration)[35]。土壤黏土矿物主要吸附极性和亲水性化合物,SOM既能吸附极性、亲水性化合物也能吸附非极性、憎水亲脂性化合物[20]。有机污染物的吸附和包埋涉及多种分子间相互作用,包括氢键和范德华力等[14, 27, 35](表2)。SOM的微孔越小,有机污染物的包埋速率越慢但强度更大[58];SOM的体积膨胀和收缩也会影响有机污染物的物理包埋[59]。
有机污染物与SOM经共价键结合生成Ⅱ型NER。共价键合是有机污染物与土壤基质间研究较多的一类结合模式,不可逆、稳定且具强键合力(>300 kJ·mol−1)[35];可由酶和自由基反应或光化学催化介导[60],在母体化合物或降解产物与腐殖质间形成酯键、醚键或碳-碳键等[61]。由表2可知,共价键的结合能远高于大部分类型的分子间作用力,因此Ⅱ型NER与SOM的结合紧密程度一般远高于Ⅰ型NER。
微生物在降解过程中利用有机污染物的碳(或其他组分,比如氮)来构建其生物质组分。微生物死亡后,生物质组分固定在SOM中,形成Ⅲ型NER。如果微生物具有相应的转化酶,有机污染物可能会经共代谢直接降解而不会被同化为生物质组分,这种情形下只会生成Ⅰ型和Ⅱ型NER。氨基酸(构成SOM中总碳的10%—20%和总氮的30%—50%[35, 62];构成细菌细胞干重的约55%[63])和脂肪酸(构成细菌生物质干重的约5%[64])分别为微生物体内蛋白质和脂质的主要成分,可作为Ⅲ型NER的生物标志物。基于13C标记有机污染物的生物降解实验表明,13C可被固定进氨基酸和脂肪酸中。比如,在添加了13C-菲的焦油污染土壤实验中,蛋白质水解后的氨基酸中检测出较高含量的13C(即Ⅲ型NER),相当于总NER的11%[65]。
-
研究NER生成随时间变化的动力学(kinetics)特征,是深入理解土壤中有机污染物环境行为的必要环节。近年来对土壤中有机污染物NER生成动力学的报道逐渐增多,但主要集中在非持久性有机污染物,对POPs的报道较为缺乏。对非持久性有机污染物NER生成特征的研究,一定程度上也可以帮助理解POPs-NER的生成动力学。
NER的生成动力学曲线可按老化时间分为三阶段[20]:(1)初始段受控于刚开始老化时(加标24小时后)土壤中污染物的可萃取性。决定污染物在初始段可萃取性的影响因素包括萃取方法、污染物的理化性质以及土壤性质等。一般情况下,初始段有机污染物快速生成的NER不超过污染物施放总量的10%;部分研究报道大于10%(比如硫丹12%[20, 66]),少数报道大于50%(比如百草枯>90%[67])。(2)中间段受控于NER生成速率。当生成速率较快时,NER生成动力学曲线会很快进入平台期,同时NER的生成比例一般也较高;当生成速率较慢时,动力学曲线进入平台期的时间相对滞后且NER生成比例通常也较低。(3)末段可被认为是NER的成熟期。该阶段中,NER生成曲线可能在进入平台期后持续稳定,也可能持续缓慢上升(有新的NER生成)或缓慢下降(有NER释放)。
前已提及,NER生成机制多种多样,包含物理吸附或包埋(Ⅰ型)、化学共价键合(Ⅱ型)和生物同化(Ⅲ型)等,而每一种机制对应的生成动力学模型可能差异较大,因此同时考虑各类NER的生成动力学报道极为有限[68]。大部分有机污染物的NER生成模式呈现出“三阶段”特征[20],其生成动力学可默认简化为一阶或多相一阶动力学模型。比如,Matthies等[68]基于一阶动力学模型研究了8种理化性质各异的非持久性农药,并分别计算了母体化合物和降解产物的NER生成速率常数。
Johnson等[69]对一系列土壤PAHs吸附与解吸的动力学模型进行了评估,推荐双相一阶动力学模型为描述土壤PAHs吸附过程的最佳动力学模型。吸附是Ⅰ型NER生成的主要机制,因此Kästner等[35]将双相一阶动力学模型应用于Ⅰ型NER生成过程的模拟,快速和缓慢吸附/锁定速率分别代表SOM表面和SOM内部的有机污染物吸附速率。除直接基于14C或13C标记的NER生成实验来获取NER生成速率外,也有研究尝试利用非同位素标记POPs间接推导得出NER生成速率。比如,Ding等[70]基于为期一年的青藏高原东缘场地试验获取土壤PAHs的总损失速率,利用表层土壤模型计算得到土壤PAHs的挥发、淋溶和生物降解速率,推导得出PAHs母体化合物的(Ⅰ+Ⅱ型)NER生成速率(双相一阶动力学)[71]。目前暂无关于Ⅱ型NER生成动力学或生成速率的文献报道[35]。
Ⅲ型NER形成于有机污染物的生物同化过程,描述微生物降解的米氏方程(Michaelis-Menten equation)和莫诺方程(Monod equation)相关参数因而被应用于Ⅲ型NER生成动力学的模拟。比如,Trapp等[72]利用热力学方法估算了微生物产率,基于产率、CO2释放与微生物生长间的关系建立了Ⅲ型NER的生成动力学模型(MTB模型),并将其应用到土壤中2种非持久性有机污染物(2,4-二氯苯氧乙酸和布洛芬;均为13C标记)的NER生成动力学模拟。结果显示,Ⅲ型NER随时间逐渐增加然后逐渐保持恒定,且多数Ⅲ型NER来源于微生物蛋白质。该方法随后被应用到40种具环境风险有机污染物(包含DDT、菲、蒽和芘等POPs)的Ⅲ型NER生成及环境归趋模拟[73],并验证基于微生物产率建立的Ⅲ型NER生成动力学模型的可靠性。然而该产率估算方法仅考虑了微生物将有机污染物作为碳源的情况,忽略了有机污染物也能作为微生物其他物质来源(比如氮源和磷源)的情况。基于此,Brock等[54]以除草剂草甘膦(13C和15N标记)作为模式化合物,将Ⅲ型NER的生成动力学和环境归趋模型拓展到考虑有机污染物被同时作为微生物碳源和氮源的情形。
-
土壤有机污染物(包括POPs)NER生成受污染物的理化性质、土壤基质性质、环境条件和微生物活动等多因素共同影响。
生物可降解性是影响NER生成类型和生成量的重要因素。对于易生物降解的非持久性有机污染物(如2,4-二氯苯氧乙酸和布洛芬,生物降解半衰期预测值均为3.55 d [19]),多个研究表明Ⅲ型NER为其土壤NER的主要构成类型[35]。Nowak等[74, 75]基于13C标记的生物降解实验发现,2,4-二氯苯氧乙酸在64 d后[74]以及布洛芬在90 d后[75]生成的土壤NER中,所有组分几乎都为生物成因的Ⅲ型NER。此外,微生物活动可显著提升NER(主要是Ⅲ型NER)的生成量或生成比例。Benoit和Barriuso[76]发现,在未经灭菌处理的堆肥中,2,4-二氯苯氧乙酸、2,4-二氯苯酚和4-氯苯酚会形成较高比例的NER;但在灭菌堆肥中,其NER生成率几乎可以忽略。
对于低分子量POPs,尽管降解难度高于非持久性有机污染物,但在环境适宜的情况下仍可能发生一定程度的生物降解(比如被广泛报道的六氯环己烷好氧降解菌Sphingobium japonicum UT26[77]和菲降解菌属Sphingomonas[78]即具备相应降解能力)。因此,Ⅲ型NER也可能构成土壤中低分子量POPs-NER的重要组成部分。Kästner等[79]发现土壤中14C-蒽(三环PAH)生成的NER中仅有一小部分为Ⅰ型NER,并推测余下NER组分中大部分为生物成因的Ⅲ型NER。Richnow等[65]随后报道了13C-菲(三环PAH)生成的NER中,基于可水解氨基酸组分换算的Ⅲ型NER比例为11%,而总的Ⅲ型NER比例可能远超过该值。
然而,对于更难降解的高分子量POPs(比如五环和六环PAHs),微生物无法或难以对其进行同化,Ⅲ型NER的贡献相对较低;同时由于生物型与非生物型NER生成是竞争过程[35],吸附(Ⅰ型NER)和共价键合(Ⅱ型NER)等非生物过程是其NER主要生成机制。此外,在不考虑Ⅲ型NER生成的情况下,高分子量、高KOW(辛醇-水分配系数)的有机污染物可能会生成更高比例的NER。比如,Northcott和Jones[80]进行了灭菌和避光条件下土壤PAHs老化的微宇宙室内实验,发现苯并[a]芘(五环PAH)的NER生成率(23%—40%)远高于芘(四环PAH;9%—18%)和菲(5%—10%),反映了重环PAHs在吸附过程作用下能生成更高比例的NER。该现象可由重环PAHs与土壤基质间更强的不可逆吸附[70, 81-82]来解释。
土壤中SOM占比也是重要影响因素之一。高SOM含量土壤中的POPs可能会生成更高比例的NER(Ⅰ型和Ⅱ型)。比如,Luo等[83]研究发现,PAHs的可萃取性与土壤总有机碳(SOM的主要成分)含量呈显著负相关。其中,不同有机碳组分的吸附能力也有区别[84]。玻璃质的硬有机碳主导了疏水性有机污染物的吸附过程,且土壤硬有机碳含量增高时,PAHs(尤其是高环PAHs)的吸附变强;橡胶质的软有机碳对PAHs的吸附较弱,其含量与PAHs的可萃取性无显著相关性[83]。然而,部分研究表明添加某些种类的有机碳无法提高NER生成比例。在Kästner等[79]对14C标记-蒽的降解实验中,向土壤额外添加堆肥后,NER生成量反而显著下降,但蒽的矿化程度增加。该现象被归因于堆肥可刺激微生物的共代谢过程[85]以及可能减弱土壤中PAHs母体化合物的吸附。此外,POPs-NER在SOM不同组分(比如富里酸、胡敏酸和胡敏素等)中的分布也有差异。Kohl和Rice[86]研究了不同粒径土壤的腐殖质(SOM主要组成部分)中PAHs-NER和PCBs-NER的分布,结果显示50%—95%的NER分布在胡敏素中。
干湿循环、冻融循环和不同氧化还原条件等环境因素也可能会影响有机污染物的NER生成。比如,White等[87]报道土壤干湿循环会增强菲的锁定(即增加Ⅰ型NER生成)。Zhao等[88]在菲和芘的老化实验中发现,在老化初始阶段(第1和8 d),冻融循环会提高PAHs的萃取效率;但在长期老化后(第30和120 d),冻融循环会降低萃取效率(即促进NER生成)。该现象被归因于,PAHs在吸附初期主要以非扩散状态集中在SOM大分子外围的橡胶质组分中,冻融循环会减慢其扩散效率,从而使萃取效率增加。在经历较长时间的吸附后,PAHs扩散进入SOM内核玻璃质组分中,冻融过程可能会促进PAHs吸附到玻璃质组分中需要高活化能的位点,从而降低萃取效率;同时,冻融使玻璃质体积扩大,可增加其对PAHs的锁定。蔡蕊等[12]发现土壤中四溴双酚A在有氧条件下比无氧条件下能生成更高比例的NER,可能是由于氧化条件下能生成更多化学性质活泼的降解中间化合物,利于与SOM共价键合生成Ⅱ型NER。
-
土壤中有机污染物NER生成后,随着土壤基质性质和环境条件改变,或受老化时长和微生物作用等因素影响,NER可能会有不同程度的释放或再活化(remobilization)。NER生成传统上被认为是污染物的去毒(detoxification)过程;作为其相反途径,NER释放可能代表污染物环境和健康风险的再次增加。
-
有机污染物Ⅰ型NER主要由物理吸附和包埋形成,其与基质结合紧密程度低于共价键合的Ⅱ型NER,因此,Ⅰ型NER可能会优先释放出来。Kästner等[53]认为在Ⅰ型NER的各种结合方式中,多数为可逆结合;腐殖质分解后,吸附和包埋在腐殖质中的NER会释放出来。尽管Ⅱ型NER与土壤基质间的共价键较为稳定,但仍可能在某些条件下再次释放。比如四溴双酚A在经历有氧-淹水-有氧条件下的培养后,其Ⅱ型NER释放量可达7%—28%[13]。Ⅲ型NER作为SOM的组成部分,其稳定性可能比Ⅰ型和Ⅱ型NER更高。大量研究在NER生成后,利用不同的土壤处理手段来评估土壤中有机污染物(包括POPs)NER的释放潜力,其中一部分研究对NER具体类型作了表征或推测(表3)。
对于具有强疏水性的中性POPs(比如重环PAHs),在NER生成量达到峰值后,一般会进入稳定阶段(即2.2小节中的NER成熟期),在长期自然老化培养中伴随着少量NER释放或者生成。比如,Umeh等[22]将含苯并[a]芘-NER的土壤经正丁醇萃取后再度进行老化培养(无物理、化学和生物处理),结果显示仅有<9%的NER能从土壤中再活化变为可萃取组分。Burauel和Führ[28]对含2种PAHs和2种PCBs的土壤连续老化培养25个月,发现NER在第4个月达到峰值,之后的21个月中PAHs-NER有微量(<4%)释放,PCBs-NER有微量释放(<4%)或者生成(<6%)。
然而,Zhu等[90]对于可电离POPs——全氟辛烷磺酸盐及其替代品6:2氯化多氟醚磺酸盐(商品名为F-53B)的实验报道结果略有不同。土壤中全氟辛烷磺酸盐和F-53B的NER生成量均在老化培养第60 d达到峰值,但NER在第60—180 d时间段内大量(>50%)释放,直到最后第180—240 d培养期趋于稳定,伴随着微量(<4%)Ⅰ型或Ⅱ型NER生成。该研究可能反映了可电离POPs新形成的NER稳定性较差,经历较长时间的老化后趋于稳定。
作为相对更稳定的Ⅲ型NER,似乎难以在无处理老化培养和经物理处理的老化培养中释放,仅在化学处理和生物处理的老化培养中有释放潜力(表3)。比如,在加入EDTA萃取时,土壤中四种PAHs[48]和三硝基甲苯[92]的Ⅲ型NER有不同程度的少量释放(<15%)。该现象也反映了用EDTA提取Ⅰ型NER(见1.3小节)的局限性,即部分Ⅲ型NER可能与Ⅰ型NER一同释放,导致对Ⅰ型NER比例的高估。接种微生物培养可能会加强非持久性有机污染物(比如三硝基甲苯[92])Ⅲ型NER的释放,但难以促进高分子量POPs(比如重环PAHs[48]和DDT[89])NER的释放。
从表3来看,在经历一定时间的老化培养(比如半年)以及NER达到稳定后,仅有少量或微量的各种类型NER(<28%)会重新释放。在无处理和物理处理老化培养中,除四溴双酚A外,仅有<10%的NER释放。该结果反映了部分POPs-NER(比如PAHs-NER和PCBs-NER)在自然环境条件下老化时,稳定性较强,释放潜力较小;但对于未被报道的其他POPs,释放潜力有待进一步研究。在化学处理和生物处理的老化培养实验中,部分有机污染物的NER显示出一定程度的释放潜力,该结果对于土壤有机污染修复(比如化学修复和生物修复)和风险评估可能具有参考价值。
-
前已述及,土壤中POPs-NER具有不同程度的释放潜力,鉴于POPs及其初级降解产物的高毒性和生物蓄积性,对于POPs-NER的环境风险评估显得极为重要。但目前国内外无单独针对POPs-NER的风险评估标准,本小节主要介绍土壤有机污染物NER环境风险的相关研究作为参考依据。
有机污染物的NER生成一直是土壤化学领域的研究热点,但其环境风险长期处于未知状态[53]。由于NER生成会导致部分污染物的生物有效性下降,传统上该过程被认为是一种降解或去毒过程。但根据ECHA[44]和Kästner等[53]的最新土壤风险监管视角,在NER详细组分信息未知的情况下,NERs须被默认视为未降解的母体化合物。针对NERs的3种类型,其风险高低也作了相应分类[44, 53]:Ⅰ型NER的释放潜力相对较高,可能有潜在风险,须纳入风险评价并视为未降解化合物;Ⅱ型NER与土壤基质以共价键形式结合,稳定度相对Ⅰ型NER更高,除非有文献或监测数据证明其释放潜力,一般情况下可在风险评价中视为已消除的污染物;Ⅲ型NER经微生物的合成代谢形成,生物毒性较低,可被视为已消除的污染物。
然而,按NERs类型区分其环境风险的评估方法在应用到实际的场地风险评估时面临许多问题和不确定性。当对NER含量或比例进行定量时(不同类型NER的定量方法详见1.3小节),其前提是先移除掉土壤中的可萃取组分。尽管ECHA[44]建议使用耗竭性萃取方法(包括索氏提取、PLE、USE和MASE等萃取方法)去除可萃取组分以避免对NER组分的高估,仍然有部分研究采用温和型提取方法[35],导致不同研究间的实验结果不具备可比性。此外,就耗竭性萃取方法而言,也需要统一萃取方法以缩小不同耗竭性方法间的萃取效率差异,比如采用Loeffler[27]推荐的萃取效率最高的PLE方法。
此外,虽然ECHA[44]和Kästner等[53]对3类NERs的风险进行了分类,但若要进一步了解NER的毒性和风险,还须对其生物有效性进行评估,包括基于化学手段和基于生物手段的评估方法[95]。比如,Harmsen等[56]利用Tenax萃取法来获取含NER土壤的生物有效浓度(bioavailable concentration),并利用蚯蚓(Eisenia fetida)回避试验、发光细菌(Vibrio fischeri)毒性试验和水蚤(Daphnia magna)毒性试验等毒理学实验评估含NER土壤的毒性。实验结果显示,在三硝基甲苯生物有效浓度降低的同时,基于发光细菌测试的毒性也同步降低;但NER生成比例的增加并未导致毒性增加,揭示了土壤毒性的来源是三硝基甲苯的生物有效组分而不是NER。该实验还反映了同位素标记化合物在评估土壤有机污染物NER毒性和风险中的重要性,基于非标记化合物的实验数据可能有较大的不确定性[56]。
尽管基于土壤污染物生物有效浓度评估其风险的方法已被土壤修复领域的学者们广泛接受[95],但在土壤风险评估的实际案例中,仍然默认使用总可提取浓度(total-extractable concentration)而不是生物有效浓度来判定污染物风险。以PAHs为例,多个研究表明其总可提取浓度与生物有效性之间无显著相关性[96],使用总可提取浓度可能会高估其风险。在NER释放出的化合物中,仅有一部分可为生物利用(即生物有效组分),应就这一部分进行评估。但Umeh等[96]认为,若将NER等残留组分引入土壤风险评估,会使土壤修复的成本变高且更难达到理想修复效果。
总体而言,虽然NER的3种类型被明确定义,但其环境风险以及相应评估方法仍存在较多争议和不确定性。在不主张将NER纳入风险评估体系的研究中,大多认为NER难以释放或仅部分Ⅰ型NER有释放潜力,因此不需要过多考虑其环境风险。然而,由“3.1”小节可知,尽管在无处理或物理处理的老化培养中,NER的释放潜力有限,但在化学处理和生物处理中显示出更高的释放潜力。场地环境条件的改变、土壤低等动物的摄取以及植物根系分泌物的刺激等因素(可被视为不同程度的“化学”和“生物”处理)都可能促进NER的释放,从而导致其环境风险升高。
-
POPs-NER 的精确定量依赖于放射性和稳定同位素标记化合物的应用,包括14C 标记和13C 标记等. 通过破坏土壤基质结构的处理(比如硅烷化和水解),可对Ⅰ型、Ⅱ型和Ⅲ型 NER 分别进行测试. Ⅰ型 NER 的生成动力学模拟通常采用基于吸附的双相一阶动力学模型,Ⅲ型 NER 的生成动力学模拟推荐使用基于生物降解相关参数(包括产率等)的 MTB 模型. 对于易生物降解的非持久性有机污染物和低分子量 POPs,Ⅲ型 NER 可能是其 NER 主要成分,且生成比例受微生物活动影响;对于难生物降解的高分子量 POPs,Ⅰ型和Ⅱ型 NER 的占比可能更高. 土壤 SOM 含量与组分类别以及环境条件的变化等因素也显示出对 NER 生成的影响. 在经历长时间无处理或物理处理的老化培养后,仅有微量 NER 释放;但化学处理和生物处理的老化培养会增加 NER 的释放潜力. 根据 ECHA[44]和 Kästner 等[53]的风险分类,Ⅰ型 NER 包含具释放潜力的母体化合物,风险较高;Ⅱ型 NER 较难释放,Ⅲ型 NER 经生物代谢后毒性较低,两者风险均相对较低.
目前有关土壤有机污染物NER的研究主要针对PAHs和非持久性农药,对于其他POPs的研究相对较少。为了增强不同NER研究结果之间的可比性,建议采用相同的耗竭性萃取方法(比如PLE方法)。关于土壤NER生成和释放的影响因素研究,当下较多偏重于分析土壤基质不同组分(比如富里酸、胡敏酸和胡敏素等)的影响;鉴于NER生成为化合物与基质间的共同作用,对于POPs不同结构(比如芳香环和长链等)、不同分子量和不同KOW等化合物性质的影响有待进一步研究。
土壤中持久性有机污染物不可提取态残留的测试方法、生成特征与环境风险研究进展
Determination, formation, and environmental risk of non-extractable residue (NER) of persistent organic pollutants (POPs) in soil: A review
-
摘要: 持久性有机污染物(persistent organic pollutants,POPs)是一类具持久性、高生物蓄积性、高毒性且具长距离迁移性的高关注度化学物质,土壤是其主要储存库。POPs进入土壤后,与土壤结合发生老化现象,一部分生成不可提取态残留(non-extractable residue,NER)。作为POPs环境归趋中的重要过程,NER的生成传统上被视为污染物的去毒或降解途径;但随着老化时间的增加或者受环境条件变化等因素的影响,土壤中NER可能会再次释放,造成环境风险。国内外有关土壤有机污染物NER的研究已有较多报道,但其中关于POPs-NER的报道相对欠缺。本文介绍了POPs-NER的同位素测试手段和生成机理,对特定组分NER的测试方法、POPs-NER生成的动力学模型与影响因素以及POPs-NER的释放与环境风险等方面的研究进展进行了综述,并简要分析了当前POPs-NER研究领域的局限和未来需要关注的问题。由于部分研究方向缺乏POPs-NER的案例,本文分析了一些非持久性有机污染物NER的相关研究,以期为今后POPs-NER的研究提供参考。Abstract: Persistent organic pollutants (POPs) are substances of very high concern (SVHC) that are persistent, bioaccumulative, toxic, and have long-range transport potential. Soil is the main terrestrial reservoir of POPs due to the large sorptive capacity in soil matrices. After POPs enter the soil, a proportion of these contaminants becomes tightly bound to soil matrices and further generate non-extractable residues (NER), with the soil-contaminant interactions increasing with time (also termed “aging”). The NER formation is an important process in environmental fate of POPs and was traditionally deemed as a pathway of detoxification or degradation of contaminants. But NER could release or remobilize from soil matrices as the aging time increases or environmental conditions change, thus may pose environmental risks. The NER of organic contaminants in soil has been studied for decades, however, the report on POPs-NER is still rare. This article (Ⅰ) introduces the determination of NER based on isotopic labelling approaches and formation mechanisms of NER, (Ⅱ) reviews the progress in the research on the characterization of three types of NER, kinetic models and contributory factors of POPs-NER formation, and the release and environmental risks of POPs-NER, and also (Ⅲ) discusses the problems that should be investigated in the future. Due to the lack of cases in the research on POPs-NER, we summarized some studies on NER of non-persistent organic contaminants to provide references for future studies on POPs-NER.
-
Key words:
- soil /
- persistent organic pollutants (POPs) /
- non-extractable residue (NER) /
- aging /
- sequestration
-

-
表 1 土壤中有机污染物3种类型NER的常见测试方法
Table 1. Attempts on determination of three types of NER of organic contaminants in soil
NER类型
Type of NER生成机理
Formation mechanism of NER土壤基质的破坏性处理方法[35, 37, 45]
Destructive treatment method
of soil matrix处理方法优势
Advantages of treatment分析测试组分
Components for determination after treatment分析测试方法
Determination methodI型NER 吸附、
包埋等硅烷化 甲硅烷基取代SOM中的氢原子,使SOM中由非共价键聚集的部分裂解 萃取液 HPLC-14C-LSC[46] a;LSC[37];
13C-NMR[37]EDTA萃取 EDTA可提取土壤金属-有机配合物中的稳定二价金属离子,使SOM空间结构破坏或使部分SOM溶解 萃取液 HPLC-UV[47] b;LSC[48] II型NER 共价键
合化学降解 氢氧化钾或氢氧化钠-甲醇(KOH/NaOH-MeOH)水解 碱性水解可断裂NER与SOM间的酯键 萃取液 GC-MS[42, 49] 三溴化硼(BBr3)处理 BBr3可断裂NER与SOM间的醚键 萃取液 GC-MS[42] 四氧化钌(RuO4)氧化 RuO4氧化可断裂NER与SOM间的碳-碳键 萃取液 GC-MS[42] 热化学降解 四甲基氢氧化铵(TMAH)热化学降解 断裂NER与SOM间的碳-碳键;且甲基化利于酸性化合物的气相色谱分析 萃取液 GC-MS[50] III型NER 生物同化 酸性水解 盐酸水解 使土壤中含III型NER的蛋白质水解为氨基酸 萃取液 LSC[51] a HPLC:高效液相色谱;b UV:紫外. 表 2 NER生成过程中有机污染物与土壤基质间的相互作用(修改自文献[53])
Table 2. Binding interactions between organic contaminants and soil matrices within formation of NER (modified from Ref. [53])
I型NER/Type I NER II型NER/Type II NER 非共价键
Type of non-covalent binding适用范围
Type of compounds related to the interaction能量/(kJ·mol−1)
Binding force共价键
Type of covalent binding典型案例
Examples能量/(kJ·mol−1)
Binding force离子-离子力 可电离化合物 100—350 C—H键 >400 离子-偶极力 广泛适用 50—200 C—C键 >300 偶极-偶极力 广泛适用 5—50 C—N键 >300 π-π堆积 芳香族化合物 <5—50 C—O键 醚键、酯键 >300 配位体交换 广泛适用 50—150 C—F键 >400 电荷转移 广泛适用 5—50 C=C键 >600 氢键 广泛适用 4—120 C=O键 酯键 >700 疏水效应 非极性化合物 5—10 C≡C键 >800 范德华力 广泛适用 0.5—5 表 3 不同老化培养条件下有机污染物NER的释放比例与类型
Table 3. The proportion and type of released NER of organic contaminants in soil under different aging or incubation conditions
化合物类型
Type of spiked chemical化合物
Spiked compound辛醇-水分配系数a
KOW化合物标
记情况
Type of isotopic labelling of spiked chemical土壤老化时长
Aging time in soil to form NER含NER土壤
处理手段
Treatment approaches of soil containing NER处理类别
Type of soil treatmentNER释放
比例
Proportion of released NERNER可能释放类型
Proposed type of released NER参考文献
Ref.POPs PAHs 苯并[a]芘 6.57 未标记 200 d 经正丁醇萃取后再度老化30 d 无处理 2%—7%或2%—9% I型NER [22] 萘、蒽、芘和苯并[a]芘 3.44、4.88、5.45和6.57 14C标记 174 d 冻融循环及常温下共老化4个月 物理处理 0% — [48] 加入EDTA萃取
90 min化学处理 15% III型NER [48] 接种腐殖质降解菌或添加碳源后培养190 d 生物处理 0% — [48] 荧蒽和苯并[a]芘 4.81和6.57 14C标记 120 d NER生成达到峰值后继续老化21个月 无处理 2%—4% I型NER [28] PCBs PCB 28和PCB 52 5.84和6.39 14C标记 120 d NER生成达到峰值后继续老化21个月 无处理 −6%—4% b I型NER [28] OCPs p,p’-DDT 7.13 14C标记 672 d 经甲醇萃取后用硫酸处理 化学处理 5%—21% I型或II型NER [89] 经甲醇萃取后接种微生物培养3个月 生物处理 0% — [89] 溴代阻燃剂 四溴双酚A 8.68 14C标记 93 d(有氧) 经甲醇萃取后添加新鲜土壤在有氧-淹水-有氧条件下共培养231 d 物理处理 24%或7% II型NER [13] 66 d(淹水) 经甲醇萃取后添加新鲜土壤在有氧-淹水-有氧条件下共培养231 d 物理处理 28%或10% II型NER [13] 全氟化合物 全氟辛烷磺酸盐 4.03 c 未标记 60 d NER生成达到峰值后继续老化180 d 无处理 >60% I型或II型NER [90] 180 d 继续老化60 d 无处理 −4% b I型或II型NER [90] F-53B 3.09 c 未标记 60 d NER生成达到峰值后继续老化180 d 无处理 >50% I型或II型NER [90] 180 d 继续老化60 d 无处理 −3% b I型或II型NER [90] 非持久性有机污染物 除草剂 扑草净 3.29 14C标记 365 d 经甲醇萃取后接种微生物培养22 d 生物处理 27% I型NER [91] 含能化合物 三硝基
甲苯2.33 14C标记 176 d 冻融循环及常温下共老化4个月 物理处理 0% — [92] 研磨破碎 物理处理 0% — [92] 加入EDTA萃取 化学处理 7%—12% III型NER [92] 接种木质素降解菌后培养42 d 生物处理 <10% III型NER [92] a 表3中所有化合物的KOW值取自UFZ-LSER数据库[93]基于多参数线性自由能关系(ppLFERs)模型[94]的预测值;b 负号代表与初始阶段相比,有部分可萃取组分转化为NER,导致NER增加(NER释放的相反情况)。c UFZ-LSER数据库无法计算带电荷化合物的KOW值,全氟辛烷磺酸盐和F-53B的KOW值分别取自全氟辛烷磺酸和6:2氯化多氟醚磺酸. -
[1] WANIA F, MACKAY D. Peer reviewed: Tracking the distribution of persistent organic pollutants [J]. Environmental Science & Technology, 1996, 30(9): 390A-396A. [2] JONES K C, de VOOGT P. Persistent organic pollutants (POPs): State of the science [J]. Environmental Pollution, 1999, 100(1/2/3): 209-221. [3] COUSINS I T, BECK A J, JONES K C. A review of the processes involved in the exchange of semi-volatile organic compounds (SVOC) across the air-soil interface [J]. Science of the Total Environment, 1999, 228(1): 5-24. doi: 10.1016/S0048-9697(99)00015-7 [4] GLOTFELTY D E, TAYLOR A W, TURNER B C, et al. Volatilization of surface-applied pesticides from fallow soil [J]. Journal of Agricultural and Food Chemistry, 1984, 32(3): 638-643. doi: 10.1021/jf00123a053 [5] FLURY M. Experimental evidence of transport of pesticides through field soils—A review [J]. Journal of Environmental Quality, 1996, 25(1): 25-45. [6] WILSON S C, JONES K C. Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): A review [J]. Environmental Pollution, 1993, 81(3): 229-249. doi: 10.1016/0269-7491(93)90206-4 [7] 李晓军, 李培军, 蔺昕, 等. 土壤中有机污染物的老化概念探讨 [J]. 应用生态学报, 2007, 18(8): 1891-1896. doi: 10.13287/j.1001-9332.2007.0294 LI X J, LI P J, LIN X, et al. About the conception of “aging” for organic contaminants in soil [J]. Chinese Journal of Applied Ecology, 2007, 18(8): 1891-1896(in Chinese). doi: 10.13287/j.1001-9332.2007.0294
[8] 李晓军, 李培军, 蔺昕. 土壤中难降解有机污染物锁定机理研究进展 [J]. 应用生态学报, 2007, 18(7): 1624-1630. doi: 10.3321/j.issn:1001-9332.2007.07.035 LI X J, LI P J, LIN X. Research advances in sequestration mechanisms of hardly biodegradable organic contaminants in soil [J]. Chinese Journal of Applied Ecology, 2007, 18(7): 1624-1630(in Chinese). doi: 10.3321/j.issn:1001-9332.2007.07.035
[9] STOKES J D, PATON G I, SEMPLE K T. Behaviour and assessment of bioavailability of organic contaminants in soil: Relevance for risk assessment and remediation [J]. Soil Use and Management, 2005, 21(S2): 475-486. [10] REID B J, JONES K C, SEMPLE K T. Bioavailability of persistent organic pollutants in soils and sediments—a perspective on mechanisms, consequences and assessment [J]. Environmental Pollution, 2000, 108(1): 103-112. doi: 10.1016/S0269-7491(99)00206-7 [11] GEVAO B, JONES K C, SEMPLE K T, et al. Peer reviewed: Nonextractable pesticide residues in soil [J]. Environmental Science & Technology, 2003, 37(7): 138A-144A. [12] 蔡蕊, 王文姬, 许航, 等. 四溴双酚A在土壤中的降解转化及残留研究进展 [J]. 环境化学, 2021, 40(1): 102-110. doi: 10.7524/j.issn.0254-6108.2020021001 CAI R, WANG W J, XU H, et al. Degradation, transformation, and residue formation of tetrabromobisphenol A (TBBPA) in soil: A review [J]. Environmental Chemistry, 2021, 40(1): 102-110(in Chinese). doi: 10.7524/j.issn.0254-6108.2020021001
[13] 王松凤, 吴玄, 王麒麟, 等. 土壤中四溴双酚A不可提取态残留的降解转化 [J]. 科学通报, 2019, 64(33): 3458-3466. WANG S F, WU X, WANG Q L, et al. The degradation and transformation of TBBPA-derived NER in soil [J]. Chinese Science Bulletin, 2019, 64(33): 3458-3466(in Chinese).
[14] GEVAO B, SEMPLE K T, JONES K C. Bound pesticide residues in soils: A review [J]. Environmental Pollution, 2000, 108(1): 3-14. doi: 10.1016/S0269-7491(99)00197-9 [15] BARRACLOUGH D, KEARNEY T, CROXFORD A. Bound residues: Environmental solution or future problem? [J]. Environmental Pollution, 2005, 133(1): 85-90. doi: 10.1016/j.envpol.2004.04.016 [16] BECK A J, WILSON S C, ALCOCK R E, et al. Kinetic constraints on the loss of organic chemicals from contaminated soils: Implications for soil-quality limits [J]. Critical Reviews in Environmental Science and Technology, 1995, 25(1): 1-43. doi: 10.1080/10643389509388473 [17] 段林, 张承东, 陈威. 土壤和沉积物中疏水性有机污染物的锁定及其环境效应 [J]. 环境化学, 2011, 30(1): 242-251. DUAN L, ZHANG C D, CHEN W. Sequestration of hydrophobic organic contaminants in soil/sediment and its environmental impact [J]. Environmental Chemistry, 2011, 30(1): 242-251(in Chinese).
[18] STEINBERG S M, PIGNATELLO J J, SAWHNEY B L. Persistence of 1, 2-dibromoethane in soils: Entrapment in intraparticle micropores [J]. Environmental Science & Technology, 1987, 21(12): 1201-1208. [19] USEPA. CompTox Chemicals Dashboard [EB/OL]. [2021-09-01].https://comptox.epa.gov/dashboard [20] BARRIUSO E, BENOIT P, DUBUS I G. Formation of pesticide nonextractable (bound) residues in soil: Magnitude, controlling factors and reversibility [J]. Environmental Science & Technology, 2008, 42(6): 1845-1854. [21] BOESTEN J J T I. Proposal for field-based definition of soil bound pesticide residues [J]. Science of the Total Environment, 2016, 544: 114-117. doi: 10.1016/j.scitotenv.2015.11.122 [22] UMEH A C, DUAN L C, NAIDU R, et al. Time-dependent remobilization of nonextractable benzo[a]Pyrene residues in contrasting soils: Effects of aging, spiked concentration, and soil properties [J]. Environmental Science & Technology, 2018, 52(21): 12295-12305. [23] UNITED NATIONS ENVIRONMENT PROGRAMME (UNEP). Stockholm Convention on Persistent Organic Pollutants [EB/OL]. [2021-09-01]. [24] EUROPEAN MONITORING AND EVALUATION PROGRAMME (EMEP). Convention on Long-Range Transboundary Air Pollution [EB/OL]. [2021-09-01]. [25] SCHÄFFER A, KÄSTNER M, TRAPP S. A unified approach for including non-extractable residues (NER) of chemicals and pesticides in the assessment of persistence [J]. Environmental Sciences Europe, 2018, 30(1): 1-14. doi: 10.1186/s12302-017-0129-6 [26] UMEH A C, DUAN L C, NAIDU R, et al. Extremely small amounts of B[a]P residues remobilised in long-term contaminated soils: A strong case for greater focus on readily available and not total-extractable fractions in risk assessment [J]. Journal of Hazardous Materials, 2019, 368: 72-80. doi: 10.1016/j.jhazmat.2019.01.030 [27] LOEFFLER D, HATZ A, ALBRECHT D, et al. Determination of non-extractable residues in soils: Towards a standardised approach [J]. Environmental Pollution, 2020, 259: 113826. doi: 10.1016/j.envpol.2019.113826 [28] BURAUEL P, FÜHR F. Formation and long-term fate of non-extractable residues in outdoor lysimeter studies [J]. Environmental Pollution, 2000, 108(1): 45-52. doi: 10.1016/S0269-7491(99)00200-6 [29] 黄焕芳. 青藏高原有机氯农药的大气长距离迁移转化研究[D]. 武汉: 中国地质大学, 2018. HUANG H F. Long-range atmospheric transport and transformations of organochlorine pesticides (OCPs) in the Qinghai-Tibet plateau[D]. Wuhan: China University of Geosciences, 2018(in Chinese).
[30] KÄSTNER M, NOWAK K M, MILTNER A, et al. (Multiple) Isotope probing approaches to trace the fate of environmental chemicals and the formation of non-extractable ‘bound’ residues [J]. Current Opinion in Biotechnology, 2016, 41: 73-82. doi: 10.1016/j.copbio.2016.05.002 [31] NOWAK K M, TELSCHER M, SEIDEL E, et al. Unraveling microbial turnover and non-extractable residues of bromoxynil in soil microcosms with 13C-isotope probing [J]. Environmental Pollution, 2018, 242: 769-777. doi: 10.1016/j.envpol.2018.07.049 [32] WEISS M, GEYER R, RUSSOW R, et al. Fate and metabolism of [15N]2, 4, 6-trinitrotoluene in soil [J]. Environmental Toxicology and Chemistry, 2004, 23(8): 1852-1860. doi: 10.1897/03-414 [33] CLARK I D, FRITZ P. Environmental isotopes in hydrogeology [M]. CRC press, 2013. DOI: 10.1201/9781482242911. [34] RICHNOW H H, ESCHENBACH A, MAHRO B, et al. Formation of nonextractable soil residues: A stable isotope approach [J]. Environmental Science & Technology, 1999, 33(21): 3761-3767. [35] KÄSTNER M, NOWAK K M, MILTNER A, et al. Classification and modelling of nonextractable residue (NER) formation of xenobiotics in soil - A synthesis [J]. Critical Reviews in Environmental Science and Technology, 2014, 44(19): 2107-2171. doi: 10.1080/10643389.2013.828270 [36] NORTHCOTT G L, JONES K C. Experimental approaches and analytical techniques for determining organic compound bound residues in soil and sediment [J]. Environmental Pollution, 2000, 108(1): 19-43. doi: 10.1016/S0269-7491(99)00199-2 [37] DEC J, HAIDER K, SCHÄFFER A, et al. Use of a silylation procedure and 13C-NMR spectroscopy to characterize bound and sequestered residues of cyprodinil in soil [J]. Environmental Science & Technology, 1997, 31(10): 2991-2997. [38] HAIDER K, SPITELLER M, REICHERT K, et al. Derivatization of humic compounds: An analytical approach for bound organic residues [J]. International Journal of Environmental Analytical Chemistry, 1992, 46(1/2/3): 201-211. [39] RICHNOW H H, ESCHENBACH A, MAHRO B, et al. The use of 13C-labelled polycyclic aromatic hydrocarbons for the analysis of their transformation in soil [J]. Chemosphere, 1998, 36(10): 2211-2224. doi: 10.1016/S0045-6535(97)10193-X [40] RICHNOW H H, SEIFERT R, HEFTER J, et al. Metabolites of xenobiotica and mineral oil constituents linked to macromolecular organic matter in polluted environments [J]. Organic Geochemistry, 1994, 22(3/4/5): 671-IN10. [41] RICHNOW H H, SEIFERT R, HEFTER J, et al. Organic pollutants associated with macromolecular soil organic matter: Mode of binding [J]. Organic Geochemistry, 1997, 26(11/12): 745-758. [42] SCHWARZBAUER J, RICKING M, LITTKE R. DDT-related compounds bound to the nonextractable particulate matter in sediments of the Teltow Canal, Germany [J]. Environmental Science & Technology, 2003, 37(3): 488-495. [43] BERNS A, VINKEN R, BERTMER M, et al. Use of 15N-depleted artificial compost in bound residue studies [J]. Chemosphere, 2005, 59(5): 649-658. doi: 10.1016/j.chemosphere.2004.10.027 [44] European Chemical Agency (ECHA). Options to address non-extractable residues in regulatory persistence assessment [EB/OL]. 2019. [2021-09-01]. https://echa.europa.eu/documents/10162/17224/bg_note_addressing_non-extractable_residues.pdf/e88d4fc6-a125-efb4-8278-d58b31a5d342 [45] ZHU X J, DSIKOWITZKY L, KUCHER S, et al. Formation and fate of point-source nonextractable DDT-related compounds on their environmental aquatic-terrestrial pathway [J]. Environmental Science & Technology, 2019, 53(3): 1305-1314. [46] CAO S Q, WANG S F, ZHAO Y Y, et al. Fate of bisphenol S (BPS) and characterization of non-extractable residues in soil: Insights into persistence of BPS [J]. Environment International, 2020, 143: 105908. doi: 10.1016/j.envint.2020.105908 [47] GOULAS A, SABOURIN L, ASGHAR F, et al. Explaining the accelerated degradation of ciprofloxacin, sulfamethazine, and erythromycin in different soil exposure scenarios by their aqueous extractability [J]. Environmental Science and Pollution Research, 2018, 25(16): 16236-16245. doi: 10.1007/s11356-018-1834-4 [48] ESCHENBACH A, WIENBERG R, MAHRO B. Fate and stability of nonextractable residues of [14C]PAH in contaminated soils under environmental stress conditions [J]. Environmental Science & Technology, 1998, 32(17): 2585-2590. [49] KALATHOOR R, ZEINER M, SCHMIDT B, et al. First evidence for covalent linkage of acidic metabolites of metalaxyl and DDT as non-extractable pesticide residues in soil and sediment [J]. Environmental Chemistry Letters, 2015, 13(4): 431-437. doi: 10.1007/s10311-015-0514-6 [50] KRONIMUS A, SCHWARZBAUER J, RICKING M. Analysis of non-extractable DDT-related compounds in riverine sediments of the Teltow Canal, Berlin, by pyrolysis and thermochemolysis [J]. Environmental Science & Technology, 2006, 40(19): 5882-5890. [51] POßBERG C, SCHMIDT B, NOWAK K, et al. Quantitative identification of biogenic nonextractable pesticide residues in soil by 14C-analysis [J]. Environmental Science & Technology, 2016, 50(12): 6415-6422. [52] SCHWARZBAUER J, RICKING M, GIEREN B, et al. Anthropogenic Organic Contaminants Incorporated into the Non-Extractable Particulate Matter of Riverine Sediments from the Teltow Canal (Berlin) [M]. Environmental Chemistry, 2005: 329-352. [53] KÄSTNER M, TRAPP S, SCHÄFFER A. Consultancy services to support ECHA in improving the interpretation of Non-Extractable Residues (NER) in degradation assessment. Discussion paper-final report [EB/OL]. 2018. [2021-09-01]. https://echa.europa.eu/documents/10162/13630/echa_discussion_paper_en.pdf/4185cf64-8333-fad2-8ddb-85c09a560f7c [54] BROCK A L, REIN A, POLESEL F, et al. Microbial turnover of glyphosate to biomass: Utilization as nutrient source and formation of AMPA and biogenic NER in an OECD 308 test [J]. Environmental Science & Technology, 2019, 53(10): 5838-5847. [55] HENNECKE D, JÖHNCKE U, WIEMANN A, et al. How to characterise non-extractable residues (NER) in PBT assessment-development of a harmonised procedure to be used in routine testing[C]. Proceedings of the SETAC Europe 29th annual meeting, 2019. [56] HARMSEN J, HENNECKE D, HUND-RINKE K, et al. Certainties and uncertainties in accessing toxicity of non-extractable residues (NER) in soil [J]. Environmental Sciences Europe, 2019, 31(1): 1-14. doi: 10.1186/s12302-018-0176-7 [57] ROBERTS T R. Non-extractable pesticide residues in soils and plants [J]. Pure and Applied Chemistry, 1984, 56(7): 945-956. doi: 10.1351/pac198456070945 [58] ALEXANDER M. Aging, bioavailability, and overestimation of risk from environmental pollutants [J]. Environmental Science & Technology, 2000, 34(20): 4259-4265. [59] SCHAUMANN G E, BERTMER M. Do water molecules bridge soil organic matter molecule segments? [J]. European Journal of Soil Science, 2008, 59(3): 423-429. doi: 10.1111/j.1365-2389.2007.00959.x [60] SENESI N. Binding mechanisms of pesticides to soil humic substances [J]. Science of the Total Environment, 1992, 123/124: 63-76. doi: 10.1016/0048-9697(92)90133-D [61] KÄSTNER M, RICHNOW H H. Formation of Residues of Organic Pollutants Within the Soil Matrix — Mechanisms and Stability[M]. Treatment of Contaminated Soil, 2001: 219-251. [62] STEVENSON F J. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties [M]. Agronomy: A series of monographs, 1982. [63] MADIGAN M, MARTINKO J, STAHL D, et al. Brock biology of microorganisms, Pearson [M]. Benjamin Cummings, San Francisco, CA. 2011. [64] BAS P, ARCHIMÈDE H, ROUZEAU A, et al. Fatty acid composition of mixed-rumen bacteria: Effect of concentration and type of forage [J]. Journal of Dairy Science, 2003, 86(9): 2940-2948. doi: 10.3168/jds.S0022-0302(03)73891-0 [65] RICHNOW H H, ANNWEILER E, KONING M, et al. Tracing the transformation of labelled [1-13C]phenanthrene in a soil bioreactor [J]. Environmental Pollution, 2000, 108(1): 91-101. doi: 10.1016/S0269-7491(99)00205-5 [66] MONTEIRO R T R, WALDER J M M, WIENDL F M, et al. Endosulfan-14C: degradation in soil [J]. Revista Brasileira de Ciência do Solo, 1989, 13: 163-168. [67] MORDAUNT C J, GEVAO B, JONES K C, et al. Formation of non-extractable pesticide residues: Observations on compound differences, measurement and regulatory issues [J]. Environmental Pollution, 2005, 133(1): 25-34. doi: 10.1016/j.envpol.2004.04.018 [68] MATTHIES M, WITT J, KLASMEIER J. Determination of soil biodegradation half-lives from simulation testing under aerobic laboratory conditions: A kinetic model approach [J]. Environmental Pollution, 2008, 156(1): 99-105. doi: 10.1016/j.envpol.2007.12.040 [69] JOHNSON M D, KEINATH T M, WEBER W J. A distributed reactivity model for sorption by soils and sediments. 14. characterization and modeling of phenanthrene desorption rates [J]. Environmental Science & Technology, 2001, 35(8): 1688-1695. [70] DING Y, LI L, WANIA F, et al. Formation of non-extractable residues as a potentially dominant process in the fate of PAHs in soil: Insights from a combined field and modeling study on the eastern Tibetan Plateau [J]. Environmental Pollution, 2020, 267: 115383. doi: 10.1016/j.envpol.2020.115383 [71] 丁洋. 青藏高原东缘土壤中典型持久性有机污染物的来源与迁移转化机制 [D]. 武汉: 中国地质大学, 2021. DING Y. Source identification and mechanisms of transportation and transformation of typical persistent organic pollutants in the soil from the Eastern Tibetan Plateau [D]. Wuhan: China University of Geosciences, 2021(in Chinese).
[72] TRAPP S, BROCK A L, NOWAK K, et al. Prediction of the formation of biogenic nonextractable residues during degradation of environmental chemicals from biomass yields [J]. Environmental Science & Technology, 2018, 52(2): 663-672. [73] BROCK A L, KÄSTNER M, TRAPP S. Microbial growth yield estimates from thermodynamics and its importance for degradation of pesticides and formation of biogenic non-extractable residues [J]. SAR and QSAR in Environmental Research, 2017, 28(8): 629-650. doi: 10.1080/1062936X.2017.1365762 [74] NOWAK K M, MILTNER A, GEHRE M, et al. Formation and fate of bound residues from microbial biomass during 2, 4-D degradation in soil [J]. Environmental Science & Technology, 2011, 45(3): 999-1006. [75] NOWAK K M, GIRARDI C, MILTNER A, et al. Contribution of microorganisms to non-extractable residue formation during biodegradation of ibuprofen in soil [J]. Science of the Total Environment, 2013, 445/446: 377-384. doi: 10.1016/j.scitotenv.2012.12.011 [76] BENOIT P, BARRIUSO E. Fate of 14C-ring-labeled 2, 4-D, 2, 4-dichlorophenol and 4-chlorophenol during straw composting [J]. Biology and Fertility of Soils, 1997, 25(1): 53-59. doi: 10.1007/s003740050279 [77] LAL R, PANDEY G, SHARMA P, et al. Biochemistry of microbial degradation of hexachlorocyclohexane and prospects for bioremediation [J]. Microbiology and Molecular Biology Reviews, 2010, 74(1): 58-80. doi: 10.1128/MMBR.00029-09 [78] WAIGI M G, KANG F X, GOIKAVI C, et al. Phenanthrene biodegradation by sphingomonads and its application in the contaminated soils and sediments: A review [J]. International Biodeterioration & Biodegradation, 2015, 104: 333-349. [79] KÄSTNER M, STREIBICH S, BEYRER M, et al. Formation of bound residues during microbial degradation of [14C]anthracene in soil [J]. Applied and Environmental Microbiology, 1999, 65(5): 1834-1842. doi: 10.1128/AEM.65.5.1834-1842.1999 [80] NORTHCOTT G L, JONES K C. Partitioning, extractability, and formation of nonextractable PAH residues in soil. 1. compound differences in aging and sequestration [J]. Environmental Science & Technology, 2001, 35(6): 1103-1110. [81] ENELL A, REICHENBERG F, EWALD G, et al. Desorption kinetics studies on PAH-contaminated soil under varying temperatures [J]. Chemosphere, 2005, 61(10): 1529-1538. doi: 10.1016/j.chemosphere.2005.04.092 [82] HAFIDI M, AMIR S, JOURAIPHY A, et al. Fate of polycyclic aromatic hydrocarbons during composting of activated sewage sludge with green waste [J]. Bioresource Technology, 2008, 99(18): 8819-8823. doi: 10.1016/j.biortech.2008.04.044 [83] LUO L, LIN S, HUANG H L, et al. Relationships between aging of PAHs and soil properties [J]. Environmental Pollution, 2012, 170: 177-182. doi: 10.1016/j.envpol.2012.07.003 [84] XING B S, PIGNATELLO J J. Dual-mode sorption of low-polarity compounds in glassy poly(vinyl chloride) and soil organic matter [J]. Environmental Science & Technology, 1997, 31(3): 792-799. [85] KÄSTNER M, MAHRO B. Microbial degradation of polycyclic aromatic hydrocarbons in soils affected by the organic matrix of compost [J]. Applied Microbiology and Biotechnology, 1996, 44(5): 668-675. doi: 10.1007/BF00172501 [86] KOHL S D, RICE J A. The binding of contaminants to humin: A mass balance [J]. Chemosphere, 1998, 36(2): 251-261. doi: 10.1016/S0045-6535(97)10005-4 [87] WHITE J C, KELSEY J W, HATZINGER P B, et al. Factors affecting sequestration and bioavailability of phenanthrene in soils [J]. Environmental Toxicology and Chemistry, 1997, 16(10): 2040-2045. doi: 10.1002/etc.5620161008 [88] ZHAO Q, XING B S, TAI P D, et al. Effect of freeze-thawing cycles on soil aging behavior of individually spiked phenanthrene and Pyrene at different concentrations [J]. Science of the Total Environment, 2013, 444: 311-319. doi: 10.1016/j.scitotenv.2012.11.062 [89] ANDRÉA M M, TOMITA R Y, LUCHINI L C, et al. Laboratory studies on volatilization and mineralization of 14c-p, p'-DDT in soil, release of bound residues and dissipation from solid surfaces [J]. Journal of Environmental Science and Health, Part B, 1994, 29(1): 133-139. doi: 10.1080/03601239409372865 [90] ZHU X J, SONG X, SCHWARZBAUER J. First insights into the formation and long-term dynamic behaviors of nonextractable perfluorooctanesulfonate and its alternative 6: 2 chlorinated polyfluorinated ether sulfonate residues in a silty clay soil [J]. Science of the Total Environment, 2021, 761: 143230. doi: 10.1016/j.scitotenv.2020.143230 [91] KHAN S U, IVARSON K C. Microbiological release of unextracted (bound) residues from an organic soil treated with prometryn [J]. Journal of Agricultural and Food Chemistry, 1981, 29(6): 1301-1303. doi: 10.1021/jf00108a052 [92] WEIß M, GEYER R, GÜNTHER T, et al. Fate and stability of 14c-labeled 2, 4, 6-trinitrotoluene in contaminated soil following microbial bioremediation processes [J]. Environmental Toxicology and Chemistry, 2004, 23(9): 2049. doi: 10.1897/03-143 [93] ULRICH N, ENDO S, BROWN T, et al. UFZ-LSER database v 3.2.1 [Internet], Leipzig, Germany: Helmholtz Centre for Environmental Research-UFZ [EB/OL]. 2017. [2021-09-01].http://www.ufz.de/lserd [94] GOSS K U. Predicting the equilibrium partitioning of organic compounds using just one linear solvation energy relationship (LSER) [J]. Fluid Phase Equilibria, 2005, 233(1): 19-22. doi: 10.1016/j.fluid.2005.04.006 [95] ORTEGA-CALVO J J, HARMSEN J, PARSONS J R, et al. From bioavailability science to regulation of organic chemicals [J]. Environmental Science & Technology, 2015, 49(17): 10255-10264. [96] UMEH A C, DUAN L C, NAIDU R, et al. Residual hydrophobic organic contaminants in soil: Are they a barrier to risk-based approaches for managing contaminated land? [J]. Environment International, 2017, 98: 18-34. doi: 10.1016/j.envint.2016.09.025 -



 下载:
下载: