-
随着工业经济的发展,大气中CO2浓度急剧上升,使得全球气候变暖从而引发各种生态环境问题[1],缓解全球气候变暖,调节大气温室气体浓度,成为各国政治、经济关注的焦点,作为主要的温室气体,CO2在全球气候调节中的作用极为关键[2]. 此外,在全球碳循环中,由于存在下落不明的碳汇(遗漏碳汇(missing sink))[3],导致碳源汇失衡[4],进而使碳汇估算失真[5];而研究证明,其中,由水循环过程引起的岩溶碳汇就占据遗漏汇的1/3[6]. 因此,研究全球碳循环,寻找遗失碳汇是控制全球气候变暖的关键.
作为连接陆地碳库和海洋碳库的关键纽带[7],河流不仅对碳的运输具有重要作用,而且也是物质循环与能量流动的重要场所[8]. 每年由河流向海洋输送的碳大约为0.8—1.2 Gt Cyr−1,其中,溶解有机碳(DOC)和溶解无机碳(DIC)分别为25%和38%,颗粒有机碳(POC)和颗粒无机碳(PIC)分别为20%和17%[9]. 河流中的DIC(主要是HCO3−),主要来源于碳酸盐岩和硅酸盐岩溶解消耗大气以及土壤中的CO2[2]. 碳酸盐岩作为全球最大的碳库,其风化过程受到多种因素的影响,如工业活动中工业废水的排放[10]、农业活动中化肥的施用[11]、土地利用类型的变更[12],都将不同程度的影响碳酸盐岩的风化溶解. 同时,碳酸盐岩溶蚀提供的无机碳源与水生光合作用(生物碳泵效应-BCP)相结合[13],将无机碳转化为有机碳(OC),从而形成一种长时间尺度的稳定碳汇[14]. 但在工农业生产过程中产生的污染物因包含大量的N、S化合物,经过一系列生化作用后形成硝酸和硫酸等外源酸,进入岩溶动力系统,积极参与碳酸盐岩的风化溶解[15];而外源酸参与的风化过程将不再消耗大气/土壤中的CO2,反而在高浓度的外源酸参与下形成碳源,导致岩溶区碳汇强度的估算结果存在差异[16-17]. 学者们对不同尺度流域内硝酸、硫酸等外源酸参与碳酸盐岩溶解的研究中发现,大溶江流域内硫酸溶蚀碳酸盐岩可贡献20%的溶解无机碳[18],硫酸/硝酸溶蚀碳酸盐岩对漓江流域无机碳的贡献达10%—30%[19],硫酸溶解碳酸盐岩对广西平果岩溶流域地下水中HCO3−的平均贡献率为11.81%[20],硫酸/硝酸在漓江流域阳朔断面洪水过程中溶解碳酸盐岩对无机碳的最高贡献率达31.7%[21]. 可见外源酸(硫酸、硝酸)风化流域碳酸盐岩对无机碳的贡献举足轻重,因此,探究外源酸对岩溶流域碳循环的影响对精确估算岩溶作用产生的碳汇量及完善全球碳循环机理具有十分重要的科学意义.
西南地区作为中国典型的岩溶地带,岩溶类型丰富,喀斯特地貌面积分布广泛,地表、地下独特的二元结构使各种外来物质流入岩溶区后加速碳酸盐岩的风化,如外源酸的参与使水体中DIC的浓度增加,影响岩溶区碳汇强度的估算. 双河流域为西南地区典型的喀斯特流域,目前对双河流域的研究大多集中于利用相关同位素来揭示主要离子来源与岩石风化类型[22-23]、环境指示意义以及对DIC的影响因素分析[24-25];虽然有对硫酸溶蚀碳酸盐岩的研究,但对硝酸、硫酸等外源酸参与碳酸盐岩溶解,对HCO3−的贡献比例鲜有深入研究.
为了进一步研究硝酸、硫酸等外源酸在流域碳循环中的作用,本文选取双河小流域进行系统研究. 分别于2019年春夏秋冬四季开展了四次野外动态监测,运用数理统计、离子比值、质量平衡模型和图解法等方法对流域水体水化学组成及主要离子来源进行分析,计算硝酸、硫酸等外源酸对流域碳酸盐岩溶蚀的贡献率,为喀斯特流域碳循环研究提供理论依据.
-
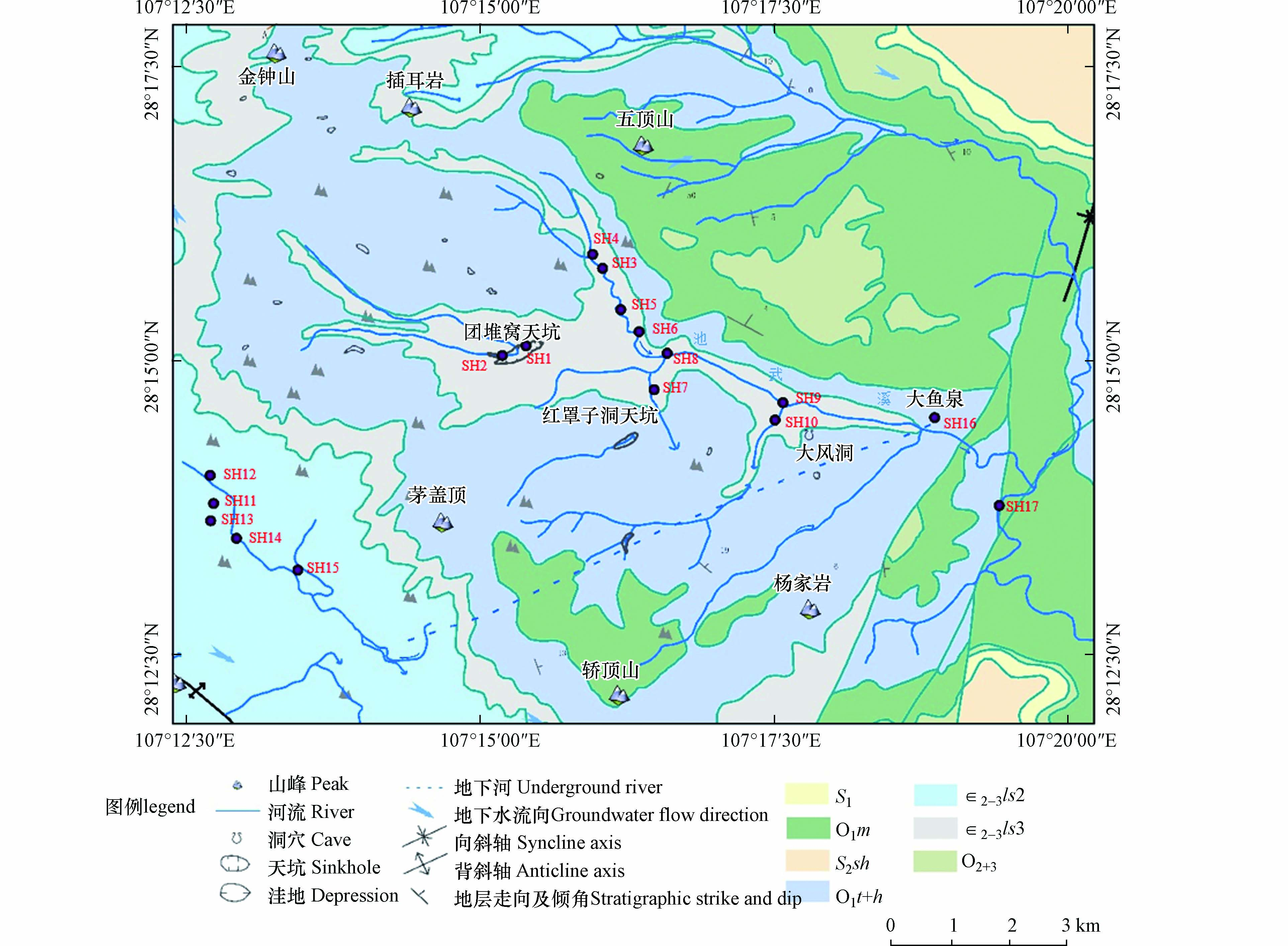
双河流域(107°02′30″—107°25′00″E,28°08′00″—28°20′00″N)位于贵州省绥阳县温泉镇境内(图1),为典型喀斯特小流域,区域内出露地层主要为寒武系中上统娄山关组、奥陶系桐梓组,二叠系中、上统、三叠系下统、志留系中、下统以及第四系地层,岩性主要以白云岩、白云质灰岩以及夹燧石和泥质的白云岩等碳酸盐岩为主[26]. 其中,由于第四纪的间歇性抬升,研究区内地形切割强烈,沟谷纵横发育,且地质作用对沟谷方向控制明显,主要为水流沿断层节理侵蚀、溶蚀以及崩塌而形成. 区域内喀斯特地貌面积分布广泛,岩溶地貌类型丰富,主要有峰丛洼地、峡谷、天坑、落水洞、洞穴以及地下暗河等[27],形成较典型、独特的岩溶区二元立体三维结构的地貌形态特征;碳酸盐岩裂隙洞穴水、碳酸盐岩夹碎屑岩裂隙洞穴水及碎屑岩裂隙水为研究区主要的地下水类型. 地下河是流域的主要补给来源,区内地下河流量大约在100 L·s−1以上,以大气降雨补给为主[28].
气候属于中亚热带季风气候,雨热同期,四季分明,昼夜温差平均小于10 ℃,年均气温为15.5 ℃,降雨主要集中在每年的4—10月,年均降雨量为1210 mm[29],充沛的降雨量为喀斯特地貌的形成,尤其是洞穴的形成提供了良好的生态环境.
-
为了分析双河流域水体基本水化学特征、主要离子来源以及外源酸对流域碳汇效应的影响,于2019年3月、6月、10月和12月对流域进行4次野外监测和样品采集. 根据研究区的水文地质背景及流域分布特征,共选取17个样点进行分析. 其中样品的pH、温度、溶解氧(dissolved oxygen DO)和电导率(electrical conductance EC),采用WTWMulti-3430便携式多参数水质分析仪(德国WTW公司)进行现场测定,测量误差分别为0.01、0.1 ℃、0.01 mg·L−1、0.1 μS·cm−1,HCO3−和Ca2+采用德国Aquamerck公司出产的便携式碱度计和硬度计进行现场测定,测量精度分别为0.1 mmol·L−1和2 mg·L−1;每一组水样重复操作滴定2—3次,误差控制低于5%. 使用聚乙烯瓶采集部分水样用于实验室分析,采样前用去离子水将瓶子重复清洗2—3次,接着用0.22 μm的醋酸纤维滤膜过滤后装入聚乙烯瓶中,装样前避免样品中滞留空气应确保无气泡装入水样,其中测试阳离子(Ca2+、Mg2+、Na+和K+)的水样需加入2—3滴1:1的优纯级浓HNO3,调至pH≤2,其余水样不做任何处理,直接装入聚乙烯瓶中密封保存,所有水样采集完成后,放置于4 ℃的冰箱中冷藏保存,尽快带回实验室进行分析. Ca2+、Mg2+、Na+和K+等阳离子用美国Varian公司生产的VISTA MPX型电感耦合等离子体-发射光谱仪进行检测,测定范围175—785 nm,分辨率0.009 nm[30],测量精度0.001 mg·L−1,相对标准偏差低于2%,NO3−、SO42−和Cl−等阴离子用美国Dionex公司生产的ICS90型离子色谱仪进行检测,测定范围0—1000 μS·cm−1,分辨率0.2 μS·cm−1,以上样品的阴阳离子测试均在中国科学院地球化学研究所完成[31].
-
双河流域基本水化学参数统计如表1所示. 其中,水体pH值变化范围为7.81—9.00,平均值为(8.46±0.18),变异系数为2%,表明流域pH值季节变化较稳定,水体偏弱碱性. 水温变化范围为9.30—20.80 ℃,平均值为(14.72±1.59) ℃,变异系数为10.75%;电导率(EC)的变化范围为209.00—356.00 μS·cm−1,平均值为(277.22±29.41) μS·cm−1,变异系数为10.75%;溶解性总固体(total dissolved solids-TDS)指水中溶解的无机盐和有机物的总称,TDS浓度变化表征水中主要离子成分也随之改变,流域TDS的变化范围为202.73—316.11 mg·L−1,平均值为255.06 mg·L−1,高于世界河流的均值(65 mg·L−1)[32],变异系数为9.25%.
-
研究区水体离子组成中,阳离子质量浓度大小顺序为Ca2+>Mg2+>K+>Na+,以Ca2+为优势阳离子,Mg2+次之;其中,Ca2+质量浓度变化介于36—60 mg·L−1之间,平均值为(44.27±4.51) mg·L−1,变异系数为10.25%,表明流域Ca2+含量变化幅度较小. Mg2+质量浓度变化介于10.74—22.58 mg·L−1之间,平均值为(14.9±2.74) mg·L−1,变异系数为18.5%,变化幅度较Ca2+大;K+质量浓度变化介于0.61—2.80 mg·L−1之间,平均值为(1.09±0.34) mg·L−1,变异系数为31.75%;Na+质量浓度介于0.17—2.91 mg·L−1之间,平均值为(0.87±0.53) mg·L−1,变异系数为61.75%;阴离子质量浓度大小顺序为HCO3−>SO42−>NO3−>Cl−,优势阴离子为HCO3−;其中,HCO3−质量浓度变化介于134.20—207.40 mg·L−1之间,平均值为(174.37±16.33) mg·L−1,变异系数为9.25%;SO42−质量浓度介于7.99—28.49 mg·L−1之间,平均值为(13.60±3.75) mg·L−1,变异系数为27.25%,最大值出现在阴河潭水(SH10)处,受人类活动影响较大;NO3−质量浓度介于2.54—8.56 mg·L−1之间,平均值为(4.81 ±1.27) mg·L−1,变异系数为25.5%,最大值位于饮水公司后(SH13),可能受该公司的日常运作的影响,导致其浓度高于其他采样点;Cl−质量浓度介于0.39—4.14 mg·L−1之间,平均值为(1.04 ±0.63) mg·L−1,变异系数为60%;综上分析可知,流域内K+、Na+和Cl−均表现为含量变化幅度较小,而变异系数变化幅度较大的特征,表明人类活动对水体变化影响较大.
-
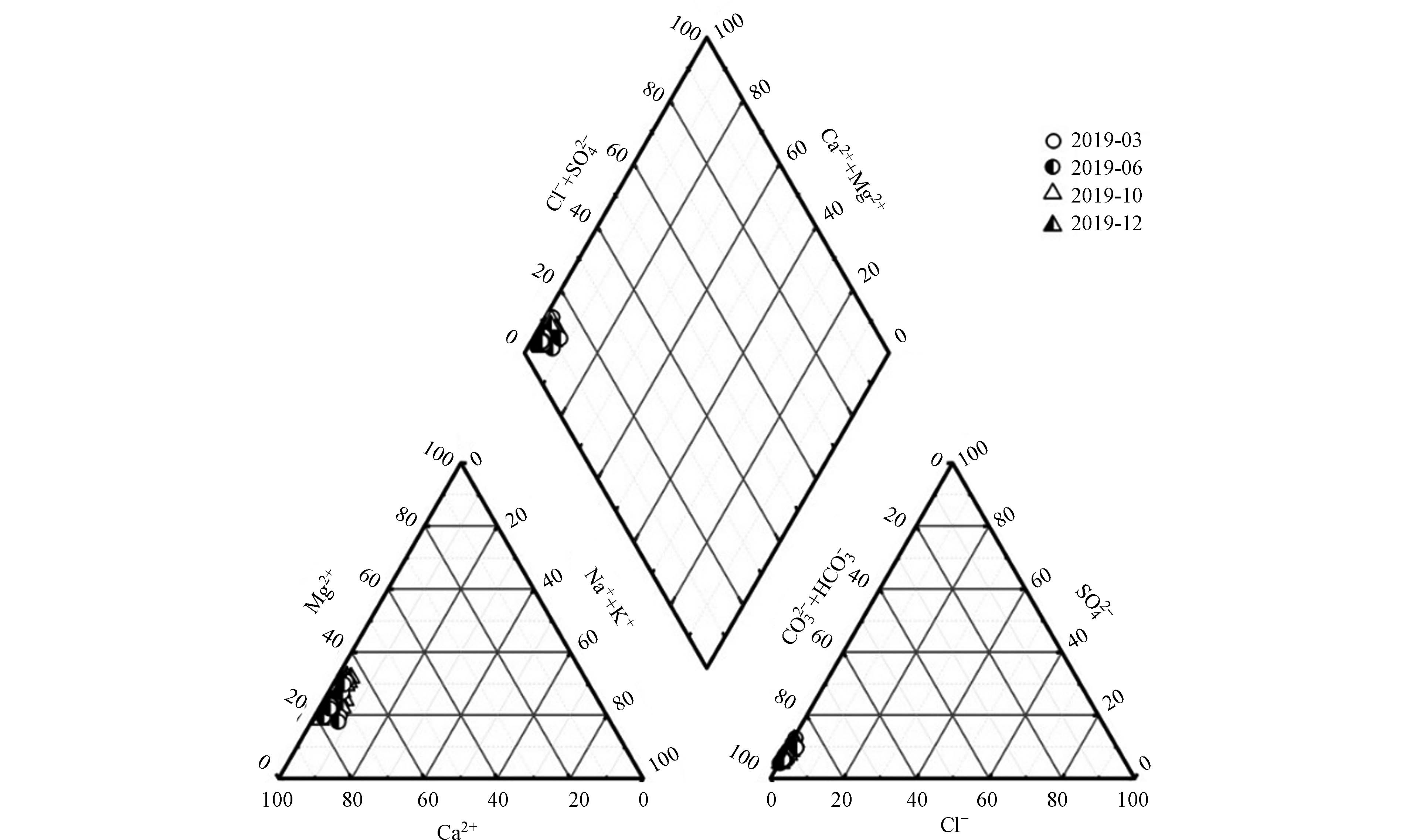
根据Piper三线图可判别流域水化学类型,从图2中可以看出,阳离子主要靠近Ca2+、Mg2+一侧,阴离子主要位于HCO3−一侧,主要阳离子Ca2+占总阳离子的72.44%;Mg2+占总阳离子的24.38%;主要阴离子HCO3−占总阴离子的89.97%,表明双河流域水化学类型为HCO3-Ca·Mg型,同时推断出研究区的岩石风化类型主要为碳酸盐岩风化.
-
通常大气沉降、岩石风化、蒸发浓缩、N、S化和物的风化与溶解以及人类活动的影响等因素会扰乱地下水和地表水的物化生过程,进而导致水体主要离子组分发生变化.
-
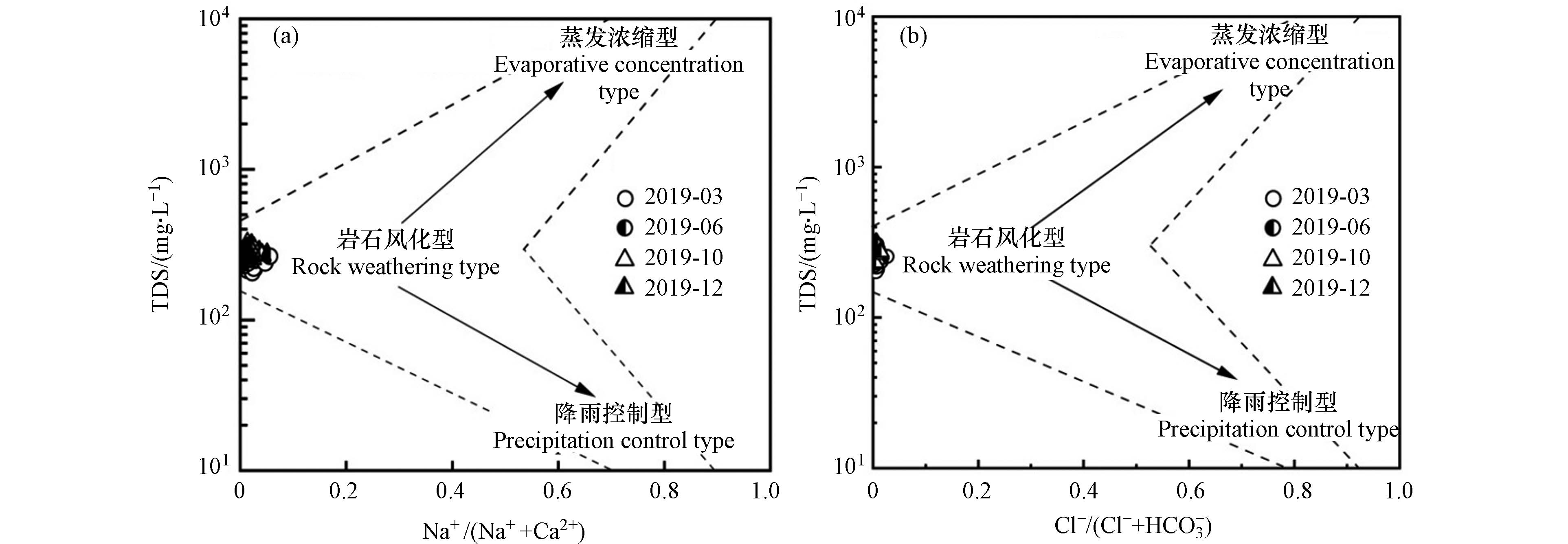
为探究流域水化学变化的控制因素,通常借助吉布斯(Gibbs)图,根据TDS与Na+/(Na++Ca2+)和Cl−/(Cl−+HCO3−)的关系来反映水中主要离子组分的成因机制,判别影响流域水化学变化的主要因素,如岩石风化、蒸发浓缩以及大气降雨的影响[33];当TDS偏低,Na+/(Na++Ca2+)和Cl−/(Cl−+HCO3−)的比值小于0.5,则说明流域水化学变化主要受岩石风化的控制[11]. 绘制双河流域的Gibbs图如图3所示,TDS与Na+/(Na++Ca2+)的比值关系图3(a)和TDS与Cl−/(Cl−+HCO3−)的比值关系图3(b)中,春、夏、秋、冬四季的水样点分布集中,均位于岩石风化控制型一侧,Na+/(Na++Ca2+)和Cl−/(Cl−+HCO3−)的比值均小于0.5,表明双河流域的水化学变化主要受岩石风化的控制,受蒸发浓缩和降雨的影响较小,与前人研究结果一致[8].
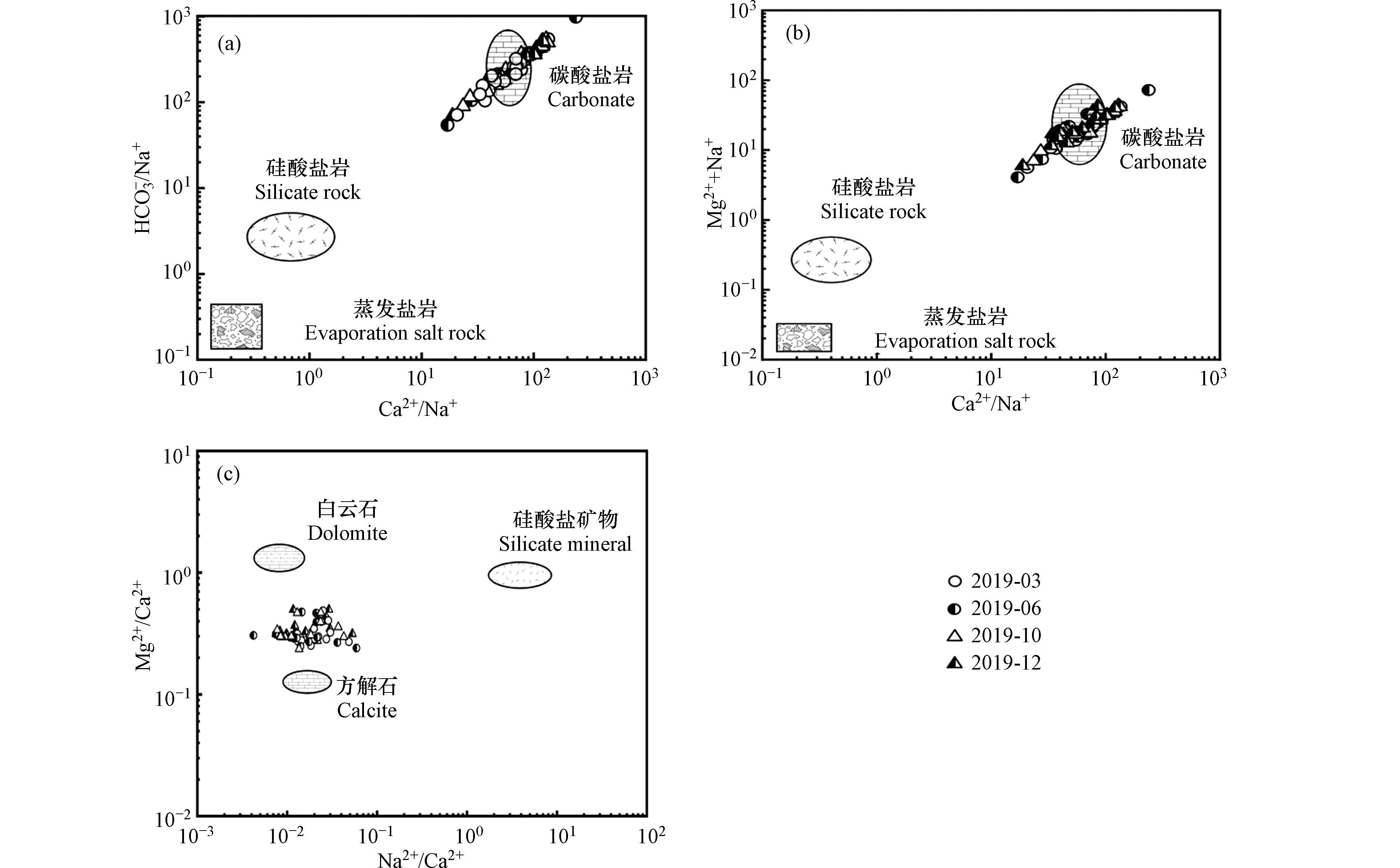
在自然地质环境中,Ca2+和Mg2+主要来源于碳酸盐岩、蒸发盐岩以及硅酸盐岩矿物的溶解,HCO3−主要来源于碳酸盐岩矿物的溶解,Na+和K+主要来源于硅酸盐岩和蒸发盐岩矿物的溶解[33]. 因岩石类型存在差异,导致其矿物溶解产物的离子比值也显著不同,因此可以通过Ca2+/Na+与HCO3−/Na+和Mg2+/Na+的比值关系来区分流域水体的岩石风化类型[34]. 当Ca2+/Na+与HCO3−/Na+和Mg2+/Na+的比值分别接近0.02、0.004、0.11时,主要受控于蒸发盐岩溶解;当比值接近0.35、2、0.24时,主要受硅酸盐岩风化控制,当比值接近50、120、10时,主要由碳酸盐岩溶解控制[35]. 由图4(a)和4(b)可知,双河流域的水样点均位于碳酸盐岩矿物溶解一侧,远离硅酸盐岩与蒸发盐岩一端,表明研究区水岩作用主要以碳酸盐岩矿物溶解为主,硅酸盐岩矿物与蒸发盐岩矿物溶解对流域离子组成的影响较小[36]. 根据流域中的Mg2+/Ca2+和Na+/Ca2+的比值关系进一步验证区域岩石风化类型[37],由图4(c)可知,研究区水样水化学组成中的Mg2+/Ca2+和Na+/Ca2+的比值位于白云石和方解石之间,远离硅酸盐岩一侧,碳酸盐岩风化占主要部分,说明双河流域的水化学环境主要受控于碳酸盐岩的风化溶解.
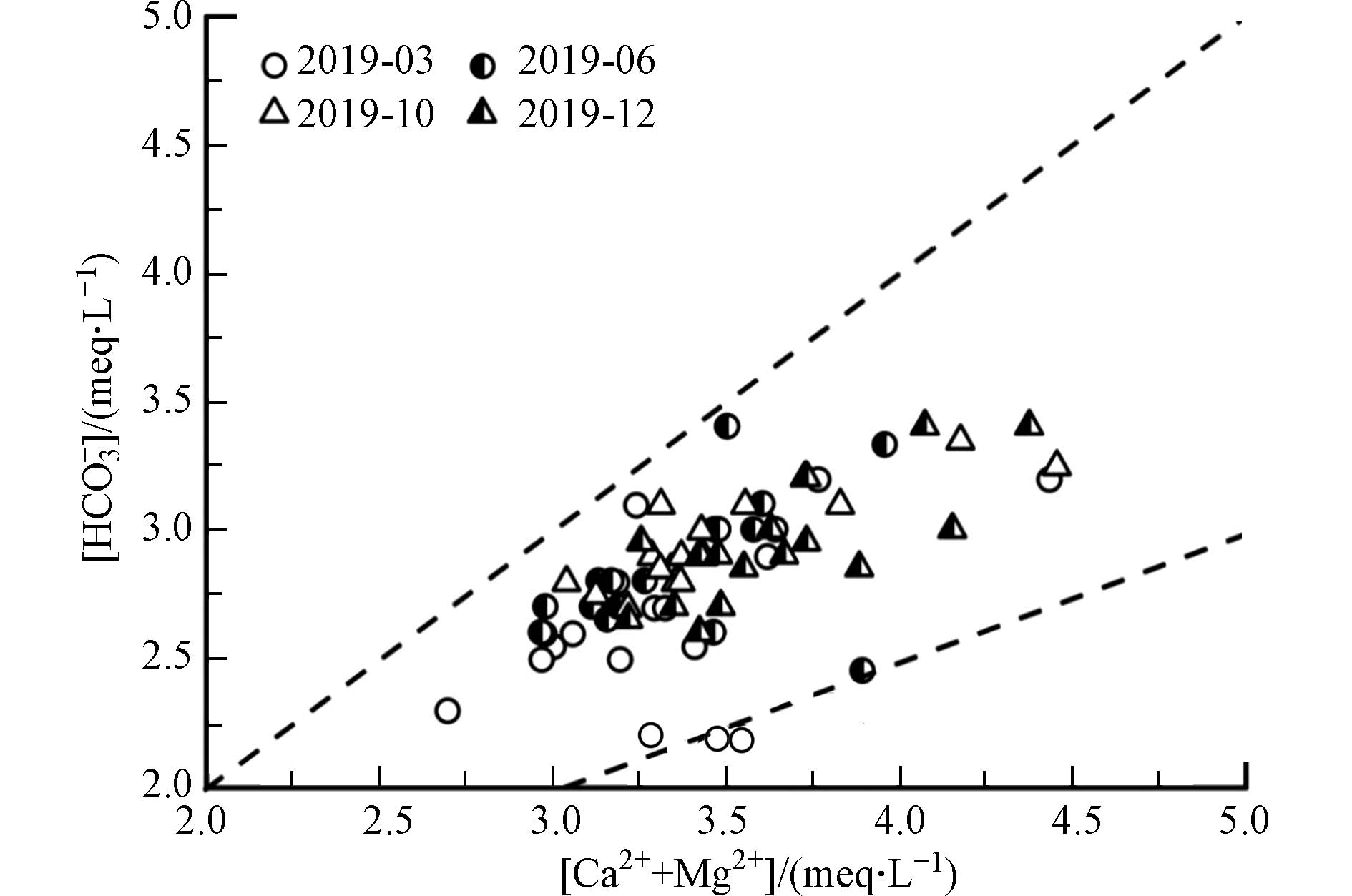
由图4可知,双河流域水体水化学组成主要受碳酸盐岩矿物溶解影响,当只有CO2与水结合生成H2CO3积极参与流域碳酸盐岩风化溶解时,[HCO3−]/[Ca2++Mg2+]比值为1:1,其溶解化学方程式为:
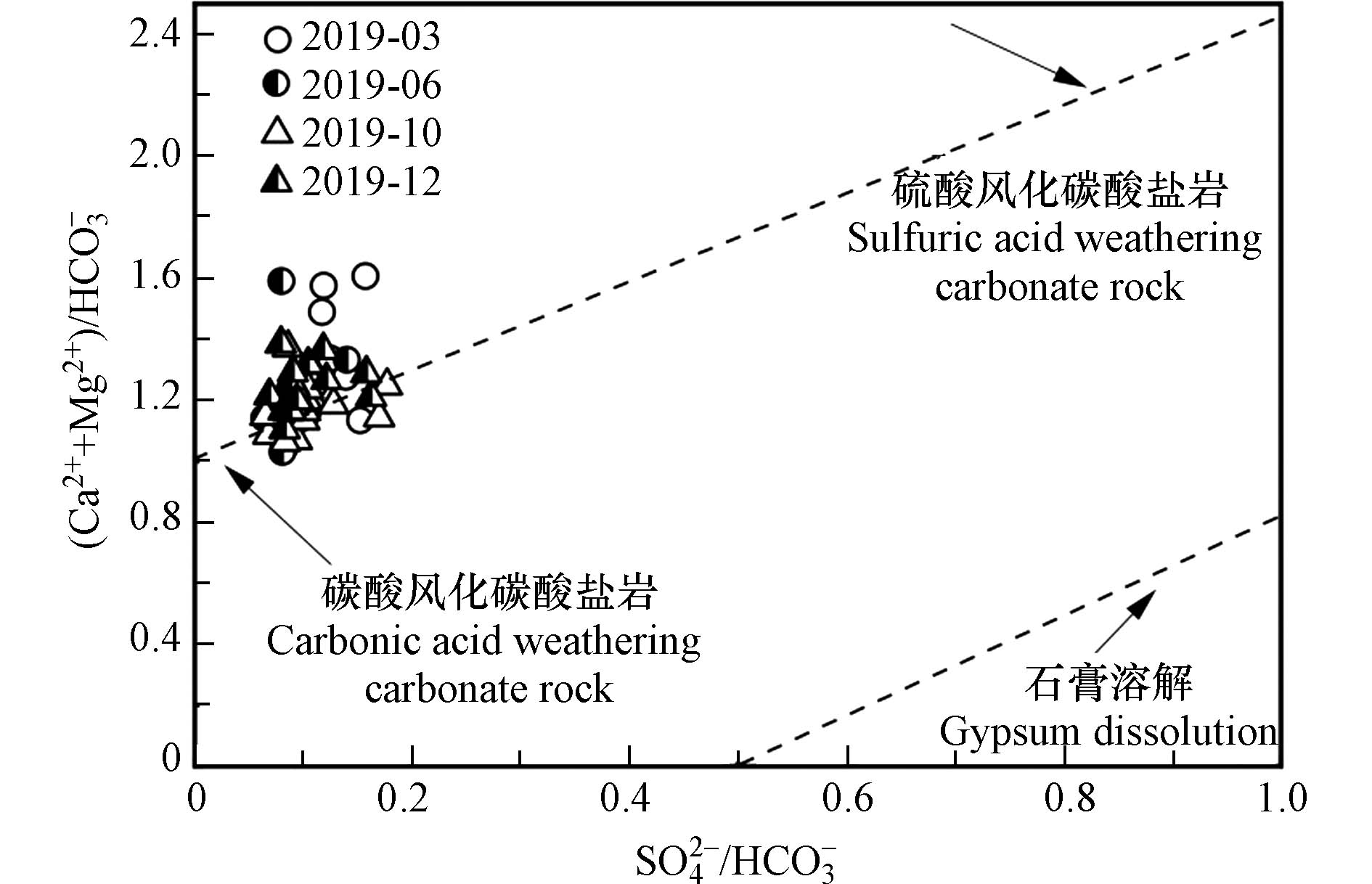
当[HCO3−]/[Ca2++Mg2+]的比值低于1:1时,表明仅由碳酸溶蚀碳酸盐岩不能平衡Ca2+和Mg2+的量,水体中过量的[Ca2++Mg2+]则需要更多的阴离子补充平衡[19],阴离子可能来源于硫酸和硝酸等外源酸参与碳酸盐岩风化溶解提供的SO42−和NO3−. 由图5可知,研究区实测水样中[HCO3−]/[Ca2++Mg2+]的比值明显低于1:1,且由图4可知,研究区主要受碳酸盐岩的影响,蒸发盐岩对流域离子组成贡献较小,表明硝酸和硫酸等外源酸积极参与了研究区碳酸盐岩的溶解,前人对双河小流域的研究中也证实有外源酸介入到碳酸盐岩的风化过程[23].
-
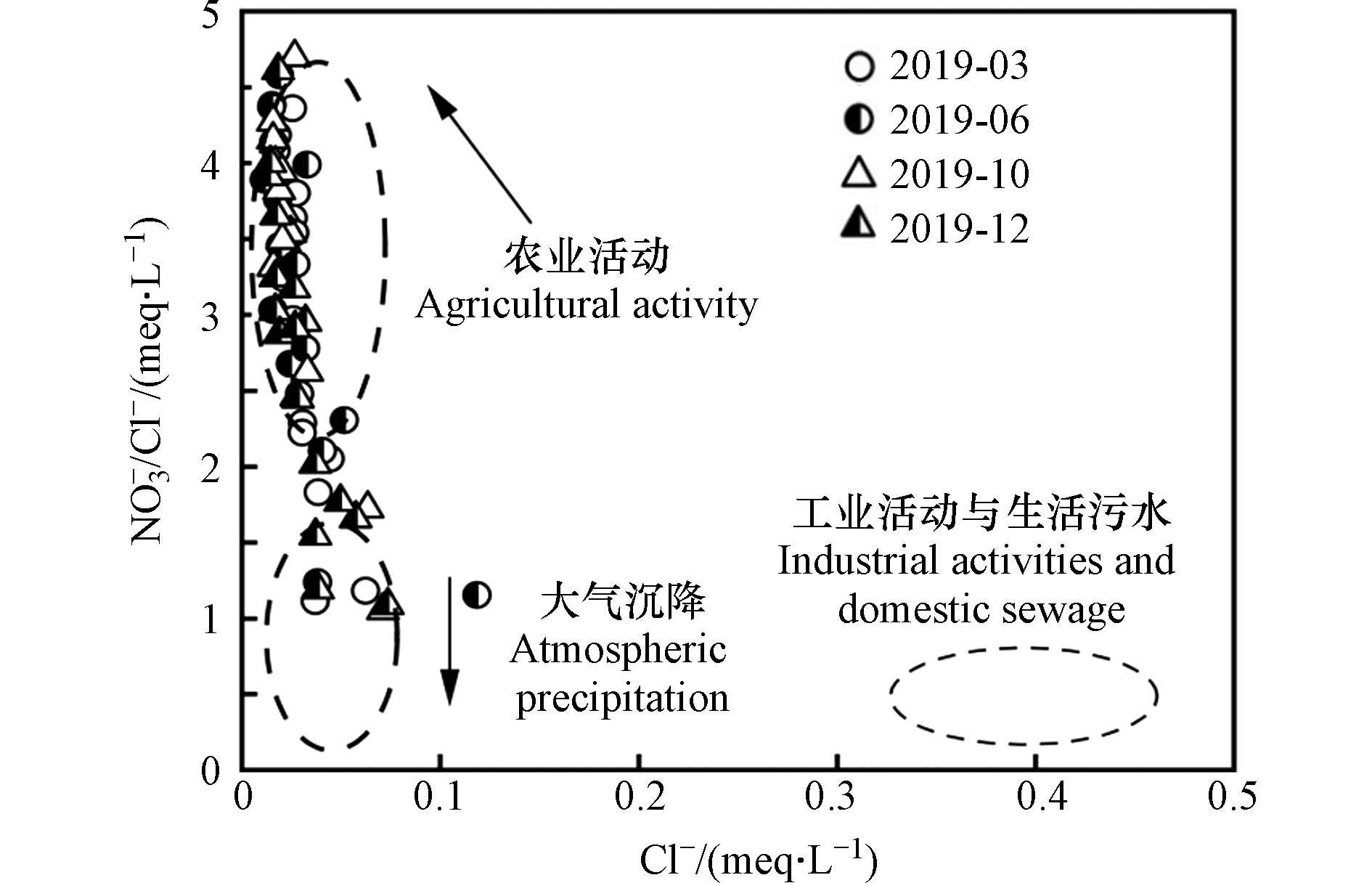
水中离子的相关性可指示水体在运移过程中的化学变化特征以及水中离子的来源[35]. 氯元素是自然界中化学性质相对稳定的元素,其生物和化学惰性极强[38],对物化生等进程敏感性极弱,因此利用NO3−/Cl−比值能有效判别硝酸盐的来源[39]. 流域中Cl−主要来源于岩石中氯化物的风化溶解、海水对地下水的补给、火山喷发物中的溶质、大气沉降、农用化肥、生活污水以及动物粪便等[40],结合图2、图3和图4的分析得知,研究区的岩石风化以碳酸盐岩为主,且矿物类型主要为白云石和方解石,所以流域中Cl−没有明显的岩性起源[41],因此,可推断出研究区Cl−主要来源于人类活动与大气沉降.
通常,岩石化学风化过程中没有NO3−离子的产生[42],流域中NO3−主要来源于土壤有机氮、大气沉降、化学氮肥以及粪肥和生活污水等[43]. 因不同来源的硝酸盐其NO3−/Cl−的比值存在差异,所以可利用NO3−/Cl−的比值来判别流域NO3−的来源[44]. 当NO3−/Cl−比值偏高,Cl−偏低,则表明流域NO3−来源于农业活动;当NO3−/Cl−比值偏低,Cl−偏高,则指示流域NO3−来源于粪肥和生活污水;当NO3−/Cl−比值与Cl−均偏低时,则说明流域NO3−主要来源于大气沉降[45]. 如图6所示,流域Cl−偏低,NO3−/Cl−比值偏高,说明双河流域NO3−主要来源于农业活动与大气沉降,与前人研究结果一致[25];NO3−来源于农业活动,主要因流域周边的土地利用类型以耕地为主,在耕种期人类活动加剧,对农作物施用的粪肥及含N化肥,经生物地球化学过程后贡献了大量的NO3−.
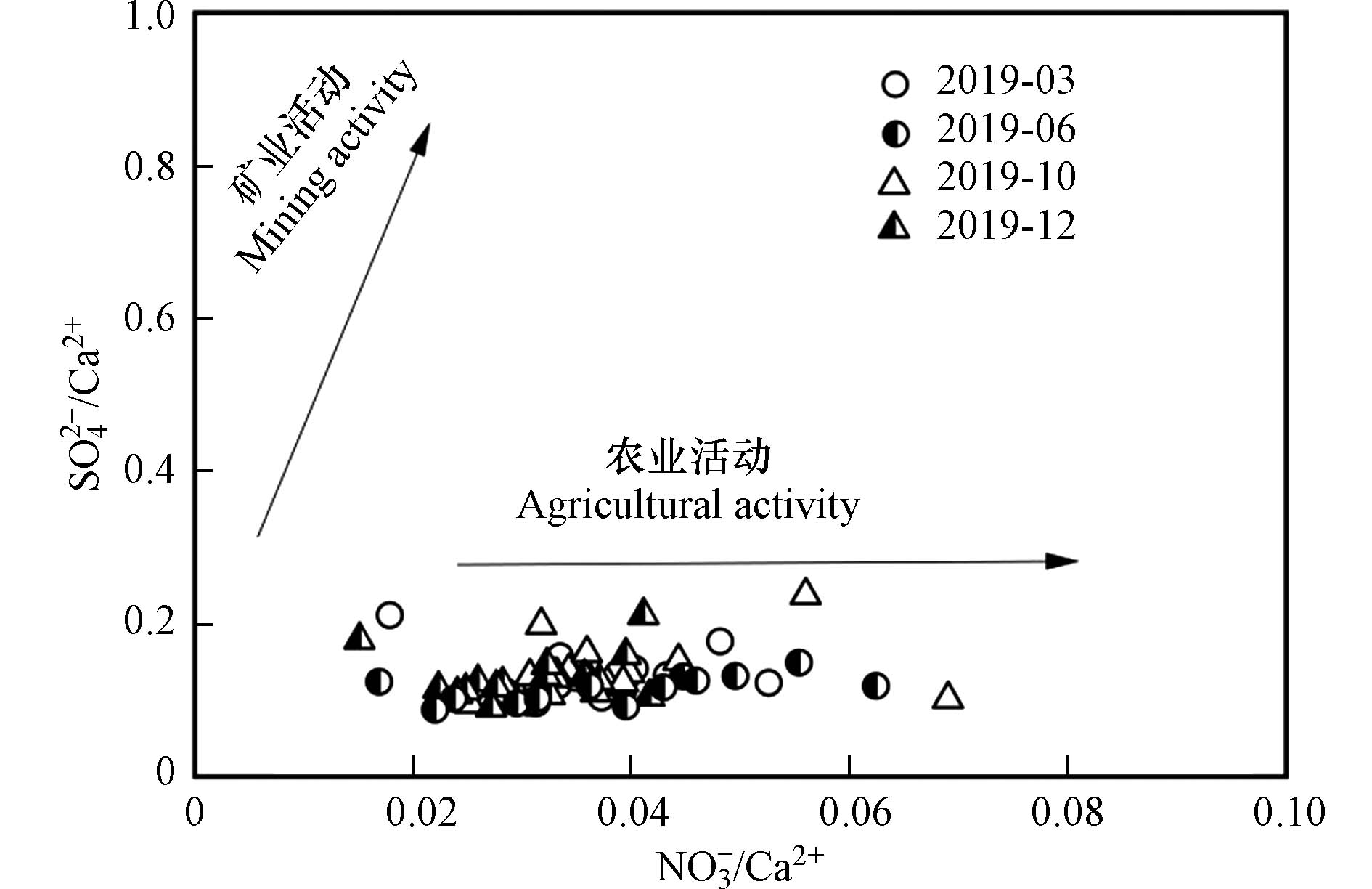
在岩溶地区流域硫酸盐主要来源于大气沉降、硫化物氧化、土壤中有机硫的矿化、石膏溶解以及人类活动的影响(如工、农业废水、农用化肥以及生产生活污水等)[46]. 由于研究区几乎不存在煤系地层以及远离城镇,因而初步判断SO42−可能主要来源于大气沉降、农业活动以及生活污水. 前人对研究区河水水化学环境的研究推断出SO42−主要以人类活动输入为主[25],为进一步明确SO42−的来源,利用NO3−/Ca2+与SO42−/Ca2+的比值关系,判别不同人类活动对地表水的影响[47]. 判别依据:当NO3−/Ca2+比值偏高,则表明主要受农业活动的影响,当SO42−/Ca2+比值偏高,则表明主要以矿业活动为主. 绘制NO3−/Ca2+与SO42−/Ca2+的比值关系图如图7所示,结果显示研究区所有采样点均位于图中靠近农业活动一侧,远离矿业活动,说明流域SO42−主要受农业活动的影响.
-
双河流域2019年春、夏、秋、冬四季SO42−的平均质量浓度分别为:(13.70 ±3.13)、(11.49 ±2.68)、(14.95±4.82)、(14.25±4.36) mg·L−1,NO3−的平均质量浓度分别为:(4.72±0.99)、(4.96±1.74)、(5.22±1.21)、(4.32±0.99) mg·L−1;HCO3−的季节变化如表2所示,结果显示流域HCO3−季节变化幅度相对较小. 季节变化幅度最大的水样点为SH14(61 mg·L−1),该点周围为居民点和农耕区,变化幅度大主要受人类活动影响(生活污水与农业活动). 流域HCO3−除来自碳酸参与碳酸盐岩风化溶蚀贡献外,硝酸和硫酸等外源酸对流域碳酸盐岩溶蚀产生的HCO3−不容忽视. 硝酸和硫酸对碳酸盐岩溶蚀的化学方程式为:
结合方程式(1)、(2)、(3)有:
根据方程式(4),假设分别有k1(mmol)的H2CO3、k2(mmol)的HNO3和k3(mmol)的H2SO4共同参与了碳酸盐岩的溶蚀,由此得到:
碳酸溶蚀流域碳酸盐岩产生的(Ca2++Mg2+)和HCO3−分别为:k1=[HCO3−]-[Ca2++Mg2+],HCO3−=2k[48]. 外源酸(硝酸和硫酸)溶蚀碳酸盐岩产生的(Ca2++Mg2+)占流域总(Ca2++Mg2+)比例为:1-(k1/[Ca2++Mg2+]总),产生的HCO3−占流域[HCO3−]总的比例为:1-(2k1/[HCO3−]总)[36]. 研究区外源酸参与流域碳酸盐岩风化对(Ca2++Mg2+)和HCO3−的贡献比例其计算结果如表3、表4所示. 2019年春、夏、秋、冬四季外源酸溶蚀产生的(Ca2++Mg2+)占[Ca2++Mg2+]总的平均占比分别为38.46%、30.55%、28.68%和38.41%;溶蚀产生的HCO3−占[HCO3−]总的平均比例分别为:25.49%、18.97%、17.16%和24.18%, 表明在进行碳汇估算时必须考虑外源酸对流域碳酸盐岩溶蚀作用的影响,且计算结果还显示,(Ca2++Mg2+)与HCO3−占[Ca2++Mg2+]总和[HCO3−]总的比例均表现为春季、冬季高,夏季、秋季低的变化趋势,这主要与人类活动和大气降雨有关;春耕时期,农业化肥与粪肥的施用使SO42−和NO3−浓度增加;冬季温度较夏季温度低,冬季燃煤向大气输入较多的硫化物,在运移过程中氧化,也会增加SO42−浓度;与大气降雨的关系,夏、秋两季降雨量较春、冬两季多,河流流量增加对流域河水进行稀释,降低了流域SO42−与NO3−的浓度,同时夏季气温高,土壤微生物活动强烈,引起土壤CO2浓度偏高,所以在大气降雨下渗过程中,会携带大量土壤和大气中的CO2进入河流,导致碳酸对基岩的溶蚀比例增加,硫酸溶蚀比例减少,从图8(Ca2++Mg2+)/HCO3−与SO42−/HCO3−的当量比值关系中也可以看出流域水样点位于碳酸溶蚀碳酸盐岩与硫酸溶蚀碳酸盐岩之间,但更靠近碳酸溶蚀一侧,说明夏、秋两季外源酸对[Ca2++Mg2+]和[HCO3−]的溶蚀比例减少主要受降雨稀释效应的影响.
-
(1)通过对双河流域内水化学组分进行分析,流域pH介于7.81—9.00之间,水体偏弱碱性;优势阳离子以Ca2+、Mg2+为主,分别占总阳离子的72.44%、24.38%;优势阴离子以HCO3−为主,占总阴离子的89.97%;流域水化学类型为HCO3-Ca·Mg型.
(2)根据Gibbs图判别出流域水化学环境受控于岩石风化,受蒸发浓缩与大气降雨影响较小;岩石风化类型以碳酸盐岩为主,流域内优势阴、阳离子(Ca2+、Mg2+、HCO3−)主要来源于碳酸盐岩溶解,蒸发盐岩与硅酸盐岩对主要离子成分贡献较小.
(3)流域内NO3−浓度在2.54—8.56 mg·L−1之间,最大值出现在饮水公司后(SH13),可能受该公司日常运作的影响,导致该点的NO3−浓度高于其他监测点,SO42−浓度在7.99—28.49 mg·L−1之间,最大值出现在阴河潭水(SH10)处,该点位于居民区与耕种区交汇处,受人类活动影响较大.
(4)通过离子比值法判别出 NO3−主要来源于农业活动和大气沉降,SO42−主要来源于农业活动;由质量平衡模型计算出外源酸(硝酸、硫酸)风化碳酸盐岩对(Ca2++Mg2+)的贡献,季节变化分别为38.46%、30.55%、28.68%、38.41%;对HCO3−的贡献,季节变化分别为25.49%、18.97%、17.16%和24.18%,贡献率均表现为春、冬两季较夏、秋两季高,这主要受人类活动和降雨稀释效应的影响.
外源酸对喀斯特流域碳汇效应的影响
Influence of exogenous acid on carbon sink effect in a karst watershed
-
摘要: 为探究喀斯特流域水化学特征变化以及外源酸对碳酸盐岩风化的贡献率,以双河流域为研究对象,分别于2019年3月、6月、10月和12月对流域进行4次监测,共选取17个水样点,通过简单数理分析、Piper图、Gibbs图、离子比值法、质量平衡模型等方法进行综合分析. 结果表明,研究区水体pH值介于7.81—9.00之间,流域整体呈弱碱性,阳离子以Ca2+为主,占总阳离子的72.44%,Mg2+次之,占总阳离子的24.38%,阴离子以HCO3-为主,占总阴离子的89.97%,流域水化学类型为HCO3-Ca·Mg型. 流域岩石风化类型以碳酸盐岩为主,Ca2+、Mg2+及HCO3-均来自碳酸盐岩的风化溶解,NO3-以农业活动与大气沉降为主要来源,而SO42-主要来源于农业活动. 外源酸(硝酸、硫酸)溶蚀流域碳酸盐岩产生的(Ca2++Mg2+)占[Ca2++Mg2+]总的季节变化分别为38.46%、30.55%、28.68%、38.41%;产生的HCO3-占[HCO3-]总的季节变化分别为25.49%、18.97%、17.16%、24.18%. 季节上,受人类活动与降雨稀释效应的影响,贡献比例均表现为春、冬两季高于夏、秋两季.Abstract: To explore the changes in hydrochemical characteristics of the Karst basin and the contribution rate of exogenous acid to the weathering of carbonate rocks, the Shuanghe River basin was taken as the research object, the basin was monitored four times in March, June, October, and December 2019, respectively, and a total of 17 water sampling points were selected. A comprehensive analysis was carried out by simple mathematical analysis, Piper diagram, Gibbs diagram, ion ratio method, and mass balance model. The results showed that the pH of water in the study area was between 7.81 and 9.00. The basin as a whole was weakly alkaline. The cations were mainly Ca2+, accounting for 72.44% of the total cations, followed by Mg2+, accounting for 24.38% of the total cations. The anion was mainly HCO3-, accounting for 89.97% of the total anions, and the hydrochemical type of the watershed was HCO3-Ca·Mg type. The rock weathering type in the watershed was dominated by carbonate rocks. Ca2+, Mg2+, and HCO3- were all derived from the weathering and dissolution of carbonate rocks. NO3- was mainly derived from agricultural activities and atmospheric deposition, while SO42- was mainly derived from agriculture activities. The total seasonal variation of (Ca2++Mg2+) produced by carbonate rocks in the basin dissolved by exogenous acid (nitric acid and sulfuric acid) was 38.46%, 30.55%, 28.68%, and 38.41%, respectively; The seasonal variation of HCO3- in the total [HCO3-] was 25.49%, 18.97%, 17.16%, and 24.18% respectively. Seasonally, due to the influence of human activities and the dilution effect of rainfall, the contribution ratio was higher in spring and winter than in summer and autumn.
-
Key words:
- karst basin /
- hydrochemical characteristics /
- ion source /
- exogenous acid /
- carbon sequestration.
-

-
表 1 研究区水化学基本参数变化
Table 1. Variation of basic parameters of hydrochemistry in the study area
时间
Time统计值
Statistic水温/
°CpH EC/
(μS·cm−1)TDS/
(mg·L−1)DO/
(mg·L−1)Ca2+/
(mg·L−1)K+/
(mg·L−1)Na+/
(mg·L−1)Mg2+/
(mg·L−1)SO42-/
(mg·L−1)Cl-/
(mg·L−1)HCO3-/
(mg·L−1)NO3-/
(mg·L−1)201903 最大值 17.30 9.00 356.00 286.68 10.90 48.00 1.49 2.13 19.99 23.37 2.18 195.20 6.84 最小值 10.70 8.29 209.00 202.73 8.75 36.00 0.61 0.55 10.74 8.93 0.60 134.20 2.54 平均值 13.64 8.58 266.00 238.31 9.72 42.47 0.91 0.91 13.79 13.70 1.06 160.75 4.72 标准差 1.69 0.17 31.65 23.60 0.52 3.54 0.26 0.41 2.70 3.13 0.46 19.85 0.99 变异系数/% 12.00 2.00 12.00 10.00 5.00 8.00 28.00 0.45 0.20 23.00 44.00 12.00 21.00 201906 最大值 20.80 8.68 345.00 295.60 9.34 56.00 2.80 2.91 20.66 17.46 4.14 207.40 8.48 最小值 14.87 8.21 248.00 222.13 8.03 39.00 0.69 0.17 11.90 7.99 0.39 149.45 2.65 平均值 16.62 8.41 278.53 252.14 8.86 43.79 1.10 0.85 14.38 11.49 1.07 174.50 4.96 标准差 1.55 0.13 29.65 21.72 0.40 4.26 0.55 0.64 3.01 2.68 0.88 15.87 1.74 变异系数/% 9.00 1.00 11.00 9.00 5.00 10.00 50.00 75.00 21.00 23.00 82.00 9.00 35.00 201910 最大值 20.07 8.69 356.00 315.71 10.10 60.00 2.04 1.96 20.20 28.49 2.49 204.35 8.56 最小值 14.20 7.81 245.00 238.28 7.85 40.00 0.78 0.31 12.50 9.10 0.55 164.70 3.62 平均值 16.70 8.31 288.35 265.34 8.73 45.18 1.08 0.81 14.91 14.95 0.96 182.28 5.22 标准差 1.68 0.25 29.86 23.67 0.59 5.80 0.32 0.48 2.46 4.82 0.57 15.12 1.21 变异系数/% 10.00 3.00 10.00 9.00 7.00 13.00 30.00 63.00 16.00 32.00 60.00 8.00 23.00 201912 最大值 14.10 9.00 339.00 316.11 11.20 60.00 1.68 2.60 22.58 25.77 2.58 207.40 5.88 201912 最小值 9.30 8.28 234.00 231.37 8.93 40.50 0.93 0.33 13.83 8.75 0.49 158.60 2.81 平均值 11.91 8.52 276.00 263.93 9.86 45.65 1.26 0.89 16.52 14.25 1.08 179.95 4.32 标准差 1.44 0.16 26.48 22.47 0.54 4.44 0.24 0.57 2.77 4.36 0.59 14.47 0.99 变异系数/% 12.00 2.00 10.00 9.00 5.00 10.00 19.00 64.00 17.00 31.00 54.00 8.00 23.00 表 2 双河流域[HCO3-]质量浓度的季节变化
Table 2. Seasonal variation of [HCO3-] mass concentration in Shuanghe River Basin
采样点
Sampling site采样时间
Sampling timeHCO3-/
(mg·L−1)采样时间
Sampling timeHCO3-/
(mg·L−1)采样时间
Sampling timeHCO3-/
(mg·L−1)采样时间
Sampling timeHCO3-/
(mg·L−1)季节变化/(mg·L−1)
Seasonal variationSH1 2019.03 134.20 2019.06 164.70 2019.10 173.85 2019.12 173.85 39.65 SH2 2019.03 134.20 2019.06 158.60 2019.10 167.75 2019.12 176.90 42.70 SH3 2019.03 155.55 2019.06 164.70 2019.10 164.70 2019.12 164.70 9.15 SH4 2019.03 158.60 2019.06 170.80 2019.10 189.10 2019.12 176.90 30.50 SH5 2019.03 158.60 2019.06 161.65 2019.10 176.90 2019.12 158.60 18.30 SH6 2019.03 152.50 2019.06 170.80 2019.10 170.80 2019.12 179.95 27.45 SH7 2019.03 164.70 2019.06 170.80 2019.10 170.80 2019.12 176.90 12.20 SH8 2019.03 164.70 2019.06 164.70 2019.10 176.90 2019.12 164.70 12.20 SH9 2019.03 155.55 2019.06 183.00 2019.10 183.00 2019.12 183.00 27.45 SH10 2019.03 195.20 2019.06 203.13 2019.10 204.35 2019.12 207.40 12.20 SH11 2019.03 140.30 2019.06 183.00 2019.10 170.80 2019.12 161.65 42.70 SH12 2019.03 189.10 2019.06 183.00 2019.10 189.10 2019.12 173.85 15.25 SH13 2019.03 195.20 2019.06 149.45 2019.10 189.10 2019.12 183.00 45.75 SH14 2019.03 134.20 2019.06 183.00 2019.10 173.85 2019.12 195.20 61.00 SH15 2019.03 170.80 2019.06 189.10 2019.10 198.25 2019.12 195.20 27.45 SH16 2019.03 176.90 2019.06 207.40 2019.10 222.65 2019.12 207.40 45.75 SH17 2019.03 152.50 2019.06 158.60 2019.10 176.90 2019.12 179.95 27.45 季节变化平均值 29.24 表 3 外源酸参与碳酸盐岩风化产生的(Ca2++Mg2+)占[Ca2++Mg2+]总的比例(%)
Table 3. The total proportion of (Ca2++Mg2+) in [Ca2++Mg2+] generated by exogenous acid in weathering of carbonate rock(%)
采样点
Sampling site201903 201906 201910 201912 SH1 65.73 18.80 29.54 39.63 SH2 73.08 24.77 24.08 30.74 SH3 30.09 26.60 32.21 38.91 SH4 25.15 21.29 12.96 33.34 SH5 29.86 32.22 23.60 48.19 SH6 31.47 23.38 33.20 18.81 SH7 35.98 28.64 33.90 42.02 SH8 37.53 30.83 28.05 45.14 SH9 50.37 27.03 25.14 34.77 SH10 23.76 31.89 39.72 44.72 SH11 29.32 27.58 15.90 35.39 SH12 8.67 32.47 38.26 53.39 SH13 30.04 74.26 25.77 55.65 SH14 75.52 35.39 27.92 28.41 SH15 24.15 28.15 54.23 28.67 SH16 39.71 6.04 11.82 33.21 SH17 43.42 49.99 31.30 42.02 表 4 外源酸参与碳酸盐岩风化产生的HCO3−占[HCO3−]总的比例(%)
Table 4. Total proportion of HCO3− in [HCO3−] generated by exogenous acid in weathering of carbonate rock(%)
采样点
Sampling site201903 201906 201910 201912 SH1 48.95 10.37 17.33 24.71 SH2 57.58 14.14 13.69 18.16 SH3 17.71 15.34 19.20 24.15 SH4 14.39 11.91 6.93 20.01 SH5 17.55 19.20 13.38 31.74 SH6 18.67 13.24 19.91 10.38 SH7 21.94 16.71 20.41 26.60 SH8 23.10 18.23 16.31 29.15 SH9 33.66 15.63 14.37 21.04 SH10 13.48 18.97 24.78 28.80 SH11 17.18 16.00 8.64 21.50 SH12 4.53 19.38 23.65 36.42 SH13 17.68 59.06 14.79 38.56 SH14 60.67 21.50 16.22 16.56 SH15 13.73 16.38 37.21 16.74 SH16 24.77 3.11 6.28 19.91 SH17 27.73 33.33 18.55 26.60 -
[1] 姚冠荣, 高全洲. 河流碳输移与陆地侵蚀-沉积过程关系的研究进展 [J]. 水科学进展, 2007, 18(1): 133-139. doi: 10.3321/j.issn:1001-6791.2007.01.022 YAO G R, GAO Q Z. Correlation between riverine carbon transport and terrestrial erosion-deposition processes [J]. Advances in Water Science, 2007, 18(1): 133-139(in Chinese). doi: 10.3321/j.issn:1001-6791.2007.01.022
[2] 刘朋雨, 张连凯, 黄奇波, 等. 外源水和外源酸对万华岩地下河系统岩溶碳汇效应的影响 [J]. 中国岩溶, 2020, 39(1): 17-23. LIU P Y, ZHANG L K, HUANG Q B, et al. Effect of exogenous water and acid on Karst carbon sink in the Wanhuayan underground river system [J]. Carsologica Sinica, 2020, 39(1): 17-23(in Chinese).
[3] 刘再华, Wolfgang Dreybrodt, 王海静. 一种由全球水循环产生的可能重要的CO2汇 [J]. 科学通报, 2007, 52(20): 2418-2422. doi: 10.3321/j.issn:0023-074x.2007.20.013 LIU Z H, WOLFGANG D, WANG H J. A potentially important CO2 sink generated by the global water cycle [J]. Chinese Science Bulletin, 2007, 52(20): 2418-2422(in Chinese). doi: 10.3321/j.issn:0023-074x.2007.20.013
[4] SCHIMEL D S, HOUSE J I, HIBBARD K A, et al. Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems [J]. Nature, 2001, 414(6860): 169-172. doi: 10.1038/35102500 [5] SPENCE J, TELMER K. The role of sulfur in chemical weathering and atmospheric CO2 fluxes: Evidence from major ions, δ13CDIC, and δ34SSO4 in rivers of the Canadian Cordillera [J]. Geochimica et Cosmochimica Acta, 2005, 69(23): 5441-5458. doi: 10.1016/j.gca.2005.07.011 [6] 袁道先. 地球系统的碳循环和资源环境效应 [J]. 第四纪研究, 2001, 21(3): 223-232. doi: 10.3321/j.issn:1001-7410.2001.03.004 YUAN D X. Carbon cycle in earth system and its effects on environment and resources [J]. Quaternary Sciences, 2001, 21(3): 223-232(in Chinese). doi: 10.3321/j.issn:1001-7410.2001.03.004
[7] 李军, 刘丛强, 李龙波, 等. 硫酸侵蚀碳酸盐岩对长江河水DIC循环的影响 [J]. 地球化学, 2010, 39(4): 305-313. doi: 10.19700/j.0379-1726.2010.04.002 LI J, LIU C Q, LI L B, et al. The impacts of chemical weathering of carbonate rock by sulfuric acid on the cycling of dissolved inorganic carbon in Changjiang River water [J]. Geochimica, 2010, 39(4): 305-313(in Chinese). doi: 10.19700/j.0379-1726.2010.04.002
[8] 汪炎林, 周忠发, 田衷珲, 等. 池武溪流域岩溶水SO42-的空间变化特征及其来源分析 [J]. 环境化学, 2017, 36(12): 2690-2700. doi: 10.7524/j.issn.0254-6108.2017030105 WANG Y L, ZHOU Z F, TIAN Z H, et al. Analysis of the spatial variation and sources of SO42- in Karst water of Chiwu River [J]. Environmental Chemistry, 2017, 36(12): 2690-2700(in Chinese). doi: 10.7524/j.issn.0254-6108.2017030105
[9] LUDWIG W, PROBST J L, KEMPE S. Predicting the oceanic input of organic carbon by continental erosion [J]. Global Biogeochemical Cycles, 1996, 10(1): 23-41. doi: 10.1029/95GB02925 [10] PU J B, YUAN D X, ZHANG C, et al. Hydrogeochemistry and possible sulfate sources in Karst groundwater in Chongqing, China [J]. Environmental Earth Sciences, 2013, 68(1): 159-168. doi: 10.1007/s12665-012-1726-8 [11] YIN C, YANG H Q, WANG J F, et al. Combined use of stable nitrogen and oxygen isotopes to constrain the nitrate sources in a karst lake [J]. Agriculture, Ecosystems & Environment, 2020, 303: 107089. [12] WANG S Q, ZHENG W B, CURRELL M, et al. Relationship between land-use and sources and fate of nitrate in groundwater in a typical recharge area of the North China Plain [J]. Science of the Total Environment, 2017, 609: 607-620. doi: 10.1016/j.scitotenv.2017.07.176 [13] LIU Z H. Review on the role of terrestrial aquatic photosynthesis in the global carbon cycle [J]. Procedia Earth and Planetary Science, 2013, 7: 513-516. doi: 10.1016/j.proeps.2013.03.003 [14] 曹建华, 王福星, 黄俊发, 等. 桂林地区石灰岩表面生物岩溶溶蚀作用研究 [J]. 中国岩溶, 1993, 12(1): 11-22. CAO J H, WANG F X, HUANG J F, et al. The erosion action of biokarst on limestone in Guilin area [J]. Carsologica Sinica, 1993, 12(1): 11-22(in Chinese).
[15] 陈波, 陈文瑾, 陆苹茹, 等. 基于CCM机制的水生碳泵效应协同富营养化缓解研究进展[J]. 贵州师范大学学报(自然科学版), 2022, 40(2): 19-26. CHEN B, CHEN W J, LU P R, et al. Research progress of aquatic carbon pump effect synergistic eutrophication mitigation based on CCM mechanism[J]. Journal of Guizhou Normal University(Natural Science Edition), 2022, 40(2): 19-26(in Chinese).
[16] 张远瞩. 外源酸(硫酸、硝酸)对岩溶碳循环的影响: 以重庆南山老龙洞地下河流域为例[D]. 重庆: 西南大学, 2017. ZHANG Y Z. Effects of allogenic acids(sulfuric acid and nitric acid)on karst carbon cycle—A study from Laolongdong subterranean catchment, Chongqing[D]. Chongqing: Southwest University, 2017(in Chinese).
[17] 任梦梦. 漓江流域外源酸(硝酸、硫酸)对岩溶碳汇的影响研究[D]. 北京: 中国地质大学(北京), 2020. REN M M. Effects of exogenous acids (nitric acid, furic acid) on karst carbon sink in Lijiang River Basin [D]. Beijing: China University of Geosciences (Beijing), 2020(in Chinese).
[18] 孙平安, 于奭, 莫付珍, 等. 不同地质背景下河流水化学特征及影响因素研究: 以广西大溶江、灵渠流域为例 [J]. 环境科学, 2016, 37(1): 123-131. SUN P A, YU S, MO F Z, et al. Hydrochemical characteristics and influencing factors in different geological background: A case study in darongjiang and Lingqu basin, Guangxi, China [J]. Environmental Science, 2016, 37(1): 123-131(in Chinese).
[19] 孙平安. 漓江流域无机碳和有机碳来源及岩溶碳循环过程[D]. 武汉: 中国地质大学, 2021. SUN P A. Sources of inorganic and organic carbon and karst carbon cycle in Lijiang River Basin[D]. Wuhan: China University of Geosciences, 2021(in Chinese).
[20] 谢银财, 朱同彬, 杨慧, 等. 硫酸对典型岩溶流域碳酸盐岩溶蚀及碳循环意义: 以广西平果岩溶流域为例 [J]. 第四纪研究, 2017, 37(6): 1271-1282. doi: 10.11928/j.issn.1001-7410.2017.06.11 XIE Y C, ZHU T B, YANG H, et al. Chemical weathering of carbonate rocks by sulfuric acid in typical karst catchment and its implication for carbon cycle: A case study in karst catchment in Pingguo, Guangxi Province, southwest China [J]. Quaternary Sciences, 2017, 37(6): 1271-1282(in Chinese). doi: 10.11928/j.issn.1001-7410.2017.06.11
[21] 原雅琼, 孙平安, 苏钊, 等. 岩溶流域洪水过程水化学动态变化及影响因素 [J]. 环境科学, 2019, 40(11): 4889-4899. doi: 10.13227/j.hjkx.201905022 YUAN Y Q, SUN P A, SU Z, et al. Dynamic changes in hydrochemical characteristics and influencing factors in the karst watershed flood process [J]. Environmental Science, 2019, 40(11): 4889-4899(in Chinese). doi: 10.13227/j.hjkx.201905022
[22] 周忠发, 张结, 潘艳喜, 等. 双河洞洞穴系统岩溶地表水-地下水主要离子化学特征及其来源分析 [J]. 科学技术与工程, 2018, 18(6): 5-13. doi: 10.3969/j.issn.1671-1815.2018.06.002 ZHOU Z F, ZHANG J, PAN Y X, et al. Chemical characteristics and source analysis of main ions in karst surface water and groundwater in Shuanghe cave system [J]. Science Technology and Engineering, 2018, 18(6): 5-13(in Chinese). doi: 10.3969/j.issn.1671-1815.2018.06.002
[23] 张结, 周忠发, 曹明达, 等. 双河洞小流域主要离子化学特征及其来源分析 [J]. 水土保持学报, 2017, 31(2): 327-332,338. doi: 10.13870/j.cnki.stbcxb.2017.02.053 ZHANG J, ZHOU Z F, CAO M D, et al. Hydrogeochemical characteristics and analysis of their sources in Karst small watershed of Shuanghe cave system [J]. Journal of Soil and Water Conservation, 2017, 31(2): 327-332,338(in Chinese). doi: 10.13870/j.cnki.stbcxb.2017.02.053
[24] 黄静, 周忠发, 丁圣君, 等. 双河洞流域氢氧同位素特征及其指示意义[J]. 地球与环境, 2022, 50(4): 516-525. HUANG J, ZHOU Z F, DING S J, et al. Characteristics of hydrogen and oxygen isotopes in Shuanghedong Basin and their implications [J]. Earth and Environment: 2022, 50(4): 516-525.
[25] 丁圣君, 周忠发, 石亮星, 等. 池武溪流域溶解无机碳特征及影响因素分析 [J]. 环境科学与技术, 2021, 44(6): 27-34. doi: 10.19672/j.cnki.1003-6504.2021.06.004 DING S J, ZHOU Z F, SHI L X, et al. Analysis of dissolved inorganic carbon characteristics and influencing factors in Chiwu River [J]. Environmental Science & Technology, 2021, 44(6): 27-34(in Chinese). doi: 10.19672/j.cnki.1003-6504.2021.06.004
[26] 张绍云, 周忠发, 田衷珲. 膏盐层下石膏晶洞水化学特征的环境指示意义 [J]. 科学技术与工程, 2017, 17(16): 13-20. doi: 10.3969/j.issn.1671-1815.2017.16.003 ZHANG S Y, ZHOU Z F, TIAN Z H. Environmental implications of hydro-chemical characteristics of gypsum holes in salt-gypsum bed [J]. Science Technology and Engineering, 2017, 17(16): 13-20(in Chinese). doi: 10.3969/j.issn.1671-1815.2017.16.003
[27] 周忠发, 张结, 殷超, 等. 岩溶洞穴地下水水化学特征及其地球化学敏感性比较: 以贵州双河洞和织金洞为例 [J]. 水利水电技术, 2017, 48(6): 98-105. ZHOU Z F, ZHANG J, YIN C, et al. Study on the water chemical characteristics and geochemical sensitivity of underground water in different lithologic development-a case study of Shuanghe cave and Zhijin cave system in Guizhou Province [J]. Water Resources and Hydropower Engineering, 2017, 48(6): 98-105(in Chinese).
[28] 李坡, 贺卫, 钱治. 双河洞地质公园研究[M]. 贵阳: 贵州人民出版社, 2008. LI P, HE W, QIAN Z. Study on Shuanghe cave geopark[M]. Guiyang: Guizhou People's Publishing House, 2008(in Chinese).
[29] 张结, 周忠发, 汪炎林, 等. 短时间高强度旅游活动下洞穴CO2的变化特征及对滴水水文地球化学的响应 [J]. 地理学报, 2018, 73(9): 1687-1701. doi: 10.11821/dlxb201809006 ZHANG J, ZHOU Z F, WANG Y L, et al. Variation of CO2 and its response to the drip hydrogeochemistry in caves under the short-time high-strength tourism activities [J]. Acta Geographica Sinica, 2018, 73(9): 1687-1701(in Chinese). doi: 10.11821/dlxb201809006
[30] 汤云涛, 周忠发, 薛冰清, 等. 岩溶洞穴水δ13CDIC时空变化及影响因素分析: 以贵州双河洞系麻黄支洞为例 [J]. 环境化学, 2020, 39(11): 3223-3234. doi: 10.7524/j.issn.0254-6108.2019081404 TANG Y T, ZHOU Z F, XUE B Q, et al. Analysis of variation characteristics and influencing factors of δ13CDIC in cave water of Mahuang Cave, Suiyang, Guizhou [J]. Environmental Chemistry, 2020, 39(11): 3223-3234(in Chinese). doi: 10.7524/j.issn.0254-6108.2019081404
[31] 安丹, 周忠发, 范宝祥, 等. 贵州大风洞洞穴空气CO2浓度及滴水水化学与洞穴通风的响应 [J]. 水土保持研究, 2020, 27(6): 338-345,352. AN D, ZHOU Z F, FAN B X, et al. Air CO2 concentration and response of dripping water chemistry to cave ventilation in Dafeng cave of Guizhou Province [J]. Research of Soil and Water Conservation, 2020, 27(6): 338-345,352(in Chinese).
[32] MEYBECK M, HELMER R. The quality of rivers: From pristine stage to global pollution [J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 1989, 75(4): 283-309. doi: 10.1016/0031-0182(89)90191-0 [33] 王琪, 于奭, 蒋萍萍, 等. 长江流域主要干/支流水化学特征及外源酸的影响 [J]. 环境科学, 2021, 42(10): 4687-4697. doi: 10.13227/j.hjkx.202012040 WANG Q, YU S, JIANG P P, et al. Water chemical characteristics and influence of exogenous acids in the Yangtze River Basin [J]. Environmental Science, 2021, 42(10): 4687-4697(in Chinese). doi: 10.13227/j.hjkx.202012040
[34] JIANG L G, YAO Z J, LIU Z F, et al. Hydrochemistry and its controlling factors of rivers in the source region of the Yangtze River on the Tibetan Plateau [J]. Journal of Geochemical Exploration, 2015, 155: 76-83. doi: 10.1016/j.gexplo.2015.04.009 [35] GAILLARDET J, DUPRÉ B, LOUVAT P, et al. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers [J]. Chemical Geology, 1999, 159(1-4): 3-30. doi: 10.1016/S0009-2541(99)00031-5 [36] 曹星星, 吴攀, 杨诗笛, 等. 贵州威宁草海流域地下水水化学特征及无机碳通量估算 [J]. 环境科学, 2021, 42(4): 1761-1771. CAO X X, WU P, YANG S D, et al. Hydrochemistry characteristics and estimation of the dissolved inorganic carbon flux in the Caohai Lake wetland catchment of Guizhou Province [J]. Environmental Science, 2021, 42(4): 1761-1771(in Chinese).
[37] HAN G L, LIU C Q. Water geochemistry controlled by carbonate dissolution: A study of the river waters draining karst-dominated terrain, Guizhou Province, China [J]. Chemical Geology, 2004, 204(1-2): 1-21. doi: 10.1016/j.chemgeo.2003.09.009 [38] XING M, LIU W G, WANG Z F, et al. Relationship of nitrate isotopic character to population density in the Loess Plateau of Northwest China [J]. Applied Geochemistry, 2013, 35: 110-119. doi: 10.1016/j.apgeochem.2013.04.002 [39] WEI Y N, FAN W, WANG W, et al. Identification of nitrate pollution sources of groundwater and analysis of potential pollution paths in loess regions: A case study in Tongchuan region, China [J]. Environmental Earth Sciences, 2017, 76(12): 1-13. [40] MENGIS M, SCHIF S L, HARRIS M, et al. Multiple geochemical and isotopic approaches for assessing ground water NO3- elimination in a riparian zone [J]. Groundwater, 1999, 37(3): 448-457. doi: 10.1111/j.1745-6584.1999.tb01124.x [41] JIANG Y J, YAN J. Effects of land use on hydrochemistry and contamination of karst groundwater from Nandong underground river system, China [J]. Water, Air, & Soil Pollution, 2010, 210(1-4): 123-141. [42] JIANG Y J, WU Y X, GROVES C, et al. Natural and anthropogenic factors affecting the groundwater quality in the Nandong Karst underground river system in Yunan, China [J]. Journal of Contaminant Hydrology, 2009, 109(1-4): 49-61. doi: 10.1016/j.jconhyd.2009.08.001 [43] CUI R Y, FU B, MAO K M, et al. Identification of the sources and fate of NO3--N in shallow groundwater around a plateau lake in southwest China using NO3- isotopes (δ15N and δ18O) and a Bayesian model [J]. Journal of Environmental Management, 2020, 270: 110897. doi: 10.1016/j.jenvman.2020.110897 [44] LIU C Q, LI S L, LANG Y C, et al. Using 15N- and 18O-values to identify nitrate sources in karst ground water, Guiyang, southwest China [J]. Environmental Science & Technology, 2006, 40(22): 6928-6933. [45] CHEN Z X, YU L, LIU W G, et al. Nitrogen and oxygen isotopic compositions of water-soluble nitrate in Taihu Lake water system, China: implication for nitrate sources and biogeochemical process [J]. Environmental Earth Sciences, 2014, 71(1): 217-223. doi: 10.1007/s12665-013-2425-9 [46] 李小倩, 刘运德, 周爱国, 等. 长江干流丰水期河水硫酸盐同位素组成特征及其来源解析 [J]. 地球科学, 2014, 39(11): 1647-1654, 1692. LI X Q, LIU Y D, ZHOU A G, et al. Sulfur and oxygen isotope compositions of dissolved sulfate in the Yangtze River during high water period and its sulfate source tracing [J]. Earth Science, 2014, 39(11): 1647-1654, 1692(in Chinese).
[47] 杨芬, 高柏, 葛勤, 等. 信江流域地下水水化学特征及形成机制 [J]. 科学技术与工程, 2021, 21(9): 3505-3512. doi: 10.3969/j.issn.1671-1815.2021.09.010 YANG F, GAO B, GE Q, et al. Hydro-chemical characteristics and formation mechanism of groundwater in Xinjiang River Basin [J]. Science Technology and Engineering, 2021, 21(9): 3505-3512(in Chinese). doi: 10.3969/j.issn.1671-1815.2021.09.010
[48] JIANG Y J. The contribution of human activities to dissolved inorganic carbon fluxes in a Karst underground river system: Evidence from major elements and δ13CDIC in Nandong, Southwest China [J]. Journal of Contaminant Hydrology, 2013, 152: 1-11. doi: 10.1016/j.jconhyd.2013.05.010 -



 下载:
下载: