-
厌氧消化(anaerobic digestion, AD)常常作为高负荷污水和固体废物处理的手段,经济高效,广泛应用于食品加工、制浆造纸、化学和石化等工业废水的处理[1]. 厌氧消化是一个多步骤的生物过程,由不同的微生物群体介导,依赖于系统中不同微生物的协调活动,微生物之间形成共生关系. 其具体过程可分为四个阶段:水解、酸化、产氢产乙酸和产甲烷,涉及的优势菌群分别为水解酸化菌与产甲烷菌[2]. 早期的研究发现水解酸化菌与产甲烷菌之间可通过氢气、甲酸等物质进行电子传递,这种间接种间电子传递(mediated interspecies electron transfer,MIET)的方式可实现能量的生成以及微生物的生长繁殖[2-4]. 进一步的研究发现两者之间存在更为高效的直接种间电子传递方式(direct interspecies electron transfer,DIET),微生物通过导电鞭毛、细胞色素c等直接完成种间电子传递[5-7]. 同时,有研究发现在厌氧消化体系中加入具有导电性能的非生物物质(如某些铁氧化物,石墨烯,生物炭等)可促进DIET,从而提高甲烷的生成速率和产量 [7-11]。这些研究聚焦于导电材料可以通过促进种间电子传递从而促进厌氧消化的宏观结果,但是对其促进种间电子传递的机理未做系统分析.
为了更深入理解厌氧消化过程中微生物种间的电子传递方式及其对厌氧消化过程的影响,本文从种间氢传递的机理入手,概述了种间电子传递如何帮助共生微生物克服其自身代谢反应的热力学障碍,随后介绍了具有导电性能的物质对DIET促进作用的研究现状,着重综述了铁氧化物对DIET促进作用和研究进展,分别从热力学、动力学以及理化反应的3个角度出发,归纳分析学者们对该作用发生机制的探究结果,指出当前研究存在的问题,并从分子水平分析DIET机制、DIET与异化铁还原过程的相互影响以及DIET工程应用等方面进行了展望,以期为此领域今后的研究提供参考.
-
传统的厌氧消化过程必涉及到水解酸化菌和产甲烷菌,这是因为:在此过程中有机物如丙酸、丁酸等物质的产氢产乙酸反应在热力学上无法自发进行(表1),这就意味着如果不改变反应的条件,厌氧消化反应将在产氢产乙酸这一步终止;而产甲烷菌能够利用氢气进行产甲烷反应,此反应符合热力学自发进行的条件(表2). 于是,当产甲烷反应发生时,部分氢气被消耗,降低了体系中的氢分压,使得产氢产乙酸反应的实际反应吉布斯自由能变成小于零,可实现产氢产乙酸反应的自发进行,进而整个厌氧消化反应有效进行. 具体而言,当氢分压小于10 Pa和100 Pa时,丙酸和丁酸的产氢产乙酸反应可分别自发进行 [12]. 因此,水解酸化菌与产甲烷菌之间的合作使得厌氧消化反应顺利进行,实现各自生命活动能量的生成. 这种合作关系中,二者缺一不可,只有嗜氢产甲烷菌“及时”地消耗氢气,系统保持较低氢分压时,产氢产乙酸过程才能顺利进行,这个过程中氢气是嗜氢产甲烷菌的电子供体,产氢菌与耗氢菌之间通过氢传递从而传递电子,由此形成了共生关系,此时两菌种间的电子传递过程称为种间氢传递(interspecies hydrogen transfer, IHT)[12]. 在有些环境中,甲酸也能够作为水解酸化菌与产甲烷菌之间的电子传递载体,即种间甲酸传递(interspecies formate transfer, IFT)[13]. 这种传递过程被称为间接种间电子传递,即在共生菌群之间,需要氢或者甲酸等作为电子传递载体,实现种群相互之间的电子传递,从而使其中某种生物化学反应得到克服热力学能垒的能量,完成系统功能.
-
近年来,有研究报道了种间电子传递效率更高的DIET. 某些细菌可以将电子直接转移到产甲烷菌,并没有通过种间氢/甲酸转移[14]. 这种独特的细胞间电子转移机制使有机物的氧化过程突破了热力学障碍可以自发进行,同时有更高的产甲烷效率. 微生物DIET主要通过导电鞭毛、细胞色素等实现[15]. 随着微生物DIET研究的进行,研究者在厌氧消化过程中加入具有导电性能的非生物物质如石墨烯、生物炭、磁铁矿、赤泥等,结果表明这些材料可以作为导体促进DIET,大大提高电子传递效率,有利于厌氧消化的进行. 由于这些非生物物质直接依靠材料的导电性实现电子传递,不需要其他能量载体的协助,因此也归结为DIET. 表3总结了近年来在厌氧消化体系中添加非铁氧化物的导电材料促进DIET的研究.
由表3可知,目前研究发现大多数具有导电性能的非铁氧化物尤其是碳基材料均可促进DIET,提高厌氧消化效率,而且导电性能强的物质促进效果更为明显. 如Chen等[40]研究发现导电性强的碳布促进了Geobacter metallireducens–Geobacter sulfurreducens 和 G. metallireducens–Methanosarcina barkeri之间的DIET,而导电性差的棉布不会促进DIET.
-
铁元素是地球上最丰富的过渡金属,以单质、矿物质或离子形式普遍存在于整个水圈和岩石圈[41]. 铁氧化物在自然界中以矿物形式为主,主要包含铁的氧化物和铁的氢氧化物。常见的铁氧化物有赤铁矿(α-Fe2O3)和磁铁矿(Fe3O4);铁的氢氧化物则主要包括针铁矿(α-FeOOH)、四方纤铁矿(β-FeOOH)、纤铁矿(γ-FeOOH)、水铁矿(Fe5HO8·4H2O)等. 在缺氧或厌氧环境中,铁元素在Fe(Ⅱ)和Fe(Ⅲ)之间循环转换,在生物地球化学氧化还原过程中起着穿梭电子的重要作用[42]. 电子循环也是现代环境生物地球化学中最重要的过程之一,因为它可以影响包括生命有机体在内的其他有机和无机物质的分布[43]. 除了介导氧化还原反应外,铁氧化物还可以促进DIET[41].
-
2010年,Summers等[44]首次报道了Geobacter metallireducens 与Geobacter sulfurreducens共培养体系中DIET现象的存在,发现它们之间主要的电子传递方式为DIET,而非种间氢传递. 紧接着,Kato等[45]首次报道了在共培养体系中磁铁矿能够促进Geobacter sulfurreducens 与 Thiobacillus denitrificans 之间的DIET,实现了乙酸的氧化与硝酸盐到亚硝酸盐的还原,据此作者认为导电的矿物有很大的潜力作为电子导体完成DIET. 同时,Kato等[46]报道了土壤中加入(半)导电的铁氧化物赤铁矿或磁铁矿,能够促进地杆菌属的生长,这是因为铁氧化物的加入加强了地杆菌属与产甲烷菌之间的共营养关系,它们之间通过DIET的方式进行电子传递. 铁氧化物能够促进DIET的发现拓展了人们对于DIET的认识,不仅丰富了种间电子传递的理论,也为强化厌氧消化处理固体废物、废水以及高效产甲烷提供了全新的思路. 不同类型的铁氧化物对整体厌氧消化效率如甲烷产量、COD去除率等有不同的影响. 表4总结了不同铁氧化物促进不同体系中DIET进而使得厌氧消化效率提升的研究.
这些研究表明,加入铁氧化物后,厌氧消化体系产生了各种积极效应,如Baek等[78]发现在乳清厌氧消化处理器中添加磁铁后,溶解性化学需氧量(soluble chemical oxygen demand, sCOD)大幅下降,在实验的最后两个月体系达到稳定并实现了出水 sCOD小于50 mg·L–1,同时甲烷产量有所提升. 根据铁元素分布的研究,添加到反应器中的磁铁矿附着在微生物细胞表面,形态基本未发生变化,形成聚集结构,以其导电性质桥接细胞连接. Cruz Viggi等[7]报道了磁铁矿对丙酸产甲烷过程的影响,他们发现在高氢气分压下(104 Pa),丙酸很快发生分解并以零级反应动力学进行,与对照组相比,丙酸,乙酸的形成与消耗速度更快,丁酸的浓度保持在较低的水平,而甲烷的产量更高,速率也更快. Lu等[79]在猪粪厌氧消化过程中加入75 mmol Fe2O3,Fe2O3通过促进DIET增强了猪粪的厌氧消化,甲烷累计生产量提高11.04%,最大生产率提高31.9%. 由此来看添加铁氧化物后系统的积极效应主要表现在底物与VFA降解效率提升,COD去除率提高,厌氧消化滞后期呈现下降趋势,甲烷产量以及生成速率有所提升,部分研究发现有关DIET的微生物富集[11, 47-79]. 这些研究不仅丰富了铁氧化物促进DIET的实例,也为厌氧消化处理效率的提高提供了新思路.
-
在没有铁氧化物加入的厌氧消化反应器中,甲烷生成的主要机理是IHT[80-82],而当铁氧化物加入到反应器中时,产甲烷反应的机理更倾向于DIET. 根据热力学第三定律,反应的吉布斯自由能变决定了反应的方向,整个反应的吉布斯自由能变为负值是反应自发进行的必要条件. 通常,底物氧化的标准反应吉布斯自由能变为正值,通过对实际反应条件的优化,反应自发进行. 一是根据范特霍夫等温公式(1),在其他条件不变的情况下,降低氢气的分压低于1—100 Pa[12, 83]. 二是在厌氧消化体系中加入铁氧化物强化氧化菌和产甲烷菌之间的DIET,直接突破热力学障碍,使反应顺利进行. 甚至在高氢分压下,该厌氧消化反应器依然有相较于普通厌氧消化反应器更良好的表现. Cruz Viggi[7]分别计算了丙酸厌氧消化IHT和DIET的反应吉布斯自由能和电流大小,当丙酸通过IHT进行厌氧消化时,可以计算得到最大H2通量约为2×10−8 nmol·s−1,理论上相当于4×10−12 A的电流;其次,作者根据(2)、(3)、(4)式计算丙酸以DIET进行厌氧消化的最大电流. 其中(2)式为丙酸氧化的半反应,(3)式为产甲烷菌利用丙酸氧化产生的电子还原碳酸氢盐产生甲烷的反应,(4)为该过程的总反应,此过程(2)式中代谢产生的电子经由Fe3O4电子导体(假设为直径100 nm的金属导线)输送到产甲烷菌. 最后计算得到当丙酸通过DIET进行厌氧消化时产生3×10−5 A的电流. 理论计算的可能与实际情况有差别,但计算结果表现出DIET介导的厌氧消化电流是IHT介导的厌氧消化电流的将近106倍. 此外磁铁矿的加入使每个周期甲烷生成速率增加了31%—33%,这说明磁铁存在的条件下DIET的种间电子传递方式避免了IHT甲烷生成过程的热力学阻力,电子传递效率有极大的提升,厌氧消化效果显著提升.
-
从厌氧消化反应来看添加铁氧化物能够加速底物代谢以及甲烷生成,大量研究比较了使用和不使用铁氧化物的甲烷产量和生成速率,以证明厌氧消化反应动力学的增强[15] 。表5为加入铁氧化物后与对照组实验相比甲烷产量增幅和最大甲烷生成速率增幅的部分研究,由表中的内容可知:铁氧化物的加入不同程度地提高了甲烷生成的动力学特征,促进甲烷化过程的潜在原因可能是铁氧化物促进了DIET反应动力学. 然而,通过宏观厌氧消化产甲烷反应的动力学特征速率或产量等来判断铁氧化物对DIET动力学的增强有一定的局限性,其间接验证了电子传递的效率,而电子传递效率或电流大小可直接说明DIET反应动力学的增强程度,如前所述磁铁的加入使得反应器电流强度是空白反应器的106倍[7]. Yin等[58]还利用循环伏安实验并根据相关公式计算了导电率、电子转移系数Kapp(Fe3O4反应器比空白反应器高84.2%)等电子转移相关的量,这些数据表明Fe3O4触发了更快的细胞外电子转移速率. 然而,虽然已有研究用到电流强度、导电率等[7, 58],但验证铁氧化物促进DIET动力学增强的手段仍然不是很多,有必要对电子转移相关的手段进行更深入的研究[84],可以借鉴较为成熟的碳基材料导电性能研究的方法等. 铁氧化物的加入使DIET效率有了极大提高,因而有机物氧化菌和产甲烷菌之间的共营养代谢更为密切,厌氧消化产甲烷量增多,有机物的消化速率也将因此提高,消化滞后时间会缩短,这些积极的改变在工程应用上有着较为重要的意义.
-
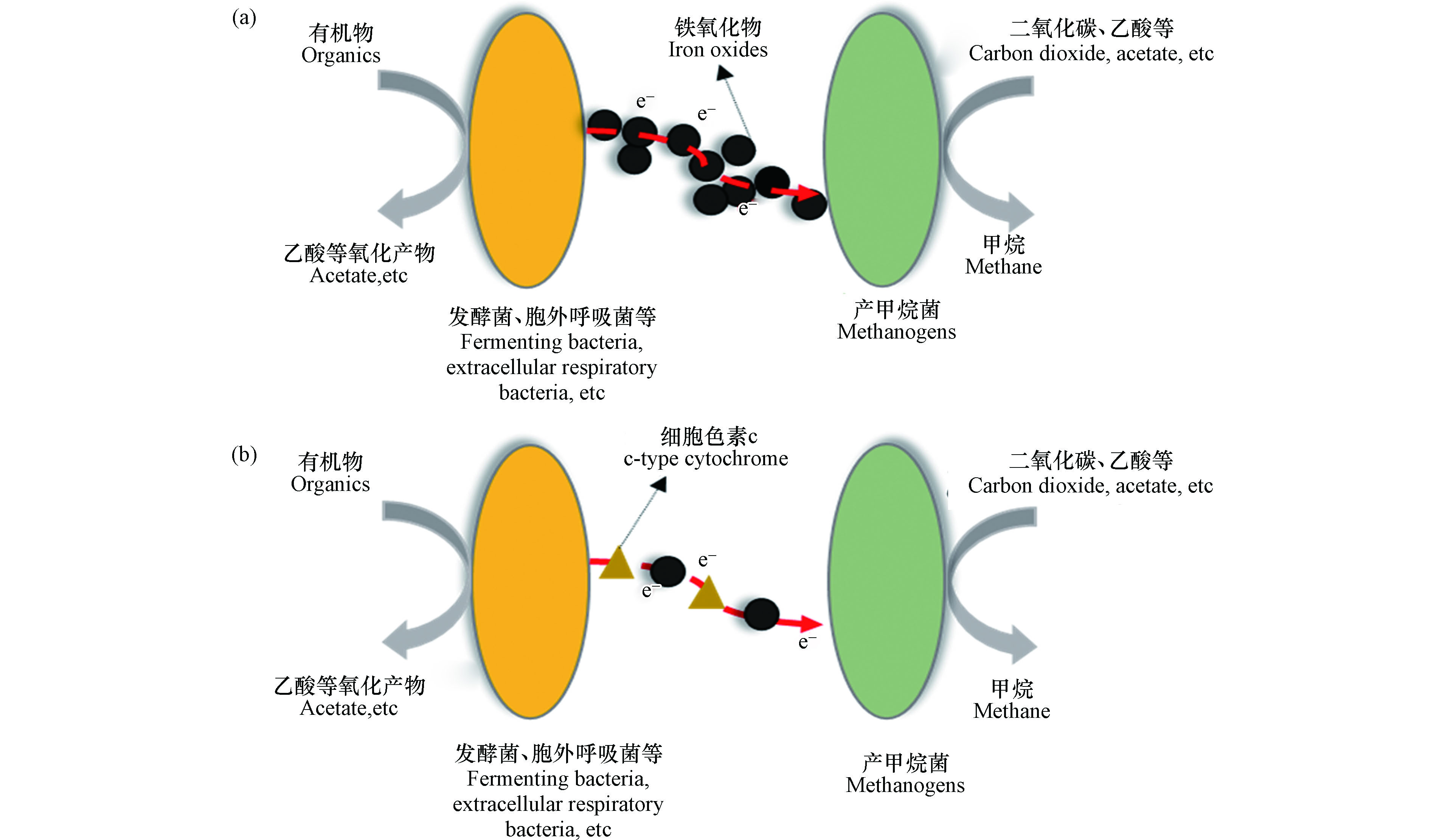
目前,铁氧化物通过促进DIET从而促进厌氧消化被广泛认可,铁氧化物促进有机物氧化菌与产甲烷菌之间的DIET,使VFA降解效率、甲烷产量、甲烷生成速率等都有显著的积极变化[11, 47-79]. 铁氧化物的作用主要是代替共生互营微生物之间的导电菌毛(如图1(a))或细胞色素c(如图1(b))[7, 85-86]. Kato等[46]认为磁铁能够通过代替导电鞭毛促进DIET. Liu等[85]发现磁铁矿能够代替细胞色素c OmcS,促进微生物之间的DIET. 因此,铁氧化物对DIET的促进作用与其导电性能息息相关. Kato等[46]使用3种不同的铁氧化物,导电的磁铁矿、半导电的赤铁矿以及不导电的水铁矿和乙酸或乙醇作为底物从稻田土壤中富集产甲烷微生物,结果发现磁铁矿和赤铁矿有利于地杆菌属的生长,而地杆菌属不会通过氢气或甲醇与嗜氢产甲烷菌进行共营养代谢,因此推测在磁铁矿和赤铁矿的存在下,地杆菌属通过铁氧化物的电子传递作用与产甲烷菌建立了更有效的DIET方式,促进了代谢使地杆菌属大量生长繁殖. 同时,Wang等[87]将芬顿铁泥于400 ℃下加热,制备磁铁矿负载的生物炭材料,其具有比单一生物炭更高的导电率,提高了DIET过程的电子转移效率,厌氧消化性能显著提高. 此外,有学者对铁氧化物代替导电鞭毛的作用作了更为细致的分类,将其分为两类[88],第一类是高导电性的铁氧化物代替导电鞭毛中的细胞色素c进行更高效的电子传递,第二类是铁氧化物相互作用形成两种共营养菌之间的电子通道,产生电子与接受电子的微生物直接与电子通道接触,二者细胞膜上的导电蛋白与电子通道进行电子交换,此时电子通道的作用相当于导电鞭毛.
-
胞外聚合物(extracellular polymeric substances,EPS)是细菌的代谢产物,由多种生物聚合物组成,主要存在于细胞表面[89-90]. EPS有很多的功能与作用如能够在细胞之间桥接使细菌种群固定,保护细胞免受外界有害物质的影响等. 更重要地是EPS可以进行胞外电子传递. EPS中含有与DIET有关的物质如细胞色素c,而且EPS促进微生物聚集体形成,缩短微生物之间的距离,这有利于微生物之间的协同作用[91]. 因此促进EPS的分泌可以促进DIET,近年来有关研究发现铁氧化物可以促进EPS分泌进而促进DIET. Ye等[74]添加赤泥到污泥的消化体系中,发现在实验的前14天EPS的浓度显著提高,不同程度地提高了电子交换容量,贡献了36.24%—45.14%的电子交换容量. 根据循环伏安图电容先增大后减小,这与电子交换容量的提高结果一致. 最后,作者认为在有和没有赤泥的两个反应器中,甲烷的生成速率都与EPS含量成正相关. 这是因为EPS包含影响DIET的细胞色素c以及其他氧化还原活性物质. 加入赤泥后,细胞色素c浓度的增加和大而致密的生物聚集体的形成以DIET方式促进了有机物氧化菌和产甲烷菌之间的电子传递. Yu等[92]将磁铁矿加入到处理餐厨垃圾乙醇发酵产物的厌氧消化反应器中,结果发现磁铁矿的加入刺激了EPS的生成. 同时根据电化学测定的结果,体系的电子交换能力有所增强. 这两个结果具有一致性,说明磁铁促进EPS的生成,而EPS的生成促进了DIET.
-
铁氧化物加入到厌氧体系中除了可能促进DIET外,还可能存在异化铁还原过程. 异化铁还原是指异化铁还原菌(如希瓦氏菌、地杆菌属)以不可溶的Fe(Ⅲ)氧化物为胞外电子受体进行代谢反应,获得生长繁殖所需要的能量的过程 [93]. 厌氧体系中产甲烷反应和异化铁还原反应都在热力学上可以自发进行,Achtnich等[94]研究发现在土壤体系中加入晶体的Fe(Ⅲ)氧化物,由于产甲烷菌与异化铁还原细菌之间的底物竞争以及热力学优先顺序[15],Fe(Ⅲ)含量较高时有利于异化铁还原,抑制产甲烷过程. 异化铁还原菌除了“抢占”部分用于通过DIET产甲烷的底物外,随着固体的Fe(Ⅲ)被还原为溶解性的Fe(Ⅱ),铁氧化物作为电子导体促进厌氧消化产甲烷过程也会进一步被削弱.
异化铁还原反应抑制DIET过程在添加无定形铁氧化物的厌氧体系中表现更明显,据报道非晶形的铁氧化物更容易作为异化铁还原中的电子受体[95]. Bodegom等[96]将无定形的Fe(OH)3加到厌氧消化池中,产甲烷菌代谢活性显著下降,这表明了该铁氧化物对厌氧消化过程的抑制. 除了底物竞争外,还存在Fe(OH)3对产甲烷菌的直接毒性作用,研究表明嗜氢产甲烷菌比嗜乙酸产甲烷菌对其毒性作用更为敏感. Zhou等[97]研究了稻田土壤中3种结晶程度不同的铁氧化物赤铁矿、磁铁矿、水铁矿对产甲烷反应的影响,结果表明赤铁矿和磁铁矿促进甲烷生成,而结晶较差的水铁矿抑制甲烷生成,其中在含水铁矿的稻田土壤中发现了大量亚铁离子的生成. 水铁矿的抑制作用主要是由于水铁矿的存在富集了大量异化铁还原菌并且消耗了一部分底物,因此甲烷产量下降.
虽然异化铁还原过程对DIET有抑制作用使得产甲烷效率下降,但该过程仍有积极的意义. 有研究表明异化铁还原过程有机物的降解效率有所提高,而且还是在产甲烷量减少的情况下,这对减少碳排放来说有重要的意义[97-99]. 除此之外,近年来关于异化铁还原反应抑制产甲烷过程的研究有新的发现,有研究表明在某些特殊情形下异化铁还原过程不仅不会抑制产甲烷反应,反而有促进作用. Peng等[28]将磁铁矿(27 g·L−1)、颗粒活性炭(27 g·L−1)、磁铁矿与颗粒活性炭的组合(各为13.5 g·L−1)分别加入到底物为市政污泥的血清瓶中,与对照相比磁铁矿组的平均甲烷产量增加了7.3%,颗粒活性炭组平均甲烷产量增加了13.1%,磁铁矿和颗粒活性炭的组合平均甲烷产量增加了20%. 这一结果表明与单独的磁铁矿和颗粒活性炭相比,组合工艺显然对厌氧消化过程的促进作用要显著. 根据VFA测定结果,与对照组相比磁铁矿反应器平均总VFA浓度增加了12.0%,组合工艺和颗粒活性炭组平均总VFA浓度分别降低了14.8%和9.8%. 说明磁铁矿有助于加速污泥的水解,从而增加了VFA的生产,而颗粒活性炭促进了甲烷的生成,从而加速了VFA的消耗. 根据微生物群落分析,单独含磁铁矿和颗粒活性炭的反应器异化铁还原菌都有所增加,但磁铁矿反应器增幅更大,而且结合其VFA浓度的增加,可以得到磁铁矿通过异化铁还原反应加速了复杂有机物的降解. 最后,复合工艺中甲烷产量的增加可能来自于水解作用的提升和DIET过程增强这两方面原因.
-
本文主要综述了铁氧化物促进厌氧消化DIET的研究进展和作用机理,着重总结了近年来铁氧化物促进DIET的研究,阐明了铁氧化物在DIET过程中的作用,同时也指出了系统中存在的异化铁还原过程可能对DIET潜在的抑制作用. 近年来铁氧化物促进厌氧消化DIET的研究越来越深入,但目前还存在一些问题. 第一,铁氧化物促进DIET的机理需要进一步深入研究. 目前的研究主要集中在宏观角度,如微生物的代谢变化,包括甲烷产量的变化、VFA生成与消耗、EPS分泌等. 而微观角度目前仅涉及到微生物群落分析和系统的循环伏安曲线变化等,后续的研究应该探究如何检测DIET过程的电流,而从生物角度则应更深入关注基因水平,通过基因测序等手段发现与DIET过程相关的基因以及它们的表达情况,从而从分子水平探究铁氧化物促进DIET的机理. 第二,研究过程中发现铁氧化物能够富集异化铁还原菌,异化铁还原过程对DIET的影响尚存在争议. 后续研究需要对铁氧化物促进DIET和异化铁还原过程的相互影响作用以及代谢的具体方式作进一步阐明. 第三,铁氧化物促进DIET的工程应用较少. 在工程应用中,处理效率和成本是两个重要的因素. 因此工艺中无论以哪种形式应用铁氧化物,与其他类似添加剂如生物炭相比,处理效率和成本方面所具备的优势还未明确,可以进行铁氧化物投加形式如直接投加、负载投加等的探索,同时进行铁氧化物循环利用等方面的探究.
铁氧化物促进微生物直接种间电子传递的机理及其研究现状
A review on enhancement of direct interspecies electron transfer induced by iron oxides and its mechanism
-
摘要: 厌氧消化常作为高负荷污水和固体废物处理的手段,经济高效,有良好的应用前景。该过程由不同的微生物群体介导,微生物之间形成共生关系,从而克服代谢过程的热力学障碍. 在共生关系中,微生物种间电子传递过程极其重要,有机物氧化菌与产甲烷菌一般通过种间氢或甲酸传递进行种间间接电子传递. 随着研究进行,人们发现了电子传递效率更高的直接种间电子传递,可实现微生物之间直接电子交换,而不需要如氢气、甲酸等作为电子传递载体. 目前研究已表明具有导电性质的材料如某些碳材料以及铁氧化物能够促进直接种间电子传递. 为加深对种间电子传递的理解以期提高厌氧消化效率,本文陈述了厌氧消化种间氢传递和直接种间电子传递的机理以及非铁氧化物促进直接种间电子传递的研究现状,着重介绍了铁氧化物促进直接种间电子传递的研究进展,并分别从热力学、动力学、理化性质三个方面进行了分析,最后对铁氧化物促进直接种间电子传递的研究进行了展望.Abstract: Anaerobic digestion is often used as an economical and efficient method for high load sewage and solid waste treatment,which has a good application prospect. Anaerobic digestion is mediated by different microbial groups, in which symbiotic relationship is formed between microorganisms so as to overcome the thermodynamic obstacles of metabolic process. It is very important of interspecies electron transfer in the symbiosis. Indirect interspecies electron transfer between organic oxidizing bacteria and methanogens is generally carried out through interspecies hydrogen or formate transfer. With the progress of research, it has been found that direct interspecies electron transfer (DIET) with higher electron transfer efficiency can finish direct electron exchange between microorganisms without hydrogen and formate as electron transfer carriers. At present, studies have shown that materials with conductive properties, such as some carbon materials and iron oxides,can promote DIET. In order to get a further insight into the interspecies electron transfer to improve the efficiency of anaerobic digestion, this review describes the mechanism of interspecies hydrogen transfer and DIET. The research situation of DIET promoted by non-iron oxides has also been summarized. Besides, the research status of iron oxides promoting DIET has been emphasized, of which the promotion mechanisms studies has been analyzed in three aspects: thermodynamics, kinetics, physical and chemical properties. Finally, the research on iron oxides promoting DIET is prospected.
-

-
表 1 丙酸和丁酸产氢产乙酸反应标准吉布斯自由能变(25 ℃)
Table 1. Standard Gibbs free-energy changes at 25 ℃ for reactions involved in propionate and butyrate oxidation in anaerobic systems
底物
Substrate反应
Reaction标准吉布斯自由能变/(kJ·mol−1)
Standard Gibbs free energy change丙酸 ${\rm{C} }{ {\rm{H} }_3}{\rm{C} }{ {\rm{H} }_2}{\rm{CO} }{ {\rm{O} }^ - }{\rm{ + 3} }{ {\rm{H} }_2}{\rm{O} } \to {\rm{C} }{ {\rm{H} }_3}{\rm{CO} }{ {\rm{O} }^ - }{\rm{ + HCO} }_3^ - {\rm{ + 3} }{ {\rm{H} }_2}{\rm{ + } }{ {\rm{H} }^ + }$ 76.1 $ {\rm{C}}{{\rm{H}}_3}{\rm{C}}{{\rm{H}}_2}{\rm{CO}}{{\rm{O}}^ - }{\rm{ + 2HCO}}_3^ - \to {\rm{C}}{{\rm{H}}_3}{\rm{CO}}{{\rm{O}}^ - }{\rm{ + 3HCO}}{{\rm{O}}^ - }{\rm{ + }}{{\rm{H}}^ + } $ 72.2 丁酸 $ {\rm{C}}{{\rm{H}}_3}{\rm{C}}{{\rm{H}}_2}{\rm{C}}{{\rm{H}}_2}{\rm{CO}}{{\rm{O}}^ - }{\rm{ + 2}}{{\rm{H}}_2}{\rm{O}} \to {\rm{2C}}{{\rm{H}}_3}{\rm{CO}}{{\rm{O}}^ - }{\rm{ + 2}}{{\rm{H}}_2}{\rm{ + }}{{\rm{H}}^ + } $ 48.1 $ {\rm{C}}{{\rm{H}}_3}{\rm{C}}{{\rm{H}}_2}{\rm{C}}{{\rm{H}}_2}{\rm{CO}}{{\rm{O}}^ - } + 2{\rm{HC}}{{\rm{O}}_3}^ - \to 2{\rm{C}}{{\rm{H}}_3}{\rm{CO}}{{\rm{O}}^ - } + 2{\rm{HCO}}{{\rm{O}}^ - } + {{\rm{H}}^ + } $ 45.5 表 2 部分底物甲烷化标准吉布斯自由能变(25 ℃)
Table 2. Standard Gibbs free-energy changes at 25 ℃ for some methanogenic reactions
底物
Substrate反应
Reaction标准吉布斯自由能变/(kJ·mol−1)
Standard Gibbs free energy change乙酸 ${\rm{C}}{{\rm{H}}_3}{\rm{CO}}{{\rm{O}}^ - } + {{\rm{H}}_2}{\rm{O}} \to {\rm{C}}{{\rm{H}}_4} + {\rm{HC}}{{\rm{O}}_3}^ -$ −31.0 氢气 $4{{\rm{H}}_2} + {\rm{HC}}{{\rm{O}}_3}^ - + {{\rm{H}}^ + } \to {\rm{C}}{{\rm{H}}_4} + 3{{\rm{H}}_2}{\rm{O}}$ −135.6 甲酸 $4{\rm{HCO}}{{\rm{O}}^ - } + {{\rm{H}}_2}{\rm{O}} + {{\rm{H}}^ + } \to {\rm{C}}{{\rm{H}}_4} + 3{\rm{HCO}}_3^ -$ −130.4 表 3 非铁氧化物促进厌氧消化DIET的研究
Table 3. Researches on direct interspecies electron transfer in anaerobic digestion
导电材料
Conductive materials底物
Substrates性能
Performance参考文献
References葡萄糖 甲烷生成速率和产量都有所提高 [16] 牛粪 甲烷生成速率提高,滞后时间缩短,挥发性脂肪酸(volatile fatty acid, VFA)和
丙酸浓度降低[17] 丁酸 滞后时间缩短,甲烷生成速率提高,避免丙酸积累引起的pH下降 [18] 生物炭 家禽粪便 此颗粒生物炭预装在高固体沼气池中90 d,可使甲烷总产量增加 69%,
每日甲烷峰值产量增加44%,与对照组相比,滞后时间缩短33%[19] 污泥 累计甲烷产量和最高甲烷生成速率分别提高了46.9%和181.6% [20] 橡果渣废料和
牛粪混合物橡果渣废料和牛粪比例为1:3时,沼气产量最大为580.9 mL·g−1;
总化学需氧量(total chemical oxygen demand, TCOD)下降了79.37%[21] 石油 粉状生物炭(< 5 μm)富集的微生物比颗粒状生物炭(0.5—1 mm)
富集的微生物多,二者均对甲烷产量有所提升[9] 碳布 垃圾焚烧
厂渗滤液碳布的加入使厌氧消化可以处理更高有机负荷的污水,
能够进行DIET的微生物大量富集在碳布表面[22] 合成废水 在高酸性和高氢分压下,DIET代替原有的IHT成为主要的工作机制,
同时代谢正常进行[23] 颗粒活性炭 人工乳制品废水 两相厌氧反应器产甲烷相中加入颗粒活性炭,醇类物质和
脂肪酸向甲烷的转化得到显着改善[24] 合成废水 底物加速降解,无活性炭的反应器甲烷累计产量第7 d达到峰值,
而加入活性炭的在4—5 d达到峰值[25] 合成废水 添加活性炭的反应器甲烷产量比没有加活性炭的反应器高1.8倍 [26] 污泥 颗粒活性炭从0到5 g,甲烷产量提高了17.4%,污泥消减率提高了6.1% [27] 污泥 颗粒活性炭反应器甲烷产量提高13.1% [28] 纳米石墨烯 合成废水 石墨烯(30 mg·L−1和120 mg·L−1)对甲烷的生成有明显的积极影响,
产量分别增加了17.0%和51.4%[29] 釜馏物 与对照组相比,纳米石墨烯反应组甲烷产量提升11%,消化滞后时间减少18.1% [30] 石墨烯 乙醇 高导电性石墨烯促进了甲烷的产量,提高了电子转移通量 [31] 活性炭 餐厨垃圾 15 g·L−1活性炭是提高甲烷产量的最佳剂量,中试实验最佳条件下
甲烷产量与对照组相比增加41%[32] 单壁碳纳米管 蔗糖 1000 mg·L-1单壁碳纳米管诱导了更快的底物利用率和甲烷产生速率 [33] 石墨毡 丙酸、丁酸 丙酸和丁酸降解期间,平均甲烷产量分别提高了19.1%和16.7%;
参与DIET的微生物丰度极大提高[34] 导电聚苯
胺纳米棒蔗糖 600 mg·L−1聚苯胺纳米棒可使甲烷产量翻倍 [35] 钨化合物(W, W2N, W18O49) 粪便尿液混合物 含钨化合物反应器沼气产量提升,消化时间减少 [36] 柚皮生物炭 乳牛粪 生物炭所在厌氧消化系统与对照相比,具有更高的累积沼气产量(525 mL·g−1 VS)和化学需氧量(chemical oxygen demand, COD)去除率(70.95%) [37] 石墨、生物炭 污泥 石墨反应器和生物炭反应器甲烷产量分别提高了38.3%和46.9%。在高H2分压下,
添加石墨和生物炭分别将甲烷产量提高了133.1%和63.7%[38] 生物炭、活性炭 葡萄糖、藻类水
热液化废水添加剂均缩短了厌氧消化滞后时间,提高了甲烷产量,
生物炭比活性炭更能提高甲烷产量[39] 石墨棒、碳布、
生物炭乙醇 COD去除率提高,均在93%以上 [10] 表 4 铁氧化物促进厌氧消化过程DIET的研究
Table 4. Study on direct interspecies electron transfer with addition of iron oxides
铁氧化物
Iron oxides底物
Substrates效应
Effects参考文献
References三氯乙烯 三氯乙烯脱氯速率提高1.5倍,其机理为磁铁促进了DIET [47] 高固体污泥 50 mg·g−1 TS磁铁矿明显减少了短链脂肪酸的积累,
并将甲烷生成速率提高了26.6%[48] 丙酸 丙酸的氧化和甲烷产量有显著的改善 [49] 乙酸、丙酸、丁酸 3种底物的降解率均有所提高 [50] 纳米Fe3O4 VFA(丁酸50%,丙酸25%,
乙酸25%)、合成废水磁铁的存在加速了丙酸降解,降低了电子传递的阻力 [11] 猪粪 75 mmol纳米磁铁矿将甲烷产量提升6%,日最大甲烷产量
提高47.8%,消化时间缩短20%以上[51] 稻草 磁铁的存在极大地促进了稻草的分解和甲烷的生成 [52] 油酸 甲烷产量有所增加,而且油酸浓度越高,甲烷产量越高
当COD为4 g·L−1时,甲烷产量增加114%[53] 煤气废水 纳米Fe3O4的加入降低了氧化还原电位以及生物毒性,
提高了污染物的去除效率[54] 乳清 文中所提到的磁铁回收与循环方法保证了长期不添加磁铁的产甲烷效率 [55] 丙酸 10 mg·L−1磁铁提高了约44%的甲烷生成速率,IHT以及DIET均起到作用 [56] 合成废水 最大甲烷生成速率提高了15.4%,滞后期缩短了13.9% [57] 合成废水 一个周期内最大甲烷产量提高了78.3%,
根据基因组分析磁铁的加入促进了DIET[58] 污泥 厌氧消化的效率提高,参加DIET的微生物大量富集 [59] 猪粪 最大甲烷产量增加16.1%,丙酸盐降解率得到改善 [60] Fe3O4 高浓度硫酸盐废水 磁铁存在的条件下不同反应器甲烷产量提高了3—10倍,污泥导电率提升了3倍 [61] 合成废水 磁铁矿的加入促进了VFA的降解和甲烷的生成 [62] 合成废水 长期补充磁铁矿可以有效减少VFA的积累,而且不同程度提高甲烷的产率 [63] 乳制品废水 更好的污泥沉降,电子传递;更高的电活性微生物丰度 [64] 猪粪 磁铁矿加速了有机物的水解,增加了Methanothrix , Methanospirillum的富集 [65] 污泥、餐厨垃圾 磁铁矿的加入增加了产甲烷性能,促进了DIET,
与此相关的微生物丰度有所提高[66] 污泥、餐厨垃圾 当污泥与餐厨垃圾的比例为1:0.5时,Fe3O4促进DIET,
VFA浓度提高,甲烷产量有所提升[67] 合成废水 磁铁矿的加入促进了厌氧硫氧化菌与产甲烷菌之间的DIET。 [68] 玉米秸秆+污泥 当Fe3O4为5 g·kg−1时,甲烷产量比对照组高60.47%;Geobacter与 Methanosarcina富集在Fe3O4表面,促进了DIET [69] 磁铁矿(Fe3O4>98%) 合成废水 除了降低氧化还原电位并促进VFA转化外,
磁铁矿的加入减少了核黄素和血红素c的合成[70] Fe3O4+ 乙醇 丙酸 Fe3O4和乙醇同时加入时,最大甲烷生成速率提高了81.4%,添加Fe3O4的
污泥电导率和电子转移系统活性分别增加了2.66倍和2.73倍[71] 纳米Fe2O3 甜菜制糖工业废水 750 mg·L−1纳米 Fe2O3 12 h后具有更高的COD去除率,甲烷生成率 [72] α-Fe 2O3 污泥、餐厨垃圾 缓冲了体系pH,增加了甲烷产量,尤其增加了丙酸、丁酸向乙酸的转化 [73] 赤泥(含45.46%
赤铁矿)污泥 甲烷产量增加了(35.52%± 2.64%),赤泥中的铁元素增加了电子转移效率 [74] 苯甲酸 赤铁矿和磁铁矿的加入,苯甲酸的降解率分别达到了81.8%和91.5% [75] 赤铁矿、
磁铁矿苯酚 二者均提高了甲烷产量以及苯酚的降解,同时产甲烷过程有较高的电子利用率 [76] 针铁矿 合成废水 增强了Syntrophomonadaceae的生长以及VFA的降解 [77] 表 5 铁氧化物促进厌氧消化反应动力学
Table 5. Kinetics of anaerobic digestion promoted by iron oxide
-
[1] STAMS A J M, SOUSA D Z, KLEEREBEZEM R, et al. Role of syntrophic microbial communities in high-rate methanogenic bioreactors [J]. Water Science and Technology, 2012, 66(2): 352-362. doi: 10.2166/wst.2012.192 [2] DEMIREL B, SCHERER P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review [J]. Reviews in Environmental Science and Bio/Technology, 2008, 7(2): 173-190. doi: 10.1007/s11157-008-9131-1 [3] MUYZER G, STAMS A J M. The ecology and biotechnology of sulphate-reducing bacteria [J]. Nature Reviews Microbiology, 2008, 6(6): 441-454. doi: 10.1038/nrmicro1892 [4] SELA-ADLER M, RONEN Z, HERUT B, et al. Co-existence of methanogenesis and sulfate reduction with common substrates in sulfate-rich estuarine sediments [J]. Frontiers in Microbiology, 2017, 8: 766. doi: 10.3389/fmicb.2017.00766 [5] ABBAS Y, YUN S N, WANG Z Q, et al. Recent advances in bio-based carbon materials for anaerobic digestion: A review [J]. Renewable and Sustainable Energy Reviews, 2021, 135: 110378. doi: 10.1016/j.rser.2020.110378 [6] GAHLOT P, AHMED B, TIWARI S B, et al. Conductive material engineered direct interspecies electron transfer (DIET) in anaerobic digestion: Mechanism and application [J]. Environmental Technology & Innovation, 2020, 20: 101056. [7] CRUZ VIGGI C, ROSSETTI S, FAZI S, et al. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation [J]. Environmental Science & Technology, 2014, 48(13): 7536-7543. [8] CAPSON-TOJO G, MOSCOVIZ R, RUIZ D, et al. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste [J]. Bioresource Technology, 2018, 260: 157-168. doi: 10.1016/j.biortech.2018.03.097 [9] LÜ F, LIU Y, SHAO L M, et al. Powdered biochar doubled microbial growth in anaerobic digestion of oil [J]. Applied Energy, 2019, 247: 605-614. doi: 10.1016/j.apenergy.2019.04.052 [10] ZHAO Z Q, ZHANG Y B, WOODARD T L, et al. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials [J]. Bioresource Technology, 2015, 191: 140-145. doi: 10.1016/j.biortech.2015.05.007 [11] CRUZ VIGGI C, CASALE S, CHOUCHANE H, et al. Magnetite nanoparticles enhance the bioelectrochemical treatment of municipal sewage by facilitating the syntrophic oxidation of volatile fatty acids [J]. Journal of Chemical Technology & Biotechnology, 2019, 94(10): 3134-3146. [12] SCHMIDT J E, AHRING B K. Effects of hydrogen and formate on the degradation of propionate and butyrate in thermophilic granules from an upflow anaerobic sludge blanket reactor [J]. Applied Environmental Microbiology, 1993, 59(8): 2546-2551. doi: 10.1128/aem.59.8.2546-2551.1993 [13] BOONE D R, JOHNSON R L, LIU Y T. Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems and its implications in the measurement of Km for H2 or formate uptake [J]. Applied and Environmental Microbiology, 1989, 55(7): 1735-1741. doi: 10.1128/aem.55.7.1735-1741.1989 [14] MORITA M, MALVANKAR N S, FRANKS A E, et al. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates [J]. mBio, 2011, 2(4): e00159-e00111. [15] WU Y, WANG S, LIANG D H, et al. Conductive materials in anaerobic digestion: From mechanism to application [J]. Bioresource Technology, 2020, 298: 122403. doi: 10.1016/j.biortech.2019.122403 [16] LUO C H, LÜ F, SHAO L M, et al. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes [J]. Water Research, 2015, 68: 710-718. doi: 10.1016/j.watres.2014.10.052 [17] JANG H M, CHOI Y K, KAN E. Effects of dairy manure-derived biochar on psychrophilic, mesophilic and thermophilic anaerobic digestions of dairy manure [J]. Bioresource Technology, 2018, 250: 927-931. doi: 10.1016/j.biortech.2017.11.074 [18] WANG G J, LI Q, GAO X, et al. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms [J]. Bioresource Technology, 2018, 250: 812-820. doi: 10.1016/j.biortech.2017.12.004 [19] INDREN M, BIRZER C H, KIDD S P, et al. Effects of biochar parent material and microbial pre-loading in biochar-amended high-solids anaerobic digestion [J]. Bioresource Technology, 2020, 298: 122457. doi: 10.1016/j.biortech.2019.122457 [20] SHEN Y W, YU Y M, ZHANG Y, et al. Role of redox-active biochar with distinctive electrochemical properties to promote methane production in anaerobic digestion of waste activated sludge [J]. Journal of Cleaner Production, 2021, 278: 123212. doi: 10.1016/j.jclepro.2020.123212 [21] WANG Z Q, YUN S N, XU H F, et al. Mesophilic anaerobic co-digestion of acorn slag waste with dairy manure in a batch digester: Focusing on mixing ratios and bio-based carbon accelerants [J]. Bioresource Technology, 2019, 286: 121394. doi: 10.1016/j.biortech.2019.121394 [22] LEI Y Q, SUN D Z, DANG Y, et al. Stimulation of methanogenesis in anaerobic digesters treating leachate from a municipal solid waste incineration plant with carbon cloth [J]. Bioresource Technology, 2016, 222: 270-276. doi: 10.1016/j.biortech.2016.10.007 [23] ZHAO Z Q, ZHANG Y B, LI Y, et al. Potentially shifting from interspecies hydrogen transfer to direct interspecies electron transfer for syntrophic metabolism to resist acidic impact with conductive carbon cloth [J]. Chemical Engineering Journal, 2017, 313: 10-18. doi: 10.1016/j.cej.2016.11.149 [24] ZHAO Z Q, LI Y, QUAN X, et al. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials [J]. Water Research, 2017, 115: 266-277. doi: 10.1016/j.watres.2017.02.067 [25] ZHAO Z Q, ZHANG Y B, YU Q L, et al. Communities stimulated with ethanol to perform direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate [J]. Water Research, 2016, 102: 475-484. doi: 10.1016/j.watres.2016.07.005 [26] LEE J Y, LEE S H, PARK H D. Enrichment of specific electro-active microorganisms and enhancement of methane production by adding granular activated carbon in anaerobic reactors [J]. Bioresource Technology, 2016, 205: 205-212. doi: 10.1016/j.biortech.2016.01.054 [27] YANG Y F, ZHANG Y B, LI Z Y, et al. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition [J]. Journal of Cleaner Production, 2017, 149: 1101-1108. doi: 10.1016/j.jclepro.2017.02.156 [28] PENG H, ZHANG Y B, TAN D M, et al. Roles of magnetite and granular activated carbon in improvement of anaerobic sludge digestion [J]. Bioresource Technology, 2018, 249: 666-672. doi: 10.1016/j.biortech.2017.10.047 [29] TIAN T, QIAO S, LI X, et al. Nano-graphene induced positive effects on methanogenesis in anaerobic digestion [J]. Bioresource Technology, 2017, 224: 41-47. doi: 10.1016/j.biortech.2016.10.058 [30] WU B T, LIN R C, KANG X H, et al. Graphene addition to digestion of thin stillage can alleviate acidic shock and improve biomethane production [J]. ACS Sustainable Chemistry & Engineering, 2020, 8(35): 13248-13260. [31] LIN R C, CHENG J, ZHANG J B, et al. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion [J]. Bioresource Technology, 2017, 239: 345-352. doi: 10.1016/j.biortech.2017.05.017 [32] ZHANG L, ZHANG J X, LOH K C. Activated carbon enhanced anaerobic digestion of food waste - Laboratory-scale and Pilot-scale operation [J]. Waste Management, 2018, 75: 270-279. doi: 10.1016/j.wasman.2018.02.020 [33] LI L L, TONG Z H, FANG C Y, et al. Response of anaerobic granular sludge to single-wall carbon nanotube exposure [J]. Water Research, 2015, 70: 1-8. doi: 10.1016/j.watres.2014.11.042 [34] ZHANG M Y, MA Y Q, JI D D, et al. Synergetic promotion of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with graphite felt in anaerobic digestion [J]. Bioresource Technology, 2019, 287: 121373. doi: 10.1016/j.biortech.2019.121373 [35] HU Q, SUN D Z, MA Y, et al. Conductive polyaniline nanorods enhanced methane production from anaerobic wastewater treatment [J]. Polymer, 2017, 120: 236-243. doi: 10.1016/j.polymer.2017.05.073 [36] WANG Z Q, YUN S N, SHI J, et al. Critical evidence for direct interspecies electron transfer with tungsten-based accelerants: An experimental and theoretical investigation [J]. Bioresource Technology, 2020, 311: 123519. doi: 10.1016/j.biortech.2020.123519 [37] WANG C, YUN S N, XU H F, et al. Dual functional application of pomelo peel-derived bio-based carbon with controllable morphologies: An efficient catalyst for triiodide reduction and accelerant for anaerobic digestion [J]. Ceramics International, 2020, 46(3): 3292-3303. doi: 10.1016/j.ceramint.2019.10.035 [38] LÜ C X, SHEN Y W, LI C, et al. Redox-active biochar and conductive graphite stimulate methanogenic metabolism in anaerobic digestion of waste-activated sludge: Beyond direct interspecies electron transfer [J]. ACS Sustainable Chemistry & Engineering, 2020, 8(33): 12626-12636. [39] SHANMUGAM S R, ADHIKARI S, NAM H, et al. Effect of bio-char on methane generation from glucose and aqueous phase of algae liquefaction using mixed anaerobic cultures [J]. Biomass and Bioenergy, 2018, 108: 479-486. doi: 10.1016/j.biombioe.2017.10.034 [40] CHEN S S, ROTARU A E, LIU F H, et al. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures [J]. Bioresource Technology, 2014, 173: 82-86. doi: 10.1016/j.biortech.2014.09.009 [41] BAEK G, KIM J, LEE C. A review of the effects of iron compounds on methanogenesis in anaerobic environments [J]. Renewable and Sustainable Energy Reviews, 2019, 113: 109282. doi: 10.1016/j.rser.2019.109282 [42] STRAUB K L, BENZ M, SCHINK B. Iron metabolism in anoxic environments at near neutral pH [J]. FEMS Microbiology Ecology, 2001, 34(3): 181-186. doi: 10.1111/j.1574-6941.2001.tb00768.x [43] 林霄涵, 杨帆, 赵峰. 微生物的胞外电子传递界面 [J]. 环境化学, 2021, 40(11): 3283-3296. doi: 10.7524/j.issn.0254-6108.2021033106 LIN X H, YANG F, ZHAO F. The interface of microbial extracellular electron transfer [J]. Environmental Chemistry, 2021, 40(11): 3283-3296(in Chinese). doi: 10.7524/j.issn.0254-6108.2021033106
[44] SUMMERS Z M, FOGARTY H E, LEANG C, et al. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria [J]. Science, 2010, 330(6009): 1413-1415. doi: 10.1126/science.1196526 [45] KATO S, HASHIMOTO K, WATANABE K. Microbial interspecies electron transfer via electric currents through conductive minerals [J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(25): 10042-10046. doi: 10.1073/pnas.1117592109 [46] KATO S, HASHIMOTO K, WATANABE K. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals [J]. Environmental Microbiology, 2012, 14(7): 1646-1654. doi: 10.1111/j.1462-2920.2011.02611.x [47] AULENTA F, FAZI S, MAJONE M, et al. Electrically conductive magnetite particles enhance the kinetics and steer the composition of anaerobic TCE-dechlorinating cultures [J]. Process Biochemistry, 2014, 49(12): 2235-2240. doi: 10.1016/j.procbio.2014.09.015 [48] WANG T, ZHANG D, DAI L L, et al. Magnetite triggering enhanced direct interspecies electron transfer: A scavenger for the blockage of electron transfer in anaerobic digestion of high-solids sewage sludge [J]. Environmental Science & Technology, 2018, 52(12): 7160-7169. [49] XIA X X, ZHANG J C, SONG T Z, et al. Stimulation of Smithella-dominating propionate oxidation in a sediment enrichment by magnetite and carbon nanotubes [J]. Environmental Microbiology Reports, 2019, 11(2): 236-248. doi: 10.1111/1758-2229.12737 [50] LEE J, KOO T, YULISA A, et al. Magnetite as an enhancer in methanogenic degradation of volatile fatty acids under ammonia-stressed condition [J]. Journal of Environmental Management, 2019, 241: 418-426. [51] ZHANG J Y, WANG Z Y, LU T D, et al. Response and mechanisms of the performance and fate of antibiotic resistance genes to nano-magnetite during anaerobic digestion of swine manure [J]. Journal of Hazardous Materials, 2019, 366: 192-201. doi: 10.1016/j.jhazmat.2018.11.106 [52] HUANG J J, MA K, XIA X X, et al. Biochar and magnetite promote methanogenesis during anaerobic decomposition of rice straw [J]. Soil Biology and Biochemistry, 2020, 143: 107740. doi: 10.1016/j.soilbio.2020.107740 [53] MOSTAFA A, IM S, SONG Y C, et al. Enhanced anaerobic digestion of long chain fatty acid by adding magnetite and carbon nanotubes [J]. Microorganisms, 2020, 8(3): 333. doi: 10.3390/microorganisms8030333 [54] MA W C, LI J X, ZHONG D, et al. New insights into enhanced anaerobic degradation of coal gasification wastewater (CGW) with the assistance of magnetite nanoparticles [J]. Chemosphere, 2021, 262: 127872. doi: 10.1016/j.chemosphere.2020.127872 [55] BAEK G, JUNG H, KIM J, et al. A long-term study on the effect of magnetite supplementation in continuous anaerobic digestion of dairy effluent - Magnetic separation and recycling of magnetite [J]. Bioresource Technology, 2017, 241: 830-840. doi: 10.1016/j.biortech.2017.06.018 [56] JING Y H, WAN J J, ANGELIDAKI I, et al. iTRAQ quantitative proteomic analysis reveals the pathways for methanation of propionate facilitated by magnetite [J]. Water Research, 2017, 108: 212-221. doi: 10.1016/j.watres.2016.10.077 [57] YIN Q D, MIAO J, LI B, et al. Enhancing electron transfer by ferroferric oxide during the anaerobic treatment of synthetic wastewater with mixed organic carbon [J]. International Biodeterioration & Biodegradation, 2017, 119: 104-110. [58] YIN Q D, YANG S, WANG Z Z, et al. Clarifying electron transfer and metagenomic analysis of microbial community in the methane production process with the addition of ferroferric oxide [J]. Chemical Engineering Journal, 2018, 333: 216-225. doi: 10.1016/j.cej.2017.09.160 [59] ZHAO Z S, LI Y, YU Q L, et al. Ferroferric oxide triggered possible direct interspecies electron transfer between Syntrophomonas and Methanosaeta to enhance waste activated sludge anaerobic digestion [J]. Bioresource Technology, 2018, 250: 79-85. doi: 10.1016/j.biortech.2017.11.003 [60] ZHANG J Y, LU T D, WANG Z Y, et al. Effects of magnetite on anaerobic digestion of swine manure: Attention to methane production and fate of antibiotic resistance genes [J]. Bioresource Technology, 2019, 291: 121847. doi: 10.1016/j.biortech.2019.121847 [61] JIN Z, ZHAO Z Q, ZHANG Y B. Potential of direct interspecies electron transfer in synergetic enhancement of methanogenesis and sulfate removal in an up-flow anaerobic sludge blanket reactor with magnetite [J]. Science of the Total Environment, 2019, 677: 299-306. doi: 10.1016/j.scitotenv.2019.04.372 [62] WANG C Q, WANG C, JIN L N, et al. Response of syntrophic aggregates to the magnetite loss in continuous anaerobic bioreactor [J]. Water Research, 2019, 164: 114925. doi: 10.1016/j.watres.2019.114925 [63] MA K L, WANG W, LIU Y Q, et al. Insight into the performance and microbial community profiles of magnetite-amended anaerobic digestion: Varying promotion effects at increased loads [J]. Bioresource Technology, 2021, 329: 124928. doi: 10.1016/j.biortech.2021.124928 [64] KIM J, CHOI H, LEE C. Formation and characterization of conductive magnetite-embedded granules in upflow anaerobic sludge blanket reactor treating dairy wastewater [J]. Bioresource Technology, 2022, 345: 126492. doi: 10.1016/j.biortech.2021.126492 [65] ZHENG S C, YANG F, HUANG W L, et al. Combined effect of zero valent iron and magnetite on semi-dry anaerobic digestion of swine manure [J]. Bioresource Technology, 2022, 346: 126438. doi: 10.1016/j.biortech.2021.126438 [66] BAEK G, KIM J, LEE C. Effectiveness of electromagnetic in situ magnetite capture in anaerobic sequencing batch treatment of dairy effluent under electro-syntrophic conditions [J]. Renewable Energy, 2021, 179: 105-115. doi: 10.1016/j.renene.2021.07.052 [67] ZHU R L, HE L Y, LI Q Y, et al. Mechanism study of improving anaerobic co-digestion performance of waste activated sludge and food waste by Fe3O4 [J]. Journal of Environmental Management, 2021, 300: 113745. doi: 10.1016/j.jenvman.2021.113745 [68] JUNG H, BAEK G, LEE C. Magnetite-assisted in situ microbial oxidation of H2S to S0 during anaerobic digestion: A new potential for sulfide control [J]. Chemical Engineering Journal, 2020, 397: 124982. doi: 10.1016/j.cej.2020.124982 [69] LI P F, WANG Q, HE X M, et al. Investigation on the effect of different additives on anaerobic co-digestion of corn straw and sewage sludge: Comparison of biochar, Fe3O4, and magnetic biochar [J]. Bioresource Technology, 2022, 345: 126532. doi: 10.1016/j.biortech.2021.126532 [70] WANG C Q, LIU Y, JIN S, et al. Responsiveness extracellular electron transfer (EET) enhancement of anaerobic digestion system during start-up and starvation recovery stages via magnetite addition [J]. Bioresource Technology, 2019, 272: 162-170. doi: 10.1016/j.biortech.2018.10.013 [71] XING L Z, WANG Z F, GU M Q, et al. Coupled effects of ferroferric oxide supplement and ethanol co-metabolism on the methanogenic oxidation of propionate [J]. Science of the Total Environment, 2020, 723: 137992. doi: 10.1016/j.scitotenv.2020.137992 [72] AMBUCHI J J, ZHANG Z H, SHAN L L, et al. Response of anaerobic granular sludge to iron oxide nanoparticles and multi-wall carbon nanotubes during beet sugar industrial wastewater treatment [J]. Water Research, 2017, 117: 87-94. doi: 10.1016/j.watres.2017.03.050 [73] ZHU R L, CHEN Y D, ZHAO T, et al. Enhanced mesophilic anaerobic co-digestion of waste sludge and food waste by using hematite (α-Fe2O3) supported bentonite as additive [J]. Bioresource Technology, 2020, 313: 123603. doi: 10.1016/j.biortech.2020.123603 [74] YE J, HU A D, REN G P, et al. Enhancing sludge methanogenesis with improved redox activity of extracellular polymeric substances by hematite in red mud [J]. Water Research, 2018, 134: 54-62. doi: 10.1016/j.watres.2018.01.062 [75] ZHUANG L, TANG Z Y, MA J L, et al. Enhanced anaerobic biodegradation of benzoate under sulfate-reducing conditions with conductive iron-oxides in sediment of Pearl River Estuary [J]. Frontiers in Microbiology, 2019, 10: 374. doi: 10.3389/fmicb.2019.00374 [76] TANG Y P, LI Y, ZHANG M Q, et al. Link between characteristics of Fe(III) oxides and critical role in enhancing anaerobic methanogenic degradation of complex organic compounds [J]. Environmental Research, 2021, 194: 110498. doi: 10.1016/j.envres.2020.110498 [77] XU S Y, ZHANG W Q, ZUO L Q, et al. Comparative facilitation of activated carbon and goethite on methanogenesis from volatile fatty acids [J]. Bioresource Technology, 2020, 302: 122801. doi: 10.1016/j.biortech.2020.122801 [78] BAEK G, KIM J, LEE C. A long-term study on the effect of magnetite supplementation in continuous anaerobic digestion of dairy effluent - Enhancement in process performance and stability [J]. Bioresource Technology, 2016, 222: 344-354. doi: 10.1016/j.biortech.2016.10.019 [79] LU T D, ZHANG J Y, WEI Y S, et al. Effects of ferric oxide on the microbial community and functioning during anaerobic digestion of swine manure [J]. Bioresource Technology, 2019, 287: 121393. doi: 10.1016/j.biortech.2019.121393 [80] THAUER R K, KASTER A K, SEEDORF H, et al. Methanogenic Archaea: Ecologically relevant differences in energy conservation [J]. Nature Reviews Microbiology, 2008, 6(8): 579-591. doi: 10.1038/nrmicro1931 [81] STAMS A J M, PLUGGE C M. Electron transfer in syntrophic communities of anaerobic bacteria and Archaea [J]. Nature Reviews Microbiology, 2009, 7(8): 568-577. doi: 10.1038/nrmicro2166 [82] ROTARU A E, SHRESTHA P M, LIU F H, et al. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane [J]. Energy & Environmental Science, 2014, 7(1): 408-415. [83] 赵智强, 李杨, 张耀斌. 厌氧消化中直接种间电子传递产甲烷机理研究与技术应用 [J]. 科学通报, 2020, 65(26): 2820-2834. doi: 10.1360/TB-2020-0661 ZHAO Z Q, LI Y, ZHANG Y B. Direct interspecies electron transfer in anaerobic digestion: Research and technological application [J]. Chinese Science Bulletin, 2020, 65(26): 2820-2834(in Chinese). doi: 10.1360/TB-2020-0661
[84] 田晓春, 吴雪娥, 赵峰, 等. 电化学联用技术研究微生物的胞外电子传递机制 [J]. 化学进展, 2018, 30(8): 1222-1227. TIAN X C, WU X E, ZHAO F, et al. Research on mechanisms of microbial extracellular electron transfer by electrochemical integrated technologies [J]. Progress in Chemistry, 2018, 30(8): 1222-1227(in Chinese).
[85] LIU F H, ROTARU A E, SHRESTHA P M, et al. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange [J]. Environmental Microbiology, 2015, 17(3): 648-655. doi: 10.1111/1462-2920.12485 [86] LOVLEY D R. Syntrophy Goes electric: Direct interspecies electron transfer [J]. Annual Review of Microbiology, 2017, 71: 643-664. doi: 10.1146/annurev-micro-030117-020420 [87] WANG M W, ZHAO Z Q, ZHANG Y B. Magnetite-contained biochar derived from Fenton sludge modulated electron transfer of microorganisms in anaerobic digestion [J]. Journal of Hazardous Materials, 2021, 403: 123972. doi: 10.1016/j.jhazmat.2020.123972 [88] KANG H J, LEE S H, LIM T G, et al. Recent advances in methanogenesis through direct interspecies electron transfer via conductive materials: A molecular microbiological perspective [J]. Bioresource Technology, 2021, 322: 124587. doi: 10.1016/j.biortech.2020.124587 [89] FLEMMING H C, WINGENDER J. The biofilm matrix [J]. Nature Reviews Microbiology, 2010, 8(9): 623-633. doi: 10.1038/nrmicro2415 [90] WANG H W, DENG H H, MA L M, et al. Influence of operating conditions on extracellular polymeric substances and surface properties of sludge flocs [J]. Carbohydrate Polymers, 2013, 92(1): 510-515. doi: 10.1016/j.carbpol.2012.09.055 [91] XIAO Y, ZHANG E H, ZHANG J D, et al. Extracellular polymeric substances are transient media for microbial extracellular electron transfer [J]. Science Advances, 2017, 3(7): e1700623. doi: 10.1126/sciadv.1700623 [92] YU Q, YANG Y F, WANG M W, et al. Enhancing anaerobic digestion of kitchen wastes via combining ethanol-type fermentation with magnetite: Potential for stimulating secretion of extracellular polymeric substances [J]. Waste Management, 2021, 127: 10-17. doi: 10.1016/j.wasman.2021.04.022 [93] WEBER K A, ACHENBACH L A, COATES J D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction [J]. Nature Reviews Microbiology, 2006, 4(10): 752-764. doi: 10.1038/nrmicro1490 [94] ACHTNICH C, BAK F, CONRAD R. Competition for electron donors among nitrate reducers, ferric iron reducers, sulfate reducers, and methanogens in anoxic paddy soil [J]. Biology and Fertility of Soils, 1995, 19(1): 65-72. doi: 10.1007/BF00336349 [95] LOVLEY D R, HOLMES D E, NEVIN K P. Dissimilatory Fe(III) and Mn(IV) reduction [J]. Advances in Microbial Physiology, 2004, 49: 219-286. [96] VAN B P M, SCHOLTEN J C M, STAMS A J M. Direct inhibition of methanogenesis by ferric iron [J]. FEMS Microbiology Ecology, 2004, 49(2): 261-268. doi: 10.1016/j.femsec.2004.03.017 [97] ZHOU S G, XU J L, YANG G Q, et al. Methanogenesis affected by the co-occurrence of iron(III) oxides and humic substances [J]. FEMS Microbiology Ecology, 2014, 88(1): 107-120. doi: 10.1111/1574-6941.12274 [98] 马金莲, 马晨, 汤佳, 等. 电子穿梭体介导的微生物胞外电子传递: 机制及应用 [J]. 化学进展, 2015, 27(12): 1833-1840. doi: 10.7536/PC150533 MA J L, MA C, TANG J, et al. Mechanisms and applications of electron shuttle-mediated extracellular electron transfer [J]. Progress in Chemistry, 2015, 27(12): 1833-1840(in Chinese). doi: 10.7536/PC150533
[99] LOVLEY D R, PHILLIPS E J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments [J]. Applied and Environmental Microbiology, 1986, 51(4): 683-689. doi: 10.1128/aem.51.4.683-689.1986 -



 下载:
下载:






















