-
氯代芳烃,包括二噁英(PCDD/Fs)、多氯联苯、五氯苯、六氯苯等[1],作为一类典型的持久性有机污染物,具有高毒性、生物累积性,难降解性和远距离迁移性[2],会对环境造成严重的危害[3],因此,包括六氯苯、PCDD/Fs以及多氯联苯等在内的氯代芳烃已被列入《关于持久性有机污染物的斯德哥尔摩公约》。研究表明,废弃物焚烧[4],铁矿石烧结,金属冶炼[5]、炼焦以及发电供热等热工业过程的非故意排放[6]是目前二噁英等氯代芳烃的主要来源[7]。Lei等[8]根据估算的二噁英排放因子以及对各类二噁英来源的调查,计算了2018年中国的二噁英排放总量为9267 g TEQ,约占世界排放总量的9.5%。在各类排放源中,金属生产、供热和发电、垃圾焚烧、垃圾处置是我国二噁英的主要排放来源,分别占国家排放总量的53.7%、22.3%、9.8%和7.1%,其它排放源仅占7.1%。陈露露等[9]的研究表明,在空间上,我国PCDD/Fs的排放主要集中在京津冀、长三角、珠三角等地区,其中京津冀和长三角地区的PCDD/Fs排放主要来源于钢铁生产,而珠三角地区主要来源于垃圾焚烧。此外,在日本[10]和葡萄牙[11],垃圾焚烧是最主要的二噁英排放来源。近年来,随着公民环保意识的增强以及国家对环境保护的重视,我国对生活垃圾焚烧二噁英的排放浓度进行了严格的控制。2016年执行的《生活垃圾焚烧污染控制标准》中规定垃圾焚烧烟气中二噁英类的排放浓度降低至0.1 ng·m−3[12]。因此,控制和削减热工业过程,尤其是垃圾焚烧行业中的二噁英等氯代芳烃的排放对于我国的履约建设及环境安全具有重要意义。
垃圾焚烧等热工业过程中,除了氯代芳烃等有机污染物的排放外,高温条件也会造成氮氧化物(NOx)的生成[13]。NOx的主要生成机理有燃料型NOx[14]、热力型NOx和瞬时型NOx[15]。垃圾焚烧炉膛温度一般为850—1000 ℃,因此NOx排放主要来自燃料型NOx,即燃料中的含氮化合物在燃烧条件下转化为NOx[14]。氮氧化物的排放会引起一系列环境问题,如臭氧层空洞[16]、酸雨、雾霾[17]、光化学烟雾等[14]。随着国家对脱硝的重视及相关政策的实施,工业氮氧化物的排放量从2010年的1523.8万t,降低至2019年的548.1万t,总体下降了64.0%[18]。虽然NOx排放量逐年下降,但工业过程的排放限值也在进一步收紧。2016年执行的《生活垃圾焚烧污染控制标准》规定,垃圾焚烧烟气中NOx的排放浓度从400 mg·m−3降低至250 mg·m−3(24 h均值)[12]。因此,对氮氧化物的削减也至关重要[19]。
目前,常见的氯代芳烃的控制技术主要有热焚烧技术、光催化氧化技术、电催化氧化技术、催化加氢脱氯技术[20]、催化氧化技术等[21-22]。其中,催化氧化技术可以将氯代芳烃深度降解为CO2、H2O、HCl等小分子物质,从而引起研究者们的广泛关注[23]。催化氧化技术的核心是催化剂,常见的催化剂主要有贵金属催化剂(如Pt、Pb等)、钙钛矿催化剂(ABO3型)和过渡金属氧化物催化剂(CrOx、MnOx、VOx等)[24]。其中,贵金属催化剂拥有优异的催化活性,但其成本高,抗氯中毒能力弱;钙钛矿型催化剂热稳定性强,具有一定的抗中毒能力,并且价格低廉,但是其活性温度高,容易产生高氯代副产物。相比而言,过渡金属氧化物具有较低的成本、较强的催化活性以及抗中毒能力,且不易产生二次污染等优势[25]。其中以二氧化钛为载体的过渡金属氧化物催化剂凭借其优异的性能在催化降解领域有着广泛的应用。
氮氧化物(NOx)的控制技术通常可以分为3类[14]:燃烧前脱氮、改进燃烧方式降低燃烧后氮氧化物的排放量、烟气脱硝技术。在这3类技术中,烟气脱硝技术在工业上的应用最为广泛,常见的烟气脱硝技术主要有选择性催化还原(SCR)、选择性非催化还原、湿式洗涤法、吸附法、电子束法、电化学还原法等。其中,选择性催化还原法降解效率较高,目前已经成为主要的NOx减排技术[26]。在SCR反应中,催化剂的选择也至关重要,通常需要满足以下特性:(1)脱硝活性好;(2)机械强度高;(3)合适的操作温度范围;(4)抗中毒能力强。常见的用于NH3-SCR反应的催化剂主要有贵金属(Pt,Pd、Au等)、贵金属/过渡金属(Pt/Al2O3、Pt/ZSM5、Pd/Al2O3、Rh/Al2O3、Rh/ZSM5等)、过渡金属氧化物(NiO、Co3O4、V2O5、Fe3O4、MnO2等)以及过渡金属(Cu、Fe、Cr、V、Mn等)。其中以钒基催化剂为代表的过渡金属氧化物由于具有良好的低温活性以及优异的抗SO2能力在工业催化中有着广泛的应用。V2O5-WO3/TiO2催化剂是目前工业上应用最广泛的商业催化剂,这类催化剂在固体废弃物焚烧等行业都表现出了优异的性能[27]。
目前工业上对污染物的控制已经由单一污染物向混合多污染物的方向发展[28],在固体废弃物焚烧、铁矿石烧结和金属冶炼等行业中会同时排放氯代芳烃和氮氧化物,这两类物质均作为PM2.5和O3的重要前驱体,容易对生态环境及人体健康造成严重威胁。因此,有必要对二者进行协同处置,从而达到排放标准。目前,关于氯代芳烃和NOx的协同控制已有了一定进展。Xu等[29]的研究发现,在有氧条件下,NOx对五氯苯的降解有促进作用,而在无氧条件下,NOx对五氯苯促进作用则忽略不计。Bertinchamps等[30]发现,对于VOx-TiO2催化剂而言,在有氧条件下,通入NO后对氯苯降解活性没有较大提升,而当催化剂中含有WO3和MoO3时,NO则对氯苯的降解有较为明显的促进作用。此外,Gallastegi-Villa等[31]还通过研究不同类型催化剂(将Cu、Fe、Mn、V负载在ZSM-5载体上)对氯代芳烃和NOx的降解活性,并与商用VOx/TiO2催化剂进行对比,结果发现在模拟焚烧厂烟气环境的条件下,与金属/沸石催化剂相比,VOx/TiO2催化剂对NO和o-DCB的协同降解表现出了最好的性能。因此,催化剂对二者协同处置的活性与反应条件和催化剂的种类息息相关。然而,目前对于氯代芳烃和NOx的协同处置的研究大多数集中于实验室自主研发的催化剂,而对商用催化剂的协同活性缺乏系统全面的评价。因此开展商用催化剂对氯代芳烃和NOx的协同处置研究能够为实际工业过程中两类污染物的协同减排提供科学有效的参考与支撑。
