-
近年来,随着经济建设的飞速发展,各种工业活动及城市生活所产生的固体废物排放量也逐年增加,其中很多固体废物都含有重金属,如有色金属生产工序中所排放的冶炼渣、尾矿,污水处理厂排放的污泥,受重金属污染的土壤,生活垃圾焚烧厂的垃圾焚烧飞灰等[1-3]. 这些固体废物堆存于环境中不仅占用了大量的土地空间,且其中所含的重金属(如Pb、Zn、Cd、Cu、Cr、Mn及类金属As等)会随着地表径流冲刷、雨水淋溶、风化等作用而浸出从而迁移到环境中,众所周知,重金属难于被生物降解,且在环境中迁移性强,易通过食物链富集,最终危害到生态环境及人体的健康[4-7]. 例如在加纳奥布阿西尾矿附近土壤种植的蔬菜中,重金属As、Pb和Hg超出了允许的限值,饮用水中As、Cd、Cr、Hg、Fe和Mn的含量均高于饮用水标准[8]. 因此,防治含重金属固废所带来的重金属污染已成为目前环境治理领域的研究热点.
固体废物的治理应遵循减量化、资源化、无害化的“三化”原则,但含重金属固体废物的环境风险严重影响了其治理. 固化/稳定化处理技术是通过一定的物理、化学或生物手段降低废物中有害元素的浸出性或生物有效性,使其达到安全稳定的一项废物处理技术[9],该技术已被广泛研究并应用于尾矿、冶炼废渣、受重金属污染土壤、垃圾焚烧灰及污泥等固体废物的处理,具有良好的处理效果. 近年来,重金属固化/稳定化技术的研究取得了很多新的成果,但未见有相关文献针对该技术进行系统性的总结. 因此,本文结合近年来相关学者的研究,综述了目前国内外最常用的几种固体废物重金属固化/稳定化技术的最新研究进展,包括水泥基固化/稳定化技术、地质聚合物基固化/稳定化技术、化学药剂稳定化技术以及微生物诱导矿物沉淀稳定化技术等,总结了各项技术的优点及存在的不足,并针对未来重金属固化/稳定化处理技术的发展提出了展望.
-
固化/稳定化技术的概念是运用物理、化学或生物的方法将固体废物中的重金属等有害物质固定起来,或者将其转化成化学性质不活泼的形态,阻止其在环境中迁移、扩散,从而降低污染物质的环境风险. 在美国环保署(EPA)[10]的定义中,固化和稳定化是两个不同的概念,固化技术指将污染物囊封入惰性基材中,或在污染物外部加入低渗透性材料,通过减少污染物暴露的淋滤面积达到限制污染物迁移的目的,而稳定化则是从污染物的生物有效性出发,通过形态转化,将污染物转化为不易溶解、迁移性或毒性更小的形式来实现无害化处理.
固化/稳定化技术起源于20 世纪50年代对放射性废物的固化处置,美国和欧洲一些国家在处理放射性废物时基本上是先用水泥等惰性材料对废物进行包封,然后再进行填埋处置[11]. 目前,该技术被广泛用于各类含重金属固体废物的处理,并取得了极大的发展与进步.
-
重金属的固化/稳定化技术,顾名思义,就是以降低废物中重金属的迁移性或是生物有效性为目标. 为了评估固体废物中重金属的浸出性,我国颁布了《固体废物浸出毒性浸出方法 水平振荡法》(HJ557—2009)[12]、《固体废物浸出毒性浸出方法 硫酸硝酸法》(HJ/T299—2007)以及《固体废物浸出毒性浸出方法 醋酸缓冲溶液法》(HJ/T300—2007)[13]三项标准,在国际上,EPA推荐的毒性特征浸出程序(TCLP)法是目前使用最为广泛的重金属毒性浸出方法[14],此外,还有欧洲国家颁布的En-12457系列浸出试验标准[15],这些方法均被用于检测固体废物中重金属元素的溶出性及迁移性.
重金属固化/稳定化效果的最重要的评价指标便是重金属的稳定化率,通过上述方法,测定固化/稳定化前后固体废物中重金属的浸出毒性,便可计算重金属稳定化率,计算公式如下:
式中,X为固体废物中重金属的稳定化率(%);C1为未经固化/稳定化样品的重金属浸出毒性(mg·L−1),C2为经固化/稳定化处理后重金属的浸出毒性(mg·L−1).
除了重金属稳定化率外,固体废物中重金属的赋存形态对其迁移性及生物有效性有着重要影响,因此分析固化/稳定化前后固体废物中重金属的形态分布变化也是评估固化/稳定化效果的重要途径. 1979年Tessier提出了颗粒中金属赋存形态的五步提取法,用于分析固体颗粒内金属元素的形态分布,该方法将重金属赋存形态划分为可交换态、碳酸盐结合态、铁(锰)氧化物结合态、有机质及硫化物结合态及残渣态5种[16],不同赋存形态重金属的迁移释放规律总结于表1. 1987年,欧共体标准局在Tessier五步法的基础上提出了BCR三步提取法[17],该方法较Tessier法流程更短,因此使用更为广泛,但两种提取方法均存在一定缺陷,在使用各种浸取剂提取重金属的过程中,重金属的赋存形态也在动态的变化,使得结果不够准确. 近年来,得益于光学器件及检测技术的发展,同步辐射-X射线荧光分析技术(SRXRF)能够更准确地分析重金属在颗粒内的分布情况及赋存形态[18].
分析出固体废物重金属的赋存形态分布情况后,便能够通过重金属形态的风险评价指数(RAC)来评估重金属的环境风险[19],RAC计算方法如下:
其中,M可交换态、M碳酸盐结合态分别为固体废物中某一重金属元素的可交换态及碳酸盐结合态含量占比.
计算出重金属的RAC值后,根据RAC值确定重金属的风险等级,RAC≤1%(无风险)、1%<RAC≤10% (低风险)、10%<RAC≤30%(中风险)、30%<RAC≤50%(高风险)、RAC>50%(极高风险). 通过分析固化/稳定化处理前后固体废物中重金属的RAC值变化,便能够评估重金属的固化/稳定化效果.
-
水泥是一种无机胶结材料,经水化反应可生成坚硬的固化体. 利用水泥处理含重金属的固体废物已有超过60年的历史,操作简单、处理成本低及适用性广的优点使得水泥成为固化工艺的良好黏合剂,针对水泥固化技术的研究也已较为广泛和成熟[20]. 最常用作固化剂的水泥材料是硅酸盐水泥,主要是因为硅酸盐水泥的成分较一致并且其凝结硬化过程被人们所熟知[21]. 但据之前的研究,传统硅酸盐水泥材料固化技术存在一些缺陷,首先,硅酸盐水泥材料由于其生产过程较高的碳排放而对环境的长远影响不利[22],据统计,每生产1 t硅酸盐水泥将产生660—820 kg的CO2,硅酸盐水泥行业的碳足迹约占全球CO2排放量的7%[23]. 其次,在固化过程中,固体废物不仅仅作为骨料,而是会与水泥成分发生反应,因此废物中的成分可能会对水泥的水化过程产生影响,比如废物中的硫酸盐会与硅酸盐水泥所含的氢氧化钙反应生成硫酸钙或进一步与水化铝酸钙反应生成钙矾石,从而使固化体积大大增加,造成固化体破裂,有害物质再度浸出[24-25],废物中的一些重金属对硅酸盐水泥的水化反应的阻滞作用也较为明显,这将影响最终固化产物的力学性能及重金属固定性能[26]. 此外,传统水泥材料的抗酸性普遍较低,因此在pH较低的环境下,水泥固定重金属的性能较差[27].
为了克服上述问题,近年来,有关水泥基固化技术的研究有了许多新的成果,具体研究进展总结于表2,其中废物掺量、力学性能及重金属稳定化率均取自最佳条件下的结果,近年来的研究主要表现在新型低碳足迹水泥固化材料的研究与应用以及对重金属稳定化机理的探究. 可以发现,一些新型水泥作为固体废物的固化材料,不仅取得了良好的重金属稳定化效果,且与硅酸盐水泥相比碳足迹更低,对重金属耐受性更强,能作为硅酸盐水泥的良好低碳替代品. 如石灰石-煅烧黏土水泥(LC3)的碳足迹相比于普通硅酸盐水泥降低了约45%[28],由高炉矿渣和普通硅酸盐水泥与富磷酸盐/硫酸盐副产物结合所生产的二元水泥(BC)碳足迹降低约47%[26],硫铝酸钙-贝利特水泥(CSAB)的碳足迹降低了35%[29]. 此外,一些水泥材料通过优异的固化性能,以较低的水泥掺量达到了良好的重金属稳定化效果,这也在一定程度上降低了水泥固化技术的碳足迹[30-31].
在重金属稳定化机理方面,目前的研究中关于水泥固定重金属的机理可以概括为以下几点:(1)水泥水化的产物(硅酸钙水凝胶(C-S-H)、钙矾石和单硫酸盐晶体对重金属的物理包封作用;(2)水泥水化产物硅酸钙水凝胶(C-S-H)较大的比表面积和层间交错结构对重金属的吸附作用;(3)重金属通过同构置换的方式取代C-S-H凝胶或是钙矾石中的Al3+或Ca2+从而进入C-S-H 或钙矾石晶格中被固定;(4)重金属与水泥的成分发生反应生成难溶性沉淀物从而被固定.
尽管目前有关水泥固化/稳定化技术的研究与应用已十分广泛,但还存在一些不足之处. 首先,水泥处理的增容率过大问题使得废物需要更多土地空间来容纳,这使得该技术的未来发展前景不被看好. 其次,水泥水化产物对重金属的稳定机制还需进一步研究,虽然目前关于水泥固定重金属机理已较为明确,但各机制的占比如何,哪种机制对重金属的稳定作用更显著等问题还属未知. 最后,已有不少研究表明某些重金属离子在固化过程中会干扰水泥的水化反应,但具体的影响机制还鲜有报道,相关的应对措施也很少. 如能解决这些问题,将使水泥基固化/稳定化技术的发展更进一步.
-
地质聚合物(GP)是由铝和硅四面体网络组成的新一代铝硅酸盐无机聚合物. 该材料由富含反应性铝硅酸盐的前体和碱活化剂之间的反应形成,具有类似于沸石的三维网络结构[36]. 20世纪70年代末,Davidovits教授开展了防火材料的研究,并引入了地质聚合物的概念[37],此后,由于GP强度高、耐酸耐热、原料来源广、生产工艺简单、成本低等优点,使其越来越受到世界各国研究人员的关注[38]. 地质聚合是一个复杂的过程,包括溶解、沉淀、重组、凝胶化和缩聚[39],Komnitsas和 Zaharaki 的研究中将地质聚合机制解释为:“固体铝硅酸盐氧化物在 MOH 溶液(M:碱金属)中溶解,溶解的 Al 和 Si 复合物从颗粒表面扩散或运输到颗粒间空间,由添加的硅酸盐溶液与铝和硅复合物之间的聚合形成凝胶相,并最终使凝胶相硬化” [40]. 已有研究表明,作为一种无机聚合物,与传统硅酸盐水泥相比,GP的碳排放更低,其碳足迹减少量高达80%以上,且能源需求以及资源需求也更低,被认为能够成为传统硅酸盐水泥的良好替代产品[41-42].
1993年,主要从事核废料和炉渣处理的德国B.P.S Engineering公司首次提出了基于GP材料的重金属固化/稳定化技术[43]. 随后,GP在核废料和重金属固化/稳定化方面表现出良好的耐久性和长期效益. GP材料的碳足迹、抗压强度、抗腐蚀性能和重金属固定效果均优于传统水泥,这些优点决定了GP在重金属固化/稳定化处理中的可行性[44]. GP对重金属的固定机理主要取决于物理包封和化学键合,还包括吸附、取代Al3+等作用,如图1所示. 在固定化过程中,重金属主要以不溶于水的形式被GP物理包裹,相反,当金属以溶解离子的形式存在时,主要作用是化学键合和吸附过程,重金属离子被键合或吸附在GP表面结构或孔隙结构上. 通常,在不同条件下,几种效应对重金属的固定作用是同时生效的,它们同时影响着GP 固定重金属的性能.
近年来,GP在重金属废物固化/稳定化中的应用越来越多,表3总结了近几年GP在重金属固化/稳定化应用中的最新研究进展,最新的研究拓展了GP的原材料来源,在传统的矿物材料(偏高岭土、高岭土、沸石、粉煤灰)的基础上,将很多受重金属污染的固体废物(如垃圾焚烧飞灰、尾矿砂、选矿废渣、高炉渣)应用于GP材料,并评估了它们用作建筑材料的可行性,同时实现了废物的减量化、无害化与资源化. 研究所关注的重金属以Pb、Zn、Cd、Cr、Cu居多,并通过外加重金属的方式评估GP材料固定化重金属的性能. 此外,一些研究还着重探讨不同的固化温度[45]、活化方式[46-47]、Si/Al比[48],碱活化剂种类及添加量[49]等因素对GP力学性能和重金属固定性能的影响.
从目前的研究中,可以看出GP在重金属固化/稳定化方面展现出了优异的性能,但仍有一些问题需要解决. 首先,富铝硅酸盐材料是制备GP的必要原料,这虽然使得大量富含铝硅酸盐矿物的工业废料可用于合成GP. 然而,这些材料的理化性质差异很大,使得GP生产的质量控制技术难以标准化,难以形成标准化操作. 这一问题也导致GP在重金属固定化过程中存在较大的异质性和潜在的不稳定性[41]. 其次,很多研究发现,重金属的种类、形态和含量都会影响GP的抗压强度,这种影响十分复杂,很难预测[42-43]. 这种复杂性将显著影响GP材料的耐久性和资源化利用.
-
化学药剂稳定化技术的原理是通过向废物中添加某些化学药剂,通过吸附、pH调节、氧化还原、离子交换、沉淀或共沉淀、络合或螯合等作用,以达到降低废物中重金属的浸出性或生物毒性的目的[57]. 与胶凝材料固化/稳定化技术相比,化学药剂稳定化技术的优势在于可使废物不增容或少增容,且无需对废物进行养护操作,能较容易地进行原位操作. 考虑到我国各种类有害固体废物庞大的产量及堆存量,化学药剂稳定化技术更加具有规模化应用前景. 表4列举了目前常用的稳定化药剂及其对各类型固体废物中重金属的稳定效果,根据所使用的药剂类型可分为无机类稳定剂,有机类稳定剂以及复合类稳定剂三大类.
-
在目前化学药剂稳定化技术的研究中,关于无机药剂的研究比较多,所使用的无机稳定剂主要是各类铁盐、硅酸盐、磷酸盐、硫化物,其中磷酸盐和硫化物的稳定化表现较好. 无机稳定剂对重金属的稳定机制主要是通过调节废物pH、对重金属的氧化/还原或是通过与废物中的重金属的沉淀或共沉淀反应来降低重金属的迁移性及生物毒性[58-60]. 无机类药剂一般具有一定的选择性,不同类型的药剂所稳定的重金属种类可能不同,如铁盐类药剂一般被用于稳定固体废物中的As元素,因为Fe(Ⅲ)能够与As反应生成难溶的砷酸铁(FeAsO4)沉淀[61-62],而磷酸盐虽然能与许多重金属形成磷酸盐沉淀物,但其对Pb的稳定效果更好,硫化物对Pb、Cd的稳定效果明显优于Zn[63].
-
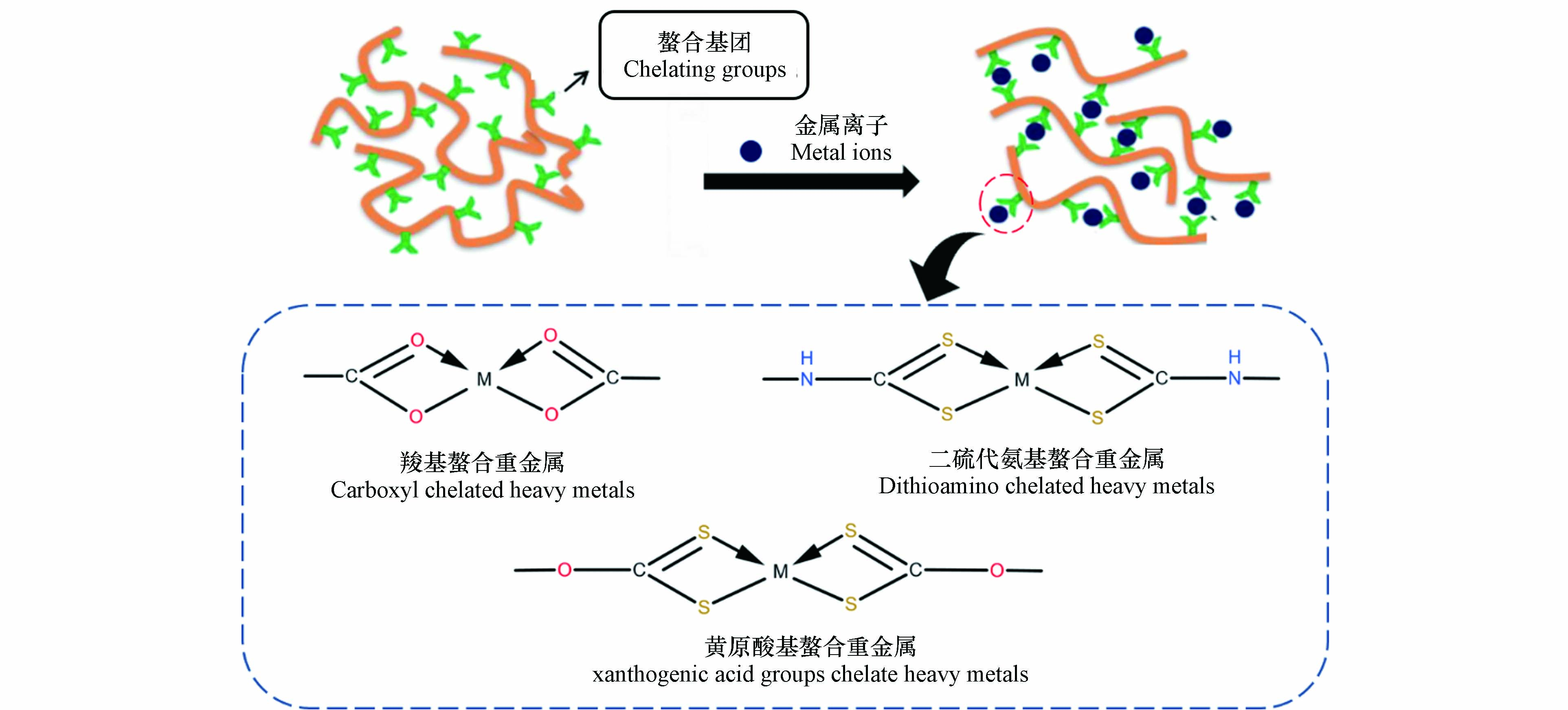
有机稳定剂主要是各类有机螯合剂,有机螯合剂是络合剂的一种形式,能通过自身含有的配位基团与金属离子结合形成金属络合物,例如羟基、羧基、巯基等,但与络合剂有所区别的是,一个螯合剂分子通常含有两个以上含孤对电子的配位基团,多个配位基团能够与单个金属离子形成极为稳定的环状物质,称为螯合物,如图2所示,稳定的环状结构使得金属螯合物相比于一般的金属配合物有着更强的稳定性[64],螯合剂的这一特性使得其有了作为固体废物重金属稳定剂的潜能.
目前常用于固体废物重金属稳定化的有机螯合剂主要有二硫代氨基甲酸盐类螯合剂(DTC)[65]、三巯基均三嗪三钠盐(TMT)[66]、乙二胺四乙酸(EDTA)[67]、六硫代胍酸(SGA)[68]、和有机磷酸盐(KOP)[69]等,其中DTC类螯合剂的研究与应用最为广泛. 清华大学的蒋建国[70]开创了国内有机螯合剂稳定化处理重金属的先河,其利用多胺类物质与二硫化碳在强碱性条件下所合成的螯合剂DCTR,用量仅0.6%时就能使垃圾焚烧飞灰中的重金属浸出浓度达到危险废物填埋标准,且螯合产物在较宽的pH范围内也能保持稳定. 该研究为我国有机螯合剂的稳定化应用开辟了道路,此后,不断有研究人员开发出新的DTC类螯合剂,并将其用于废物的稳定化处理. 相比于无机药剂的稳定化处理,有机螯合剂不仅对重金属的稳定效果更好,能稳定废物中的大多数重金属,同时在较宽的pH范围内重金属都不容易再度浸出,且所需加药量较无机药剂要少得多.
-
由于无机药剂在重金属稳定化方面的短板以及有机药剂较高的成本,有的研究人员采用将无机药剂和有机药剂复配的方式来稳定化处理重金属,旨在保证较好重金属稳定化效果的同时进一步降低药剂添加量,从而降低药剂处理的成本. 如在朱杰民等[71]的研究中,采用硫化钠、磷酸二氢钠、乙硫氮、丁铵黑药和3,4,6-三巯基均三嗪三钠盐处理某垃圾焚烧厂飞灰,在单一药剂稳定化实验中,使飞灰重金属浸出浓度满足《生活垃圾填埋场污染控制标准》的药剂添加量分别为,8%硫化钠、8%磷酸二氢钠、4.2%乙硫氮、4.2%丁铵黑药和4.2%3,4,6-三巯基均三嗪三钠盐,而根据复合药剂正交实验,所需加药量仅为1.2%硫化钠、1.2磷酸二氢钠和0.8%丁铵黑药,成本显著降低.
对于化学药剂稳定化技术,无论是无机药剂、有机药剂或是复合类药剂,均存在一定的不足之处. 无机药剂对固体废物中的重金属一般具有一定的选择性,对于来源复杂、含多种重金属元素的固体废物,单一的一种无机药剂往往无法解决全部的重金属问题,且无机药剂在酸性环境下稳定效果较差,重金属容易二次浸出,为达到稳定效果往往需要大量的加药量,这可能会给周围环境带来一定的影响. 有机螯合剂虽克服了无机药剂的这些缺陷,但是目前有机螯合剂的成本普遍较高,这使其应用受到限制. 对于复合类药剂,根据现有的研究结果来看确实取得了较好的稳定化效果,但并不能保证复合药剂的成本及效果就一定优于单一药剂,对于种类繁多的稳定化药剂与固体废物,需要大量的研究与数据来平衡重金属的稳定化效果与处理成本. 除上述各类药剂的不足之外,化学药剂稳定化技术还存在以下两点不可忽视的问题:第一,某些化学药剂在稳定化应用过程中将可能对环境造成二次污染,如Na2S在溶于水或吸潮后将会释放出有毒的H2S气体,磷酸盐类药剂的大量使用可能造成周围水体的富营养化,DTC类药剂在其生产过程中可能会释放CS2气体等. 第二,化学药剂稳定化技术虽然能够使废物达到不增容的效果,但由于缺少固化材料的物理包封效果,废物也不可避免地更容易受到外界环境条件变化的影响,如pH、氧化还原电位等条件的变化均可能导致重金属的再度释放,因此化学药剂稳定化技术常需要与固化技术协同使用才能达到更长效的稳定化效果. 这些问题导致目前无机药剂稳定化技术的发展与应用受到极大的阻碍.
-
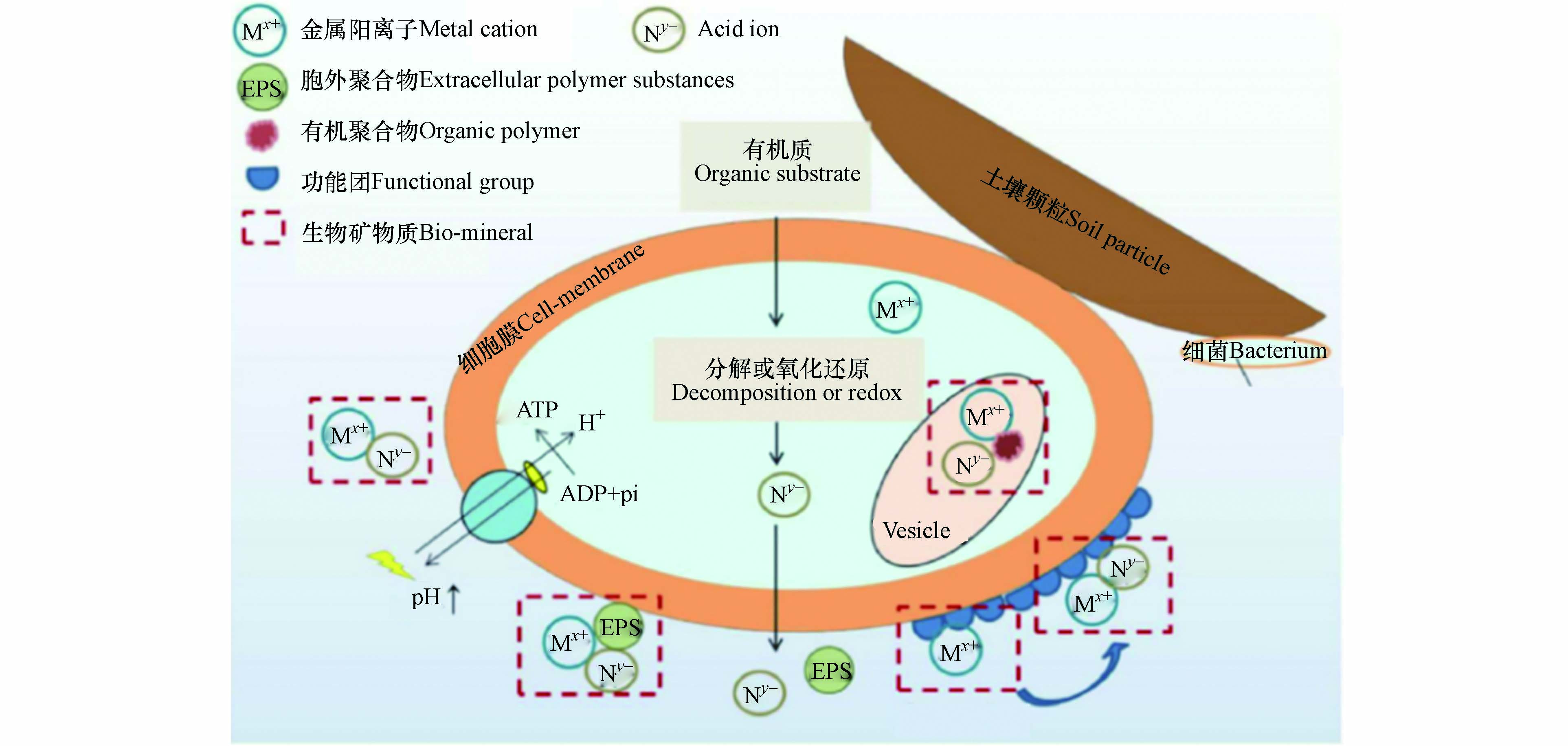
微生物诱导矿物沉淀(MIMP)是生物圈中常见的现象,自然界中的一些微生物在合适的条件下(如营养、pH、温度)的生命活动将会产生酸性阴离子(CO32-、PO43-、SO42-等)与重金属离子结合形成稳定的金属盐沉淀. 在这个过程中微生物也会改变其周围的微环境,产生适合矿物质沉淀的物理化学条件[84-85]. 该过程的机制如图3所示,首先,它们分解有机底物,产生沉淀所需的酸自由基,而这一过程增加了细胞外环境的碱度,随后促进了沉淀. 其次,微生物细胞外表面和细胞外分泌的聚合物物质中的各种负离子基团,如羟基、胺、酰胺、羧酸等,会络合带正电的金属离子,酸性离子一旦分泌到细胞膜外,就能及时与这些络合的金属离子结合,形成稳定的沉淀,从而有效降低了晶体成核的自由能,此外,少量(重金属)离子会在膜电位作用下进入细胞,在细胞内积聚或矿化[86].
一些研究人员利用这一过程开发出了基于MIMP的重金属稳定化技术,与其他固化/稳定化技术相比,MIMP重金属稳定化技术最为绿色环保,在过去10年中该技术主要用于修复受重金属污染的土壤,并取得了长足的进步[86-87],近几年,该技术逐渐被评估用于处理其他一些重金属废物的可行性,如尾矿、垃圾焚烧飞灰[88-89]. 目前,使用最广泛的微生物主要有产脲酶细菌(UPB)、磷酸盐溶解菌(PSB)和硫酸盐还原菌(SRB)三大类,与它们相对应的重金属稳定化技术分别是微生物诱导碳酸盐沉积技术(MICP)、微生物诱导磷酸盐沉积技术(MIPP)和微生物硫酸盐还原技术(MSR). 表5概述了它们的矿化机制以及适宜的诱导条件. 可以看出通过细菌分泌的酶水解相应的底物(尿素、有机磷酸盐)是UPB和PSB的核心机制,而对于SRB而言,通过有机碳的能量交换诱导的氧化还原反应在生物矿化中发挥重要作用.
表6总结了MIMP在重金属稳定化方面的一些研究案例,MIMP对重金属的稳定主要通过3种形式实现:沉淀或共沉淀、吸附或络合以及氧化还原反应,其中,沉淀或共沉淀起主要作用[96-97]. 从表中可以看出MIMP对重金属的稳定化率普遍可达到90%以上,但重金属浓度的增加对稳定化有不利影响,这主要是因为高浓度的金属会抑制细菌细胞生长[98]. 此外,同一细菌对不同重金属的稳定作用因其性质不同而存在差异,如在包含 Pb、Cd、Cu 的重金属混合物中,因Pb2+的电负性和离子半径更大,由于空间位阻效应导致吸附位点迅速饱,使得更多的Pb2+被稳定[97]. 在使用的微生物方面,UPB是MIMP技术中最受欢迎的细菌,主要由于其较快的矿化速度以及尿素相对较低的水解能和成本,但UPB诱导产生的碳酸盐与重金属结合能力和稳定性不如PSB与SRB形成的磷酸盐和硫化物[90],而SRB在近两年逐渐被应用于处理各种硫化重金属尾矿,主要原因是这些尾矿中丰富的硫酸盐可以作为SRB的硫酸盐源[88].
尽管目前MIMP技术在重金属稳定化方面取得了令人满意的效果,且近两年逐渐被研究用于处理除土壤外的一些含重金属的固体废物,但由于该技术的发展历史相对较短,还面临一些问题需要解决. 首先,目前的研究普遍是在实验室环境中给微生物提供最适宜的生长代谢条件,但在实际应用中固体废物可能处于高pH、高温、高盐浓度、干燥缺水、营养匮乏等环境,复杂的环境条件将使MIMP技术难以施展. 其次,与其他固化/稳定化技术相比,MIMP处理技术耗时较长,漫长的处理周期使得MIMP技术的实际应用面临挑战. 最后,若废物中缺乏MIMP技术所需的矿化材料源,那么就需要外加大量MIMP所需的材料,处理成本将大幅增加.
-
上文综述了目前常用的固化/稳定化技术的最新研究进展,并总结了现阶段各项技术存在的不足之处,根据现有技术的不足,可以针对性地提出各项技术未来的发展方向. 总结如下:
(1)水泥基固化/稳定化技术:在当前碳达峰、碳中和背景下,开发高性能绿色低碳水泥材料仍应作为该领域的研究重点,以在保持良好力学性能及重金属稳定化性能的同时,进一步降低水泥用量,提高废物掺量,以达到降低水泥固化/稳定化处理的碳足迹及废物增容率的目的. 同时,应重点关注水泥固化过程中及固化后重金属的微观行为,如过程中重金属的相变情况,分布情况等,有利于进一步系统性地掌握水泥固定重金属的机理以及重金属对水泥水化反应的影响机制,并采取针对性措施提高水泥固定重金属的性能.
(2)地质聚合物基固化/稳定化技术:针对铝硅酸盐材料的物理化学特性进行大规模的调查和研究,将有助于拓宽地质聚合物的原材料来源,使GP材料的标准化、规模化生产和稳定化应用成为可能. 此外,领域内的研究人员应聚焦于重金属对GP材料抗压强度的影响,掌握不同种类、形态及含量的重金属对GP材料抗压强度的影响机制及规律,并采取一定措施规避这一负面影响.
(3)化学药剂稳定化技术:化学药剂稳定化技术应朝着绿色、高效的方向发展,因此在未来化学药剂的研究与应用中应尽量减少甚至消除化学药剂可能对环境造成的二次污染,同时应进一步提升稳定化产物应对环境条件变化的能力,鉴于重金属螯合物较强的稳定性,可着手研发成本较低同时螯合性能优异的新型有机螯合剂,或是针对不同固体废物、不同重金属种类,通过大量实验研究寻找无机药剂和有机药剂的最优配比,以实现成本的最低化及重金属的长效稳定化.
(4)微生物诱导矿化稳定化技术:①筛选出更多对复杂环境条件具有良好耐受性的矿化细菌或是通过人工途径提高矿化细菌的耐受性,确保该技术能适用于固体废物所处的复杂环境条件. ②采取一定措施加速微生物的代谢或是通过预先培养的方式提取微生物所分泌的酶并将其用于MIMP稳定化处理,或可缩短该技术的处理周期. ③根据固体废物中所含的矿化材料源情况选择合适的微生物进行矿化稳定处理,如利用SRB处理硫酸盐含量很高的重金属尾矿,用UPB处理Ca含量很高的垃圾焚烧灰,将使该技术的处理成本大幅降低.
以上分别阐述了各项固化/稳定化技术未来的发展方向,总的来说,目前固化/稳定化技术仍有很大的发展空间. 在我国,除水泥基固化/稳定化技术外,其余技术大多停留在实验室研究阶段,未实现规模化的实际应用,主要原因是受限于这些技术现阶段下较高的处理成本或是在实际应用场景中较复杂的操作性. 同时,所有固化/稳定化技术仍缺少在环境中的长期稳定性数据,这使得稳定化产物在复杂环境中的长期稳定性还是未知的. 因此,未来所有的固化/稳定化技术都应从这两方面着手展开相关研究.
-
固化/稳定化处理技术是解决含重金属固体废物环境问题的重要技术手段,本文首先对固化稳定化技术的概念及效果评价方法进行了简要的介绍,再结合近年来的相关研究综述了目前常用的固体废物重金属固化/稳定化技术的研究进展,包括水泥基固化/稳定化技术、地质聚合物基固化/稳定化技术、化学药剂稳定化技术以及微生物诱导矿化稳定化技术,总结了各项技术的优点、最新研究成果和现阶段存在的不足之处,并针对各项技术提出了未来的发展方向. 根据现有研究,目前各项固化/稳定化技术均能实现较好的重金属稳定化效果,但还需从成本,实际操作性以及在环境中的长期稳定性等角度着手开展相关研究,旨在推动各项固化/稳定化技术实现规模化的应用,对固体废物的“三化”处理以及防治重金属污染具有重要意义.
固体废物中重金属的固化/稳定化技术研究进展
Review on solidification/stabilization of heavy metals in solid waste
-
摘要: 含重金属固体废物的大量堆存不仅占用了大量的土地资源,且其中的重金属元素将会在地表径流冲刷、雨水淋溶、风化等作用下浸出从而对周遭环境生态造成巨大威胁. 固化/稳定化处理技术能够降低固体废物中重金属的浸出毒性或是生物有效性,是应对固体废物重金属环境污染的重要技术手段. 本文首先简要介绍了固化/稳定化技术的概念及效果评价方法,再结合近年来相关研究总结了现阶段常用的固化/稳定化技术的研究进展,包括水泥基固化/稳定化技术,地质聚合物基固化/稳定化技术,化学药剂稳定化技术以及微生物诱导矿化稳定化技术. 总结了各项技术的优点及存在的不足,并针对未来重金属固化/稳定化技术的发展提出了一定的看法.Abstract: The massive accumulation of solid waste containing heavy metals not only occupies a large amount of land resources, but also the heavy metal elements contained in solid waste will be leached out under the action of surface runoff erosion, rainwater leaching, weathering, and so on, which will pose a huge threat to the surrounding environment and ecology. The solidification/stabilization treatment technology can reduce the leaching toxicity or bioavailability of heavy metals in solid waste, and it’s an important technical means to deal with the environmental pollution of heavy metals in solid waste. First of all, this paper briefly introduces the concept and effect evaluation method of solidification/stabilization technology, and then summarizes the research progress of solidification/stabilization technology commonly used at the present stage, including cement-based solidification/stabilization technology, geopolymer-based solidification /stabilization technology, chemical agents stabilization technology and microbial-induced mineralization stabilization technology. The advantages and disadvantages of each technology are summarized, and some opinions are put forward for the development of solidification/stabilization technology in the future.
-
Key words:
- solid waste /
- heavy metal /
- leaching toxicity /
- solidification /
- stabilization.
-

-
表 1 固体废物中不同赋存形态重金属的迁移释放规律
Table 1. Migration and release of heavy metals in different forms
重金属赋存形态
Occurrence forms
of heavy metals迁移释放规律
Migration and release迁移性
Ability to
migrate可交换态 可进行离子交换及专性吸附,可在阳离子溶液中被释放,也可直接被生物所吸收利用 ★★★★★ 碳酸盐结合态 与碳酸盐结合的沉淀或共沉淀形态重金属,通过较温和的酸便能够从尾矿中溶出释放 ★★★★ 锰氧化物结合态 重金属被铁锰氧化物专性吸附或与之共沉淀,在还原条件下将会溶出释放 ★★★ 有机结合态 与有机质及配位基团相结合,较为稳定,但在强氧化及强碱性条件下将会溶出释放 ★★ 残渣态 重金属被包裹于矿物晶格中,较难迁移和被生物利用,仅在强酸条件下或是经特定微生物作用才会释放 ★ 表 2 水泥基固化处理含重金属固体废物研究进展
Table 2. Research progress of cement-based solidification treatment of solid waste containing heavy metals
水泥材料
Cement material固体废物
Solid waste废物掺量及
抗压强度
Waste content and the compressive strength重金属稳定化率
Stabilization rate of heavy metals固化/稳定化机理
Solidification/stabilization mechanism参考文献
ReferenceLC3 镍铁渣 — Cr :98.96—99.65%
Mn :85.8—90.43%LC3主要水化产物水合硅酸铝钙(C-A-S-H)和钙矾石(AFt) 形成低孔隙率的致密结构对重金属的封装作用 [28] 污泥焚烧渣基磷酸镁钾水泥(SIR-MKPC) 污泥焚烧渣 废物掺量:5%
抗压强度:40.32 MPaCr : 92.3%
Cu : 96.6%
Zn : 96.3%
Ni : 91.4%
Cd : 87.5%
Pb : 90.9%重金属在固化过程中以形成磷酸盐沉淀的形式被固定 [32] BC 高铅污染土壤 废物掺量50%
1.25—50 MPaPb : 99.98% BC丰富的C-S-H产物对重金属的吸附作用,重金属与磷酸盐或硫酸盐形成沉淀 [26] CSAB 金尾矿 废物掺量50%
12—20 MPaAs : >17%
Cr :>80%
Ni : >90%
Sb : >50%
Ba : >44%CSAB的水化反应生成C-S-H和单硫酸盐晶体对重金属的物理封装作用,重金属通过离子交换进入钙矾石晶格中 [33] Ⅰ型硅酸盐水泥 重金属污染黏土 废物掺量82%
1.2—1.7 MPaPb : 68%—95% 水解反应产生的高碱度溶液将SiO2和Al2O3溶解在黏土中,Pb2+离子被SiO2和Al2O3吸收并形成Pb3SiO5 [30] Ⅱ型硅酸盐水泥 含铅蒙脱石土壤 废物掺量80%
0.6—1.7 MPaPb :99.98% 固化/稳定过程中添加 NaOH能够消除 Pb对水泥水化的阻滞作用,促进了C-S-H的生成并封装住可溶性Pb离子 [31] (LC3) 铅锌冶炼渣 废物掺量90%
3.539 MPaPb : 99.14%
Zn :85%
Cd :99.82%LC3水化形成的低孔隙率结构对重金属的物理封装,重金属形成氢氧化物沉淀 [34] 氯氧镁水泥和磷酸镁水泥 电弧炉粉尘 — Hg : >99.99%
Pb : >98.46%
Se :>58%镁质水泥提供的高pH环境使大部分金属阳离子的溶解性降低,水解MgO产物的表面正电荷对金属类氧阴离子具有较高吸附性 [35] 表 3 GP在固体废物重金属固化/稳定化中的应用研究进展
Table 3. Research progress in application of GP in solidification/stabilization of heavy metals in solid waste
固体废物/GP原材料
Solid waste /GP
raw materials重金属稳定化率
Stabilization rate
of heavy metals抗压强度
compressive
strength固化/稳定化机理
Solidification/stabilization
mechanism参考文献
Reference城市垃圾焚烧飞灰
粉煤灰`Zn :99.4%
Pb :99.8%
Cd :99%14.3—22.4 MPa MSWI、粉煤灰地质聚合反应中生成了大量C-S-H以及Friedel盐、水铝钙石等新的相,表现出更致密的结构、优异的力学性能和对重金属的强吸附作用 [50] 铅锌冶炼渣(LZSS)
粉煤灰
高炉矿渣Pb: 97.26%
Zn: 99.3%
Cu: 84.46%
Cr: 99.43%15—47.39 MPa 重金属的固定是吸附和化学键合共同作用的结果 [51] 城市垃圾焚烧飞灰
Shell煤气化飞灰
偏高岭土
钢渣Pb: 93.12%—99.29%
Zn: 93.85%—96.74%
Cr: 95.44%—99.45%— Cr主要残留在铝硅酸盐晶格中. Pb和Zn以M—O—Al和M—O—Si(M=Pb或Zn)的形式嵌入到铝硅酸盐的三维结构中. [52] 金矿尾矿
偏高岭土
高炉矿渣Cr: >99.2%
Cu: >97.25%
Ni: >98.4%
Zn: >98.73%
Mn: >99.22%— 偏高岭土的加入提升了GP的Si/Al和Na/Al,导致形成了更多的凝胶相及更致密的结构,对重金属形成极强的包封作用 [53] 电解锰渣
粉煤灰
偏高岭土Pb: >99%
Cd: >99%18.4—31.4 MPa Pb2+和Cd2+可能主要通过取代Na+或Ca2+而固定在GP中 [45] 污水厂污泥
炼钢炉渣Cu: >95%
Zn: >95%
Pb: >95%46.96—89.03 MPa 炼钢炉渣中的铝硅酸盐容易与Zn和Cu等重金属形成硅酸盐金属化合物 [54] 稀土尾矿
偏高岭土Pb、Ba浸出浓度远低于危险废物浸出毒性标准 最高35 MPa Pb2+/Ba2+参与Si/Al凝胶相的缩聚,并与-[TOT]-(T:Si或Al)中的Si/Al或O结合形成PbO/BaSiO3固定框架 [55] 焚烧炉底灰
偏高岭土Pb: >99.99%
Cu: 99.91—99.95%
Sn: > 99.99%— Pb和Cr可能通过化学方式结合到材料基体上. 然而,无法说明金属是化学结合还是物理结合到结构上,因为这两种选择都可以是该过程的一部分 [49] 电厂粉煤灰 Pb; >99.99%
Cd: >99.99%
Mn: >99.99%
Cr: >99.99%35.98—49.34 MPa 通过取代Na+和Ca2+等金属离子,重金属可以潜在地固定在粉煤灰基地质聚合物中 [56] 表 4 不同化学药剂对固体废物中重金属的稳定效果
Table 4. Stabilization effects of different chemicals on heavy metals in solid waste
化学药剂
Chemicals固体废物
Solid waste药剂添加量
Dosage of
reagent重金属稳定化率
Stabilization rate
of heavy metals参考文献
Reference无机类 Na2S 不锈钢酸洗污泥 5wt% Cr(Ⅵ) 87.4%;Ni 93.5% [72] FeS Cr污染土壤 2.5 mg·g−1 Cr(Ⅵ)15% [73] Na2S 垃圾焚烧飞灰 0.1 mol·kg−1 Cd72%;Cr72%;Cu82%;Ni77%;Pb78%;Zn81% [68] Na2S 垃圾焚烧飞灰 10%wt Cd 86.22%;Se 97.19 [63] Na2HPO4 垃圾焚烧飞灰 10%wt Pb 99.25%;Zn 81.76% NaH2PO4 铅锌尾矿 10%wt Pb 98.42%;Zn 79.08% [67] KH2PO4 Pb污染土壤 — Pb70% [74] Na3PO4 垃圾焚烧飞灰 10%wt Pb85% [75] H3PO4 垃圾焚烧飞灰 45 mL·kg−1 Pb99.87%;Zn94.7% [76] 羟基磷灰石(HAP) 重金属污染沉积物 10%wt Zn76%;Mn28%;Pb78%;Cd85% [77] Ca(H2PO4)2 重金属污染沉积物 10%wt Zn66%;Mn16%;Pb86%;Cd82% FeCl2 含砷尾矿废渣 Fe/As物质的
量比大于1pH在6.5—7.5范围内As稳定化率大于96% [78] FeSO4 雄黄尾矿 20%wt As98.15% [61] 硅灰 垃圾焚烧灰 20%wt Cu84%;Pb89%;Zn97% [79] 有机类 四硫代二氨基甲酸(TBA) 垃圾焚烧飞灰 0.1 mol·kg−1 Cd大于99.99%;Cr60%;Cu95%;Ni92%;Pb82%;Zn74% [68] 六硫代胍酸(SGA) 垃圾焚烧飞灰 0.1 mol·kg−1 Cd81%;Cr84%;Cu95%;Ni82%;Pb80%;Zn78% 二甲基二硫代氨基甲酸钠(SDD) 垃圾焚烧飞灰 0.1 mol·kg−1 Cd64%;Cr64%;Cu90%;Ni86%;Pb60%;Zn61% 三巯基均三嗪三钠盐(TMT) 铅锌尾矿 4%wt Pb:99.31%;Zn80.92% [67] 二异丙基二硫代磷酸钾(DDP) 垃圾焚烧飞灰 1%wt Pb、Zn、Cu的稳定化率均大于95% [80] 巯基官能化树枝状聚合物
(TEPA-SNa)垃圾焚烧飞灰 3%wt Pb接近100% [75] 硫脲 垃圾焚烧飞灰 10%wt Pb94.6% 二甲基二硫代氨基甲酸钠(SDD) 垃圾焚烧飞灰 5%wt Pb97%;Cd88% [81] 二硫代羧基功能化四乙基五胺(TEPA-DTC) 垃圾焚烧飞灰 5%wt Pb98.7%;Cd99.8% 二硫代羧基功能化聚氨基酰胺树枝状聚合物(PAMAM-0G-DTC) 垃圾焚烧飞灰 1%wt Pb98.4%;Cd97% 复合类 1.2%Na2S+1.2%NaH2PO4+0.8% DDTP 垃圾焚烧飞灰 — 可使Cu、Zn、Cd、Cr、Ni、Pb达到填埋标准 [66] DTC+ NaH2PO4+ Na2S 垃圾焚烧飞灰 3%wt 对Cd和Pb稳定化率大于98% [82] Na2S+EDTA二钠+ NaH2PO4
(2:1:2)垃圾焚烧飞灰 0.4%wt 可使Pb、Zn达到填埋标准 [83] 表 5 常用于重金属稳定化的微生物概述
Table 5. Overview of microorganisms commonly used for stabilization of heavy metals
类型
Type典型菌株
Typical strains诱导矿化机制
Mechanism of induced mineralization适宜条件
Conditions参考文献
ReferenceUPB 芽孢杆菌
克雷白氏杆菌
柠檬酸杆菌
赖氨酸芽胞杆菌CO(NH2)2+3H2O $ \xrightarrow{\;\;\;{\text{脲酶}}\;\;\;} $
M2+ (aq)+CO32−(aq)→MCO3↓温度:25—35 ℃;
pH:7—9;[90-91] PSB 巨大芽孢杆菌
蜡样芽孢杆菌
阴沟肠杆菌
醋菌属有机磷酸盐+H2O $\xrightarrow{\;\;\;{\text{磷酸酶}}\;\;\;} $
M2+(aq)+PO43−/HPO42−(aq)→MPO4OH/MHPO4/M3(PO4)2↓温度:20—40 ℃;
pH:约7;
适合富含不溶性磷的环境[92-93] SRB 脱硫弧菌
脱硫单胞菌属CH3COO−+2H++SO42−→HS−+2H2O+2CO2
M2+(aq)+S2−(aq)→MS↓
M2+(aq)+CO32−(aq)→MCO3↓中温细菌:30—40 ℃;
高温细菌:55—60 ℃;
pH:5—10;
厌氧环境[94-95] 表 6 MIMP在重金属稳定化方面的研究进展
Table 6. Research progress in heavy metal stabilization by MIMP
类型
Type微生物
microbial固体废物
Solid waste重金属稳定化率
Stabilization rate of heavy metals稳定化机理/产物
Stabilization mechanism/product参考文献
ReferenceUPB 枯草芽孢杆菌 重金属污染土壤 Cr(Ⅲ): 99.95%;
Cu(Ⅱ): 95.90%;
Zn(Ⅱ): 86.59%沉淀和共沉淀;形成
Cu2(OH)2CO3、ZnCO3、NiCr2O4、FeCr2O3、ZN5(CO3)2(OH)6、CaCO3[87] 黏质沙雷氏菌 重金属污染土壤 Cd:65%—98% 形成CdCO3沉淀 [98] 阴沟肠杆菌 重金属污染土壤 Cd:80%—99% Cd与CaCO3的共沉淀 [98] 膨胀土杆菌 重金属污染土壤 Pb:100%; Cd100%; Zn:96%; Co:92%; Ni:90%; Cu:90% 形成ZnCO3、CuCO3、PbCO3、CdCO3、NiCO3和CoCO3沉淀. 土壤颗粒间隙有CaCO3晶体生成 [99] 蜡样芽孢杆菌NS4 重金属污染土壤 Ni可交换态降低了90% Ni在CaCO3的晶格中沉淀,并以 NiCO3的形式被稳定. [100] 巴氏杆菌 垃圾焚烧飞灰 Cu: 55.5%—93.5%;
Hg: 52.9%—60.6%
Pb: 56.9%—86.7%
Zn: 22.6%—35.2%
Ni: 34.9%—35.8%
Cr: 6.9%—11.7%
Cd: 13.3%—30.4%重金属转化为碳酸盐沉淀或是被大量形成的碳酸钙矿物所物理包封 [89] PSB 拉恩氏菌属 重金属污染土壤 Cu: 58.2%—75.8% Cu转化为棒状Cu3(OH)3PO4晶体 [101] 赖氨酸芽孢杆菌 重金属污染土壤 Pb:97.9% 形成Pb3(PO4)2、Pb5(PO4)3OH沉淀 [102] 植酸酶 铅锌尾矿 Pb;97.7% 形成了新辉石MgHPO4(H2O)3固化尾矿颗粒;Pb转化为Pb9(PO4)6 [103] SRB 脱硫孢子粉
氧化亚铁硫杆菌黄铜尾矿
铅锌尾矿Cu: 100%; Zn: 100%; Pb:84.62% 重金属形成了黑色金属硫化物沉淀 [94] 蜡样芽孢杆菌Cd01 重金属污染土壤 Cd:29.25% 细菌对Cd有生物吸附作用;
Cd形成CdS和Cd·xH3PO4沉淀[96] 梭状芽胞杆菌
脱硫肠状菌属废弃矿区尾矿 As残渣态比例从29%增加至69%;
Pb残渣态比例从49%增加至90%Pb和As转化为稳定的方铅矿(PbS)、雄黄(AsS)或雌黄(As2S3)等矿物 [88] 丙酸脱硫杆菌
脱硫弧菌金属冶炼厂污水沉积物 Cu: 100%; Pb: 100%
Cd: 98.5%; Zn: 90.69%重金属与硫化物形成金属硫化物沉淀 [104] 嗜酸氧化硫硫杆菌
氧化亚铁硫杆菌铅锌尾矿 Pb: 30%; Zn: 28% 两种微生物加速了尾矿中黄铁矿和黑云母类矿物的风化,促进了次生黄钾铁矾类矿物的生成,Pb和Zn被该类矿物固定 [105] -
[1] AKCIL A, ERUST C, OZDEMIROGLU S, et al. A review of approaches and techniques used in aquatic contaminated sediments: Metal removal and stabilization by chemical and biotechnological processes [J]. Journal of Cleaner Production, 2015, 86: 24-36. doi: 10.1016/j.jclepro.2014.08.009 [2] BOLAN N, KUNHIKRISHNAN A, THANGARAJAN R, et al. Remediation of heavy metal(loid)s contaminated soils - To mobilize or to immobilize? [J]. Journal of Hazardous Materials, 2014, 266: 141-166. doi: 10.1016/j.jhazmat.2013.12.018 [3] FAN C C, WANG B M, ZHANG T T. Review on cement stabilization/solidification of municipal solid waste incineration fly ash [J]. Advances in Materials Science and Engineering, 2018: 5120649. [4] 梁雅雅, 易筱筠, 党志, 等. 某铅锌尾矿库周边农田土壤重金属污染状况及风险评价 [J]. 农业环境科学学报, 2019, 38(1): 103-110. doi: 10.11654/jaes.2018-0252 LIANG Y Y, YI X Y, DANG Z, et al. Pollution and risk assessment of heavy metals in agricultural soils around a Pb-Zn tailing pond [J]. Journal of Agro-Environment Science, 2019, 38(1): 103-110(in Chinese). doi: 10.11654/jaes.2018-0252
[5] LI Z Y, MA Z W, van der KUIJP T J, et al. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment [J]. Science of the Total Environment, 2014, 468/469: 843-853. doi: 10.1016/j.scitotenv.2013.08.090 [6] JIANG X W, LIU W H, XU H, et al. Characterizations of heavy metal contamination, microbial community, and resistance genes in a tailing of the largest copper mine in China [J]. Environmental Pollution, 2021, 280: 116947. doi: 10.1016/j.envpol.2021.116947 [7] ZHANG M J, SUN M J, WANG J L, et al. Geographical distribution and risk assessment of heavy metals: A case study of mine tailings pond [J]. Chemistry and Ecology, 2020, 36(1): 1-15. doi: 10.1080/02757540.2019.1676420 [8] BEMPAH C K, EWUSI A. Heavy metals contamination and human health risk assessment around Obuasi gold mine in Ghana [J]. Environmental Monitoring and Assessment, 2016, 188(5): 261. doi: 10.1007/s10661-016-5241-3 [9] 竹涛. 矿山固体废物处理与处置工程[M]. 北京: 冶金工业出版社, 2016. ZHU T. Mine solid waste treatment and disposal project [M]. Beijing: Metallurgical Industry Press, 2016(in Chinese).
[10] SAWYER S. International Waste Technologies/Geo-Con in situ stabilization/solidification: Applications analysis report[R]. Tech. Rep. EPA/540/A5-89/004, United States Environmental Protection Agency, Office of Research and Development, Cincinnati, Ohio, USA, 1990. [11] BARTH E F. An overview of the history, present status, and future direction of solidification/stabilization technologies for hazardous waste treatment [J]. Journal of Hazardous Materials, 1990, 24(2/3): 103-109. [12] 环境保护部. 固体废物浸出毒性浸出方法 水平振荡法: HJ 557—2010[S]. 北京: 中国环境科学出版社, 2010. Ministry of Environmental Protection of the People's Republic of China. Solid waste-Extraction procedure for leaching toxicity-Horizontal vibration method: HJ 557—2010[S]. Beijing: China Environment Science Press, 2010(in Chinese).
[13] 刘锋, 王琪, 黄启飞, 等. 固体废物浸出毒性浸出方法标准研究 [J]. 环境科学研究, 2008, 21(6): 9-15. doi: 10.13198/j.res.2008.06.11.liuf.015 LIU F, WANG Q, HUANG Q F, et al. Study on the standard methods of leaching toxicity of solid waste [J]. Research of Environmental Sciences, 2008, 21(6): 9-15(in Chinese). doi: 10.13198/j.res.2008.06.11.liuf.015
[14] EPA U S. Toxicity Characteristics Leaching Procedure, Method 1311[S]. 1992. [15] INSTITUTION B S. En 12457 Leaching - Compliance Test For Leaching Of Granular Waste Materials[S]. 1996. [16] TESSIER A, CAMPBELL P G C, BISSON M. Sequential extraction procedure for the speciation of particulate trace metals [J]. Analytical Chemistry, 1979, 51(7): 844-851. doi: 10.1021/ac50043a017 [17] URE A M, QUEVAUVILLER P, MUNTAU H, et al. Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of the European communities [J]. International Journal of Environmental Analytical Chemistry, 1993, 51(1/2/3/4): 135-151. [18] LIU J X, JIANG X M, ZHANG Y C, et al. Size segregation behavior of heavy metals in superfine pulverized coal using synchrotron radiation-induced X-ray fluorescence [J]. Fuel, 2016, 181: 1081-1088. doi: 10.1016/j.fuel.2016.04.115 [19] JIANG L, SUN H J, PENG T J, et al. Comprehensive evaluation of environmental availability, pollution level and leaching heavy metals behavior in non-ferrous metal tailings [J]. Journal of Environmental Management, 2021, 290: 112639. doi: 10.1016/j.jenvman.2021.112639 [20] RAZZELL W E. Chemical fixation, solidification of hazardous waste [J]. Waste Management & Research, 1990, 8(2): 105-111. [21] CONNER J R, HOEFFNER S L. A critical review of stabilization/solidification technology [J]. Critical Reviews in Environmental Science and Technology, 1998, 28(4): 397-462. doi: 10.1080/10643389891254250 [22] 段荣国. 中国水泥生产的物质消耗和环境排放分析 [J]. 工程技术(文摘版)·建筑, 2017(1): 92. DUAN R G. Material consumption and environmental emission analysis of Cement production in China [J]. Engineering Technology (Abstract Edition)· Architecture, 2017(1): 92(in Chinese).
[23] GRÜNHÄUSER SOARES E, CASTRO-GOMES J. Carbonation curing influencing factors of Carbonated Reactive Magnesia Cements (CRMC) - A review [J]. Journal of Cleaner Production, 2021, 305: 127210. doi: 10.1016/j.jclepro.2021.127210 [24] CHEN I A, HARGIS C W, JUENGER M C G. Understanding expansion in calcium sulfoaluminate-belite cements [J]. Cement and Concrete Research, 2012, 42(1): 51-60. doi: 10.1016/j.cemconres.2011.07.010 [25] ZHANG H Q, YANG Y Y, YI Y C. Effect of sulfate erosion on strength and leaching characteristic of stabilized heavy metal contaminated red clay [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(3): 666-675. doi: 10.1016/S1003-6326(17)60074-8 [26] WANG L, YU K Q, LI J S, et al. Low-carbon and low-alkalinity stabilization/solidification of high-Pb contaminated soil [J]. Chemical Engineering Journal, 2018, 351: 418-427. doi: 10.1016/j.cej.2018.06.118 [27] LI Z D, LI T H, SHI L Z, et al. The rainfall effect onto solidification and stabilization of heavy metal-polluted sediments [J]. Royal Society Open Science, 2020, 7(7): 192234. doi: 10.1098/rsos.192234 [28] GU Y C, LI J L, PENG J K, et al. Immobilization of hazardous ferronickel slag treated using ternary limestone calcined clay cement [J]. Construction and Building Materials, 2020, 250: 118837. doi: 10.1016/j.conbuildmat.2020.118837 [29] GÁLVEZ-MARTOS J L, VALENTE A, MARTÍNEZ-FERNÁNDEZ M, et al. Eco-efficiency assessment of calcium sulfoaluminate clinker production [J]. Journal of Industrial Ecology, 2020, 24(3): 695-706. doi: 10.1111/jiec.12967 [30] TEERAWATTANASUK C, VOOTTIPRUEX P, HORPIBULSUK S. Improved heavy metal immobilization of compacted clay by cement treatment [J]. Heliyon, 2021, 7(4): e06917. doi: 10.1016/j.heliyon.2021.e06917 [31] OUHADI V R, YONG R N, DEIRANLOU M. Enhancement of cement-based solidification/stabilization of a lead-contaminated smectite clay [J]. Journal of Hazardous Materials, 2021, 403: 123969. doi: 10.1016/j.jhazmat.2020.123969 [32] CAO X, MA R, ZHANG Q S, et al. The factors influencing sludge incineration residue (SIR)-based magnesium potassium phosphate cement and the solidification/stabilization characteristics and mechanisms of heavy metals [J]. Chemosphere, 2020, 261: 127789. doi: 10.1016/j.chemosphere.2020.127789 [33] KIVENTERÄ J, PIEKKARI K, ISTERI V, et al. Solidification/stabilization of gold mine tailings using calcium sulfoaluminate-belite cement [J]. Journal of Cleaner Production, 2019, 239: 118008. doi: 10.1016/j.jclepro.2019.118008 [34] REDDY V A, SOLANKI C H, KUMAR S, et al. Comparison of limestone calcined clay cement and ordinary Portland cement for stabilization/solidification of Pb-Zn smelter residue [J]. Environmental Science and Pollution Research International, 2022, 29(8): 11393-11404. doi: 10.1007/s11356-021-16421-w [35] PANTAZOPOULOU E, NTINOUDI E, ZOUBOULIS A I, et al. Heavy metal stabilization of industrial solid wastes using low-grade magnesia, Portland and magnesia cements [J]. Journal of Material Cycles and Waste Management, 2020, 22(4): 975-985. doi: 10.1007/s10163-020-00985-9 [36] FAHIM HUSEIEN G, MIRZA J, ISMAIL M, et al. Geopolymer mortars as sustainable repair material: A comprehensive review [J]. Renewable and Sustainable Energy Reviews, 2017, 80: 54-74. doi: 10.1016/j.rser.2017.05.076 [37] DAVIDOVITS J. Geopolymers [J]. Journal of Thermal Analysis, 1991, 37(8): 1633-1656. doi: 10.1007/BF01912193 [38] ALBITAR M, MOHAMED ALI M S, VISINTIN P. Experimental study on fly ash and lead smelter slag-based geopolymer concrete columns [J]. Construction and Building Materials, 2017, 141: 104-112. doi: 10.1016/j.conbuildmat.2017.03.014 [39] NATH S K, MUKHERJEE S, MAITRA S, et al. Kinetics study of geopolymerization of fly ash using isothermal conduction calorimetry [J]. Journal of Thermal Analysis and Calorimetry, 2017, 127(3): 1953-1961. doi: 10.1007/s10973-016-5823-x [40] KOMNITSAS K, ZAHARAKI D. Geopolymerisation: A review and prospects for the minerals industry [J]. Minerals Engineering, 2007, 20(14): 1261-1277. doi: 10.1016/j.mineng.2007.07.011 [41] PÉREZ-VILLAREJO L, BONET-MARTÍNEZ E, ELICHE-QUESADA D, et al. Biomass fly ash and aluminium industry slags-based geopolymers [J]. Materials Letters, 2018, 229: 6-12. doi: 10.1016/j.matlet.2018.06.100 [42] ZHANG Z H, ZHU H J, ZHOU C H, et al. Geopolymer from Kaolin in China: An overview [J]. Applied Clay Science, 2016, 119: 31-41. doi: 10.1016/j.clay.2015.04.023 [43] (法)约瑟夫·戴维德维斯. 地聚合物化学及应用[M]. 北京: 国防工业出版社, 2011. JOSEPH D. Geopolymer chemistry & applications[M]. Beijing: National Defense Industry Press, 2011(in Chinese).
[44] HAJIMOHAMMADI A, NGO T, KASHANI A. Glass waste versus sand as aggregates: The characteristics of the evolving geopolymer binders [J]. Journal of Cleaner Production, 2018, 193(aug.20): 593-603. [45] LI J, LI J X, WEI H, et al. Alkaline-thermal activated electrolytic manganese residue-based geopolymers for efficient immobilization of heavy metals [J]. Construction and Building Materials, 2021, 298: 123853. doi: 10.1016/j.conbuildmat.2021.123853 [46] LI Y C, MIN X B, KE Y, et al. Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation [J]. Waste Management, 2019, 83: 202-208. doi: 10.1016/j.wasman.2018.11.019 [47] ZHANG X L, ZHANG S Y, LIU H, et al. Disposal of mine tailings via geopolymerization [J]. Journal of Cleaner Production, 2021, 284: 124756. doi: 10.1016/j.jclepro.2020.124756 [48] KRÄNZLEIN E, HARMEL J, PÖLLMANN H, et al. Influence of the Si/Al ratio in geopolymers on the stability against acidic attack and the immobilization of Pb2+ and Zn2+ [J]. Construction and Building Materials, 2019, 227: 116634. doi: 10.1016/j.conbuildmat.2019.08.015 [49] BOCA SANTA R A A, SOARES C, RIELLA H G. Geopolymers with a high percentage of bottom ash for solidification/immobilization of different toxic metals [J]. Journal of Hazardous Materials, 2016, 318: 145-153. doi: 10.1016/j.jhazmat.2016.06.059 [50] FAN C C, WANG B M, AI H M, et al. A comparative study on solidification/stabilization characteristics of coal fly ash-based geopolymer and Portland cement on heavy metals in MSWI fly ash [J]. Journal of Cleaner Production, 2021, 319: 128790. doi: 10.1016/j.jclepro.2021.128790 [51] XIA M, MUHAMMAD F, ZENG L H, et al. Solidification/stabilization of lead-zinc smelting slag in composite based geopolymer [J]. Journal of Cleaner Production, 2019, 209: 1206-1215. doi: 10.1016/j.jclepro.2018.10.265 [52] CHEN Y C, CHEN F Y, ZHOU F, et al. Early solidification/stabilization mechanism of heavy metals (Pb, Cr and Zn) in Shell coal gasification fly ash based geopolymer [J]. Science of the Total Environment, 2022, 802: 149905. doi: 10.1016/j.scitotenv.2021.149905 [53] KIVENTERÄ J, LANCELLOTTI I, CATAURO M, et al. Alkali activation as new option for gold mine tailings inertization [J]. Journal of Cleaner Production, 2018, 187: 76-84. doi: 10.1016/j.jclepro.2018.03.182 [54] SUN S C, LIN J H, ZHANG P X, et al. Geopolymer synthetized from sludge residue pretreated by the wet alkalinizing method: Compressive strength and immobilization efficiency of heavy metal [J]. Construction and Building Materials, 2018, 170: 619-626. doi: 10.1016/j.conbuildmat.2018.03.068 [55] HU S X, ZHONG L L, YANG X J, et al. Synthesis of rare earth tailing-based geopolymer for efficiently immobilizing heavy metals [J]. Construction and Building Materials, 2020, 254: 119273. doi: 10.1016/j.conbuildmat.2020.119273 [56] WANG Y G, HAN F L, MU J Q. Solidification/stabilization mechanism of Pb(II), Cd(II), Mn(II) and Cr(III) in fly ash based geopolymers [J]. Construction and Building Materials, 2018, 160: 818-827. doi: 10.1016/j.conbuildmat.2017.12.006 [57] DERAKHSHAN NEJAD Z, JUNG M C, KIM K H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology [J]. Environmental Geochemistry and Health, 2018, 40(3): 927-953. doi: 10.1007/s10653-017-9964-z [58] LEI C, CHEN T, ZHANG Q Y, et al. Remediation of lead polluted soil by active silicate material prepared from coal fly ash [J]. Ecotoxicology and Environmental Safety, 2020, 206: 111409. doi: 10.1016/j.ecoenv.2020.111409 [59] YUAN X Z, XIONG T, WANG H, et al. Immobilization of heavy metals in two contaminated soils using a modified magnesium silicate stabilizer [J]. Environmental Science and Pollution Research, 2018, 25(32): 32562-32571. doi: 10.1007/s11356-018-3140-6 [60] BAKER M R, COUTELOT F M, SEAMAN J C. Phosphate amendments for chemical immobilization of uranium in contaminated soil [J]. Environment International, 2019, 129: 565-572. doi: 10.1016/j.envint.2019.03.017 [61] WANG X, ZHANG H, WANG L L, et al. Transformation of arsenic during realgar tailings stabilization using ferrous sulfate in a pilot-scale treatment [J]. Science of the Total Environment, 2019, 668: 32-39. doi: 10.1016/j.scitotenv.2019.02.289 [62] CARLSON L, BIGHAM J M, SCHWERTMANN U, et al. Scavenging of As from acid mine drainage by schwertmannite and ferrihydrite: A comparison with synthetic analogues [J]. Environmental Science & Technology, 2002, 36(8): 1712-1719. [63] 刘引, 杨为中, 孙晓龙, 等. 无机试剂无害化处理垃圾焚烧飞灰的研究 [J]. 环境科学导刊, 2015, 34(5): 72-77. doi: 10.3969/j.issn.1673-9655.2015.05.020 LIU Y, YANG W Z, SUN X L, et al. Experimental study of heavy metal stabilization in fly ash of waste combustion using inorganic reagents [J]. Environmental Science Survey, 2015, 34(5): 72-77(in Chinese). doi: 10.3969/j.issn.1673-9655.2015.05.020
[64] 殷景华, 王雅珍, 鞠刚. 功能材料概论[M]. 哈尔滨: 哈尔滨工业大学出版社, 2017. YIN J H, WANG Y Z, JU G. An introduction to functional materials[M]. Harbin: Harbin Institute of Technology Press, 2017(in Chinese).
[65] ZHENG L, WANG W, LI Z F, et al. Immobilization of heavy metal using dithiocarbamate agent [J]. Journal of Material Cycles and Waste Management, 2019, 21(3): 652-658. doi: 10.1007/s10163-019-00829-1 [66] ZHU J M, HAO Q J, CHEN J J, et al. Distribution characteristics and comparison of chemical stabilization ways of heavy metals from MSW incineration fly ashes [J]. Waste Management, 2020, 113: 488-496. doi: 10.1016/j.wasman.2020.06.032 [67] LUO Z T, TANG C B, HAO Y H, et al. Solidification/stabilization of heavy metals and its efficiency in lead-zinc tailings using different chemical agents [J]. Environmental Technology, 2020: 1-11. [68] WANG F H, ZHANG F, CHEN Y J, et al. A comparative study on the heavy metal solidification/stabilization performance of four chemical solidifying agents in municipal solid waste incineration fly ash [J]. Journal of Hazardous Materials, 2015, 300: 451-458. doi: 10.1016/j.jhazmat.2015.07.037 [69] XU Y. Stabilization of heavy metal-contaminated sediment with a Chelator and humic acid mixture [J]. Water, Air, & Soil Pollution, 2016, 228(1): 1-11. [70] 蒋建国, 王伟, 李国鼎, 等. 重金属螯合剂处理焚烧飞灰的稳定化技术研究 [J]. 环境科学, 1999, 20(3): 13-17. doi: 10.3321/j.issn:0250-3301.1999.03.004 JIANG J G, WANG W, LI G D, et al. Experimental study on the chemical stabilization technology in treating with fly ash using heavy metal chelating agent [J]. Environmental Science, 1999, 20(3): 13-17(in Chinese). doi: 10.3321/j.issn:0250-3301.1999.03.004
[71] 朱节民, 李梦雅, 郑德聪, 等. 重庆市垃圾焚烧飞灰中重金属分布特征及药剂稳定化处理 [J]. 环境化学, 2018, 37(4): 880-888. doi: 10.7524/j.issn.0254-6108.2017091407 ZHU J M, LI M Y, ZHENG D C, et al. Distribution and chemical stabilization of heavy metals in municipal solid waste incineration fly ash of Chongqing [J]. Environmental Chemistry, 2018, 37(4): 880-888(in Chinese). doi: 10.7524/j.issn.0254-6108.2017091407
[72] SU P D, ZHANG J K, LI Y D. Investigation of chemical associations and leaching behavior of heavy metals in sodium sulfide hydrate stabilized stainless steel pickling sludge [J]. Process Safety and Environmental Protection, 2019, 123: 79-86. doi: 10.1016/j.psep.2019.01.001 [73] LYU H H, ZHAO H, TANG J C, et al. Immobilization of hexavalent chromium in contaminated soils using biochar supported nanoscale iron sulfide composite [J]. Chemosphere, 2018, 194: 360-369. doi: 10.1016/j.chemosphere.2017.11.182 [74] OSBORNE L R, BAKER L L, STRAWN D G. Lead immobilization and phosphorus availability in phosphate-amended, mine-contaminated soils [J]. Journal of Environmental Quality, 2015, 44(1): 183-190. doi: 10.2134/jeq2014.07.0323 [75] ZHANG B R, ZHOU W X, ZHAO H P, et al. Stabilization/solidification of lead in MSWI fly ash with mercapto functionalized dendrimer Chelator [J]. Waste Management, 2016, 50: 105-112. doi: 10.1016/j.wasman.2016.02.001 [76] 郑鹏, 刘建国, 刘锋, 等. 垃圾焚烧飞灰磷酸洗涤对重金属的固定效应研究 [J]. 环境工程学报, 2007, 1(1): 121-125. doi: 10.3969/j.issn.1673-9108.2007.01.031 ZHENG P, LIU J G, LIU F, et al. Effects of phosphoric acid washing on immobilization of heavy metals in municipal solid waste incineration fly ash [J]. Chinese Journal of Environmental Engineering, 2007, 1(1): 121-125(in Chinese). doi: 10.3969/j.issn.1673-9108.2007.01.031
[77] ZHANG Z B, SHI X H, ZHANG Y H, et al. Study on immobilization of diatomite, Ca(H2PO4)2, CaCO3, HAP and nano-HAP for heavy metal contaminated sediment [J]. Water Quality Research Journal, 2020, 55(4): 370-381. doi: 10.2166/wqrj.2020.117 [78] 陆俏利, 瞿广飞, 吴斌, 等. 矿区含砷尾矿及废渣稳定化研究 [J]. 环境工程学报, 2016, 10(5): 2587-2594. doi: 10.12030/j.cjee.201412257 LU Q L, QU G F, WU B, et al. Study on stabilization of arsenic tailings and waste residue [J]. Chinese Journal of Environmental Engineering, 2016, 10(5): 2587-2594(in Chinese). doi: 10.12030/j.cjee.201412257
[79] LI X Y, CHEN Q Y, ZHOU Y S, et al. Stabilization of heavy metals in MSWI fly ash using silica fume [J]. Waste Management, 2014, 34(12): 2494-2504. doi: 10.1016/j.wasman.2014.08.027 [80] XU Y, CHEN Y, FENG Y Y. Stabilization treatment of the heavy metals in fly ash from municipal solid waste incineration using diisopropyl dithiophosphate potassium [J]. Environmental Technology, 2013, 34(9/10/11/12): 1411-1419. [81] ZHANG M L, GUO M R, ZHANG B R, et al. Stabilization of heavy metals in MSWI fly ash with a novel dithiocarboxylate-functionalized polyaminoamide dendrimer [J]. Waste Management, 2020, 105: 289-298. doi: 10.1016/j.wasman.2020.02.004 [82] 李静, 周斌, 易新建, 等. 垃圾焚烧飞灰重金属稳定化药剂处理效果 [J]. 环境工程学报, 2016, 10(6): 3242-3248. doi: 10.12030/j.cjee.201501090 LI J, ZHOU B, YI X J, et al. Treatment efficiencies of heavy metals in municipal solid waste incineration fly ash with stabilization agents [J]. Chinese Journal of Environmental Engineering, 2016, 10(6): 3242-3248(in Chinese). doi: 10.12030/j.cjee.201501090
[83] 刘元元, 王里奥, 林祥, 等. 城市垃圾焚烧飞灰重金属药剂配伍稳定化实验研究 [J]. 环境工程学报, 2007, 1(10): 94-99. doi: 10.3969/j.issn.1673-9108.2007.10.022 LIU Y Y, WANG L, LIN X, et al. Experimental study of the stabilization of heavy metals in municipal solid waste incineration fly ash by chemical agent matching [J]. Chinese Journal of Environmental Engineering, 2007, 1(10): 94-99(in Chinese). doi: 10.3969/j.issn.1673-9108.2007.10.022
[84] CHOI S G, CHANG I, LEE M, et al. Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers [J]. Construction and Building Materials, 2020, 246: 118415. doi: 10.1016/j.conbuildmat.2020.118415 [85] KRAJEWSKA B. Urease-aided calcium carbonate mineralization for engineering applications: A review [J]. Journal of Advanced Research, 2018, 13: 59-67. doi: 10.1016/j.jare.2017.10.009 [86] HAN L J, LI J S, XUE Q, et al. Bacterial-induced mineralization (BIM) for soil solidification and heavy metal stabilization: A critical review [J]. Science of the Total Environment, 2020, 746: 140967. doi: 10.1016/j.scitotenv.2020.140967 [87] MAITY J P, CHEN G S, HUANG Y H, et al. Ecofriendly heavy metal stabilization: Microbial induced mineral precipitation (MIMP) and biomineralization for heavy metals within the contaminated soil by indigenous bacteria [J]. Geomicrobiology Journal, 2019, 36(7): 612-623. doi: 10.1080/01490451.2019.1597216 [88] VALENZUELA E I, GARCÍA-FIGUEROA A C, AMÁBILIS-SOSA L E, et al. Stabilization of potentially toxic elements contained in mine waste: A microbiological approach for the environmental management of mine tailings [J]. Journal of Environmental Management, 2020, 270: 110873. doi: 10.1016/j.jenvman.2020.110873 [89] CHEN P, ZHENG H, XU H, et al. Microbial induced solidification and stabilization of municipal solid waste incineration fly ash with high alkalinity and heavy metal toxicity [J]. PLoS One, 2019, 14(10): e0223900. doi: 10.1371/journal.pone.0223900 [90] QIAN C X, YU X N, WANG X. Potential uses and cementing mechanism of bio-carbonate cement and bio-phosphate cement [J]. AIP Advances, 2018, 8(9): 095224. doi: 10.1063/1.5040730 [91] WANG Z Y, ZHANG N, CAI G J, et al. Review of ground improvement using microbial induced carbonate precipitation (MICP) [J]. Marine Georesources & Geotechnology, 2017, 35(8): 1135-1146. [92] LI C K, LI Q S, WANG Z P, et al. Environmental fungi and bacteria facilitate lecithin decomposition and the transformation of phosphorus to apatite [J]. Scientific Reports, 2019, 9(1): 15291. doi: 10.1038/s41598-019-51804-7 [93] YU X N, JIANG J G. Phosphate microbial mineralization consolidation of waste incineration fly ash and removal of lead ions [J]. Ecotoxicology and Environmental Safety, 2020, 191: 110224. doi: 10.1016/j.ecoenv.2020.110224 [94] LIU X Y, ZHANG M J, LI Y B, et al. In situ bioremediation of tailings by sulfate reducing bacteria and iron reducing bacteria: Lab- and field-scale remediation of sulfidic mine tailings [J]. Solid State Phenomena, 2017, 262: 651-655. doi: 10.4028/www.scientific.net/SSP.262.651 [95] SHEORA N A S, SHEORAN V, CHOUDHARY R P. Bioremediation of acid-rock drainage by sulphate-reducing prokaryotes: A review [J]. Minerals Engineering, 2010, 23(14): 1073-1100. doi: 10.1016/j.mineng.2010.07.001 [96] LI F, WANG W, LI C C, et al. Self-mediated pH changes in culture medium affecting biosorption and biomineralization of Cd2+ by Bacillus cereus Cd [J]. Journal of Hazardous Materials, 2018, 358: 178-186. doi: 10.1016/j.jhazmat.2018.06.066 [97] KANG C H, KWON Y J, SO J S. Bioremediation of heavy metals by using bacterial mixtures [J]. Ecological Engineering, 2016, 89: 64-69. doi: 10.1016/j.ecoleng.2016.01.023 [98] BHATTACHARYA A, NAIK S N, KHARE S K. Harnessing the bio-mineralization ability of urease producing Serratia marcescens and Enterobacter cloacae EMB19 for remediation of heavy metal cadmium (Ⅱ) [J]. Journal of Environmental Management, 2018, 215: 143-152. doi: 10.1016/j.jenvman.2018.03.055 [99] LI M, CHENG X H, GUO H X, et al. Biomineralization of carbonate by Terrabacter tumescens for heavy metal removal and biogrouting applications [J]. Journal of Environmental Engineering, 2016, 142(9): 1-5. [100] ZHU X J, LI W L, ZHAN L, et al. The large-scale process of microbial carbonate precipitation for nickel remediation from an industrial soil [J]. Environmental Pollution, 2016, 219: 149-155. doi: 10.1016/j.envpol.2016.10.047 [101] ZHAO X M, DO H, ZHOU Y, et al. Rahnella sp. LRP3 induces phosphate precipitation of Cu (Ⅱ) and its role in copper-contaminated soil remediation [J]. Journal of Hazardous Materials, 2019, 368: 133-140. doi: 10.1016/j.jhazmat.2019.01.029 [102] ZHANG K J, ZHANG D W, WU X J, et al. Continuous and efficient immobilization of heavy metals by phosphate-mineralized bacterial consortium [J]. Journal of Hazardous Materials, 2021, 416: 125800. doi: 10.1016/j.jhazmat.2021.125800 [103] HAN L J, LI J S, XUE Q, et al. Enzymatically induced phosphate precipitation (EIPP) for stabilization/solidification (S/S) treatment of heavy metal tailings [J]. Construction and Building Materials, 2022, 314: 125577. doi: 10.1016/j.conbuildmat.2021.125577 [104] LI X, WU Y E, ZHANG C, et al. Immobilizing of heavy metals in sediments contaminated by nonferrous metals smelting plant sewage with sulfate reducing bacteria and micro zero valent iron [J]. Chemical Engineering Journal, 2016, 306: 393-400. doi: 10.1016/j.cej.2016.07.079 [105] LIU Y J, WU S L, SOUTHAM G, et al. Bioaugmentation with Acidithiobacillus species accelerates mineral weathering and formation of secondary mineral cements for hardpan development in sulfidic Pb-Zn tailings [J]. Journal of Hazardous Materials, 2021, 411: 124988. doi: 10.1016/j.jhazmat.2020.124988 -




 下载:
下载: