-
随着我国经济的迅速发展,能源消耗持续增加,工业挥发性有机化合物(VOCs)排放加剧了细颗粒物(PM2.5)和臭氧的形成,导致区域大气复合污染问题日趋严峻,给人体健康和生态环境造成了极大危害[1-3]. 我国生态环境部在“十四五”规划中,明确将VOCs纳入环境保护税征收范围. 苯系物作为工业源VOCs中的一类典型污染物,其工业排放源涉及有机化工、石油化工、包装印刷、涂料等多个重点行业,其光化学活性高且毒性强,排放浓度限制低,治理难度大[4-5]. 以低浓度为主要特征的苯系VOCs治理是污染控制领域的重点和难点.
为了满足日益严格的大气污染物排放标准,学术界和产业界开发了吸附法、催化燃烧法、吸收法、生物法、光催化氧化法、过硫酸盐氧化法等多种VOCs控制技术[6-8]. 然而,这些技术存在催化剂失活、成本高、二次污染等问题. 虽然湿法洗涤工艺被认为是一种方便经济的VOCs去除技术,但这种物理方法不能使VOCs矿化,而湿法洗涤工艺结合特定的高级氧化工艺(AOP)常用于低浓度VOCs的去除[9-10]. 耦合工艺利用了AOP技术具有能产生高氧化还原电位的活性物质,可促进VOCs在水相中氧化为H2O、CO2和其他无机物[11]. 近年来,基于真空紫外(VUV)协同过一硫酸盐(PMS)的高级氧化技术因其效率高、反应条件温和、投资成本低等优点而被广泛用于有机污染物的去除[12-13]. 真空紫外灯能发射约占总能量8.0%左右的185 nm高能光子(185 nm波长相当于6.7 eV的光子能量),不仅可以打破大多数物质的化学键,还能与空气中的O2和H2O作用生成O(1D)、O(3P)、·OH等活性物质(式1—2)[14-16]. 其中,O(3P)和O2反应生成O3,O3和H2O在254 nm的光子作用下生H2O2(式3—4). 此外,254 nm的光子可在水存在的情况下分解O3和H2O2,产生超氧自由基(
${\rm{O}}_2^{\cdot -} $ )和羟基过氧自由基(·HO2)活性氧物质(式5—9)[17]. 基于过一硫酸盐或者过二硫酸盐(PS)活化产生硫酸根自由基(${\rm{SO}}_4^{\cdot -} $ )的高级氧化技术因其催化效率高、较宽的pH适应范围、高的矿化程度及对环境更加友好等特点,在环境修复领域受到越来越多的关注[18],PMS与PS活化特性相比,PMS中的O—O键更容易被激活,更有利于水中有机污染物降解. 研究学者发现,采用PMS联合VUV新型高级氧化体系,可以进一步提高污染物去除效率和减少副产物的产生[19-20]. Xie等[11]采用紫外(UV)活化PMS在自制反应装置中去除水中气相乙酸乙酯和甲苯,发现在UV/PMS最优体系下,98.3%的乙酸乙酯和96.5%的甲苯可以被高效去除. Amiri等[21]采用VUV/PMS工艺处理天然有机物(NOM)和细菌消毒,在20 min内将TOC浓度从3.83 mg·L−1降至0.15 mg·L−1,实现完全消毒. 总的来说,VUV/PMS呈现出设计和操作相对简单的优势,在去除水中污染物方面具有很大潜在的应用前景. 然而,如何根据真空紫外及PMS的特性选择合适的氧化剂或催化剂从而提高污染物的降解效率是VUV/PMS体系研究的重点之一.ZSM-5分子筛具有比表面积大、优异的择形催化性和较高的水热稳定性等特点,作为催化剂或载体被广泛应用于VOCs的催化降解[22-23]. Aziz等[24]通过构建Fe-ZSM-5/UV体系去除不同苯系VOCs(苯、甲苯、乙苯和二甲苯),发现Fe-ZSM-5/UV体系具有较好的催化性能和化学稳定性,且可以有效减少有毒副产物的产生. 而通过构建Fe-ZSM-5/VUV/PMS体系消除低浓度苯系VOCs还有待深入研究.
因此,本研究针对单一VUV或PMS技术消除苯系VOCs过程中产物降解不彻底和效率不高的问题,通过构建Fe-ZSM-5系列催化剂,并将其耦合VUV/PMS湿法去除低浓度甲苯污染物. 借助比表面积和孔隙分析仪(BET)、X射线衍射(XRD)、氨程序升温脱附(NH3-TPD)、吡啶红外(Py-FTIR)等表征手段分析了催化剂的物化结构特征. 同时,利用电子顺磁共振(EPR)和液相色谱质谱(LC-MS)检测VUV/PMS湿法体系降解甲苯过程中主要活性物种及产生的中间产物,阐明甲苯降解机理,为VUV/PMS湿法处理工业低浓度苯系VOCs工艺的开发提供新思路.
-
甲苯、硫酸(H2SO4)均购自广州化学试剂厂,硫酸亚铁(FeSO4·7H2O)购自天津市大茂化学试剂厂,过硫酸氢钾(2KHSO5·KHSO4·K2SO4, PMS)购自上海麦克林生化科技有限公司,ZSM-5分子筛(硅铝比为120)购自天津市大茂化学试剂厂,以上试剂均为分析纯.
-
在烧杯中加入250 mL去离子水,并用0.1 mol·L−1 H2SO4溶液调节pH值为3;称取0.95 g的ZSM-5分子筛和0.25 g FeSO4·7H2O溶入上述溶液中. 将上述悬浮液在氮气氛围下磁力搅拌6 h,随后洗涤并置于真空干燥箱中在110 ℃下干燥. 最后在管式炉中以3 ℃·min−1升温到550 ℃,在550 ℃下保持5 h,自然冷却得到Fe-ZSM-5催化剂.
-
使用比表面积与孔隙度分析仪(BET, Tristar I II 3020型)来测定催化剂的比表面积. 使用X射线衍射仪(XRD, SmartLab 3KW型)测定催化剂的晶相结构,使用能量色散X射线能谱(EDS)分析催化剂的元素分布. 利用全自动化学吸附分析仪(Micromertics AUTOCHEM II 2920型)进行NH3程序升温脱附来测定催化剂的酸碱性质,得到催化剂的强酸和弱酸情况. 利用电子顺磁共振谱仪(EPR, Bruker A320)以5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)作为捕获试剂来研究参加反应自由基(
${\rm{SO}}_4^{\cdot -} $ 和·OH)的存在. 利用傅立叶变换红外光谱仪(FTIR, Thermo Nicolet 380)在300 ℃下进行吡啶-红外实验来测定催化剂的酸种类和含量. -
反应装置如图1显示,首先打开气瓶阀门,空气进入甲苯瓶和缓冲瓶. 通过鼓泡法,经质量流量计来控制污染物甲苯的流速,体系总流速为550 mL·min−1,甲苯浓度为188.5 mg·m−3. 反应器是由石英玻璃所制,反应溶液体积为500 mL,甲苯和空气的混合气体在反应器底部鼓泡. 5 W的UV灯和4 W的VUV灯(广东雪莱特光电科技股份有限公司)按实验需要置于反应器顶部的石英玻璃管中. 反应后的气体进入气相色谱仪(GC, 福立GC-9790 II)测定出气中的甲苯浓度和CO2浓度,甲苯的去除率由公式(12)计算,矿化率由公式(13)计算. 利用液相色谱质谱联用仪(LC-MS, Agilent QQQ-6410)检测反应过程溶液中的中间体. 实验PMS的量为5 mmol·L−1,催化剂的量为0.10 g,所有实验均在25 ℃下进行.
-
图2显示了ZSM-5和Fe-ZSM-5的XRD图谱. 由图2可以看出,所有样品的XRD谱图都有典型MFI型结构的衍射峰. 引入Fe改性之后,仍可清晰观察到ZSM-5的衍射峰,表明负载后其MFI骨架结构没有受到破坏.
同时在Fe-ZSM-5催化剂样品的谱图中没有明显关于Fe或者铁氧化物的衍射峰,这可能是因为Fe物种含量过低或者分散性高,也有可能是因为Fe2O3在2θ为33.00°和36.00°处的衍射峰与ZSM-5在32.87°和36.15°处的衍射峰重叠[25- 26]. 为了表明Fe成功负载到ZSM-5,进行了对催化剂中的Si、Fe和Al进行了EDS Mapping,结果由图3所示,可以清楚地看出Fe成功负载到ZSM-5上. 由BET分析数据可知,负载Fe金属后,材料的比表面积由335 m2·g−1增大到364 m2·g−1,较大的比表面积可能有利于甲苯的吸附及产生更多的活性位点,促进甲苯的进一步降解.
-
通过测试NH3-TPD研究改性前后催化剂的酸碱性,结果如图4所示. 由图4可以看出,两种催化剂均具有两个氨气脱附峰,分别是在150—250 ℃范围内的低温脱附峰(弱酸位点)和在350—550 ℃范围内的高温脱附峰(强酸位点),这是MFL结构的典型特征[27]. 随着Fe金属的引入,ZSM-5催化剂脱附峰中心向低温方向移动,同时,强酸脱附峰减弱,说明强酸位点的强度与酸量都在减弱.
-
采用FTIR光谱法对Fe-ZSM-5样品在不同处理温度下吸附吡啶,研究其酸性位点的性质. 如图5所示,当温度由40 ℃升到200 ℃时,1488 cm−1处的峰强度急剧下降,在300 ℃时消失,表明材料存在较弱的H酸性位点.
在1446 cm−1处的吸附带对应Lewis(L)酸位点,在1545 cm−1处的吸附带对应Brönsted(B)酸位点,而在1488 cm−1处的谱带对应于两者的组合.
图5展示了ZSM-5和Fe-ZSM-5样品在300 ℃下的吡啶吸附红外光谱. 对于ZSM-5,在1545 cm−1有一个低峰,而1446 cm−1处有较强的峰,这说明了ZSM-5中B酸的量较高. 而随着Fe的引入,在B酸的峰面积减少,而L酸的峰面积增加. 这表明引入少量的Fe会导致与桥羟基相关的B酸损失,而L酸显著增加. 表1显示了不同催化剂的Brønsted位/Lewis位(IB/IL)的比较. 由表1可知,Fe-ZSM-5样品的IB/IL比降低. 这可能由于部分Fe原子进入沸石孔隙和Brønsted位点,这与Li等的研究结果相似[28]. 吡啶红外的结果与NH3-TPD结果基本一致.
-
不同体系对甲苯去除率的结果见图6. 从图中可以看到,在0到70 min内,空白实验的甲苯去除率从开始的40.0%左右逐渐下降至0,这可能是因为水对甲苯的吸附作用[11],而到70 min时,溶液的甲苯浓度接近饱和. 而单独加入UV灯照射或者5 mmol·L−1 PMS后,甲苯去除率与空白实验相比略有上升,说明体系里单独加入UV或者5 mmol·L−1 PMS对去除率的提升作用不大. 在UV+Fe-ZSM-5体系中,去除率曲线略高于单独加入UV灯,说明在UV照射下,催化剂对提高去除率的作用不大. 而在单独VUV的照射下,去除率有明显的提升,这主要是因为VUV紫外光内185 nm所含有的高能光子直接光解甲苯和激发体系中的氧气和水产生活性氧物种. 在UV+PMS体系中,前70 min内甲苯去除率能达到100.0%,到70 min后去除率开始下降,这可能是因为体系中PMS被消耗,所产生的活性物质不足以维持高的去除率. 而在VUV+PMS体系中,当反应时间延长到190 min,体系仍保持接近100.0%的甲苯去除率能维持到190 min,这可能是因为在VUV和PMS耦合作用下,促进了体系中甲苯的去除. 同时,从图中可以看出,在VUV+PMS+Fe-ZSM-5体系下,甲苯去除率在240 min依然能维持100.0%,说明体系中Fe-ZSM-5催化剂的加入能够提升体系的可持续性,有利于实现工业中VOCs治理的长期运作.
一般来说,VUV+PMS体系中生成的自由基数量与PMS的浓度成正比,PMS浓度越大,则生成的自由基数量越大. 这是因为紫外光能活化PMS,导致更多的
${\rm{SO}}_4^{\cdot -} $ 和·OH生成[29]. 在甲苯的降解体系中,VUV+PMS体系比在UV+PMS体系好,很可能是因为较低波长的VUV光子(185 nm)比较高波长的UV光子(254 nm)对活化PMS更好. 研究发现,紫外光子的波长越低,PMS分子对辐射的吸收率越高,产生的自由基也就越多(式(14—16))[30]. 通过Fe改性的ZSM-5分子筛,一方面可能由于其比表面积增大,强酸位点减少,材料表面和孔道内更有利于甲苯吸附;另一方面由于体系中Fe的存在,Fe与甲苯发生化学吸附,促进了反应的进行. 同时,在VUV+PMS+Fe-ZSM-5体系中,VUV激发空气中O2产生臭氧,臭氧在水和254 nm的波长条件下反应生成H2O2(式(5—6)),当体系中加入Fe-ZSM-5时,Fenton反应会进一步产生更多的自由基(式(17—18)),提高了甲苯的降解效率. 实验表明,Fe-ZSM-5对VUV+PMS体系起到了协同作用. -
不同体系出口CO2的浓度和矿化率结果如图7所示. 从图7可以看到,空白实验和5 mmol·L−1 PMS出口的CO2浓度基本为0,说明单独PMS体系无法将甲苯矿化成CO2和CO. 而在空白实验中加入UV照射后,CO2浓度有了细微的增加,说明紫外光作用下可以直接光解小部分甲苯开环生成CO2. 在UV+Fe-ZSM-5体系中,产生的CO2略高于单独加入UV灯,说明催化剂的加入可以促进污染物的矿化. 当利用UV进一步耦合PMS降解甲苯时,发现甲苯矿化率有了明显的提升;这可能由于UV+PMS体系生成了更多的自由基,促进了甲苯深度降解. 在单独VUV照射下,CO2浓度最高可以达到144.3 mg·m−3左右,而矿化率最高只有22.9%. 同样当VUV体系中加入5 mmol·L−1 PMS后,出口的CO2浓度显著增加,最高可以达到324.6 mg·m−3,矿化率达51.4%. 相比于ZSM-5,当VUV+PMS体系中加入Fe-ZSM-5催化剂后,可以发现,CO2浓度和矿化率比上述体系都有了明显的提高,CO2浓度最高能达421.9 mg·m−3,矿化率达67.0%. 说明VUV+PMS+Fe-ZSM-5体系更有利于甲苯的深度氧化.
-
由于Fe-ZSM-5催化剂有更高的甲苯矿化率,测试VUV+PMS体系加入ZSM-5和Fe-ZSM-5前后的EPR图谱,结果如图8示. VUV+PMS+Fe-ZSM-5体系在反应过程中产生了大量的
${\rm{SO}}_4^{\cdot -} $ 和·OH,而ZSM-5的加入能促进PMS在VUV照射下产生更多的自由基,说明Fe-ZSM-5的加入对PMS的活化起到很大的作用. 体系中的甲苯去除率和矿化率的提高可能是由于生成的${\rm{SO}}_4^{\cdot -} $ 和·OH能迅速氧化水体中的甲苯及其降解过程中产生的大多数有机化合物. -
实验中GC没有检测到出气中有气态中间产物,而LC-MS中检测到溶液中有多种中间产物,如表2所示,包括醋酸、丙酸、2-戊酮等. 这说明反应体系能将降解产物转移到液相中进行下一步处理,极大地减少了空气的二次污染. 图9展示了VUV+PMS+Fe-ZSM-5体系下自由基的形成途径和可能的甲苯降解路径.
如图9(a)所示,在紫外光的照射下,PMS上的O—O键断开生成
${\rm{SO}}_4^{\cdot -} $ 和·OH,水分子上的O—H键断开生成·H和·OH. Fe-ZSM-5的存在可以加速这个产生自由基的过程,甲苯在自由基的攻击下不断矿化,最终变成CO2和H2O. 如图9(b)所示,首先,溶解的甲苯上富电子甲基的电子转移到${\rm{SO}}_4^{\cdot -} $ ,形成碳自由基阳离子(A). 这种不稳定化合物会与${\rm{SO}}_4^{\cdot -} $ 和·OH反应,通过自由基加成反应生成碳中心自由基(B)和苯甲醇(E). 碳中心自由基(B)在氧气的作用下,生成2-羟基甲苯(C)和4-羟基甲苯(D)两种同分异构体,而苯甲醇(E)在·OH的作用下生成苯甲醛(F)和苯甲酸(G). 这些化合物可以继续被${\rm{SO}}_4^{\cdot -} $ 和·OH氧化,导致苯环开环,生成一些短链化合物,如丙酸(H)、醋酸(I)、2-戊酮(J),短链化合物进一步在${\rm{SO}}_4^{\cdot -} $ 和·OH作用下被深度氧化成CO2和H2O. -
(1)本研究构建了新型真空紫外联合过硫酸盐的高级氧化体系(VUV+PMS+Fe-ZSM-5). 在VUV、5 mmol·L−1 PMS和Fe-ZSM-5共存的情况下,该体系能够在长时间高效降解甲苯,240 min内去除率接近100.0%,矿化率高达67.0%;证实了该体系能很好地活化PMS,实现甲苯的高效降解和深度矿化. 一方面可能由于Fe改性后的ZSM-5分子筛其比表面积增大,强酸位点减少,材料表面和孔道内更有利于活性物种生成和甲苯吸附;另一方面由于体系中Fe的存在,Fe与甲苯发生化学吸附,促进了反应的进行.
(2)
${\rm{SO}}_4^{\cdot -} $ 和·OH自由基是VUV+PMS+Fe-ZSM-5体系的主要活性物种. 该体系可能的甲苯降解历程为:甲苯首先通过电子转移形成碳自由基阳离子,然后通过${\rm{SO}}_4^{\cdot -} $ 和·OH自由基的加成反应生成碳中心自由基和苯甲醇;碳中心自由基在氧气的作用下,生成2-羟基甲苯和4-羟基甲苯,而苯甲醇在·OH的作用下生成苯甲醛和苯甲酸;接着通过苯环开环,生成丙酸、醋酸、2-戊酮等短链化合物,再进一步被氧化成CO2和H2O.(3)GC检测出口气体中没有气态中间产物,而LC-MS里检测到多种中间产物,说明该工艺能有效避免空气的二次污染.
Fe-ZSM-5协同真空紫外和过硫酸盐催化氧化甲苯的强化工艺与机制
A study on enhanced process and mechanism for toluene remocal by combining Fe-ZSM-5 with vacuum ultraviolet and persulfate
-
摘要: 近年来,真空紫外(VUV)协同过硫酸盐的高级氧化工艺已被逐渐开发用于处理低浓度挥发性有机污染物(VOCs). 然而,如何根据VUV及PMS的特性选择合适的催化剂从而提高工业低浓度苯系VOCs的降解和矿化效率成为当前的挑战. 因此,本研究从改性ZSM-5的酸性入手,通过浸渍湿法成功制备了Fe-ZSM-5催化剂. 利用Fe-ZSM-5耦合真空紫外和过硫酸盐的高级氧化体系在湿法条件下降解低浓度甲苯污染物,并对其催化性能和降解机制进行了探讨. 结果表明,VUV+PMS+Fe-ZSM-5体系在240 min内仍能保持接近100.0%的甲苯去除率,矿化率高达67.0%. 采用氨程序升温脱附(NH3-TPD)和吡啶红外(Py-FTIR)等表征对样品酸类型和酸量进行定性和定量分析,结果表明Fe改性减少了ZSM-5催化剂Brønsted酸的量,而显著增加了Lewis酸的量,有利于提高反应的活性和稳定性. 电子顺磁共振谱(EPR)结果表明,在VUV+PMS+Fe-ZSM-5体系下,硫酸根自由基(
${\rm{SO}}_4^{\cdot -} $ )和羟基自由基(·OH)作为主要活性物种参与了甲苯的降解. 液相色谱-质谱(LC-MS)检测到苯甲醇、苯甲醛、苯甲酸、醋酸、丙酸、2-戊酮等多种中间产物,而气相色谱仪(GC)没有检测到出口气体中的气态中间产物,表明该工艺能有效避免空气的二次污染并将中间产物转移到液相进行下一步处理. 研究结果为VUV/PMS湿法处理工业低浓度苯系VOCs工艺的开发提供新思路.Abstract: In recent years, the advanced oxidation process of vacuum ultraviolet (VUV) synergistic peroxymonosulfate has been gradually developed for the treatment of low concentration of volatile organic pollutants (VOCs). However, it is urgent to select an appropriate catalyst for coupling with VUV/PMS system to further enhance the degradation efficiency and mineralization efficiency of industrial low-concentration benzene series of VOCs. Therefore, based on the acidity characteristics of ZSM-5, this study aims to fabricate Fe-ZSM-5 catalyst through wet impregnation method, and evaluates the catalytic activity and degradation mechanism of VUV+PMS+Fe-ZSM-5 system on toluene removal under wet condition. The results showed that nearly 100.0% of toluene was degraded within 240 min in VUV+PMS+Fe-ZSM-5 system, and the mineralization rate was as high as 67.0%. Ammonia temperature programmed desorption (NH3-TPD) and pyridine infrared (FTIR) were utilized to analyze the type and amount of acid species of the catalysts. It can be achieved that Fe modification reduces the amount of Brønsted acid in ZSM-5 catalyst, but increases the amount of Lewis acid, which may be beneficial to improve the activity and stability of the reaction. Electron paramagnetic resonance (EPR) spectrum demonstrated that sulfate radicals ($ {\rm{SO}}_4^{\cdot -}$ ) and hydroxyl radicals (·OH) are the main active species in the VUV+PMS+Fe-ZSM-5 for toluene removal. Several intermediate products including benzyl alcohol, benzaldehyde, benzoic acid, acetic acid, propionic acid, 2-pentanone were detected during the degradation process by liquid chromatography-mass spectrometry (LC-MS), while no gaseous intermediates were found in the outlet by gas chromatographic (GC), indicating that this process can effectively avoid secondary air pollution and transfer intermediates to the liquid phase for further treatment. The study provides a new idea for low-concentration benzene series of VOCs control by catalyst+VUV/PMS wet process.-
Key words:
- vacuum ultraviolet /
- peroxymonosulfate /
- photocatalysis /
- Fe-ZSM-5 /
- toluene
-

-
表 1 300 ℃下ZSM-5和Fe-ZSM-5催化剂的酸度
Table 1. Acidity of ZSM-5 and Fe-ZSM-5 catalysts at 300 ℃
催化剂
CatalystBrønsted位置/cm−1
Brønsted sitesLewis位置/cm−1
Lewis sitesBrønsted/
(μmol·g−1 cat)Lewis/
(μmol·g−1 cat)IB/IL ZSM-5 1545 1448 26.00 7.48 3.48 Fe-ZSM-5 1540 1446 18.55 16.38 1.13 表 2 VUV+PMS+Fe-ZSM-5体系在氧化过程中产生的中间产物
Table 2. Intermediate products produced in the oxidation process of VUV+PMS+Fe-ZSM-5 system
质荷比
m/z化学式
Chemical formula名称
Name结构
Structure60 C2H4O2 醋酸 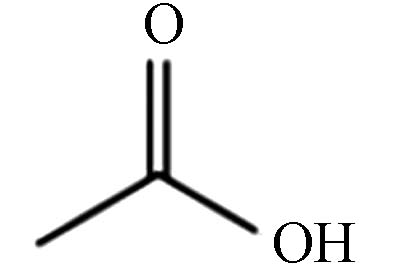
74 C3H6O2 丙酸 
86 C5H10O 2-戊酮 
108 C7H8O 苯甲醇 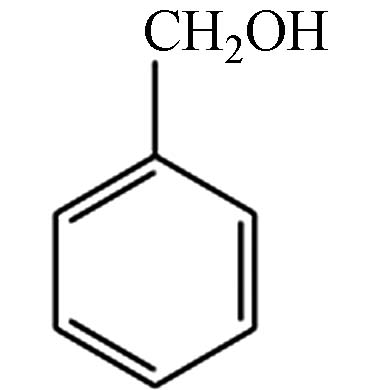
108 C7H8O 2-羟基甲苯 
108 C7H8O 4-羟基甲苯 
107 C7H6O 苯甲醛 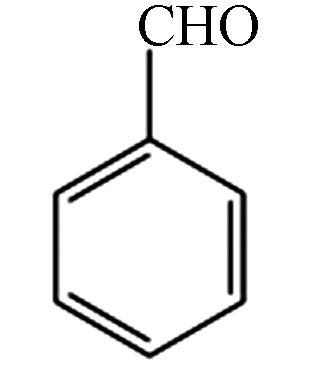
122 C7H6O2 苯甲酸 
-
[1] WU P, JIN X J, QIU Y C, et al. Recent progress of thermocatalytic and photo/thermocatalytic oxidation for VOCs purification over Manganese-based oxide catalysts [J]. Environmental Science & Technology, 2021, 55(8): 4268-4286. [2] 黎维彬, 龚浩. 催化燃烧去除VOCs污染物的最新进展 [J]. 物理化学学报, 2010, 26(4): 885-894. doi: 10.3866/PKU.WHXB20100436 LI W B, GONG H. Recent progress in the removal of volatile organic compounds by catalytic combustion [J]. Acta Physico-Chimica Sinica, 2010, 26(4): 885-894(in Chinese). doi: 10.3866/PKU.WHXB20100436
[3] GAN Q, FU M L, LIU P, et al. Synergistic catalytic ozonation of toluene with Manganese and cerium varies at low temperature [J]. Chinese Chemical Letters, 2022, 33(5): 2726-2730. doi: 10.1016/j.cclet.2021.09.006 [4] SIMAYI M, SHI Y Q, XI Z Y, et al. Emission trends of industrial VOCs in China since the clean air action and future reduction perspectives [J]. Science of the Total Environment, 2022, 826: 153994. doi: 10.1016/j.scitotenv.2022.153994 [5] ZHANG X M, ZHAO W J, NIE L, et al. A new classification approach to enhance future VOCs emission policies: Taking solvent-consuming industry as an example [J]. Environmental Pollution, 2021, 268: 115868. doi: 10.1016/j.envpol.2020.115868 [6] HUANG H B, HUANG H L, FENG Q Y, et al. Catalytic oxidation of benzene over Mn modified TiO2/ZSM-5 under vacuum UV irradiation [J]. Applied Catalysis B:Environmental, 2017, 203: 870-878. doi: 10.1016/j.apcatb.2016.10.083 [7] LIU B Y, ZHAN Y J, XIE R J, et al. Efficient photocatalytic oxidation of gaseous toluene in a bubbling reactor of water [J]. Chemosphere, 2019, 233: 754-761. doi: 10.1016/j.chemosphere.2019.06.002 [8] 汪涵, 郭桂悦, 周玉莹, 等. 挥发性有机废气治理技术的现状与进展 [J]. 化工进展, 2009, 28(10): 1833-1841. WANG H, GUO G Y, ZHOU Y Y, et al. Status and progress of treating volatile organic compounds [J]. Chemical Industry and Engineering Progress, 2009, 28(10): 1833-1841(in Chinese).
[9] XIE R J, LIU G Y, LIU D P, et al. Wet scrubber coupled with heterogeneous UV/Fenton for enhanced VOCs oxidation over Fe/ZSM-5 catalyst [J]. Chemosphere, 2019, 227: 401-408. doi: 10.1016/j.chemosphere.2019.03.160 [10] XIE X W, XIE R J, SUO Z Y, et al. A highly dispersed Co-Fe bimetallic catalyst to activate peroxymonosulfate for VOC degradation in a wet scrubber [J]. Environmental Science:Nano, 2021, 8(10): 2976-2987. doi: 10.1039/D1EN00547B [11] XIE R J, JI J, GUO K H, et al. Wet scrubber coupled with UV/PMS process for efficient removal of gaseous VOCs: Roles of sulfate and hydroxyl radicals [J]. Chemical Engineering Journal, 2019, 356: 632-640. doi: 10.1016/j.cej.2018.09.025 [12] AMANOLLAHI H, MOUSSAVI G, GIANNAKIS S. VUV/Fe(II)/H2O2 as a novel integrated process for advanced oxidation of methyl tert-butyl ether (MTBE) in water at neutral pH: Process intensification and mechanistic aspects [J]. Water Research, 2019, 166: 115061. doi: 10.1016/j.watres.2019.115061 [13] CAO T T, XU T F, ZHAO M N, et al. Application of vacuum-ultraviolet (VUV) for phenolic homologues removal in humic acid solution: Efficiency, pathway and DFT calculation [J]. Journal of Hazardous Materials, 2020, 384: 121464. doi: 10.1016/j.jhazmat.2019.121464 [14] HUANG H B, HUANG H L, ZHANG L, et al. Enhanced degradation of gaseous benzene under vacuum ultraviolet (VUV) irradiation over TiO2 modified by transition metals [J]. Chemical Engineering Journal, 2015, 259: 534-541. doi: 10.1016/j.cej.2014.08.057 [15] LIANG S M, SHU Y J, LI K, et al. Mechanistic insights into toluene degradation under VUV irradiation coupled with photocatalytic oxidation [J]. Journal of Hazardous Materials, 2020, 399: 122967. doi: 10.1016/j.jhazmat.2020.122967 [16] MOUSSAVI G, REZAEI M. Exploring the advanced oxidation/reduction processes in the VUV photoreactor for dechlorination and mineralization of trichloroacetic acid: Parametric experiments, degradation pathway and bioassessment [J]. Chemical Engineering Journal, 2017, 328: 331-342. doi: 10.1016/j.cej.2017.07.006 [17] WU M Y, HUANG H B, LEUNG D Y C. A review of volatile organic compounds (VOCs) degradation by vacuum ultraviolet (VUV) catalytic oxidation [J]. Journal of Environmental Management, 2022, 307: 114559. doi: 10.1016/j.jenvman.2022.114559 [18] GIANNAKIS S, LIN K Y A, GHANBARI F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs) [J]. Chemical Engineering Journal, 2021, 406: 127083. doi: 10.1016/j.cej.2020.127083 [19] AMANOLLAHI H, MOUSSAVI G, GIANNAKIS S. Enhanced vacuum UV-based process (VUV/H2O2/PMS) for the effective removal of ammonia from water: Engineering configuration and mechanistic considerations [J]. Journal of Hazardous Materials, 2021, 402: 123789. doi: 10.1016/j.jhazmat.2020.123789 [20] LIN Z, QIN W L, SUN L, et al. Kinetics and mechanism of sulfate radical- and hydroxyl radical-induced degradation of Bisphenol A in VUV/UV/peroxymonosulfate system [J]. Journal of Water Process Engineering, 2020, 38: 101636. doi: 10.1016/j.jwpe.2020.101636 [21] AMIRI Z, MOUSSAVI G, MOHAMMADI S, et al. Development of a VUV-UVC/peroxymonosulfate, continuous-flow Advanced Oxidation Process for surface water disinfection and Natural Organic Matter elimination: Application and mechanistic aspects [J]. Journal of Hazardous Materials, 2021, 408: 124634. doi: 10.1016/j.jhazmat.2020.124634 [22] LI J H, XIAO G F, GUO Z Y, et al. ZSM-5-supported V-Cu bimetallic oxide catalyst for remarkable catalytic oxidation of toluene in coal-fired flue gas [J]. Chemical Engineering Journal, 2021, 419: 129675. doi: 10.1016/j.cej.2021.129675 [23] XU W C, CHEN B X, JIANG X D, et al. Effect of calcium addition in plasma catalysis for toluene removal by Ni/ZSM-5: Acidity/basicity, catalytic activity and reaction mechanism [J]. Journal of Hazardous Materials, 2020, 387: 122004. doi: 10.1016/j.jhazmat.2019.122004 [24] AZIZ A, KIM K S. Synergistic effect of UV pretreated Fe-ZSM-5 catalysts for heterogeneous catalytic complete oxidation of VOC: A technology development for sustainable use [J]. Journal of Hazardous Materials, 2017, 340: 351-359. doi: 10.1016/j.jhazmat.2017.07.019 [25] QI G, GATT J E, YANG R T. Selective catalytic oxidation (SCO) of ammonia to nitrogen over Fe-exchanged zeolites prepared by sublimation of FeCl3 [J]. Journal of Catalysis, 2004, 226(1): 120-128. doi: 10.1016/j.jcat.2004.05.023 [26] ROMERO-SÁEZ M, DIVAKAR D, ARANZABAL A, et al. Catalytic oxidation of trichloroethylene over Fe-ZSM-5: Influence of the preparation method on the iron species and the catalytic behavior [J]. Applied Catalysis B:Environmental, 2016, 180: 210-218. doi: 10.1016/j.apcatb.2015.06.027 [27] WAN Z J, WU W, LI G, et al. Effect of SiO2/Al2O3 ratio on the performance of nanocrystal ZSM-5 zeolite catalysts in methanol to gasoline conversion [J]. Applied Catalysis A:General, 2016, 523: 312-320. doi: 10.1016/j.apcata.2016.05.032 [28] LI J Q, HAN D Z, HE T, et al. Nanocrystal H[Fe, Al]ZSM-5 zeolites with different silica-alumina composition for conversion of dimethyl ether to gasoline [J]. Fuel Processing Technology, 2019, 191: 104-110. doi: 10.1016/j.fuproc.2019.03.029 [29] VERMA S, NAKAMURA S, SILLANPÄÄ M. Application of UV-C LED activated PMS for the degradation of anatoxin-A [J]. Chemical Engineering Journal, 2016, 284: 122-129. doi: 10.1016/j.cej.2015.08.095 [30] KARIMIAN S, MOUSSAVI G, FANAEI F, et al. Shedding light on the catalytic synergies between Fe(II) and PMS in vacuum UV (VUV/Fe/PMS) photoreactors for accelerated elimination of pharmaceuticals: The case of metformin [J]. Chemical Engineering Journal, 2020, 400: 125896. doi: 10.1016/j.cej.2020.125896 -






 下载:
下载:






