本研究选取了5类商用SCR蜂窝催化剂为研究对象,选取氯苯(CB)作为氯代芳烃的模型污染物,一氧化氮(NO)作为NOx的模型污染物,分别探究在不同温度条件下商用SCR催化剂对CB、NO以及CB和NO协同降解活性。采用XRD、SEM-EDX、XPS、BET等表征技术对催化剂的晶型、微观形貌、元素组成以及元素价态、比表面积进行深入分析,探究不同催化剂协同降解活性差异的主导因素,利用热脱附-GC/MS联用技术全面研究催化降解过程中的中间产物,揭示CB与NO协同控制机制。
-
实验试剂:高纯氮气(纯度0.99999,海科元昌实用气体有限公司);高纯空气(氧气20%、氮气80%,纯度0.99999,海科元昌实用气体有限公司);氯苯(1841 mg·m−3,平衡气氮气,北京兆格气体科技有限公司);商用SCR脱硝催化剂(某催化剂生产公司);一氧化氮(972 mg·m−3,平衡气氮气);氨气(730 mg·m−3,平衡气氮气)。
-
为了对催化剂的形貌、结构和元素组成等进行分析,利用荷兰PANalytical X´pert Pro 型粉末衍射仪进行X射线衍射(XRD)分析。采用Cu、Kα射线,管电压40 kV,管电流40 mA,λ=0.15406 nm,扫描范围(2θ)为5°—80°;采用日本日立公司的SU-8020型场发射扫描电子显微镜(配有EDX附件)进行SEM-EDX表征;采用单色Al、Kα作为X射线源,在ESCALAB250仪器上进行X射线光电子能谱(XPS)表征;在Micromertics ASAP 2460仪器上进行BET表征。
-
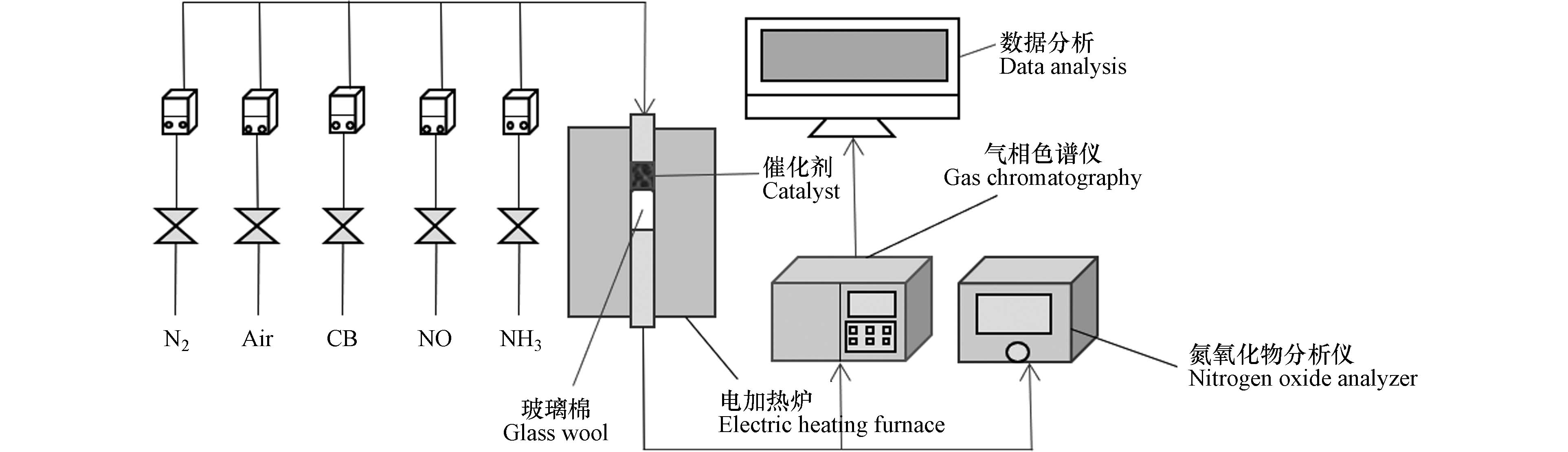
利用自主搭建的微型反应装置与气相色谱-FID/FID联用设备(Aglient 7820A, USA)对催化剂的活性进行评价,该装置的示意图如图1所示。
称取商用催化剂粉末100 mg,放入反应器中,装填完成后将玻璃管的两端连接气路,反应前通1 h氮气清洗管路,并做好气密性检验。随后将其置于加热炉进行加热,温度设定为250 ℃、300 ℃。反应气体的组成如下:O2含量5%,CB、NO、NH3浓度分别为921、168 、82 mg·m−3,总流量80 mL·min−1。反应前后的CB浓度可以通过配有双FID的安捷伦7820A气相色谱仪进行在线分析,气相色谱仪的条件如下:DB-624色谱柱:60 m×320 μm×1.8 μm,定量环体积为1 mL,柱箱温度初始值为60 ℃,保持1 min,随后以10 ℃·min−1的速率升到150 ℃,再以10 ℃·min−1的速率升到180 ℃,总运行时间为13 min,前FID检测器加热温度300 ℃,空气流量400 mL·min−1,氢气流量40 mL·min−1,尾吹气流量25 mL·min−1,后FID检测器加热温度300 ℃,空气流量400 mL·min−1,氢气流量30 mL·min−1。CB的降解效率和CO2选择性计算公式如下:
其中,DECB为CB降解效率,CBin和CBout分别指进口和出口处CB的浓度。
其中,
${S_{{\text{C}}{{\text{O}}_{\text{2}}}}} $ 为CO2选择性,CBin和CBout分别指进口和出口处CB的浓度,CO2out为实际产生的CO2浓度。在对NO进行降解时,NO反应前后的浓度由型号为42i的NO-NO2-NOx分析仪(Thermo Fisher Scientific, USA)进行检测。根据反应前后NO的浓度计算NO的转化率,计算公式如下:
其中,CENO为NO的转化率,NOin和NOout分别为进口和出口处NO的浓度。
-
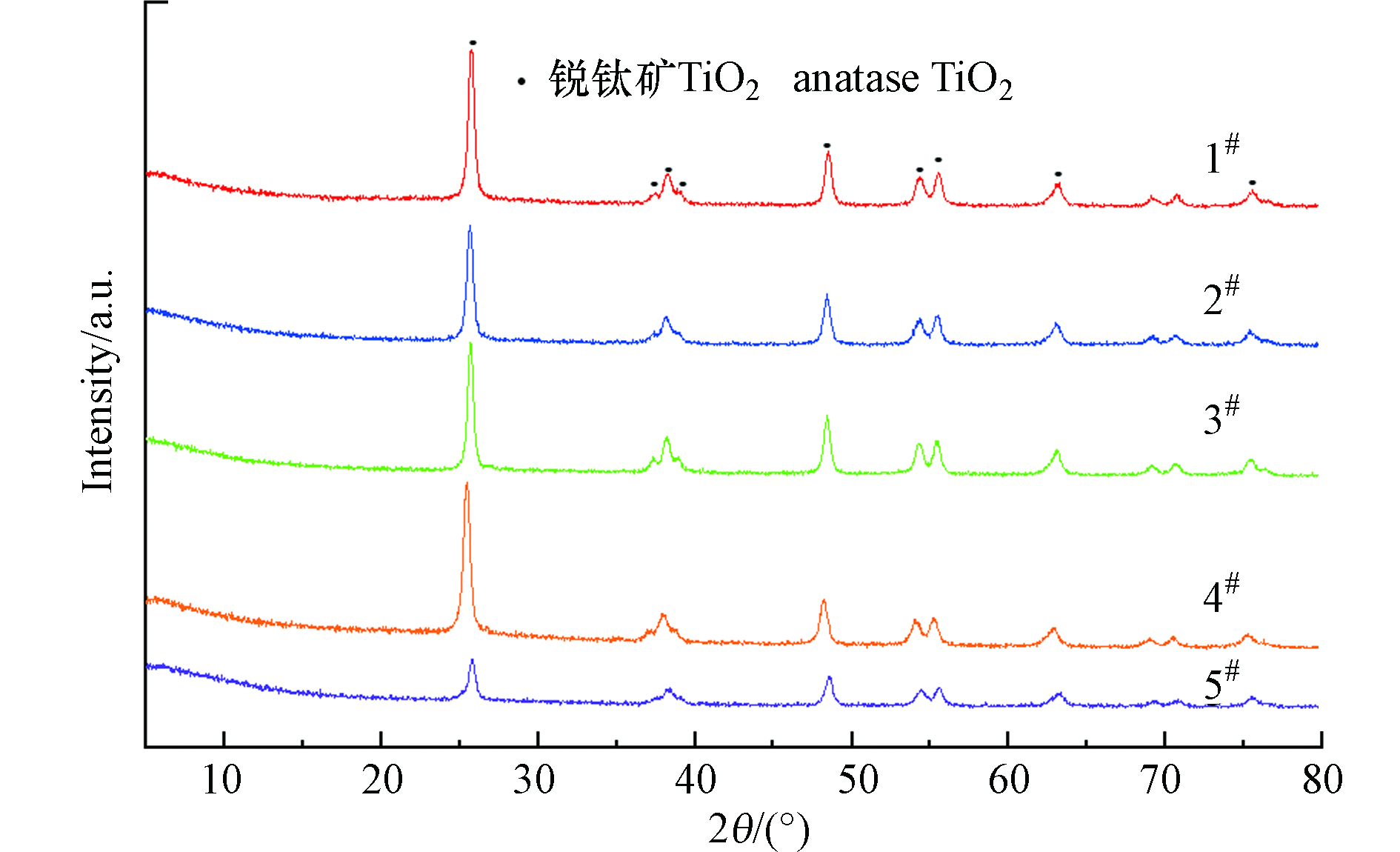
本文对1#—5#商用催化剂进行了XRD、SEM-EDX、XPS、BET的全面表征。其中图2为5类商用催化剂的XRD图谱。从图2可以看出,5类商用催化剂均为锐钛矿型TiO2(JCPDS 00-002-0406)。XRD图谱中未检测到其它元素晶型的存在,这可能是由于其它元素含量较少,或在TiO2表面较为分散[27]。
为了进一步探究5类商用催化剂的物理化学性质,利用SEM和EDX分别对它们的微观形貌和元素组成进行探究,结果如图3和表1所示。5类商用催化剂的微观形貌类似,均由球形粒子组装而成。其中1#催化剂的颗粒直径约为20 nm,主要组成元素包括Ti、O、V,活性组分V的质量百分比为1.02%。2#催化剂颗粒直径较大,约为500 nm,其元素组成与1#催化剂类似,主要含有Ti、O、V等元素,V元素的质量比例低于1#催化剂,为0.66%。3#催化剂的颗粒直径与2#催化剂相近,约为400 nm,除了Ti、O、V等元素以外,3#催化剂还含有一定量的W元素,且W与V的质量百分比接近,分别为0.46%和0.55%。4#催化剂的颗粒直径在5类催化剂中最大,约为1 μm,主要元素与3#催化剂类似,均包括Ti、O、V、W,但与3#催化剂相比,其W含量更高,质量比为1.77%,V含量略有降低,质量比为0.42%。5#催化剂与1#催化剂类似,颗粒直径大约为20 nm,主要组成元素为Ti、O、V。但与1#催化剂相比,5#催化剂V的质量百分比略高,为1.30%。
结合5类商用催化剂的微观形貌与元素组成可以得出,5类商用催化剂均含有Ti、O、V等3种元素,3#催化剂与4#还含有W元素。为了进一步探究5类商用催化剂的比表面积、孔容、孔径等信息,利用N2吸附-脱附技术进行了BET表征,结果如表2所示。1#、2#、4#和5#催化剂的比表面积相差不大,分别为67.87、72.48、70.79、76.21 m2·g−1,3#催化剂的比表面积最小,仅为49.78 m2·g−1。 5种商用催化剂的总孔容和平均孔径较为接近,分布范围分别为0.24—0.27 cm3·g−1和13.49—19.56 nm。较大的比表面积有利于污染物与活性位点的接触,进而促进催化剂对污染物的降解。Cao等[32]的研究表明,较大的比表面积有利于活性物种的分散,从而产生更多活性位点,提高催化剂的活性。
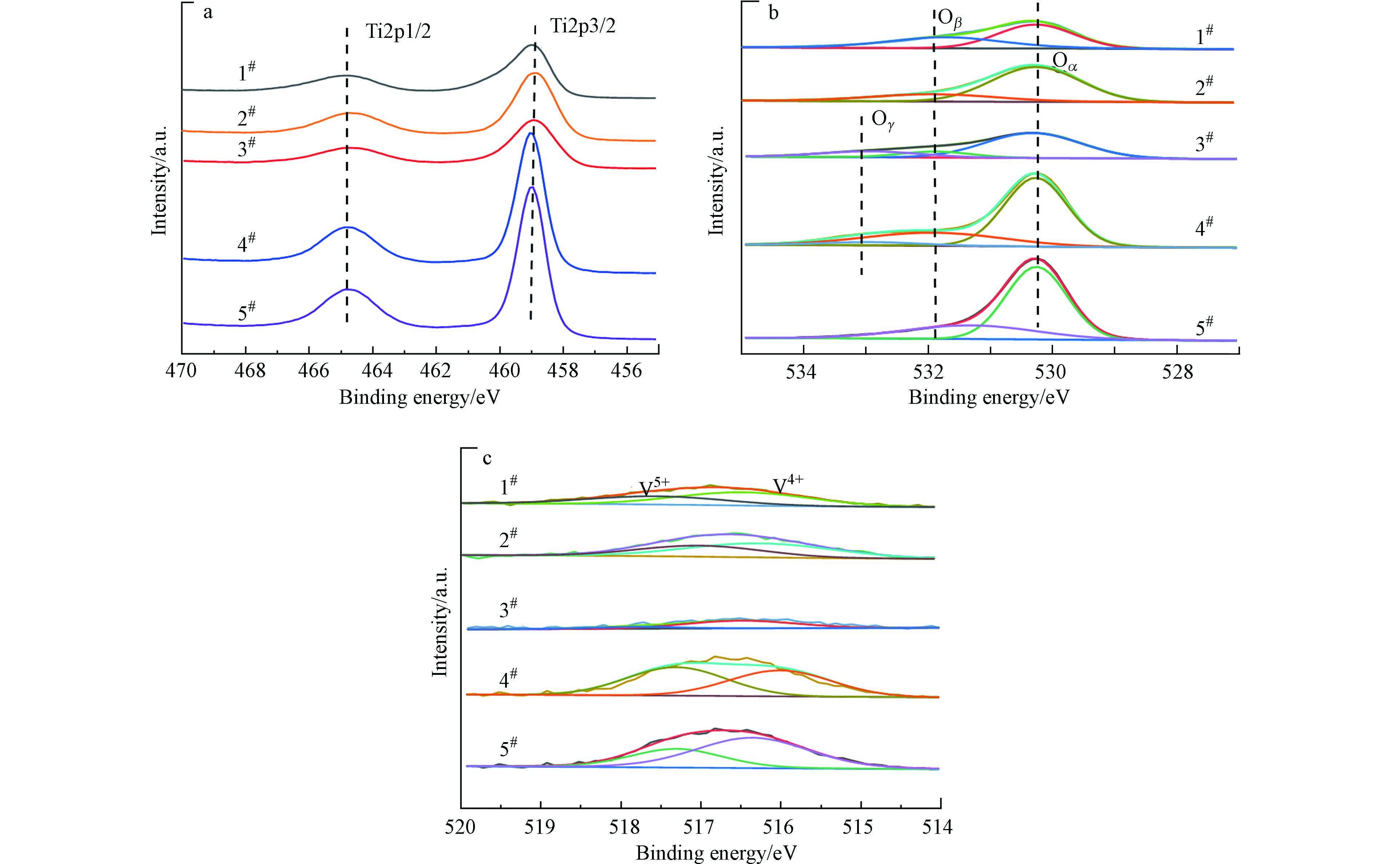
此外,本研究还利用XPS表征技术深入分析了催化剂表面Ti、O和V的化学价态,如图4所示。其中图4(a) 为5类催化剂Ti2p的XPS谱图,可以看出,每类催化剂的Ti2p谱图均由结合能位于459.0 eV左右和464.6 eV左右的两个峰组成,分别代表Ti2p3/2和Ti2p1/2[33],表明催化剂中的Ti元素均以Ti4+形式存在[34]。图4(b)显示了5类催化剂O1s的XPS谱图,其中结合能位于530.2—530.4 eV处的峰代表晶格氧(记为Oα)[35],结合能位于531.3—531.9 eV处的峰代表表面吸附氧(记为Oβ),结合能位于532.8—533.0 eV处的峰代表化学吸附的水或羟基物种(记为Oγ)[36]。研究表明,Oβ属于亲电性氧物种,与Oα和Oγ相比,具有更强的反应活性[20],可以促进污染物的氧化[37],因此较高比例的Oβ有利于氯苯的氧化和SCR反应的发生。Li等[38]的研究表明,Oβ含量高,代表催化剂氧化能力强,CB更容易深度氧化为CO2和H2O。Xu等[39]的研究表明,Oβ具有更高的活性,对于SCR反应至关重要。为了评价5类商用催化剂的活性氧物种比例,计算了5类催化剂Oβ的占比,结果如表3所示。从表3可以看出,1#催化剂的表面吸附氧占比最高,为44.10%,其次是5#、2#催化剂,表面吸附氧占比分别为29.02%、23.95%,4#催化剂和3#催化剂中表面吸附氧占比较低,分别为13.22%和12.46%。
图4(c)显示了5类催化剂V2p的XPS谱图,其中结合能位于516.28—516.49 eV处的峰代表V4+[32],结合能位于517.01—517.80 eV处的峰代表V5+[40]。研究表明,V5+是SCR反应中的主要活性物质,V5+含量的高低决定了催化剂的活性的强弱[41]。Xiao等[41]的研究发现,SCR催化剂中引入NaO后,催化剂中V5+占比降低,从而导致催化剂的SCR活性降低。本文计算了5类催化剂中V5+的占比,结果如表3所示。1#催化剂的V5+占比最高,为38.95%,其次为5#、2#和4#催化剂,V5+比例分别为38.05%、37.17%和25.55%,3#催化剂V5+占比最低,仅为19.85%。上述结果与活性氧的分布结果一致,表明具有较高活性氧物种和V5+比例的1#催化剂可能对CB和NO有较高的催化活性和协同处置性能。
-
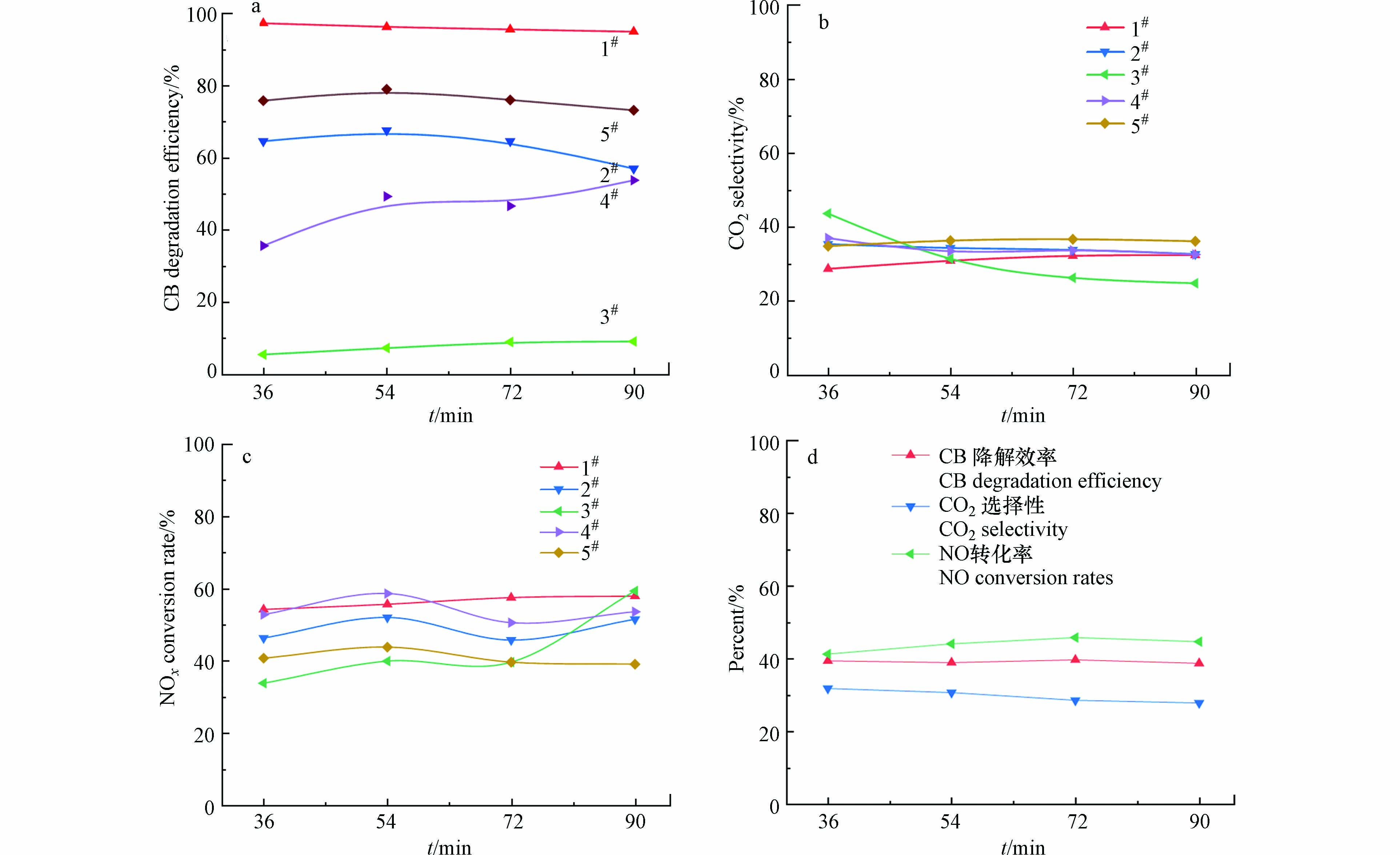
图5(a)显示了300 ℃条件下5类商用催化剂对CB (921 mg·m−3)的降解效率。可以看出,1#催化剂对CB的降解效率最高,在90 min的反应周期内CB的降解效率均能保持在95%左右,其次是5#、2#和4#催化剂,对CB的降解效率分别为75%、60%和40%左右,3#催化剂的降解效率最低,仅有5%左右。图5(b)为 300 ℃条件下5类商用催化剂对CO2的选择性,从图中可以看出,5类商用催化剂对CO2的选择性差异较小,均在30%左右。其中3#催化剂随着反应的进行,CO2的选择性有较为明显的下降。图5(c) 为300 ℃条件下5类商用催化剂对NO的转化率(NO 134 mg·m−3 ,NH3 82 mg·m−3)。与氯苯的降解活性规律类似,1#催化剂对NO的转化率较为突出,在90 min的反应周期内均能保持在57%左右,且具有较好的稳定性。2#、4#和5#催化剂对NO的转化率相差不大,在40%—50%之间,3#催化剂对NO转化率波动较大。
催化剂的活性主要与材料表面活性位点的数量有关,而活性位点的数量又取决于材料的物理化学性质[42]。研究表明,催化剂较大的比表面积有利于污染物的吸附与去除。Huang等[43]的研究发现,纳米棒状CeO2与纳米块状CeO2和纳米多面体CeO2相比,有更大的比表面积,更有利于活性位点的分散,因此更有利于污染物的催化降解。Park等[44]的研究发现,中空NiO-NiS比表面积较大,具有更多活性位点,因此对CO2具有优异的光催化转化性能。除了比表面积,活性组分的种类与价态也与催化活性息息相关。Li等[38]的研究发现,V4/TiO2比表面积最大,但是由于活性组分含量较少,催化活性并非最高。基于上述的XPS表征结果,1#催化剂拥有较大的比表面积,较多的活性氧物种和活性钒物种,其次是5#、2#、4#和3#催化剂。各催化剂的活性氧物种比例和V5+占比的分布趋势与其对CB的催化活性顺序一致,表明活性氧物种和活性钒物种是决定催化剂活性的主要因素。
由图5(a) 和图5(c) 可以看出,1#催化剂对CB和NO具有较好的催化活性,因此,选用1#催化剂为研究对象,探究其在250 ℃较低温度条件下分别对CB的降解效率、CO2的选择性和NO的转化率,结果如图5(d) 所示。可以看出,当温度从300 ℃下降到250 ℃时,1#催化剂对CB的降解效率大幅下降,从95%左右下降到40%左右,CO2的选择性没有明显变化;对NO转化率也有所下降,从57%左右下降到45%左右。上述结果表明商用催化剂对CB和NO的催化活性随着温度的降低均有所下降。
-
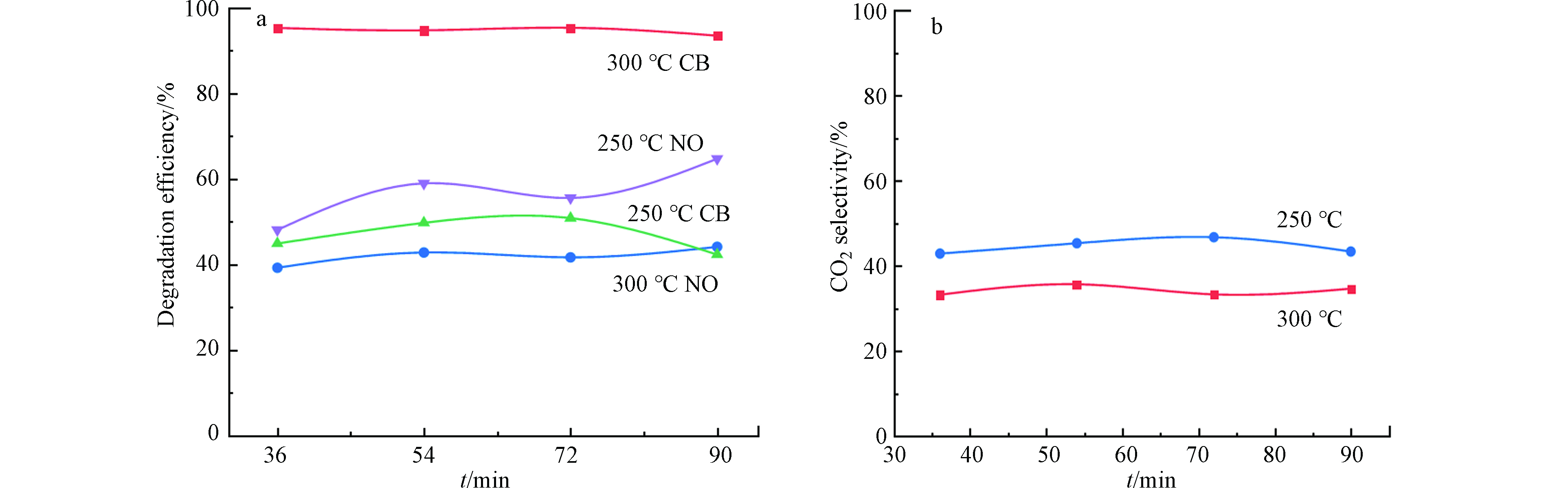
为了探究商用催化剂对CB和NO的协同处置活性,本研究选取了活性较为突出的1#催化剂,分别探究了在不同温度条件下催化剂对CB和NO的协同处置能力。图6展示了在300 ℃和250 ℃条件下1#催化剂对CB和NO的协同处置活性和CO2选择性。结果发现,在300 ℃下,与其在单污染物处置体系中的降解活性相比,CB的降解效率仍能保持在95%左右,同时CO2选择性有着微弱的提高。而NO转化效率有一定幅度的降低,基本维持在40%左右。当温度降至250 ℃时,协同处置体系中的CB降解效率为45%左右,与250 ℃下单污染物处置时的活性基本一致,CO2选择性有所提高,达到40%。相比而言,较低温度下,两类污染物共存时,催化剂对NO的降解活性为60%左右,比NO单独降解体系活性提升了10%—20%。上述结果表明,在250 ℃和300 ℃条件下,NO的引入均能够在一定程度上促进催化剂对CB的降解。然而对于NO来说,在高温条件下,CB的存在会使催化剂对NO的转化活性降低;当温度降低时,催化剂对NO的转化活性有所提升,这是由于NO在金属氧化物的表面能够被氧化生成NO2。利用NO-NO2-NOx分析仪对降解过程中的NO2进行了检测分析,发现1#催化剂在300 ℃和250 ℃下协同降解CB和NO过程中均有NO2生成,浓度分别为7.41 mg·m−3和5.06 mg·m−3。NO2的氧化性能大于O2,能够参与到氯苯的氧化反应中。Bertinchamps等[45]发现在NO与CB的协同降解过程中,CB在VOx表面发生氧化,而NO主要在WOx和MoOx表面与O2反应生成NO2,NO2与O2相比有更强的氧化能力,可以参与V4+Ox位点的再氧化,导致反应循环加速,从而促进氧化反应的进行。Gan等[46]的研究发现,CB的加入抑制了SCR的主反应以及副反应,主要原因是Cl可以通过亲电以及亲核反应从CB中分离,在催化剂表面沉积,从而影响SCR反应的进行。Wang等[47]的研究发现,CB降解过程中解离出的Cl能够在催化剂表面提供额外的Brønsted酸位点,消耗吸附的NH4+,从而形成非活性物质NH4Cl,导致SCR反应活性降低。而Gan等[48]发现在200—300 ℃的范围内,CB能够在NO还原为N2的同时转化为CO2,抑制MnOx-CeO2催化剂上NH3过度氧化,从而提高了N2的选择性,拓宽了NO的活性温度窗口。因此,1#催化剂在协同处置CB和NO过程中,NO被氧化,生成了NO2,从而对CB降解有促进作用。而对NO来说,在不同温度下CB的存在对其转化有不同的影响:较高温度下,NO2生成量相对较多,C—Cl键解离程度较大,会在一定程度上影响SCR反应的进行,导致NO的转化率较低;当温度降低时,CO2选择性提高,可能会抑制NH3的过度氧化,同时NO2生成量和C—Cl键的解离均变弱,进而使得SCR反应相对增强。
目前有关商用催化剂对CB和NO的协同降解的研究较少,仅有少量相关报道,如浙江大学蒋威宇[28]所采用的商用催化剂在300 ℃、协同降解条件下对NO的转化率可以达到90%,CB的转化率可以达到100%,CO2选择性保持在50%—60%范围内。在250 ℃条件下,氯苯的转化率在83%—90%之间波动,NO的转化率能较好地稳定在90%附近,CO2选择性略有下降,在40%—60%之间。与之相比,本文中商用催化剂对CB和NO的协同处置效果较差。可见,不同品牌的商用催化剂对CB和NO的协同处置能力存在较为明显的差异,可能与催化剂的生产厂家有关。
与商用催化剂相比,目前有关实验室制备的催化剂对CB和NO协同处置的研究较多。Zhai等[49]探究了湿式浸渍法制备的VOx/TiO2催化剂对CB和NOx的协同降解活性。结果表明,8V/TiO2催化剂对CB的转化率随着温度升高逐渐提高,250 ℃条件下CB转化率为50%,300 ℃时CB转化率达到100%,NO转化率随着温度升高先提高再降低,200—350 ℃范围内,NO转化率保持在80%以上,CO2选择性随着温度升高逐渐提升,当温度大于250 ℃时,CO2选择性保持在50%左右。Gao等[50]探究了湿式浸渍法制备的V2O5-WO3/TiO2催化剂在100—500 ℃条件下对CB和NOx的协同降解活性,发现在225—350 ℃温度区间,SCR活性较高,均达到90%以上;相比之下,催化剂对CB的低温活性较低,在250℃时降解效率为10%,随着温度升高降解效率快速增长,在300℃和325 ℃时分别达到50%和91.34%,整个温度区间CO2选择性波动不大,平均在60%左右。除了V-W-Ti体系催化剂,Ce-Mn、贵金属负载型等催化体系也有较多研究,且在实验室条件下对两类污染物有着较高的协同处置活性。如Gan等[13]研究了共沉淀法合成的MnOx-CeOx催化剂对CB和NOx的协同降解活性,结果表明,在250 ℃下CB的转化率为70%左右,CO2选择性接近100%,NOx转化率在150 ℃条件下最高,接近100%,在250 ℃和300 ℃条件下的转化率分别为90%和85%。实验室制备的催化剂体系多样,且对CB和NOx协同降解活性存在较大差异,这可能与材料种类和制备条件有关。综上所述,目前商用催化剂对污染物协同控制活性需要进一步提高,同时开发具有优异低温活性催化剂的生产工艺是推进实际工业应用的重要研究方向。
-
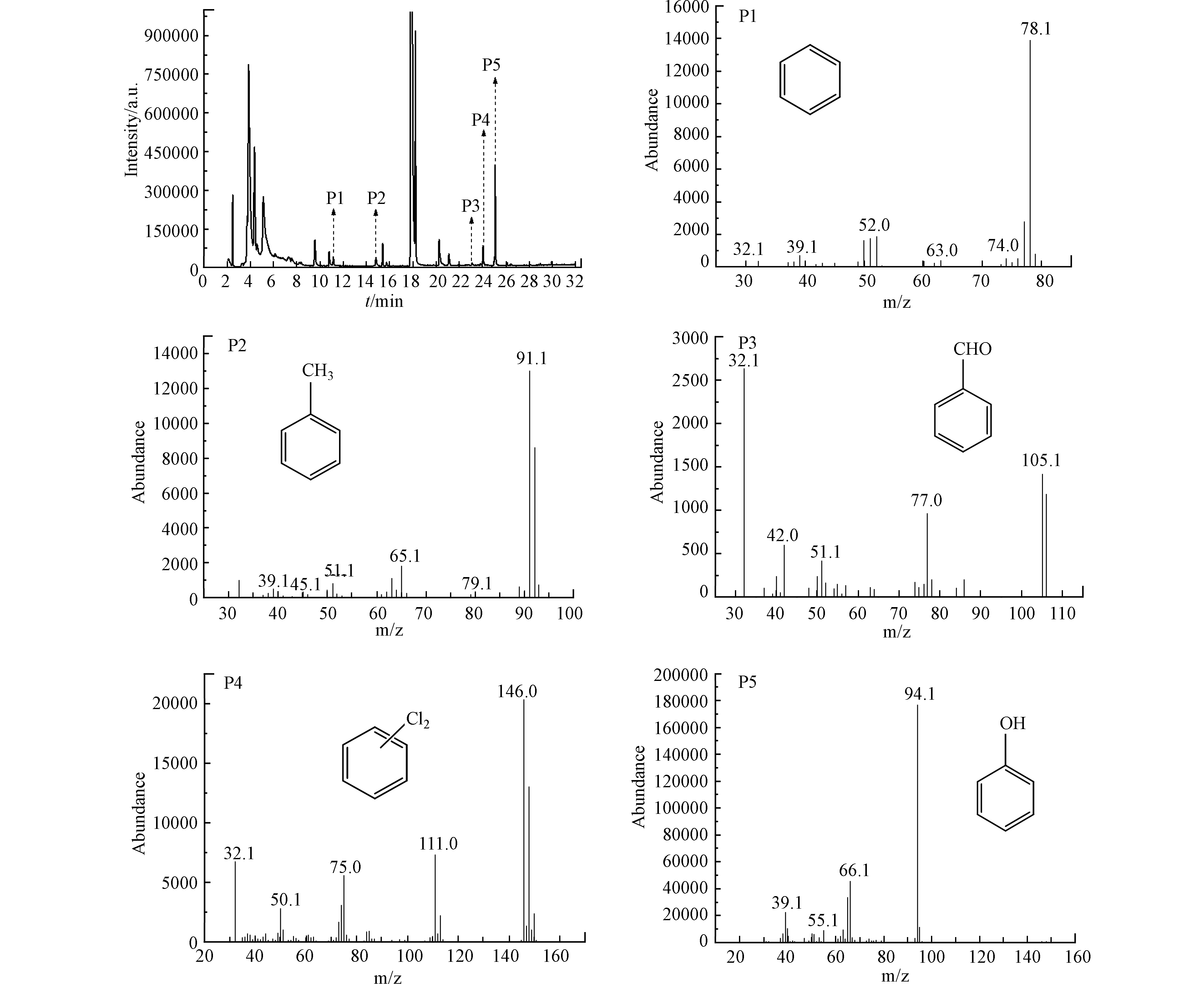
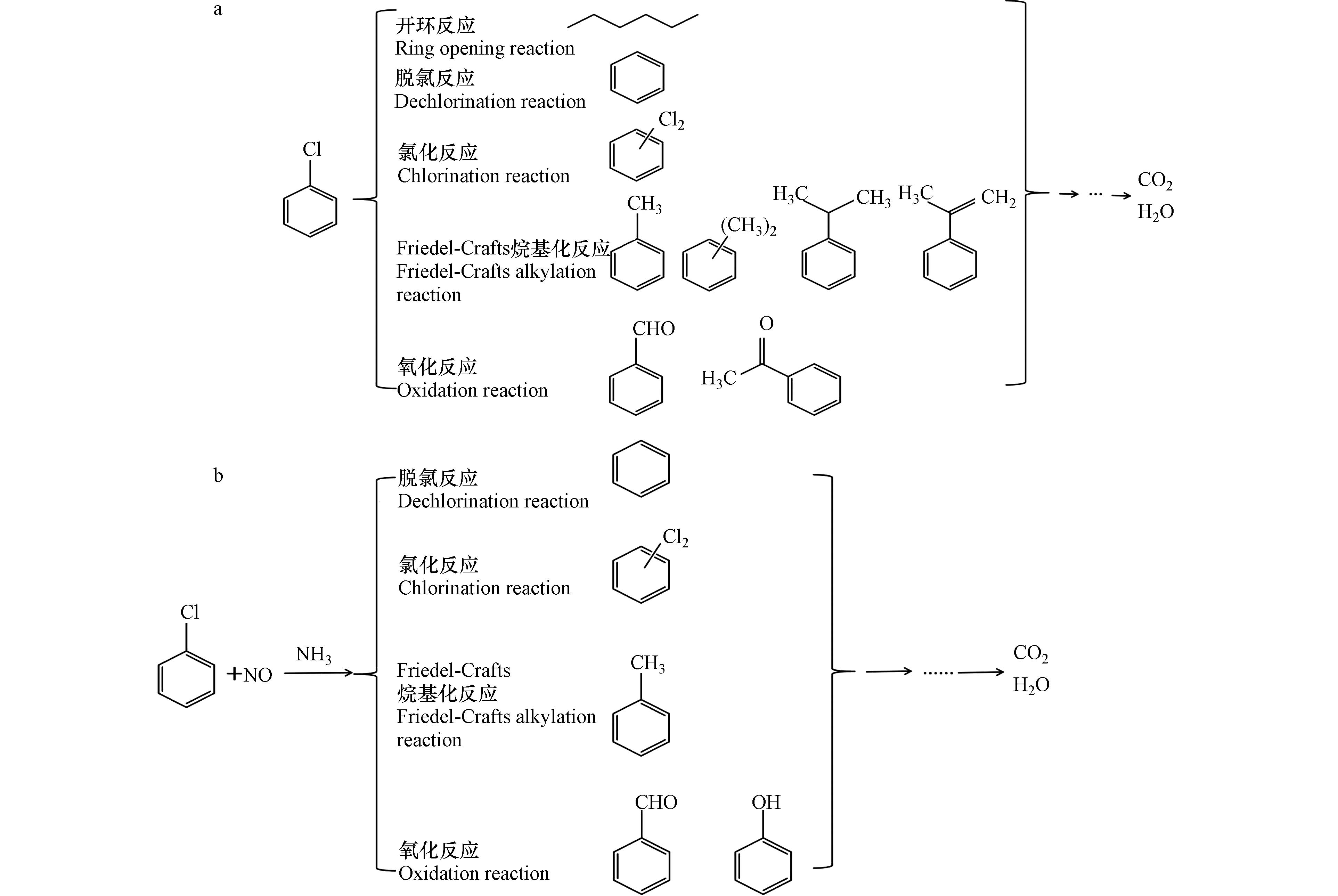
为了探究催化剂单独降解CB与协同处置CB和NO之间的机理,本研究利用热脱附与GC-MS联用仪对上述两个反应体系降解后的产物进行了全面分析。图7为300 ℃条件下1#催化剂对CB单独降解20 min后产物的色谱图和各个色谱峰对应的质谱图。基于对各个质谱图中的分子离子峰、碎片离子峰、同位素丰度比例分析,并将结果与NIST18标准谱库进行对比,定性了P1-P9共9种中间产物,分别为正己烷、苯、甲苯、二甲苯、异丙苯、α-甲基苯乙烯、苯甲醛、二氯苯、苯乙酮。如P1峰的质谱图中,m/z 86.0[M]+为分子离子峰,在m/z为57.1[M-CH3-CH2]+和41.1[M-CH3-CH2-CH2]+处出现明显的碎片离子簇,证明P1峰为正己烷。P8峰的质谱结果显示,在m/z 145.9 [M]+处为分子离子峰,在m/z 111 [M-Cl]+、75[M-Cl2]+处为碎片离子簇,且在m/z [M]+ 145.9、147.9和149.9处的Cl同位素比例为9:6:1,符合2个Cl原子的同位素分布规律,证明P8峰为二氯苯产物。上述二氯苯、苯甲醛、苯等含有苯环类产物及正己烷等直链烷烃类产物的检出表明CB在降解反应中发生了氯化、氧化及开环反应。
图8为300 ℃条件下1#催化剂对CB和NO协同降解20 min后的色谱图和各个色谱峰对应的质谱图,与图7的分析方法一致,对协同降解过程中产生的中间物种进行定性分析。由色谱图的结果可以看出,协同降解过程中中间产物种类较少,共鉴定出5种中间产物,分别为苯、甲苯、苯甲醛、二氯苯、苯酚。例如,将P1峰与相应的质谱图进行匹配,发现在m/z 78 [M]+处为它的分子离子峰,在m/z 51和39处为出现明显的特征碎片离子簇,可证实其为苯。在P3峰的质谱图中,m/z 105 [M]+为分子离子峰,在m/z 77.0 [M-CHO]+处为碎片离子簇,证实该物质为苯甲醛。上述二氯苯、苯甲醛、苯等含有苯环类产物的检出表明氯苯在协同降解反应中也发生了氯化、氧化及脱氯反应。与单独降解CB体系相比,协同降解体系缺少开环中间产物的检出,可能是由于开环产物被彻底降解为CO2或含量低于检出限无法被检出。以上结果显示1#催化剂协同处置CB与NO反应体系产生的中间产物种类明显少于单独降解CB反应体系,表明协同降解反应可以有效减少中间产物的生成,与CO2选择性的变化趋势相一致。
基于对商用催化剂物理化学性质的探究及其对CB和NO单独降解及二者协同处置中间产物的检测,本研究提出了1#催化剂对CB单污染物及CB和NO协同处置的机理,如图9所示。在300 ℃反应温度下,1#催化剂因具有较多的活性氧物种而对CB具有较好的处置活性。在对CB单独催化降解体系中,CB在催化剂表面发生亲核取代反应,被活性氧攻击,从而导致C—Cl键发生解离,生成的苯基被氢取代形成苯[51],随后开环生成正己烷。甲苯、二甲苯等物质可通过Friedel-Crafts烷基化反应生成,即甲基碳正离子通过亲电取代反应攻击苯环,从而生成甲苯。生成的甲苯可以发生过度烷基化,产生二甲苯。二氯苯通常是由CB降解过程中解离的氯物种与未反应的CB发生亲电氯化反应产生的[52] 。苯甲醛是甲苯发生氧化反应而生成的,Subramanian和Murthy等提出了两步氧化反应原理[53],即吸附于催化剂表面的物质被活性氧物种氧化,接着被还原的催化剂被再次氧化从而回到初始状态再参与反应。在本研究中,吸附于催化剂表面的中间产物甲苯被活性氧物种氧化生成苯甲醛,随后被还原的催化剂被气态氧再次氧化继续参与下一次的反应。上述中间产物会被活性氧物种进一步攻击生成H2O和CO2。相比而言,在NO和CB协同处置反应体系中,NO会转化为NO2,具有较强的氧化性能,促进了对CB的降解活性和CO2选择性,进而使中间产物的种类和生成量均有显著降低,仅检测到了苯、甲苯、苯甲醛和苯酚等中间产物。同时氯化反应生成的二氯苯副产物的强度也明显减弱,表明NO的引入不仅能促进CB的深度氧化,也能在一定程度上抑制多氯代副产物的生成。
-
本文以CB作为氯代芳烃的典型代表,探究了商用SCR脱硝催化剂对CB和NO的协同处置活性。实验结果表明,1#催化剂对CB和NO的降解活性均最高,在300 ℃温度下90 min的反应周期内,CB的降解效率均能保持在95%左右,NO的转化效率均能维持在57%左右。一系列的物理化学性质表征结果显示,1#催化剂具有较大的比表面积、较高的活性氧物种和较多的活性钒物种,上述性质赋予了其较优异的催化活性。1#催化剂对CB和NO的协同降解实验结果表明,在250 ℃和300 ℃条件下NO的引入均能在一定程度上促进催化剂对CB的降解,这是因为NO在金属氧化物表面被氧化成NO2,有利于氧化反应的进行。然而,对于NO而言,300 ℃条件下,NO2生成量相对较多,可能会竞争性地参与NO的NH3-SCR,导致NO的选择性降低,同时C—Cl键解离程度也较大,氯物种在催化剂表面发生沉积,从而影响SCR反应的进行,上述反应均会导致NO的转化率较低;当温度降到250 ℃时,CB降解过程中的CO2选择性增强,可能会抑制NH3的过度氧化,同时NO2生成量和C—Cl键的解离均变弱,进而使得SCR反应相对增强。本研究探究了1#催化剂对CB单独降解以及CB和NO协同降解的反应机理,发现CB可通过脱氯、烷基化、氧化开环、氯化等一系列反应生成苯、甲苯、正己烷、二氯苯等中间物种。协同体系产生的中间产物明显少于单独降解体系,且氯苯的氯化程度大大降低,表明协同反应可以有效减少中间产物的生成。本研究可为促进CB和NO的协同处置催化剂的研发提供理论依据。
商用催化剂对氯代芳烃和氮氧化物的协同处置研究
The research on the synergistic elimination of chlorinated aromatics and nitrogen oxides over commercial catalysts
-
摘要: 多污染物的协同控制是环境催化研究的前沿,氯代芳烃和氮氧化物(NOx)是生活垃圾焚烧等热工业过程中共存的典型污染物,目前商用催化剂能否实现二者的协同处置尚不清楚。因此,本文研究了5类商用SCR催化剂对氯苯(CB)和一氧化氮(NO)的协同处置活性。结果表明,1#催化剂在300 ℃下对CB和NO具有较好的降解活性,且在90 min的反应周期内具有较高的稳定性,这是由于1#催化剂具有较大比表面积、较高比例的表面吸附氧(Oβ)以及V5+。1#催化剂对CB和NO的协同降解实验显示,在250 ℃和300 ℃下,NO的引入能够生成NO2,可促进CB的降解。300 ℃时CB的存在抑制了NO的转化,而当温度降到250 ℃时,CB对NO的转化有促进作用。这可能是因为在较高温度下NO2生成量相对较多,C—Cl键解离程度较大,在一定程度上会影响SCR反应的进行,导致NO的转化率较低;而温度降低时,CO2选择性增强,可能会抑制NH3的过度氧化,同时NO2生成量和C—Cl键的解离均变弱,进而使得SCR反应相对增强。利用热脱附/GC-MS联用系统全面分析了降解过程中产生的中间产物,发现CB和NO协同处置体系中间产物种类显著减少,经过脱氯、烷基化、氧化等一系列反应生成苯、甲苯、苯甲醛和苯酚等中间产物,最终矿化生成H2O和CO2。可见NO的引入不仅能促进CB的深度氧化,还能在一定程度上抑制多氯代副产物的生成。Abstract: The synergistic control of various pollutants has attracted enormous interest in recent years. Both nitrogen oxides (NOx) and chlorinated aromatic hydrocarbon are typical pollutants coexisting in municipal solid waste incineration and other thermal industrial processes. However, it is unclear whether commercial catalysts can achieve the synergistic control of the NOx and chlorinated aromatic hydrocarbon at present. Hence, the synergistic treatment of chlorobenzene (CB) and nitric oxide (NO) over five typical commercial catalysts at 250 ℃ and 300 ℃ was investigated in this study. The results indicated that 1# catalyst exhibited the best degradation activity and excellent stability within 90 min reaction period, which was attributed to its larger specific surface area, higher proportion of surface-adsorbed oxygen(Oβ) and V5+. The synergistic elimination of CB and NO over 1# catalyst demonstrated that the addition of NO could generate NO2 at 250 ℃ and 300 ℃, thus promoting the degradation of CB. In contrast, in the presence of CB, the conversion of NO was inhibited at 300 ℃ but was promoted as the temperature dropped to 250 ℃. Since at the condition of higher temperature, a large amount of NO2 was generated, and the C—Cl bond was greatly dissociated, which could affect the process of the SCR reaction, resulting in a lower conversion rate of NO. Nevertheless, as the temperature dropped, the enhancement of CO2 selectivity could inhibit the over-oxidation of NH3. Meanwhile, both the generation of NO2 and the dissociation of C—Cl were weakened, which in turn facilitated the SCR reaction. In addition, the intermediate products during the degradation process were comprehensively analyzed by thermal desorption/GC-MS. The results implied that the types of intermediate products in the synergistic control system of CB and NO were significantly reduced. A series of intermediate products such as benzene, toluene, benzaldehyde and phenol were generated via the reaction of dechlorination, alkylation and oxidation, and finally mineralized to CO2 and H2O. It was noted that the introduction of NO could not only promote the deep oxidation of CB but also inhibit the formation of polychlorinated by-products to a certain extent.
-

-
图 5 5类商用催化剂对CB的(a)降解效率和(b) CO2的选择性(T=300 ℃, CB 921 mg·m−3);(c)5类商用催化剂对NO的转化率(T=300 ℃, NO 134 mg·m−3, NH3 82 mg·m−3) (d) 1#催化剂分别对CB的降解效率、CO2选择性以及对NO的转化率(T=250 ℃, CB 921 mg·m−3, NO 125 mg·m−3, NH3 82 mg·m−3)
Figure 5. (a) The degradation efficiency of CB and (b) the selectivity of CO2 over five typical commercial catalysts (T=300 ℃, CB 921 mg·m−3), (c) The conversion rate of NO over five typical commercial catalyst (T=300 ℃, NO 134 mg·m−3, NH3 82 mg·m−3) (d) The degradation efficiency of CB, the selectivity of CO2 and the conversion rate of NO over 1# catalyst (T=250 ℃, CB 921 mg·m−3, NO 125 mg·m−3, NH3 82 mg·m−3)
表 1 5类商用催化剂的元素组成
Table 1. Element composition of the five typical commercial catalysts
催化剂 Catalysts 元素 Elements 质量 Weight 1# O 55.91 Ti 43.07 V 1.02 2# O 60.75 Ti 38.59 V 0.66 3# O 61.30 Ti 37.69 V 0.55 W 0.46 4# O 60.12 Ti 37.68 W 1.77 V 0.42 5# Ti 60.42 O 38.28 V 1.30 表 2 5类商用催化剂的比表面积和孔径分布
Table 2. Specific surface area and pore diameter of five typical commercial catalysts
催化剂
Catalysts比表面积/( m2·g−1)
Specific surface area总孔容/( cm3·g−1)
Total pore volume平均孔径/nm
Average pore diameter1# 67.87 0.25 14.79 2# 72.48 0.27 15.01 3# 49.78 0.24 19.56 4# 70.79 0.26 14.92 5# 76.21 0.26 13.49 表 3 5类商用催化剂的XPS表征结果
Table 3. The XPS results of five typical commercial catalysts
催化剂
Catalysts结合能 /eV Binding energy V5+/V Oβ/O V5+ V4+ Oα Oβ Oγ 1# 517.53 516.47 530.25 531.70 — 38.95% 44.10% 2# 517.01 516.28 530.25 531.90 — 37.17% 23.95% 3# 517.80 516.49 530.30 531.86 532.92 19.85% 12.46% 4# 517.50 516.40 530.25 531.58 532.88 25.55% 13.22% 5# 517.20 516.30 530.24 531.30 — 38.05% 29.02% -
[1] FAN Y, ZHANG H J, REN M H, et al. Low-temperature catalytic degradation of chlorinated aromatic hydrocarbons over bimetallic Ce-Zr/UiO-66 catalysts [J]. Chemical Engineering Journal, 2021, 414: 128782. doi: 10.1016/j.cej.2021.128782 [2] 刘莎, 黄学敏, 黄林艳, 等. 酸碱气体对氯代芳烃削减的影响 [J]. 环境化学, 2014, 33(5): 731-738. doi: 10.7524/j.issn.0254-6108.2014.05.015 LIU S, HUANG X M, HUANG L Y, et al. Influence of acid and basic gases on the reduction of chlorinated aromatics [J]. Environmental Chemistry, 2014, 33(5): 731-738(in Chinese). doi: 10.7524/j.issn.0254-6108.2014.05.015
[3] XU C H, LIU C Q, ZHONG Y, et al. Study on the active sites of Cu-ZSM-5 in trichloroethylene catalytic combustion with air [J]. Chinese Chemical Letters, 2008, 19(11): 1387-1390. doi: 10.1016/j.cclet.2008.07.010 [4] WEIDEMANN E, LUNDIN L. Behavior of PCDF, PCDD, PCN and PCB during low temperature thermal treatment of MSW incineration fly ash [J]. Chemical Engineering Journal, 2015, 279: 180-187. doi: 10.1016/j.cej.2015.05.015 [5] BA T, ZHENG M H, ZHANG B, et al. Estimation and characterization of PCDD/Fs and dioxin-like PCBs from secondary copper and aluminum metallurgies in China [J]. Chemosphere, 2009, 75(9): 1173-1178. doi: 10.1016/j.chemosphere.2009.02.052 [6] JIN R, ZHENG M H, LAMMEL G, et al. Chlorinated and brominated polycyclic aromatic hydrocarbons: Sources, formation mechanisms, and occurrence in the environment [J]. Progress in Energy and Combustion Science, 2020, 76: 100803. doi: 10.1016/j.pecs.2019.100803 [7] BA T, ZHENG M H, ZHANG B, et al. Estimation and characterization of PCDD/Fs and dioxin-like PCB emission from secondary zinc and lead metallurgies in China [J]. Journal of Environmental Monitoring:JEM, 2009, 11(4): 867-872. doi: 10.1039/b818555g [8] LEI R R, XU Z C, XING Y, et al. Global status of dioxin emission and China's role in reducing the emission [J]. Journal of Hazardous Materials, 2021, 418: 126265. doi: 10.1016/j.jhazmat.2021.126265 [9] 陈露露, 黄韬, 陈凯杰, 等. 我国PCDD/Fs网格化大气排放清单 [J]. 环境科学, 2020, 41(2): 510-519. CHEN L L, HUANG T, CHEN K J, et al. Gridded atmospheric emission inventory of PCDD/fs in China [J]. Environmental Science, 2020, 41(2): 510-519(in Chinese).
[10] Japan Ministry of The Environment, 2016. Dioxin Emission Inventory, Dioxin Emission Inventory[EB/OL].http://www.env.go.jp/press/files/jp/102407.pdf. [11] QUINA M J, PEDRO R S, GANDO-FERREIRA L M, et al. A national inventory to estimate release of polychlorinated dibenzo-p-dioxins and dibenzofurans in Portugal [J]. Chemosphere, 2011, 85(11): 1749-1758. doi: 10.1016/j.chemosphere.2011.09.028 [12] 中华人民共和国生态环境部. 生活垃圾焚烧污染控制标准[EB/OL]. [2014-05-16] .https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/gthw/gtfwwrkzbz/201405/W020140530531389708182.pdf Ministry of Ecology and Environment of the People's Republic of China. Standard for pollution control on the municipal solid waste incineration[EB/OL]. [2014-05-16].https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/gthwgtfwwrkzbz/201405/W020140530531389708182.
[13] GAN L N, SHI W B, LI K Z, et al. Synergistic promotion effect between NOx and chlorobenzene removal on MnOx-CeO2 catalyst [J]. ACS Applied Materials & Interfaces, 2018, 10(36): 30426-30432. [14] GHOLAMI F, TOMAS M, GHOLAMI Z, et al. Technologies for the nitrogen oxides reduction from flue gas: A review [J]. Science of the Total Environment, 2020, 714: 136712. doi: 10.1016/j.scitotenv.2020.136712 [15] RAO A, MEHRA R K, DUAN H, et al. Comparative study of the NOx prediction model of HCNG engine [J]. International Journal of Hydrogen Energy, 2017, 42(34): 22066-22081. doi: 10.1016/j.ijhydene.2017.07.107 [16] SHANG X S, HU G R, HE C, et al. Regeneration of full-scale commercial honeycomb monolith catalyst (V2O5-WO3/TiO2) used in coal-fired power plant [J]. Journal of Industrial and Engineering Chemistry, 2012, 18(1): 513-519. doi: 10.1016/j.jiec.2011.11.070 [17] BONINGARI T, SMIRNIOTIS P G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement [J]. Current Opinion in Chemical Engineering, 2016, 13: 133-141. doi: 10.1016/j.coche.2016.09.004 [18] 中华人民共和国生态环境部. 2016-2019年全国生态环境统计公报[EB/OL]. [2020-12-14]. http://www.mee.gov.cn/hjzl/sthjzk/sthjtjnb/202012/P020201214580320276493.pdf. Ministry of Ecology and Environment of the People's Republic of China. National Bulletin of Ecological Environment Statistics[EB/OL]. [2020-12-14]. http://www.mee.gov.cn/hjzl/sthjzk/sthjtjnb/202012/P020201214580320276493.pdf.
[19] 纪瑞军. 烧结烟气氧化吸收脱硝工艺研究[D]. 北京: 中国石油大学(北京), 2018. JI R J. The study of oxidation and absorption of NOx in sintering gas[D]. Beijing: China University of Petroleum (Beijing), 2018(in Chinese).
[20] WANG X Y, KANG Q, LI D. Catalytic combustion of chlorobenzene over MnOx-CeO2 mixed oxide catalysts [J]. Applied Catalysis B:Environmental, 2009, 86(3/4): 166-175. [21] 刘建胜. 基于机械化学原理的卤代芳烃脱卤降解研究[D]. 杭州: 浙江工业大学, 2016. LIU J S. Studies on degradation and dehalogenation of halogenated aromatics by mechanochemical treatment[D]. Hangzhou: Zhejiang University of Technology, 2016(in Chinese).
[22] 罗邯予. 铈钛基催化剂上氯苯的催化燃烧性能研究[D]. 北京: 北京化工大学, 2019. LUO H Y. Catalytic combustion performance of chlorobenzene over ceria-titania-based complex metal oxides[D]. Beijing: Beijing University of Chemical Technology, 2019(in Chinese).
[23] 陈立. Ru基催化剂对氯代挥发性有机物(CVOCs)的催化氧化研究[D]. 贵阳: 贵州大学, 2018. CHEN L. Catalytic oxidation of chlorinated volatile organic compounds over ruthenium-based catalysts[D]. Guiyang: Guizhou University, 2018(in Chinese).
[24] 赵日晓. 改性钛基催化剂催化氧化气相氯苯以及二噁英的基础研究[D]. 杭州: 浙江大学, 2017. ZHAO R X. Fundamental research of gaseous chlorobenzenes and dioxins catalytic decomposition over modified titanium-based catalysts[D]. Hangzhou: Zhejiang University, 2017(in Chinese).
[25] TIAN W, FAN X Y, YANG H S, et al. Preparation of MnOx/TiO2 composites and their properties for catalytic oxidation of chlorobenzene [J]. Journal of Hazardous Materials, 2010, 177(1/2/3): 887-891. [26] 卢朋. Mn、CeWOx/TiO2催化剂的制备及NH3-SCR性能研究[D]. 杭州: 浙江工业大学, 2017. LU P. Preparation of Mn, CeWOx/TiO2 catalyst and its NH3-SCR activity[D]. Hangzhou: Zhejiang University of Technology, 2017(in Chinese).
[27] CHEN L, LI J H, GE M F. Promotional effect of Ce-doped V2O5-WO3/TiO2 with low vanadium loadings for selective catalytic reduction of NOx by NH3 [J]. The Journal of Physical Chemistry C, 2009, 113(50): 21177-21184. doi: 10.1021/jp907109e [28] 蒋威宇. V2O5-WO3/TiO2催化剂协同净化NOx与氯代芳香化合物的反应特征与副产物研究[D]. 杭州: 浙江大学, 2020. JIANG W Y. Reaction characteristics and byproducts analyses over V2O5-WO3/TiO2 catalyst in the synergistic elimination of NOx and chloroaromatics[D]. Hangzhou: Zhejiang University, 2020(in Chinese).
[29] XU Z Z, DENG S B, YANG Y, et al. Catalytic destruction of pentachlorobenzene in simulated flue gas by a V2O5-WO3/TiO2 catalyst [J]. Chemosphere, 2012, 87(9): 1032-1038. doi: 10.1016/j.chemosphere.2012.01.004 [30] BERTINCHAMPS F, TREINEN M, BLANGENOIS N, et al. Positive effect of NOx on the performances of VOx/TiO2-based catalysts in the total oxidation abatement of chlorobenzene [J]. Journal of Catalysis, 2005, 230(2): 493-498. doi: 10.1016/j.jcat.2005.01.009 [31] GALLASTEGI-VILLA M, ARANZABAL A, GONZÁLEZ-MARCOS J A, et al. Metal-loaded ZSM5 zeolites for catalytic purification of dioxin/furans and NOx containing exhaust gases from MWI plants: Effect of different metal cations [J]. Applied Catalysis B:Environmental, 2016, 184: 238-245. doi: 10.1016/j.apcatb.2015.11.006 [32] CAO J, LIU W Z, KANG K K, et al. Effects of the morphology and crystal-plane of TiO2 on NH3-SCR performance and K tolerance of V2O5-WO3/TiO2 catalyst [J]. Applied Catalysis A:General, 2021, 623: 118285. doi: 10.1016/j.apcata.2021.118285 [33] JIANG Y, GAO X, ZHANG Y X, et al. Effects of PbCl2 on selective catalytic reduction of NO with NH3 over vanadia-based catalysts [J]. Journal of Hazardous Materials, 2014, 274: 270-278. doi: 10.1016/j.jhazmat.2014.04.026 [34] WU Z B, JIN R B, WANG H Q, et al. Effect of ceria doping on SO2 resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature [J]. Catalysis Communications, 2009, 10(6): 935-939. doi: 10.1016/j.catcom.2008.12.032 [35] QU Z P, BU Y B, QIN Y, et al. The improved reactivity of manganese catalysts by Ag in catalytic oxidation of toluene [J]. Applied Catalysis B:Environmental, 2013, 132/133: 353-362. doi: 10.1016/j.apcatb.2012.12.008 [36] HUANG L Y, SU G J, ZHANG A Q, et al. Degradation of polychlorinated biphenyls using mesoporous iron-based spinels [J]. Journal of Hazardous Materials, 2013, 261: 451-462. doi: 10.1016/j.jhazmat.2013.07.064 [37] PAN Y X, ZHAO W, ZHONG Q, et al. Promotional effect of Si-doped V2O5/TiO2 for selective catalytic reduction of NOx by NH3 [J]. Journal of Environmental Sciences, 2013, 25(8): 1703-1711. doi: 10.1016/S1001-0742(12)60181-8 [38] LI G B, SHEN K, WANG L, et al. Synergistic degradation mechanism of chlorobenzene and NOx over the multi-active center catalyst: The role of NO2, Brønsted acidic site, oxygen vacancy [J]. Applied Catalysis B:Environmental, 2021, 286: 119865. doi: 10.1016/j.apcatb.2020.119865 [39] XU J Q, ZOU X L, CHEN G R, et al. Tailored activity of Ce-Ni bimetallic modified V2O5/TiO2 catalyst for NH3-SCR with promising wide temperature window [J]. Vacuum, 2021, 191: 110384. doi: 10.1016/j.vacuum.2021.110384 [40] JIANG W Y, YU Y L, BI F, et al. Synergistic elimination of NOx and chloroaromatics on a commercial V2O5-WO3/TiO2 catalyst: Byproduct analyses and the SO2 effect [J]. Environmental Science & Technology, 2019, 53(21): 12657-12667. [41] XIAO H P, CHEN Y, QI C, et al. Effect of Na poisoning catalyst (V2O5-WO3/TiO2) on denitration process and SO3 formation [J]. Applied Surface Science, 2018, 433: 341-348. doi: 10.1016/j.apsusc.2017.10.048 [42] SUN B H, LI Q Q, ZHENG M H, et al. Recent advances in the removal of persistent organic pollutants (POPs) using multifunctional materials: A review [J]. Environmental Pollution, 2020, 265: 114908. doi: 10.1016/j.envpol.2020.114908 [43] HUANG H, DAI Q G, WANG X Y. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene [J]. Applied Catalysis B:Environmental, 2014, 158/159: 96-105. doi: 10.1016/j.apcatb.2014.01.062 [44] PARK B H, KIM M, PARK N K, et al. Single layered hollow NiO-NiS catalyst with large specific surface area and highly efficient visible-light-driven carbon dioxide conversion [J]. Chemosphere, 2021, 280: 130759. doi: 10.1016/j.chemosphere.2021.130759 [45] BERTINCHAMPS F, TREINEN M, ELOY P, et al. Understanding the activation mechanism induced by NOx on the performances of VOx/TiO2 based catalysts in the total oxidation of chlorinated VOCs [J]. Applied Catalysis B:Environmental, 2007, 70(1/2/3/4): 360-369. [46] GAN L N, WANG Y, CHEN J J, et al. The synergistic mechanism of NOx and chlorobenzene degradation in municipal solid waste incinerators [J]. Catalysis Science & Technology, 2019, 9(16): 4286-4292. [47] WANG D, CHEN J J, PENG Y, et al. Dechlorination of chlorobenzene on vanadium-based catalysts for low-temperature SCR [J]. Chemical Communications (Cambridge, England), 2018, 54(16): 2032-2035. doi: 10.1039/C7CC08705E [48] GAN L N, LI K Z, XIONG S C, et al. MnOx-CeO2 catalysts for effective NOx reduction in the presence of chlorobenzene [J]. Catalysis Communications, 2018, 117: 1-4. doi: 10.1016/j.catcom.2018.08.008 [49] ZHAI S Y, SU Y T, WENG X L, et al. Synergistic elimination of NOx and chlorinated organics over VOx/TiO2 Catalysts: A combined experimental and DFT study for exploring vanadate domain effect [J]. Environmental Science & Technology, 2021, 55(19): 12862-12870. [50] GAO C, YANG G P, HUANG X, et al. Key intermediates from simultaneous removal of NOx and chlorobenzene over a V2O5–WO3/TiO2 catalyst: A combined experimental and DFT study [J]. Catalysis Science & Technology, 2021, 11(22): 7260-7267. [51] LICHTENBERGER J, AMIRIDIS M D. Catalytic oxidation of chlorinated benzenes over V2O5/TiO2 catalysts [J]. Journal of Catalysis, 2004, 223(2): 296-308. doi: 10.1016/j.jcat.2004.01.032 [52] DAI Q G, BAI S X, WANG X Y, et al. Catalytic combustion of chlorobenzene over Ru-doped ceria catalysts: Mechanism study [J]. Applied Catalysis B:Environmental, 2013, 129: 580-588. doi: 10.1016/j.apcatb.2012.10.006 [53] SUBRAMANIAN P, MURTHY M S. Mechanism of vapor-phase oxidation of anthracene over vanadium pentoxide catalyst [J]. Industrial & Engineering Chemistry Process Design and Development, 1974, 13(2): 112-115. -



 下载:
下载: