-
汞离子(Hg2+)是一种广泛存在于工业废水、土壤、农作物等多种介质中的有毒金属离子,易于通过生物放大在生物体或人体内富集,对人类生命健康构成潜在的威胁[1]. 环境中该类污染物的控制措施和环保管理,均需要精确获得环境介质中污染物浓度,因此建立能够精确检测环境介质中的Hg2+含量的方法具有极其重要的意义. 目前,常用环境介质中Hg2+浓度检测方法很多,如原子吸收光谱法(AAS)[2]、原子发射光谱法(AES)[3]、原子荧光光谱法等(AFS)[4]、电感耦合等离子体-质谱(ICP-MS)[5]等方法,但这些方法均需要复杂的、耗时的样品预处理过程和无法实现便携式操作. 因而,开发一种能够高效、快速、便捷式金属Hg2+检测方法迫在眉睫.
碳量子点(CQDs)和贵金属纳米簇(CNs)是目前常用的两类荧光纳米材料. 由于它们的制备成本低廉、操作简单、灵敏度高和选择性好等优点,已被广泛应用于各种介质中金属离子的检测. 如Cao等[6]利用水热法合成了掺硅量子点并对血清中尿酸和痕量汞进行荧光检测. 铜纳米簇(CuCNs)因制备原料的成本较低,被广泛用于制备各种荧光材料. 如XU [7]等以牛血清蛋白为铜纳米簇适配体,实现CuCNs对汞离子的检测. 但单一荧光响应检测污染物的含量易受荧光探针周围不稳定条件(如温度、溶剂极性、探针分布及目标物浓度)、仪器参数、探针分子的局部浓度和光漂白的影响[8-9]. 比率型荧光检测是一种对比双荧光响应的荧光分析方法[10],可通过不同纳米荧光材料的荧光响应比值来反应目标物的浓度. 比率型荧光探针作为一种有效的内部基准,响应比值可以极大地消除外部干扰,并更精确地对目标物进行定性和定量分析[11]. CQDs和CNs是比率型荧光探针的常见组合形式[12]. 如Liu等[13]构建了CQDs/金纳米簇(AuCNs)比率型荧光探针,能高灵敏地检测精氨酸.
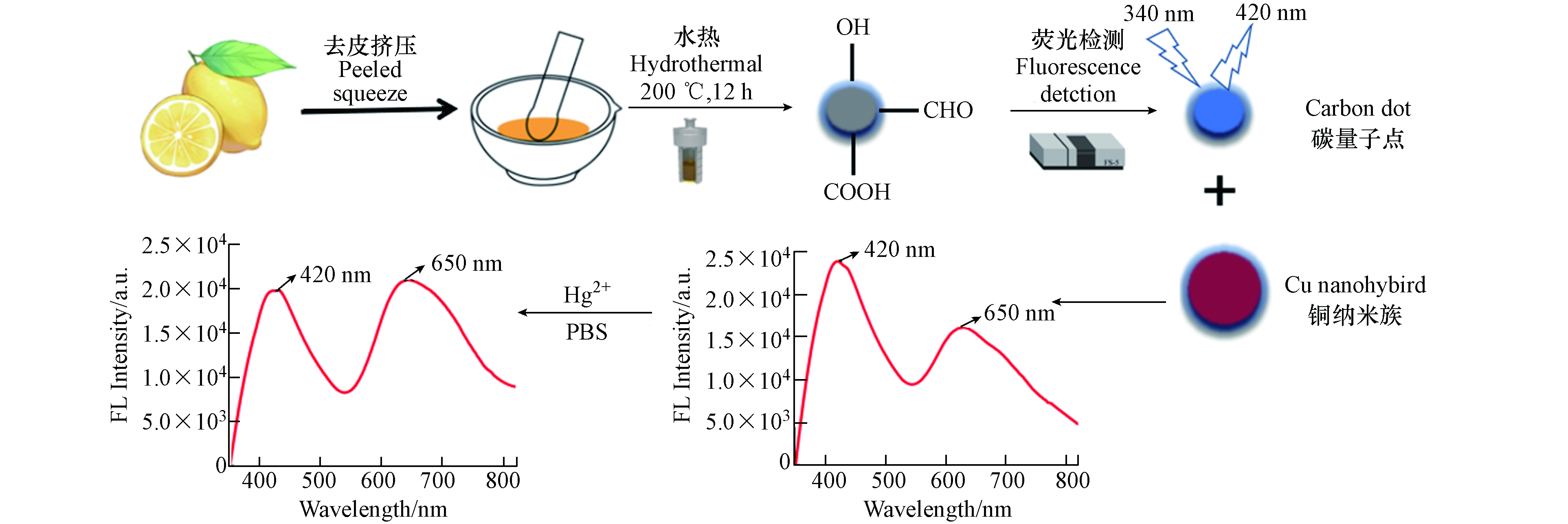
本文利用绿色柠檬汁,通过一步水热法合成碳量子点(CQDs)及谷胱甘肽修饰的铜纳米簇(CuCNs)两种荧光材料,按比例混合出一种能够快速、准确和具有现场检测潜力的Hg2+比率荧光传感器. 相比于单一荧光探针,比率荧光探针具有水分散性好、检测下限低、不需要复杂的修饰和较长的反应时间等优点,同时本文也为其它生物质制成碳点构成比率型荧光提供思路.
-
新鲜柠檬(美国新奇士)购买于苏州某超市;谷胱甘肽(GSH)、硫酸铜、氢氧化钠、醋酸和醋酸钠均为分析纯且使用过程不加任何处理. 超纯水由Milli-Q超纯水系统(>18.25 MΩ)制备. YHG-9050A电热恒温鼓风干燥箱(上海新苗医疗器械制造有限公司生产)、KQ-500DE型超声波清洗器(昆山市超声仪器有限公司)、UV-5500PC紫外可见光分光光度计(上海元析仪器有限公司)、JEOL JEM 2100F高倍透射电子显微镜(日本日立仪器公司)、IR Prestige-21傅立叶变换红外光谱仪(美国赛默飞世尔科技公司)、FS-5荧光分光光度计(英国爱丁堡有限公司)、KH19A型飞鸽离心机(上海安亭科学仪器厂).
-
首先,参照已有文献[14],利用水热法合成CQDs,具体如下:将清洗干净的新鲜柠檬挤压出汁液,取 15 mL无浆柠檬汁与10 mL超纯水混合,之后转移至100 mL的高压反应釜中,并将反应釜置于200 ℃烘箱(YHG-9050A)中反应12 h,冷却至室温,将反应液转移至50 mL尖底离心管中,8000 r.min−1转速下离心10 min,将上清液用0.22 μm滤膜过滤,之后转移至透析袋(MW=1000 Da)中纯化12 h. 利用高倍透射电子显微镜(HRTEM)分析CQDs的微观结构,利用傅立叶变换红外光谱仪(FTIR)和X射线光电子能谱仪(XPS)分析CQDs的表面官能团.
-
根据已有文献[15]合成铜纳米簇. 具体如下:将10 mL浓度为0.163 mol.L−1的GSH水溶液缓慢滴加到10 mL浓度为10 mmol.L−1 CuSO4水溶液中,混合液在37 ℃恒温搅拌1 h,之后再逐滴加入浓度为 1 mmol.L−1 的NaOH溶液,当溶液变成浅黄色后停止,在37 ℃恒温搅拌2 h,该浅黄色溶液即为GSH-CuNCs,将GSH-CuNCs避光保存于4 ℃冰箱,待用.
-
将CQDs与CuNCs按照一定比例混合(图1),用醋酸和醋酸钠缓冲溶液调节该体系pH值至5.5,磁力搅拌反应10 min,形成CQDs-CuNCs复合体系,并将其避光保存于4 ℃冰箱. 为了使该荧光探针具有良好的荧光性能,对该体系中主要参数进行了优化,如两类荧光材料的体积比(1∶1、1∶2、1∶3、1∶4和1∶5)、体系pH(3.0、3.5、4.0、4.5、5.0、5.5、6.0、6.5和7.0)、体系温度(10、20、30、40、50 ℃)和加入Hg2+的反应时间(0、2、4、6、8、10、12、14、16、18、20 min).
在最优条件下,取100 μL的CQDs-CuNCs复合溶液,然后分别加入不同浓度的Hg2+的标准溶液. 用1 mol.L−1的PBS缓冲液(pH=5.5)将溶液定容到2.4 mL,之后于30 ℃恒温水浴反应4 min. 在340 nm激发波长下,测试体系的荧光强度.
-
采集苏州市太湖2个监测点的地表水和苏州市某城镇污水厂出水,取样1 L,带回实验室后,经0.45 μm滤膜过滤后,8000 r.min−1下离心10 min以去除悬浮颗粒物. 后续操作步骤参照1.4节,检测加标前后Hg2+对荧光探针的荧光响应,计算加标回收率,并平行测试3次,计算标准偏差.
-
方法的检出限利用检测限LOD = 3 δ/S公式来计算. 式中,δ为无Hg2+时(空白样品)荧光探针的I650/I420比值的标准偏差,S即工作曲线的斜率.
利用标准的Stern-Volmer淬灭方程来描述添加不同浓度的Hg2+时比率型荧光体系中CQDs的荧光淬灭. (F1/F2) = Ksv C + 1. 式中F1表示加Hg2+时荧光强度比值,F2表示未加Hg2+时荧光强度比值,Ksv 表示Stern-Volmer淬灭常数,C为添加Hg2+的浓度,单位为nmol.L−1.
-
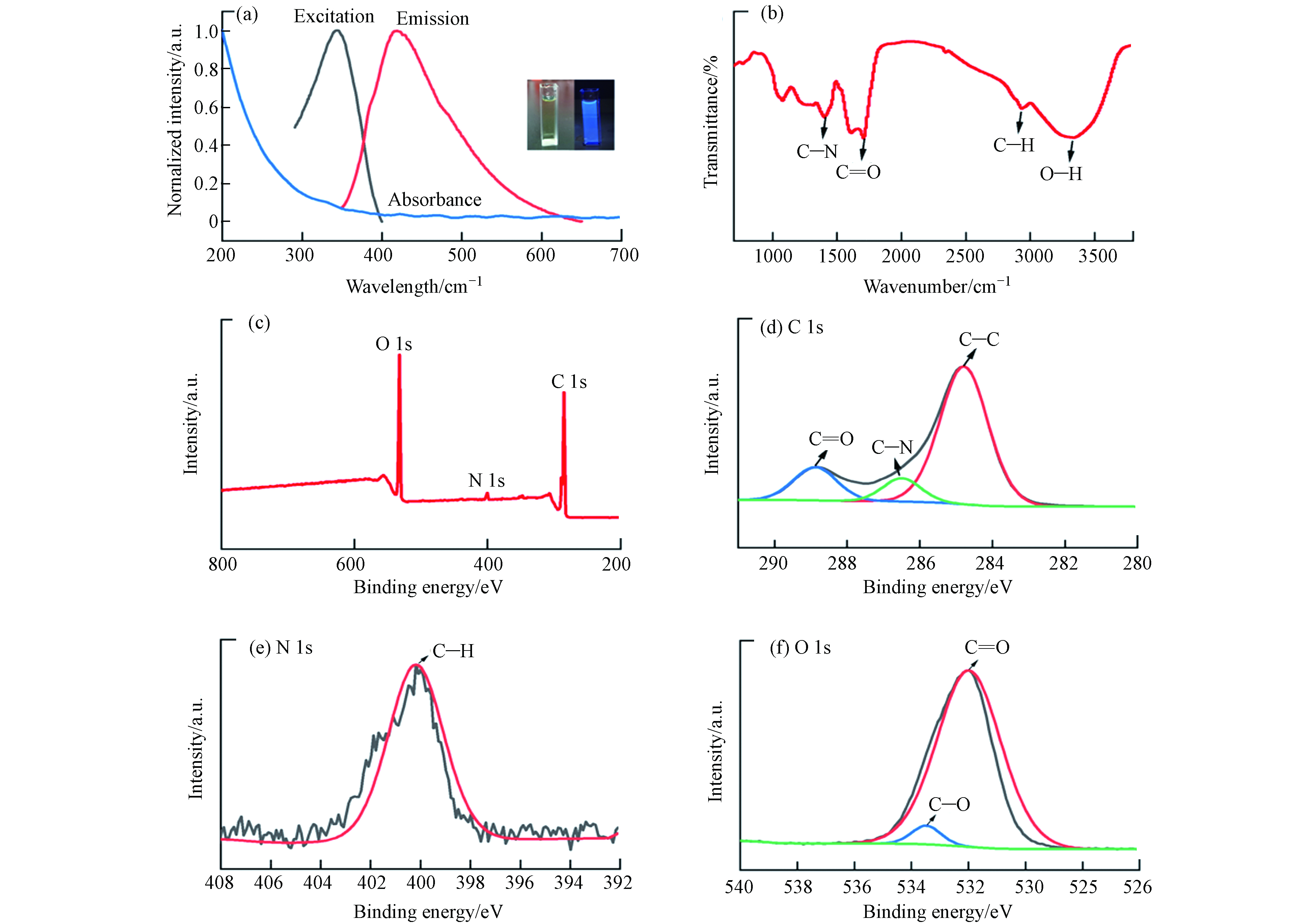
CQDs的HRTEM如图2(a)所示,CQDs的粒径均匀,呈球形,分散性良好,粒径范围在2.5—5.5 nm之间,呈正态分布(图2(b)),平均粒径为(3.88±0.26)nm,图2(a)插图高倍TEM表明条纹间距为0.26 nm的晶格平面,类石墨碳结构[16].
CQDs的紫外-可见吸收光谱如图3(a)插图所示,柠檬基CQDs溶液呈明显的淡黄色,在365 nm的紫外光下,溶液呈现出明亮的蓝色荧光,同时图3(a)蓝色曲线表明CQDs在紫外光区显示较强的光吸收,尾部延伸到可见光区. 此外,在激发波长为340 nm时,发射光谱中发现最大发射峰在420 nm处,这可能是由C=O键sp2的n-π*跃迁引起的[17-18].
CQDs的红外光谱见图3(b),在1385 cm−1处吸收峰属于C—N的伸缩振动峰[19],1654 cm−1强吸收峰是C=O双键伸缩振动峰[20],说明存在羰基. 在2940 cm−1 处吸收峰属于C—H的伸缩振动峰,在3385cm−1吸收峰属于O—H的伸缩振动峰[21]. 该结果表明,CQDs表面含有—OH、C=O等亲水性基团,说明CQDs具有良好的水溶性. 为了确定CQDs的元素组成和表面化学性质,进一步用XPS对其进行了表征. 由图3(c)所示,3个吸收峰分别对应C1s、N1s、O1s,键能分别为284.50、531.9 、399.9 eV,3种元素含量分别为64.92%、29.90%、2.96%. 对XPS全谱图进行分峰,图3(d)是C1s的分峰图,对应的键能为284.5、286.8、288.5 eV,分别对应为C—C、C—O、C=O. 图3(e)是N1s的分峰图,键能401.8 eV,对应为C—N。图3(f)是O1s的分峰图,对应的键能为532.1 、533.5 eV,分别对应C=O、C—OH. 这些结果与FT-IR的结果一致,说明CQDs表面有羟基和羰基等亲水性基团.
-
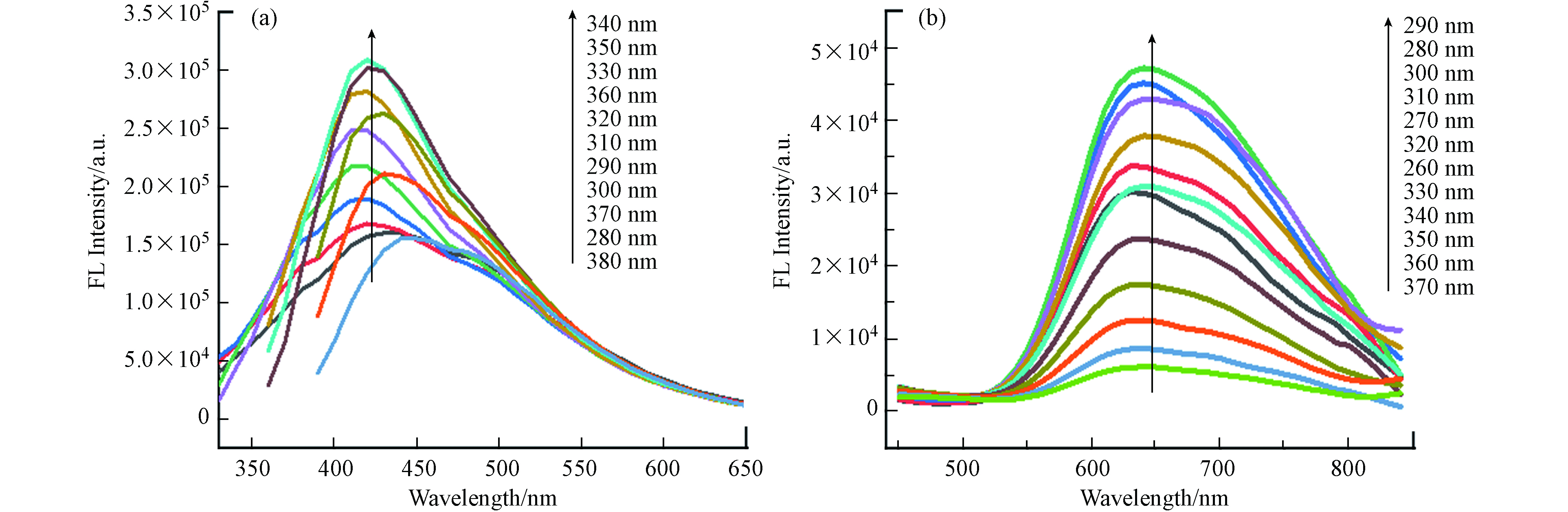
如图4所示,在340 nm的激发波长下,CQDs和CuNCs的荧光发射峰分别在420 nm和650 nm处,荧光强度达到最大. 图4(a)呈现将激发波长从280 nm增加到380 nm,会导致 CQDs的发射峰出现红移且荧光发射强度减弱,说明柠檬基CQDs具有随着激发波长变化而改变发射峰位置的特性和明显的波长可调性[22]. 可能是由于CQDs表面发光位点不同或不同粒径的CQDs尺寸效应形成的[23]. 图4(b)显示CuNCs在激发波长从260 nm增加到370 nm,发射波长没有明显的红移,说明制备的CuNCs颗粒比较均匀[24].
-
CQDs-CuNCs复合体系中主要因素如CQDs与CuNCs的比例、溶液pH、温度及反应时间将影响CQDs-CuNCs复合体系荧光响应. 图5(a)表明从CQDs与CuNCs比例1:1增加到1:5时,420 nm处(对应于CQDs)的荧光响应强度下降,而650 nm处(对应CuNCs)的荧光响应强度增加,当体积比为1:4时,二者荧光强度相近,为了后续构建的比率型荧光探针在两波长处的荧光强度的变化便于观察,因此选择CQDs与CuNCs的体积比1:4为最佳比例.
非常有趣地是在CQDs-CuNCs复合体系中,随着Hg2+浓度的增加,CQDs在420 nm处的蓝色发光缓慢下降,CuNCs在650 nm处的红色发光缓慢上升(图6),表明Hg2+能够使复合体系中CQDs的蓝光被淬灭,并且使CuNCs的红光被激活,这可能由于CQDs与CuNCs之间存在内滤光效应. 这可能是由于Hg2+与CQDs主要的表面官能团之间发生配位作用. 从而构建了对Hg2+响应的CQDs-CuNCs比率型荧光探针,利用波长650 nm和420 nm处二者的荧光发射强度的比值(I650/I420)来表示两峰的荧光强度变化.
由于pH值、温度对CQDs-CuNCs复合体系本身的荧光响应也会产生较大影响,因此对比加Hg2+与无Hg2+时的I650/I420比值,获得CQDs-CuNCs复合体系对Hg2+强响应的最佳pH、温度和反应时间. 利用F1-F2(F1与F2分别表示添加50 nmol.L−1浓度的Hg2+和无Hg2+时I650/I420比值)来表示CQDs-CuNCs复合体系对Hg2+实际荧光响应. 图5(b)表明pH从3.0到5.5时,F1-F2值快速增加,这可能是由于酸度浓度变小,CQDs的质子或非质子化并不明显,因此酸度浓度变小时,CQDs不容易团聚[25],继续增加pH值,F1-F2值则下降,可能是因为pH的变化导致荧光探针表面官能团解离出负电荷,会影响CQDs-CuNCs复合体系带电性[26],故反应最佳pH值为5.5. 图5(c)表明温度由10 ℃上升至30 ℃时,F1-F2值逐渐上升,30 ℃时F1-F2值达到最大,继续增加温度 ,F1-F2反而下降,故最佳反应温度为30 ℃. 图5(d)表明加入Hg2+从0 min到4 min,F1-F2值不断增加,4 min之后F1-F2值略有起伏,但基本趋于稳定,故最佳反应时间为4 min.
-
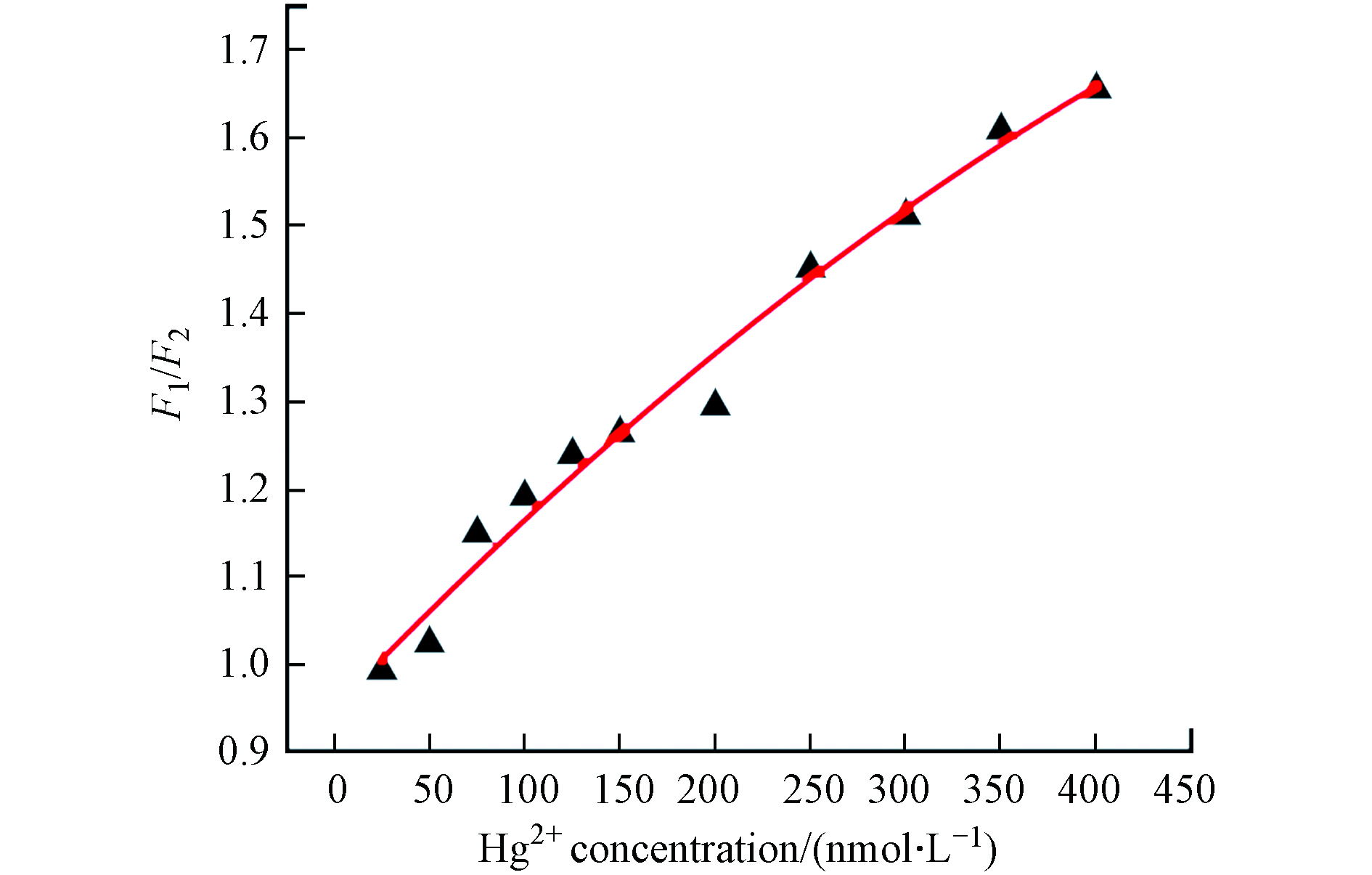
在最优的条件下,将不同浓度的Hg2+加入到CQDs-CuNCs复合体系中,Hg2+对CQDs和CuNCs的荧光响应如图6(a),随Hg2+浓度的增加,在420 nm处(CQDs)的荧光强度下降,在650 nm处(CuNCs)的荧光强度增加,并且将I650/I420比值与Hg2+浓度进行线性分析,结果表明CQDs-CuNCs比率型荧光探针对Hg2+浓度在25—400 nm.mol−1范围内有较好的线性关系(R2=0.9965)(见图6(b)). 利用LOD的计算公式推算出该方法对Hg2+的检测限为13.0 nmol.L−1.
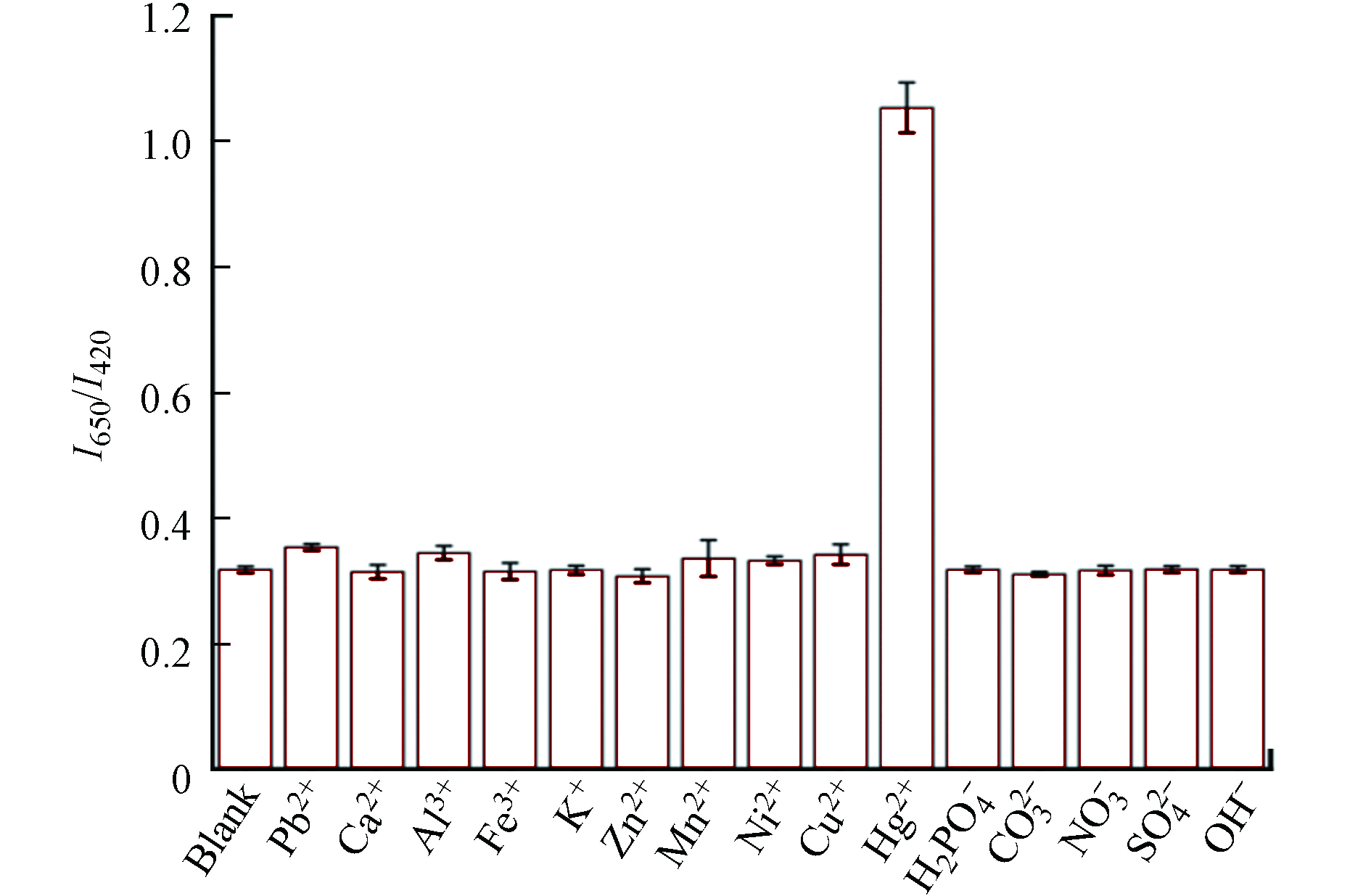
CQDs-CuNCs荧光探针特异性是判断该荧光探针能否高选择性检测Hg2+的重要指标之一. 水体常见的金属阳离子和阴离子与Hg2+共存时,对CQDs-CuNCs复合体系的I650 /I420比值的影响,如图7所示,Ca2+、Mg2+、Al3+、Ni2+、Pb2+、Ni2+、Fe3+、Mn2+、K+、Zn2+、Cu2+,浓度均为0.4 μmol.L−1的金属阳离子,仅有Hg2+对CQDs-CuNCs混合体系I650 /I420比值的变化明显,而
${\rm{H}}_2{\rm{PO}}_4^{-} $ 、${\rm{NO}}_2^{-} $ 、${\rm{CO}}_3^{2-} $ 、${\rm{SO}}_4^{2-} $ 和OH−等阴离子对I650 /I420比值略微发生变化,但基本稳定. 因此,CQDs-CuNCs比率型荧光探针对Hg2+具有较好的选择性. -
为了证实该荧光探针能用于实际水样中Hg2+的检测,按照1.4节步骤对2个湖水样品和1个污水厂出水样品进行Hg2+的荧光检测,结果均未检出. 因此在湖水和污水水样中加入浓度分别为10、50、100 nmol·L−1的汞离子溶液进行加标回收实验,平行测试3次. 结果见表1,实际水样中Hg2+加标回收率在94.12%—103.31%之间,相对标准偏差(RSD)均小于4.52%,说明该荧光探针对Hg2+检测方法具有较高的准确性和重现性.
与国家标准方法(GB/T 5750—2006)[27]规定的Hg2+测定方法相比,该比率型荧光探针的检测限和RSD均小于国标规定(50 nmol·L−1和6.8%),该方法回收率也在国标法的范围内(86.7%—120%). 因此,本方法的准确度和精密度均满足国标要求. 由于目前有关荧光探针检测Hg2+方法较多,本实验比较了部分已报道的单一探针与比率型探针对Hg2+检测的线性范围和检出限. 表2表明,本文的双荧光探针比单独探针对Hg2+检测,具有更低的检出限. 同时该双荧光探针具有合成方法简单、绿色环保、无二次污染物等优点,使其具有更广泛的应用前景.
-
已有报道认为比率型荧光探针的荧光性能主要是基于CQDs和CuNCs之间存在内滤光效应(IFE)[35-37],即随着CuNCs比例的增加,CQDs的荧光被略微淬灭(见图5(a)). 而随着Hg2+的加入,CQDs的荧光被淬灭,Hg2+浓度越大猝灭程度越强. 可能是由于Hg2+与CQDs的表面基团(C=O、C—O—C、—OH等)之间形成配合物,改变了CQDs的电子结构,使CQDs导带激发态的电子通过表面官能团转移给Hg2+,产生有效的电子转移,促进CQDs激发光的非辐射复合,导致CQDs荧光猝灭[38-39]. 由于CQDs的荧光下降,降低了CQDs与CuNCs之间的IFE效应,导致CuNCs的荧光恢复,同时可能Hg2+与CuNCs之间聚集诱导发射增强(aggregate -induced emission enhancement, AIEE),Hg2+增强了CuNCs的荧光[40-42],CuNCs与CQDs荧光强度的比值与Hg2+浓度在一定范围内呈较好的线性,这证实了双荧光探针能对Hg2+浓度产生荧光响应.
同时为了验证Hg2+对该双荧光探针中QCDs的荧光淬灭机理. 分别添加Hg2+浓度为25、50、75、100、125、150、200、250、300、350、400 nmol.L−1,利用F1/F2(F1与F2分别表示加Hg2+和无Hg2+时I650/I420比值)与Hg2+浓度进行Stern-Volmer方程作图,图8表明该F1/F2与Hg2+浓度之间并不符合传统的线性Stern-Volmer方程,这说明该双荧光体系同时存在动态和静态猝灭[43-44].
-
本文利用简单易得的水果柠檬为碳源,通过简单的一步水热法制成了柠檬基的CQDs,并与GSH-CuNCs按照一定比例组成了双发射荧光探针,该探针Hg2+浓度在25—400 nmol.L−1的范围内具有良好的线性关系,相关系数 R2=0.9965,最低检测限为13.0 nmol.L−1. 该方法利用绿色、简单、成本低的荧光探针实现了水中痕量Hg2+的检测,该方法简便、快捷、高效,样品仅需简单的过滤,具有便携式检测或在线检测的前景,将该荧光探针应用于实际水样中Hg2+的检测,获得较好的加标回收率,较低的相对标准偏差,该结果表明该双荧光探针具有良好的抗干扰性和稳定性,较好地适用于实际水体中Hg2+的检测.
柠檬基碳量子点/铜纳米团簇比率型荧光探针对环境水中Hg2+检测的应用
Application of ratiometric fluorescence probe composed of copper nanoclusters and carbon quantum dots based fresh lemon juice on Hg2+ detection in environmental water
-
摘要: 本文以新鲜柠檬汁为碳源,利用一步水热法合成了具有荧光性能良好的荧光碳量子点(CQDs)和谷胱甘肽封端的铜纳米簇(CuNCs). 由荧光光谱分析可知,在同一激发下,CQDs和CuNCs分别在420 nm和650 nm处有明显特征荧光发射峰值. 将二者按一定比例构建了CQDs-CuCNs双荧光探针,并用于水环境中汞离子的荧光检测. 结果发现,Hg2+加入CQDs-CuCNs双荧光体系后,该荧光探针在420 nm处荧光发射峰值下降,但在650 nm处荧光发射峰值略微上升. 将该双荧光探针的荧光发射峰值的比值I650/I420(在650 nm处与420 nm处的荧光发射峰值的比值)与汞离子浓度进行线性回归,结果在Hg2+浓度25—400 nmol·L-1范围内呈现良好线性(R2 = 0.9965),检出限为13.0 nmol·L-1. 该荧光探针对湖水和污水厂出水中Hg2+均未检出,但实际样品加标回收率在94.12%—103.31%之间,相对标准偏差均小于4.52%,表明该方法适用于实际水样中汞离子的快速检测.Abstract: In this paper, fluorescent carbon quantum dots (CQDs) with good fluorescence performance were synthesized by a one-step hydrothermal method using fresh lemon juice as the carbon source, and copper nanoclusters (CuCNs) were synthesized using glutathione and copper sulfate as the raw materials, and the fluorescence spectra analysis showed that both CQDs and CuCNs had obvious characteristic fluorescence emission peaks at 420 nm and 650 nm, respectively, under the same excitation wavelength. The dual fluorescent probes of CQDs-CuCNs were constructed in a certain proportion and applied to the fluorescence detection of mercury ions in the actual environmental water. During mercury ion spiked into the dual fluorescent system of CQDs-CuCNs, the fluorescence emission peak at 420 nm (luminescence of CQDs) was quenched, but the peak at the 650 nm (luminescence of CuCNs) was stable. Moreover, the fluorescence intensity of the CQDs based lemon was quenched gradually with increasing amounts of Hg(Ⅱ). The ratio of fluorescence emission intensity I650/I420, which is defined as the intensity ratio of fluorescence emission peak at 650 nm to that at 420 nm, was found to be good linear relationship (R2=0.9965) with Hg(Ⅱ) concentration in the range of 25—400 nmol·L−1, and the detection limit was 13.0 nmol·L−1. The fluorescent probe for detection Hg(Ⅱ) in lake water and effluent of wastewater treatment plant were not detected, but the standard addition recovery rate of Hg(Ⅱ) in the actual water were between 94.12% and 103.31%, and relative standard deviation (RSD) were less than 4.52%. These results show that the ratiometric fluorescent probes are suitable for the rapid detection of mercury ions in real water samples.
-

-
图 6 不同浓度Hg2+存在下CQDs-CuNCs体系的荧光光谱对比图(a)及其相应的荧光发射强度比( I650 /I420 )与 Hg2+的线性关系(b)
Figure 6. Fluorescence spectra of CQDs-CuNCs probe after the addition of different concentration of Hg2+(a)The linear relationship between the fluorescence ratio ( I650/I420 ) of CQDs-CuNCs probe and the concentration of Hg2+(b)
表 1 3种实际加标水样中 Hg2+加标回收率和相对标准偏差(n = 3)
Table 1. Recovery ratio and relative standard deviation of Hg2+ in three actual spiked water samples (n = 3)
样品来源
Sample source加标量/(nmol·L−1)
Spiked concentration测试值/(nmol·L−1)
Tested concentration回收率/%
Recovery(n=3)相对标准偏差/%
RSD(n=3)太湖监测点1湖水 20 18.82 94.12 3.76 50 51.26 102.51 4.02 100 8.78 98.65 3.57 太湖监测点2湖水 20 19.54 97.52 2.68 50 50.89 101.86 3.99 100 1102.31 102.26 4.52 污水厂出水 20 19.93 99.63 2.56 50 51.65 103.31 1.73 100 98.56 98.36 4.16 表 2 不同荧光分析法对汞离子检测效果的对比
Table 2. The comparison of different fluorescence analysis methods for detection of mercury ion
-
[1] LI H L, ZHAI J F, TIAN J Q, et al. Carbon nanoparticle for highly sensitive and selective fluorescent detection of mercury(II) ion in aqueous solution [J]. Biosensors and Bioelectronics, 2011, 26(12): 4656-4660. doi: 10.1016/j.bios.2011.03.026 [2] LEE Y F, NAN F H, CHEN M J, et al. Detection and removal of mercury and lead ions by using gold nanoparticle-based gel membrane [J]. Analytical Methods, 2012, 4(6): 1709. doi: 10.1039/c2ay05872c [3] HAN F X, PATTERSON W D, XIA Y J, et al. Rapid determination of mercury in plant and soil samples using inductively coupled plasma atomic emission spectroscopy, a comparative study [J]. Water Air and Soil Pollution, 2006, 170(1/2/3/4): 161-171. [4] ZAIB M, ATHAR M M, SAEED A, et al. Electrochemical determination of inorganic mercury and arsenic—A review [J]. Biosensors and Bioelectronics, 2015, 74: 895-908. doi: 10.1016/j.bios.2015.07.058 [5] RASTOGI L, SASHIDHAR R B, KARUNASAGAR D, et al. Gum kondagogu reduced/stabilized silver nanoparticles as direct colorimetric sensor for the sensitive detection of Hg2+ in aqueous system [J]. Talanta, 2014, 118: 111-117. doi: 10.1016/j.talanta.2013.10.012 [6] CAO D, LUO Y X, LIU W P, et al. Enzyme-free fluorescence determination of uric acid and trace Hg(Ⅱ) in serum using Si/N doped carbon dots [J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2021, 263: 120182. doi: 10.1016/j.saa.2021.120182 [7] XU J, HAN B Y. Synthesis of protein-directed orange/red-emitting copper nanoclusters via hydroxylamine hydrochloride reduction approach and their applications on Hg2+ sensing [J]. NANO, 2016, 11: 1650108. doi: 10.1142/S1793292016501083 [8] ZANG J C, LI C G, ZHOU K, et al. Nanomolar Hg2+ detection using β-lactoglobulin-stabilized fluorescent gold nanoclusters in beverage and biological media [J]. Analytical Chemistry, 2016, 88(20): 10275-10283. doi: 10.1021/acs.analchem.6b03011 [9] AGARWALLA H, MAHAJAN P S, SAHU D, et al. A switch-on NIR probe for specific detection of Hg2+ ion in aqueous medium and in mitochondria [J]. Inorganic Chemistry, 2016, 55(22): 12052-12060. doi: 10.1021/acs.inorgchem.6b02233 [10] YANG Y, XING X X, ZOU T, et al. A novel and sensitive ratiometric fluorescence assay for carbendazim based on N-doped carbon quantum dots and gold nanocluster nanohybrid [J]. Journal of Hazardous Materials, 2020, 386: 121958. doi: 10.1016/j.jhazmat.2019.121958 [11] WANG Y Y, MAO L, LIU W, et al. A ratiometric fluorometric and colorimetric probe for the β-thalassemia drug deferiprone based on the use of gold nanoclusters and carbon dots [J]. Mikrochimica Acta, 2018, 185(9): 442. doi: 10.1007/s00604-018-2982-4 [12] WANG X Y, DUAN Q Q, ZHANG B Y, et al. Ratiometric fluorescence detection of Cd2+ based on N, S co-doped carbon quantum dots/Au nanoclusters [J]. Microchemical Journal, 2021, 167: 106269. doi: 10.1016/j.microc.2021.106269 [13] LIU T, LI N, DONG J X, et al. A colorimetric and fluorometric dual-signal sensor for arginine detection by inhibiting the growth of gold nanoparticles/carbon quantum dots composite [J]. Biosensors and Bioelectronics, 2017, 87: 772-778. doi: 10.1016/j.bios.2016.08.098 [14] HU X T, LI Y X, XU Y W, et al. Green one-step synthesis of carbon quantum dots from orange peel for fluorescent detection of Escherichia coli in milk [J]. Food Chemistry, 2021, 339: 127775. doi: 10.1016/j.foodchem.2020.127775 [15] ZHANG Q, MEI H, ZHOU W T, et al. Cerium ion(III)-triggered aggregation-induced emission of copper nanoclusters for trace-level p-nitrophenol detection in water [J]. Microchemical Journal, 2021, 162: 105842. doi: 10.1016/j.microc.2020.105842 [16] NIU W J, LI Y, ZHU R H, et al. Ethylenediamine-assisted hydrothermal synthesis of nitrogen-doped carbon quantum dots as fluorescent probes for sensitive biosensing and bioimaging [J]. Sensors and Actuators B:Chemical, 2015, 218: 229-236. doi: 10.1016/j.snb.2015.05.006 [17] AMJADI M, MANZOORI J L, HALLAJ T, et al. Application of the chemiluminescence system composed of silicon-doped carbon dots, iron(II) and K2S2O8 to the determination of norfloxacin [J]. Microchimica Acta, 2017, 184(6): 1587-1593. doi: 10.1007/s00604-017-2139-x [18] JIA X F, LI J, WANG E K. One-pot green synthesis of optically pH-sensitive carbon dots with upconversion luminescence [J]. Nanoscale, 2012, 4(18): 5572-5575. doi: 10.1039/c2nr31319g [19] DING L H, GONG Z J, YAN M, et al. Determination of glucose by using fluorescent silicon nanoparticles and an inner filter caused by peroxidase-induced oxidation of o-phenylenediamine by hydrogen peroxide [J]. Microchimica Acta, 2017, 184(11): 4531-4536. doi: 10.1007/s00604-017-2445-3 [20] YANG Y, HYO D, WU H, et al. N, P-doped carbon quantum dots as a FL sensing platform for carbendazim detection based on FL resonance energy transfer [J]. Sens Actuators B, 2018, 274: 296-303. doi: 10.1016/j.snb.2018.07.130 [21] WANG W J, PENG J W, LI F M, et al. Phosphorus and chlorine co-doped carbon dots with strong photoluminescence as a fluorescent probe for ferric ions [J]. Mikrochimica Acta, 2018, 186(1): 32. [22] SK M P, CHATTOPADHYAY A. Induction coil heater prepared highly fluorescent carbon dots as invisible ink and explosive sensor [J]. RSC Advances, 2014, 4(60): 31994-31999. doi: 10.1039/C4RA04264F [23] SONG J P, LI J, GUO Z Y, et al. A novel fluorescent sensor based on sulfur and nitrogen co-doped carbon dots with excellent stability for selective detection of doxycycline in raw milk [J]. RSC Advances, 2017, 7(21): 12827-12834. doi: 10.1039/C7RA01074E [24] VINCI J C, FERRER I M, SEEDHOUSE S J, et al. Hidden properties of carbon dots revealed after HPLC fractionation [J]. The Journal of Physical Chemistry Letters, 2013, 4(2): 239-243. doi: 10.1021/jz301911y [25] 王学川, 白鹏霞, 罗晓民, 等. 基于明胶制备碳量子点及其光学性能的研究 [J]. 光谱学与光谱分析, 2019, 39(4): 1154-1161. WANG X C, BAI P X, LUO X M, et al. Synthesis of carbon quantum dots based on gelatin and study on it's optical property [J]. Spectroscopy and Spectral Analysis, 2019, 39(4): 1154-1161(in Chinese).
[26] LI L L, NI G, WANG J N, et al. Synthesis of nitrogen-doped carbon quantum dots and its application as fluorescent sensor for Hg2+ [J]. Spectroscpy and Spectral Analysis, 2016, 36(9): 2846-2851. [27] 国家环保总局. 水和废水监测分析方法[M]. 北京: 中国环境科学出版社, 2006: 354. State Environmental Protection Administration. Water and wastewater monitoring and analysis methods [M]. Beijing: China Environmental Science Press, 2006: 354.
[28] WANG K, DONG E F, FANG M, et al. Construction of ratio fluorescence sensor based on CdTe quantum dots and benzocoumarin-3-carboxylic acid for Hg2+ detection [J]. Chinese Journal of Analytical Chemistry, 2022, 50(4): 100070. doi: 10.1016/j.cjac.2022.100070 [29] CAO X T, MA J, LIN Y P, et al. A facile microwave-assisted fabrication of fluorescent carbon nitride quantum dots and their application in the detection of mercury ions [J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2015, 151: 875-880. doi: 10.1016/j.saa.2015.07.034 [30] CHAI F, WANG C G, WANG T T, et al. L-cysteine functionalized gold nanoparticles for the colorimetric detection of Hg2+ induced by ultraviolet light [J]. Nanotechnology, 2010, 21(2): 025501. doi: 10.1088/0957-4484/21/2/025501 [31] LI D Y, WANG S P, AZAD F, et al. Single-step synthesis of polychromatic carbon quantum dots for macroscopic detection of Hg2+ [J]. Ecotoxicology and Environmental Safety, 2020, 190: 110141. doi: 10.1016/j.ecoenv.2019.110141 [32] SINGH V K, SINGH V, YADAV P K, et al. Nitrogen doped fluorescent carbon quantum dots for on-off-on detection of Hg2+ and glutathione in aqueous medium: Live cell imaging and IMPLICATION logic gate operation [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2019, 384: 112042. doi: 10.1016/j.jphotochem.2019.112042 [33] LIU T, LI N, DONG J X, et al. Fluorescence detection of mercury ions and cysteine based on magnesium and nitrogen co-doped carbon quantum dots and IMPLICATION logic gate operation [J]. Sensors and Actuators B:Chemical, 2016, 231: 147-153. doi: 10.1016/j.snb.2016.02.141 [34] WANG S, CHEN H Y, XIE H L, et al. A novel thioctic acid-carbon dots fluorescence sensor for the detection of Hg2+ and thiophanate methyl via S-Hg affinity [J]. Food Chemistry, 2021, 346: 128923. doi: 10.1016/j.foodchem.2020.128923 [35] 祝艳, 鲁应光, 母昭, 等. 硅掺杂碳点荧光猝灭法检测废水中钴离子 [J]. 环境化学, 2020, 39(12): 3517-3523. ZHU Y, LU Y G, MU Z, et al. Determination of cobalt by silicon doped carbon dots fluorescence spectrophotometer [J]. Environmental Chemistry, 2020, 39(12): 3517-3523(in Chinese).
[36] KUMARI A, KUMAR A, SAHU S K, et al. Synthesis of green fluorescent carbon quantum dots using waste polyolefins residue for Cu2+ ion sensing and live cell imaging [J]. Sensors and Actuators B:Chemical, 2018, 254: 197-205. doi: 10.1016/j.snb.2017.07.075 [37] WANG X F, YANG Y X, HUO D Q, et al. A turn-on fluorescent nanoprobe based on N-doped silicon quantum dots for rapid determination of glyphosate [J]. Mikrochimica Acta, 2020, 187(6): 341. doi: 10.1007/s00604-020-04304-9 [38] ZHOU W S, LI C H, SUN C, et al. Simultaneously determination of trace Cd2+ and Pb2+ based on l-cysteine/graphene modified glassy carbon electrode [J]. Food Chemistry, 2016, 192: 351-357. doi: 10.1016/j.foodchem.2015.07.042 [39] LI Y H, CAI J B, LIU F J, et al. Construction of a turn off-on fluorescent nanosensor for cholesterol based on fluorescence resonance energy transfer and competitive host-guest recognition [J]. Talanta, 2019, 201: 82-89. doi: 10.1016/j.talanta.2019.03.110 [40] GUAN R T, TAO L X, HU Y Y, et al. Selective determination of Ag + in the presence of Cd2+, Hg2+ and Cu2+ based on their different interactions with gold nanoclusters [J]. RSC Advances, 2020, 10(55): 33299-33306. doi: 10.1039/D0RA05787H [41] 孙雪花, 张锦婷, 赵李艳, 等. 基于氮掺杂碳量子点的制备及其对Hg2+的响应 [J]. 环境化学, 2021, 40(1): 321-326. doi: 10.7524/j.issn.0254-6108.2020061504 SUN X H, ZHANG J T, ZHAO L Y, et al. Preparation of nitrogen-doped carbon quantum dots and its response to Hg2+ [J]. Environmental Chemistry, 2021, 40(1): 321-326(in Chinese). doi: 10.7524/j.issn.0254-6108.2020061504
[42] HUA J H, MU Z, HUA P, et al. Ratiometric fluorescence nanoprobe for monitoring of intracellular temperature and tyrosine based on a dual emissive carbon dots/gold nanohybrid [J]. Talanta, 2020, 219: 121279. doi: 10.1016/j.talanta.2020.121279 [43] CHAN Y H, CHEN J X, LIU Q S, et al. Ultrasensitive copper(II) detection using plasmon-enhanced and photo-brightened luminescence of CdSe quantum dots [J]. Analytical Chemistry, 2010, 82(9): 3671-3678. doi: 10.1021/ac902985p [44] LIANG G X, LIU H Y, ZHANG J R, et al. Ultrasensitive Cu2+ sensing by near-infrared-emitting CdSeTe alloyed quantum dots [J]. Talanta, 2010, 80(5): 2172-2176. doi: 10.1016/j.talanta.2009.11.025 -



 下载:
下载: