-
土壤砷(As)污染问题在世界范围内面临着严峻形势. 据报道,欧洲的矿山土壤平均As含量超过500 mg·kg−1[1],南美洲土壤平均As含量为34 mg·kg−1[2],我国湖北省某磷肥厂周围的农业土壤As含量最高可达
6402 mg·kg−1[3]. 在土壤环境中生物与非生物因素影响下,As会进入土壤溶液中并发生形态转化[4 − 5],其中当无机态的砷酸盐(As(Ⅴ))还原为亚砷酸盐(As(Ⅲ))时,其毒性增加约60倍,且移动性显著提高[6],容易向水体中迁移从而对动植物及人类健康产生严重的危害,因此土壤中As迁移与转化过程中的影响因素成为研究重点.土壤中广泛存在的砷还原菌被认为在As(Ⅴ)还原的过程发挥着重要作用. 砷还原菌一般通过细胞质砷还原或异化砷还原两种途径还原As(Ⅴ)[7],一些同时具有铁还原能力的砷还原菌还会对铁(氢)氧化物进行还原溶解[8 − 9],使As释放后再对其还原. 除此之外,土壤中的有机质对As迁移转化的影响也受到研究者的关注. 土壤有机质主要是指土壤中的动植物及微生物残体,经由复杂的微生物和物理化学转化过程形成的有机化合物,包括约70%—80%的腐殖质和约20%—30%的非腐殖质[10]. 腐殖质中常见的有胡敏酸、富里酸和胡敏素,非腐殖质则主要是小分子有机酸、糖类、含氮化合物等[11]. 多项研究证实,有机质可以通过各种官能团与As直接络合,如腐殖质的酚羟基、羧基、巯基[12 − 13],还会与As竞争土壤上的吸附位点使As释放[14]. 有机酸类一般通过溶解铁(氢)氧化物,使吸附态As成为溶解态,也可以通过竞争吸附[15]、与铁形成络合物或将铁还原促使其溶解[16]而导致As的释放. 此外,有机质还可以影响土壤微生物的生长代谢,加快As释放到土壤溶液中并发生转化,如Qiao等[17]发现胡敏酸、富里酸可以促进与As还原有关的微生物生长为优势种群. 但目前所研究的土壤有机质组分多集中于胡敏酸、富里酸等腐殖质类,其次是根系分泌物中的小分子有机酸,如草酸、柠檬酸等,而对糖类、含氮有机质等组分以及外源添加作为土壤调理剂的有机质,如生物炭和聚合氨基酸等[18 − 19]的关注较少,不同类型有机质组分对As的迁移转化作用尚不明确. 此外,在厌氧状态下砷还原菌会使土壤铁矿物发生还原溶解,导致更多的As进入土壤溶液[20],而厌氧状态下有机质组分对砷还原菌介导的As形态转化的影响机制并不明确.
本研究选择以下6种典型土壤有机质组分作为研究对象:腐殖酸(HM)、胡敏酸(HA),腐殖质中的代表性物质;柠檬酸(CA),小分子有机酸中的代表性物质;聚天冬氨酸(PA),农业中常用作肥料增效剂的多肽聚合物;多聚半乳糖醛酸(PGA),糖类中的代表性物质;谷氨酸(Glu),氨基酸中的代表性物质,并利用已筛选出的砷还原菌Desulfitobacteriumsp. DJ-3,进行如下研究:(1)无砷还原菌存在时,有机质组分对As(Ⅴ)、As(Ⅲ)的吸附/络合作用及还原/氧化作用;(2)有机质组分对砷还原菌还原As(Ⅴ)的影响及其机制. 研究结果可为探究土壤中无机As的环境行为提供理论基础.
-
所有试剂均为分析纯及以上纯度. 腐殖酸(HM)购自中国国药集团化学试剂有限公司,胡敏酸(HA)、柠檬酸(CA)、多聚半乳糖醛酸(PGA)、谷氨酸(Glu)购自Sigmar-Aldrich试剂公司,聚天冬氨酸(PA)购自上海麦克林生化科技有限公司. 培养基所需的无机盐购自美国阿拉丁工业公司,酵母提取物购自OXOID试剂公司,乳酸钠溶液购自中国国药集团化学试剂有限公司,L-半胱氨酸购自Sigmar-Aldrich试剂公司. 砷酸钠(NaAs3O4·12H2O)、亚砷酸钠(NaAsO2)购自中国国药集团化学试剂有限公司.
-
分别称取约0.2 g的HM、HA、CA、PA、PGA、Glu粉末于锥形瓶中,加入200 mL超纯水,配制成质量浓度为0.1%的6种有机质溶液,用1 mol·L−1 NaOH溶液或HCl调节pH至7—7.2,盖铝箔过夜稳定,4 ℃保存待用. 用NaAs3O4·12H2O和NaAsO2粉末分别配制浓度为80 mmol·L−1 的As(Ⅴ)和As(Ⅲ)储备液,加入0. 1 mol·L−1 HCl调节pH为7—7.2,4 ℃保存待用,使用时根据需要逐级稀释.
在厌氧瓶中分别将质量终浓度为0.1%的HM、HA、CA、PA、PGA、Glu溶液与终浓度为8 μmol·L−1 的As(Ⅴ)或As(Ⅲ)溶液混匀,同时设置不加入有机质溶液的对照组,厌氧瓶加盖丁基橡胶塞,并用铝盖密封,在黑暗中于水平摇床(150 r·min−1,37 ℃)中振荡. 在0、12、24、36、48、84、132 h取样,每次取2 mL,过0.22 μm 滤膜,与1 mol·L−1 HNO3(去氧)按体积1:2 混合,放入-20 ℃冰箱保存,待后续测定As总量与形态. 实验以及取样过程均在厌氧手套箱中进行.
-
分别称取约0.4 g的HM、HA、CA、PA、PGA、Glu粉末于锥形瓶中,加入200 mL超纯水,配制成质量浓度为0.2%的6种有机质溶液,用1 mol·L−1 NaOH溶液或HCl调节pH至7—7.2, 盖铝箔过夜稳定,4℃保存待用. 用NaAs3O4·12H2O粉末配制浓度80 mmol·L−1 的As(Ⅴ)溶液,4 ℃保存待用,使用时根据需要逐级稀释.
所选用的砷还原菌Desulfitobacteriumsp.DJ-3在厌氧状态下培养,使用的基础盐培养基中无机盐浓度如下:KH2PO4(0.14 g·L−1)、NH4Cl(0.25 g·L−1)、KCl(0.5 g·L−1)、CaCl2(0.113 g·L−1)、NaCl(1.0 g·L−1)和MgCl2·6H2O(0.62 g·L−1),培养基中加入无菌的酵母提取物(0.1 g·L−1)作为碳源、乳酸钠溶液(2 mL·L−1)作为电子供体、L-半胱氨酸(0.1 g·L−1)作为还原剂和5 mmol·L−1 As(Ⅴ)溶液,在厌氧手套箱中将菌株DJ-3接种到培养基中,随后加盖丁基橡胶塞,并用铝盖密封. 在摇床37 ℃、150 r·min−1黑暗环境下培养. 传代培养两次后,经过离心、洗涤、重悬等步骤后,得到砷还原菌悬液,用1 mol·L−1 HCl调节pH至7—7.2.
在厌氧瓶中分别将质量终浓度为0.1%的HM、HA、CA、PA、PGA、Glu溶液和砷还原菌悬液混匀,并添加5 mmol·L−1的As(Ⅴ),同时设置不添加有机质的对照组. 以上操作均在厌氧箱中进行,厌氧瓶加盖丁基橡胶塞,并用铝盖密封,在黑暗中于水平摇床(150 r·min−1,37 ℃)中振荡. 在12、24、36、48、84 h取样,每次取2 mL,过0.22 μm滤膜,与1mol·L−1 HNO3按体积1:2混合,放入-20 ℃冰箱保存,待后续测定As总量与形态. 在84 h取1 mL样品测定细菌细胞个数的变化.
-
实验设置同1.3节,从砷还原菌悬液中去除酵母提取物、乳酸钠和L-半胱氨酸,仅添加6种有机质组分作为唯一碳源. 在24、48、84、120 h取样,每次取2 mL,过0.22 μm滤膜,与1 mol·L−1 HNO3按体积1:2混合,放入-20 ℃冰箱保存,待后续测定As形态.
-
有机质的官能团组成使用衰减全反射傅里叶变换红外光谱(ATR-FTIR,vertex 70v)进行表征.
As总量使用电感耦合等离子体质谱(ICP-MS,iCAPQ,Thermo)测定;As形态使用高效液相色谱-电感耦合等离子体质谱联用(HPLC-ICP-MS,iCAPQ,Thermo;色谱柱使用Hamilton PRP-X100)测定.
细菌细胞个数变化的测定使用平板计数法.
-
本实验所有数据用Microsoft Excel 2010软件进行数据整理,用Origin 2019b软件作图.
-
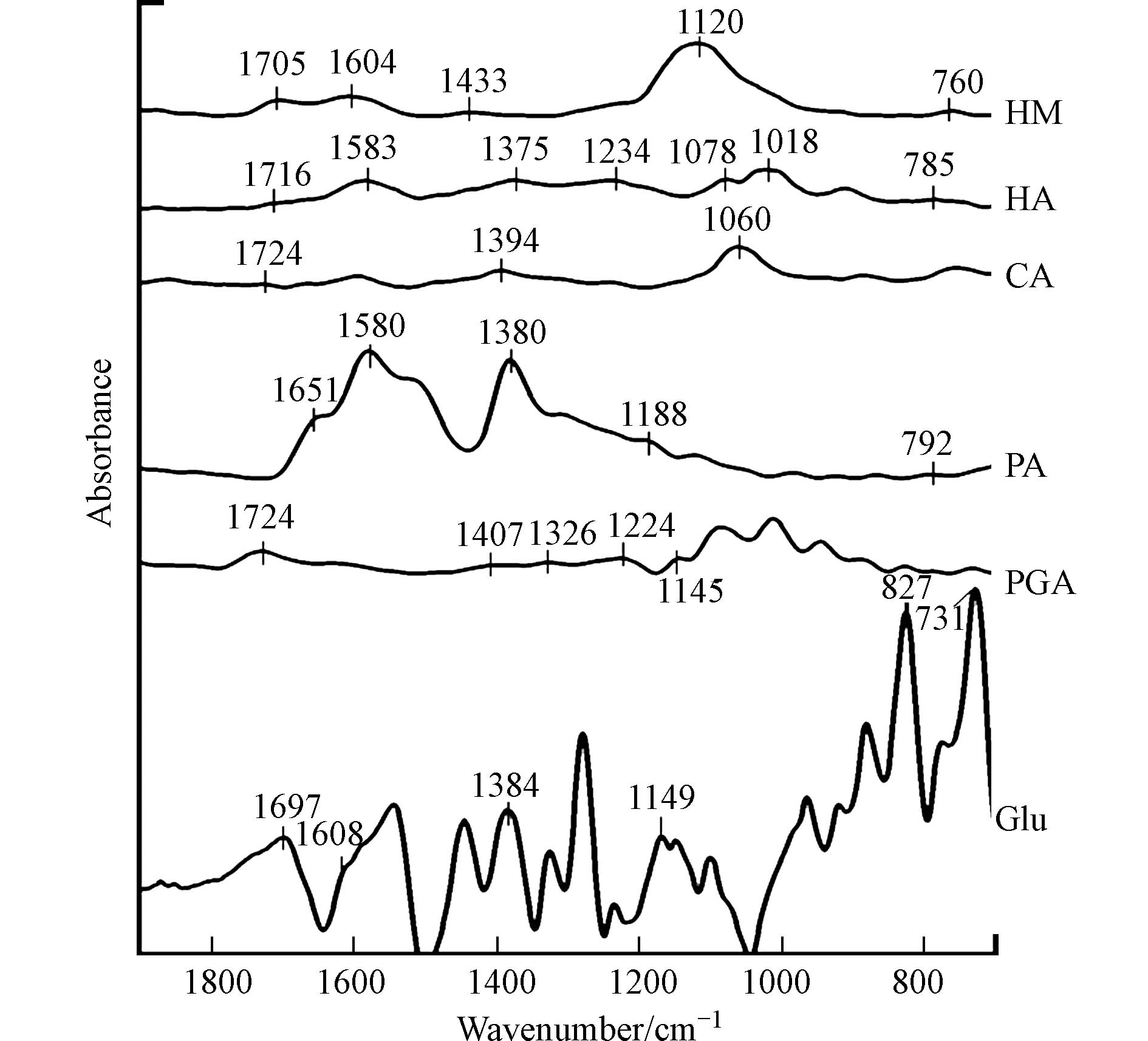
6种有机质组分的红外光谱谱图如图1所示. HM、HA、PA、Glu均含有氨基(
1650 —1550 cm−1,1230 —1020 cm−1和850—700 cm−1)和羧基(1800 —1650 cm−1,1450 —1300 cm−1),此外,HM还含有脂肪醚结构(1300 —1000 cm−1,1150 —1060 cm−1),HA还含有酯或酮,即羰基(1790 —1650 cm−1,1350 —1000 cm−1),以及芳香醚结构(1300 —1000 cm−1,1270 —1230 cm−1和1050 —1000 cm−1). CA主要含有羟基(1275 —1000 cm−1)和羧基(1800 —1650 cm−1,1450 —1300 cm−1),PGA主要含有羧基(1800 —1650 cm−1,1450 —1300 cm−1)和醛基(1765 —1645 cm−1和1407 cm−1)和脂肪醚结构(1300 —1000 cm−1,1150 —1060 cm−1). -
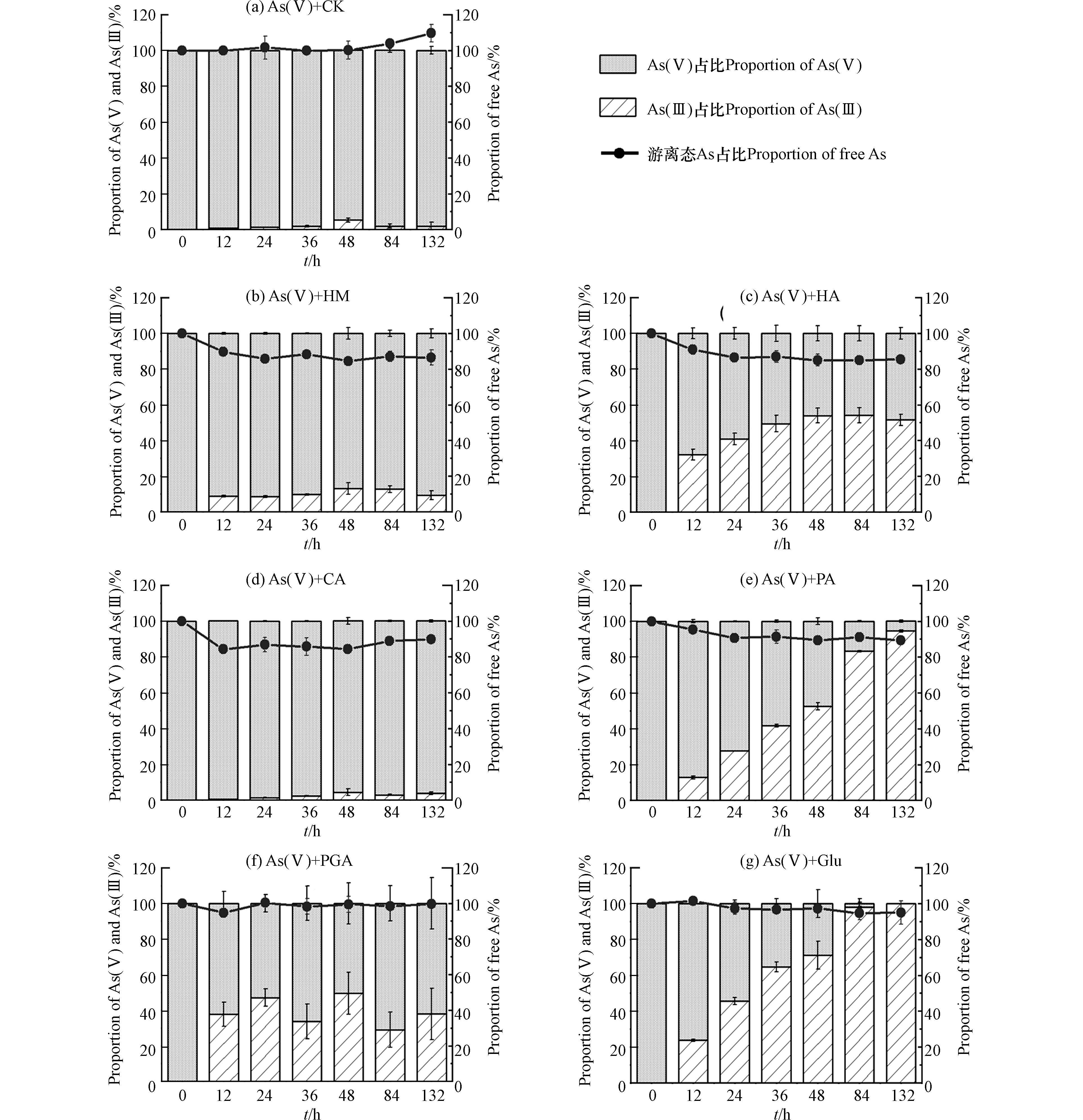
6种有机质组分与As(Ⅴ)溶液混合后,液相中游离态As总量占初始As浓度的比例以及As(Ⅴ)、As(Ⅲ)的占比随时间的变化如图2所示. 由图2((b)—(e)可知,HM、HA、CA、PA处理中132 h后游离态As浓度明显降低,最终分别为初始As浓度的86%、85%、90%及89%;由图2(g)可知,Glu处理中132 h后游离态As终浓度轻微下降,约为初始As浓度的95%;由图2(f)可知,PGA处理中游离态As浓度在实验过程中基本没有变化. 此外,图2(g)、(e)显示,Glu、PA处理中132 h后As(Ⅲ)比例最高,在132 h后分别达到100%和94.5%,还原能力较强;图(b)、(c)、(e)显示,HM、HA、PGA处理中As(Ⅲ)在132 h后分别占总As的8.3%、51.5%、37.8%;图2(d)显示,CA处理中As(Ⅲ)比例最低,在132 h后仅为4%,还原能力较弱.
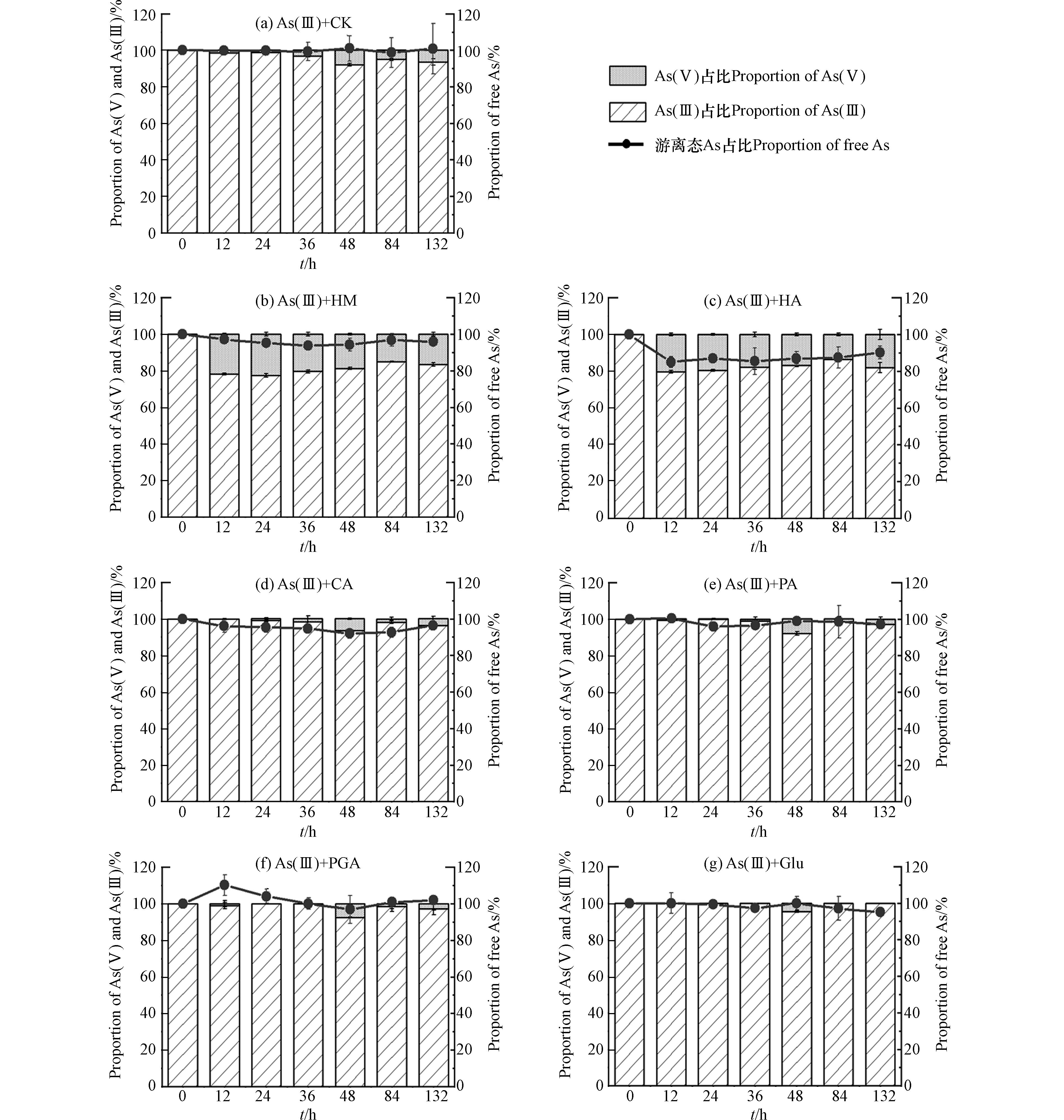
6种有机质组分与As(Ⅲ)溶液混合后,液相中游离态As总量占初始As浓度的比例以及As(Ⅴ)、As(Ⅲ)的占比随时间的变化如图3所示. 由图3(c)可知,HA处理中游离态As浓度明显下降,132 h后约为初始As浓度的90%;由图3(b)、(d)、(e)、(g)可知,HM、CA、PA、Glu处理中游离态As浓度轻微下降,132 h后约为初始As浓度的95%—97%;由图3(f)可知,PGA处理中游离态As浓度在培养过程中无明显变化. 此外,图3(b)、(c)显示HM、HA处理中生成了约20%的As(Ⅴ),说明这2种有机质组分对As(Ⅲ)有氧化作用,而图3(d)—(g)显示CA、PA、PGA、Glu处理中132 h内液相中均未检测到As(Ⅴ),说明这4种有机质组分对As(Ⅲ)基本没有氧化作用.
在pH=7的中性条件下,As(Ⅴ)主要以带负电的HAsO42−形式存在,As(Ⅲ)则主要为中性的As(OH)3和HAsO2[21]. 本次实验中,添加HM、HA后As(Ⅴ)溶液中游离态As浓度的下降程度大于As(Ⅲ)溶液中(图2(b)、(c),图3(b)、(c)),和一些研究者的结果一致[12,21 − 22]. HM、HA含有的氨基在形成腐殖质-As络合物中起重要作用[22],因为这类基团在中性溶液中带正电,能够同时络合As(Ⅴ)、As(Ⅲ),同时有研究者推测As(Ⅴ)中心因具有五个单位正电荷,能与腐殖质中的一些官能团如酚羟基发生配体交换[12],因此与腐殖质的络合程度比As(Ⅲ)大,导致As(Ⅴ)溶液中As总量减少程度更大. 此外,腐殖质本身含有一定量的Fe、Al等金属,还能够通过金属桥联机制与As形成腐殖质-桥联金属-As的三元络合物[23],因此对砷的络合作用相比其他有机质更为明显. 添加CA、PA的情况相似,在As(Ⅲ)溶液中未检测到As(Ⅴ),但游离态As浓度有轻微下降(图3(d)、(e)),说明CA、PA可以吸附/络合少量的As(Ⅲ);而在As(Ⅴ)溶液中,部分As(Ⅴ)被还原为As(Ⅲ),且游离态As浓度下降程度比As(Ⅲ)溶液中明显(图2(d)、(e)),说明这2种有机质也可以吸附/络合As(Ⅴ),且与As(Ⅴ)吸附/络合的能力比As(Ⅲ)大. 有研究显示中性条件下As(Ⅴ)、As(Ⅲ)可以通过离子交换或取代反应与CA中的羧基络合[24]. 此外Haque指出,由于HAsO42−的中心As原子带正电荷,还可能通过物理作用力与羧基进行结合[25],这可能是CA在As(Ⅴ)溶液中使游离态As浓度下降程度比As(Ⅲ)溶液明显的原因. PA可能是由于含有带正电的氨基,更易于与带负电的As(Ⅴ)络合. 添加PGA后,在As(Ⅴ)、As(Ⅲ)溶液中均没有出现游离态As浓度的明显变化(图2(f),图3(f)),说明PGA均无法吸附/络合As(Ⅴ)、As(Ⅲ). PGA和CA同样含有羧基,但没有络合As(Ⅴ)、As(Ⅲ)的能力,Deiana等的研究结果也显示PGA不能与As(Ⅴ)络合[26]. 一些研究表明,羧基的数量、排列方式会影响所形成的络合物的稳定性[27 − 28],因此可能是由于CA与PGA结构上存在差异性,导致PGA与As(Ⅴ)、As(Ⅲ)无法形成稳定的络合物. 添加Glu后,液相As(Ⅲ)未被氧化,游离态As浓度有轻微下降(图3(g)),说明Glu可以与少量的As(Ⅲ)络合,因为Glu在中性溶液中带一个单位的负电荷[29],可以络合显中性的As(Ⅲ). 在As(Ⅴ)溶液体系中生成大量As(Ⅲ),且游离态As浓度有轻微下降(图2(g)),考虑到该条件下Glu和As(Ⅴ)同样带负电,应当存在排斥作用,因此可以认为游离态砷浓度的下降是由于Glu与As(Ⅴ)还原后产生的As(Ⅲ)络合.
已有研究者证实腐殖质类在液相中可以氧化As(Ⅲ)[30],也可以还原As(Ⅴ)[31]. 从本次实验红外光谱的结果分析,HM、HA包含氨基、羰基,CA包含羟基、PA、Glu包含氨基,PGA包含醛基,都属于还原性官能团,因此能够还原As(Ⅴ). 有研究指出羧基和醌基属于得电子基团,与氧化性有关[32],其中醌基的得电子能力较大[33],而醌基是腐殖质类有机质中常见的基团之一[34],因此尽管6种有机质组分均具有羧基,但只有HM、HA表现出氧化As(Ⅲ)的能力.
-
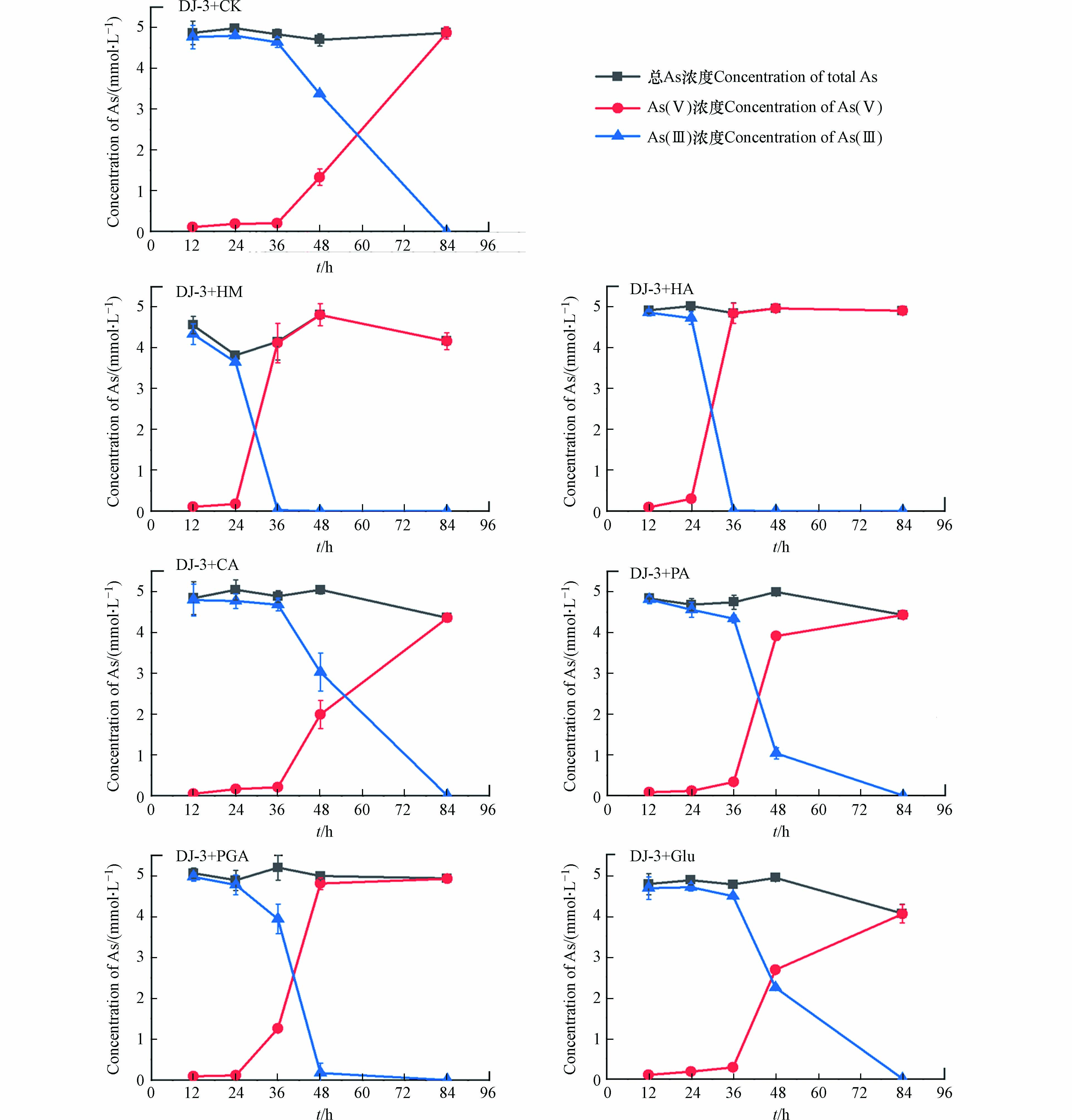
砷还原菌培养过程中,添加6种有机质组分后,液相中As总量及其形态随时间的变化均表现出显著差异(图4). 在不添加有机质组分的对照组中,84 h后液相中只含有As(Ⅲ),表明砷还原菌能在84 h后将As(Ⅴ)全部还原;添加HM、HA的处理中在36 h后液相中均未检测到As(Ⅴ),说明添加HM、HA能够显著促进砷还原菌对As(Ⅴ)的还原;与对照组相比,添加CA、PA、PGA、Glu的处理中48 h后液相中As(Ⅲ)的比例均有所增加,且84 h后As(Ⅴ)被完全还原,说明添加CA、PA、PGA、Glu能够在36—84 h加速砷还原菌对As(Ⅴ)的还原. 此外,从液相中As总量结果上看,添加CA、PA、Glu 后液相As的浓度低于其他处理组.
为了探究上述不同处理中砷还原菌对液相As(Ⅴ)的还原速率有显著差异的原因,对84 h每种处理中砷还原菌的细胞个数进行分析,结果如图5所示. 在不添加有机质组分的对照组中,84 h后细菌细胞个数约为4.35×106·mL−1. CA、Glu处理中的细菌细胞个数分别约为7.07×106、7.51×106·mL−1,说明添加CA、Glu能够显著促进砷还原菌的生长,这可能是导致这两组处理中36—84 h液相As(Ⅴ)还原速率增加的原因. 与其他4种有机质相比,CA、Glu属于小分子有机质,是更容易被微生物利用的碳源,因此能够促进细菌细胞个数的增加. 同时结合上述游离As总量的结果,CA、Glu两组处理在84 h砷总量下降的原因可能是细菌细胞个数的增多对液相As产生了一定的吸附. HM、HA、PA、PGA处理中细菌细胞个数(0.91×106 —3.31×106·mL−1)明显低于对照组,说明添加这4种有机质组分不能促进砷还原菌的生长,可见PA处理中液相砷总量下降可能是由于PA本身对As(Ⅴ)的络合作用. 此外,HM、HA、PA、PGA组中As(Ⅴ)还原速率增加,说明除细胞个数外,还原速率还受到其他因素的影响,因此进一步探究了6种有机质组分作为砷还原菌电子供体的能力.
-
在砷还原菌培养过程中,分别添加6种有机质组分作为唯一碳源,液相中As(Ⅲ)占比变化如图6. 培养120 h后,不添加有机质组分的对照组中仅有5%的As(Ⅴ)被还原,说明当不存在酵母提取物、乳酸钠、L-半胱氨酸时,因培养基中无电子供体,砷还原菌不能介导As(Ⅴ)还原. 添加6种有机质组分120 h后,液相中As(Ⅲ)比例均有所升高,分别达到总砷含量的13%—27%,说明6种有机质组分均可作为砷还原菌的电子供体. 由此可见,尽管细胞个数结果显示添加HM、HA、PA、PGA并未促进砷还原菌的生长,但这几种有机质组分可以作为电子供体促进砷还原菌对液相As(Ⅴ)的还原. 再结合图4的结果,添加HM、HA、PA、PGA比添加CA、Glu能更快地还原As(Ⅴ),特别是HM、HA仅在36 h就将As(Ⅴ)全部还原,推测这几种有机质除了作为电子供体外,还可以通过其他途径来促进砷还原菌对As(Ⅴ)的还原. 近年有学者已证实腐殖质类可以刺激As呼吸基因arrA的转录[17],该基因与异化As还原过程有关,而PA、PGA是否也有类似的作用还需进行后续的探究. 另一方面,Qiao、Cai等的研究证实[17,35],腐殖质类有机质由于具有醌基,能够作为电子穿梭体在微生物胞外进行电子传递,因而相对于其他土壤有机质组分,HM、HA能加快砷还原菌对As(Ⅴ)的还原.
-
(1)在中性pH条件下,HM、HA、CA、PA能同时络合As(Ⅴ)和As(Ⅲ), Glu能络合微量的As(Ⅲ),但几乎无法络合As(Ⅴ), PGA则对As(Ⅴ)、As(Ⅲ)基本无络合作用;HM、HA既能氧化As(Ⅲ)也能还原As(Ⅴ),其他4种有机质组分仅能还原As(Ⅴ).
(2)在砷还原菌还原As(Ⅴ)过程中,6种有机质组分均能促进其对As(Ⅴ)的还原,它们都能作为砷还原菌的电子供体,小分子的CA、Glu还能作为易利用的碳源促进菌数增加,此外HM、HA还能作为电子穿梭体加快As(Ⅴ)的还原.
典型土壤有机质组分对砷还原菌还原砷的影响机制
Impact of typical soil organic matter components on reduction of arsenic by As(Ⅴ)-reducing bacteria and its mechanism
-
摘要: 为了探究土壤中不同类型有机质组分对砷还原菌还原As(Ⅴ)的影响及其机制,选择了腐殖酸(humus acid,HM)、胡敏酸(humic acid,HA),柠檬酸(citric acid,CA)、聚天冬氨酸(polyaspartic acid,PA)、多聚半乳糖醛酸(polygalacturonic acid,PGA)、谷氨酸(glutamic acid,Glu)6种典型土壤有机质组分作为研究对象,首先研究了这几类物质在中性pH条件下对液相As(Ⅴ)、As(Ⅲ)的吸附/络合作用及形态转化,并进一步探究了其对砷还原菌Desulfitobacterium sp. DJ-3还原液相As(Ⅴ)的影响及机制. 结果表明,HM、HA、CA、PA能够络合As(Ⅴ)和As(Ⅲ),Glu仅能够络合As(Ⅲ),而PGA对As(Ⅴ)及As(Ⅲ)均无络合作用. 此外,除了HM、HA能够还原As(Ⅴ)并氧化As(Ⅲ),其余有机质组分仅对As(Ⅴ)存在还原作用. 在砷还原菌还原As(Ⅴ)的过程中,6种有机质组分可以作为电子供体促进砷还原菌对As(Ⅴ)的还原,CA、Glu还可以作为易利用的碳源促进细菌细胞个数增加,而HM、HA可能还起到电子穿梭体的作用,因而能更快地促进液相As(Ⅴ)被还原. 本研究有助于明确不同土壤有机质组分对砷还原菌还原As(Ⅴ)的影响并为土壤中无机砷的环境行为预测提供理论基础.Abstract: To explore the impact of different types of soil organic matter components on arsenic speciation transformation and its mechanisms, six kinds of typical soil organic matter components, including humus acid (HM), humic acid (HA), citric acid (CA), polyaspartic acid (PA), polygalacturonic acid (PGA) and glutamic acid (Glu), were selected. Their effects on the adsorption/complexation and speciation transformation of aqueous As(Ⅴ) and As(Ⅲ) under neutral pH condition were investigated, and the impact of different soil organic matter on the reduction of aqueous As(Ⅴ) by As(Ⅴ)-reducing bacteria Desulfitobacterium sp. DJ-3 and the relevant mechanisms were further explored. The results showed that HM, HA, CA and PA can form complexes with both As(Ⅴ) and As(Ⅲ), Glu can form complexes only with As(Ⅲ), while PGA can’t form complexes with As(Ⅴ) and As(Ⅲ). In addition, except that HM and HA can reduce As(Ⅴ) and oxidize As(Ⅲ), other organic matter components can only reduce As(Ⅴ). In the process of As(Ⅴ) reduction by As(Ⅴ)-reducing bacteria, all six organic matter components can be used as electron donors to promote microbial As(Ⅴ) reduction. As easily available carbon sources, CA and Glu can also increase the cell number of bacteria, while HM and HA may serve as electron shuttle to accelerate the reduction of aqueous As(Ⅴ). Our study can help to clarify the impact of different soil organic matter components on As(Ⅴ) reduction by As(Ⅴ)-reducing bacteria, which will provide a theoretical basis for the prediction of environmental behavior of inorganic arsenic in soil.
-
Key words:
- arsenic /
- organic matter component /
- As(Ⅴ)-reducing bacteria /
- electron donor /
- carbon source /
- electron shuttle.
-

-
-
[1] RAPANT S, DIETZOVÁ Z, CICMANOVÁ S. Environmental and health risk assessment in abandoned mining area, Zlata Idka, Slovakia[J]. Environmental Geology, 2006, 51(3): 387-397. doi: 10.1007/s00254-006-0334-x [2] TAPIA J, MURRAY J, ORMACHEA M, et al. Origin, distribution, and geochemistry of arsenic in the Altiplano-Puna plateau of Argentina, Bolivia, Chile, and Perú[J]. Science of the Total Environment, 2019, 678: 309-325. doi: 10.1016/j.scitotenv.2019.04.084 [3] JIA X Y, CAO Y N, O’CONNOR D, et al. Mapping soil pollution by using drone image recognition and machine learning at an arsenic-contaminated agricultural field[J]. Environmental Pollution, 2021, 270: 116281. doi: 10.1016/j.envpol.2020.116281 [4] SU Y H, McGRATH S P, ZHAO F J. Rice is more efficient in arsenite uptake and translocation than wheat and barley[J]. Plant and Soil, 2010, 328(1): 27-34. [5] OREMLAND R S, STOLZ J F. Arsenic, microbes and contaminated aquifers[J]. Trends in Microbiology, 2005, 13(2): 45-49. doi: 10.1016/j.tim.2004.12.002 [6] MÜLLER K, CIMINELLI V S T, DANTAS M S S, et al. A comparative study of As(Ⅲ) and As(Ⅴ) in aqueous solutions and adsorbed on iron oxy-hydroxides by Raman spectroscopy[J]. Water Research, 2010, 44(19): 5660-5672. doi: 10.1016/j.watres.2010.05.053 [7] SILVER S, PHUNG L T. Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic[J]. Applied and Environmental Microbiology, 2005, 71(2): 599-608. doi: 10.1128/AEM.71.2.599-608.2005 [8] HUANG J H, VOEGELIN A, POMBO S A, et al. Influence of arsenate adsorption to ferrihydrite, goethite, and boehmite on the kinetics of arsenate reduction by Shewanella putrefaciens strain CN-32[J]. Environmental Science & Technology, 2011, 45(18): 7701-7709. [9] CAI X L, ZHANG Z N, YIN N Y, et al. Comparison of arsenate reduction and release by three As(Ⅴ)-reducing bacteria isolated from arsenic-contaminated soil of Inner Mongolia, China[J]. Chemosphere, 2016, 161: 200-207. doi: 10.1016/j.chemosphere.2016.06.102 [10] 张璐. 土壤矿物对非腐殖质活性组分的吸附及稳定性的影响[D]. 昆明: 昆明理工大学, 2021. ZHANG L. Adsorption of soil minerals on non-humus active components and its stability effects [D]. Kunming: Kunming University of Science and Technology, 2021 (in Chinese).
[11] 李海防, 夏汉平, 熊燕梅, 等. 土壤温室气体产生与排放影响因素研究进展[J]. 生态环境, 2007(6): 1781-1788. LI H F, XIA H P, XIONG Y M, et al. Mechanism of greenhouse gases fluxes from soil and its controlling factors: A review[J]. Ecology and Environment, 2007(6): 1781-1788 (in Chinese).
[12] BUSCHMANN J, KAPPELER A, LINDAUER U, et al. Arsenite and arsenate binding to dissolved humic acids: Influence of pH, type of humic acid, and aluminum[J]. Environmental Science & Technology, 2006, 40(19): 6015-6020. [13] XU Y F, WANG K L, ZHOU Q H, et al. Effects of humus on the mobility of arsenic in tailing soil and the thiol-modification of humus[J]. Chemosphere, 2020, 259: 127403. doi: 10.1016/j.chemosphere.2020.127403 [14] LI J P, DING Y, WANG K L, et al. Comparison of humic and fulvic acid on remediation of arsenic contaminated soil by electrokinetic technology[J]. Chemosphere, 2020, 241: 125038. doi: 10.1016/j.chemosphere.2019.125038 [15] ZHANG P, YAO W Y, YUAN S H. Citrate-enhanced release of arsenic during pyrite oxidation at circumneutral conditions[J]. Water Research, 2017, 109: 245-252. doi: 10.1016/j.watres.2016.11.058 [16] LEE J C, KIM E J, KIM H W, et al. Oxalate-based remediation of arsenic bound to amorphous Fe and Al hydrous oxides in soil[J]. Geoderma, 2016, 270: 76-82. doi: 10.1016/j.geoderma.2015.09.015 [17] QIAO J T, LI X M, LI F B, et al. Humic substances facilitate arsenic reduction and release in flooded paddy soil[J]. Environmental Science & Technology, 2019, 53(9): 5034-5042. [18] ZHU M, LV X F, FRANKS A E, et al. Maize straw biochar addition inhibited pentachlorophenol dechlorination by strengthening the predominant soil reduction processes in flooded soil[J]. Journal of Hazardous Materials, 2020, 386: 122002. doi: 10.1016/j.jhazmat.2019.122002 [19] 查文文. 聚合氨基酸对北方水稻土中氧化铁转化与活性有机碳组分的影响[D]. 沈阳: 沈阳农业大学, 2017. ZHA W W. Effects of amino acids on the transformation of iron oxides and active organic carbon fractions in paddy soils of North China [D]. Shenyang: Shenyang Agricultural University, 2017 (in Chinese).
[20] TAKAHASHI Y, MINAMIKAWA R, HATTORI K H, et al. Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods[J]. Environmental Science & Technology, 2004, 38(4): 1038-1044. [21] WARWICK P, INAM E, EVANS N. Arsenic’s interaction with humic acid[J]. Environmental Chemistry, 2005, 2(2): 119. doi: 10.1071/EN05025 [22] THANABALASINGAM P, PICKERING W F. Arsenic sorption by humic acids[J]. Environmental Pollution Series B, Chemical and Physical, 1986, 12(3): 233-246. doi: 10.1016/0143-148X(86)90012-1 [23] LI F L, GUO H M, ZHOU X Q, et al. Impact of natural organic matter on arsenic removal by modified granular natural siderite: Evidence of ternary complex formation by HPSEC-UV-ICP-MS[J]. Chemosphere, 2017, 168: 777-785. doi: 10.1016/j.chemosphere.2016.10.135 [24] SHAKOOR M B, NIAZI N K, BIBI I, et al. Arsenic removal by natural and chemically modified water melon rind in aqueous solutions and groundwater[J]. Science of the Total Environment, 2018, 645: 1444-1455. doi: 10.1016/j.scitotenv.2018.07.218 [25] HAQUE M N, MORRISON G M, PERRUSQUÍA G, et al. Characteristics of arsenic adsorption to sorghum biomass[J]. Journal of Hazardous Materials, 2007, 145(1/2): 30-35. [26] DEIANA S, DEIANA L, PREMOLI A, et al. Accumulation and mobilization of arsenate by Fe(Ⅲ) polyions trapped in a Ca-polygalacturonate network[J]. Plant Physiology and Biochemistry, 2009, 47(7): 615-622. doi: 10.1016/j.plaphy.2009.02.004 [27] TAM S C, McCOLL J G. Aluminum- and calcium-binding affinities of some organic ligands in acidic conditions[J]. Journal of Environmental Quality, 1990, 19(3): 514-520. [28] BOLAN N S, NAIDU R, MAHIMAIRAJA S, et al. Influence of low-molecular-weight organic acids on the solubilization of phosphates[J]. Biology and Fertility of Soils, 1994, 18(4): 311-319. doi: 10.1007/BF00570634 [29] 刘文涵, 单伟光, 高云芳, 等. 硫化锌法原子吸收间接测定谷氨酸络合反应的机理研究[J]. 光谱学与光谱分析, 2003, 23(6): 1191-1193. LIU W H, SHAN W G, GAO Y F, et al. Studies of complexing action mechanism in the indirect determination of glutamic acid by FAAS with ZnS[J]. Spectroscopy and Spectral Analysis, 2003, 23(6): 1191-1193 (in Chinese).
[30] KO I, KIM J Y, KIM K W. Arsenic speciation and sorption kinetics in the As–hematite–humic acid system[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2004, 234(1/2/3): 43-50. [31] PALMER N E, FREUDENTHAL J H, von WANDRUSZKA R. Reduction of arsenates by humic materials[J]. Environmental Chemistry, 2006, 3(2): 131. doi: 10.1071/EN05081 [32] KLÜPFEL L, KEILUWEIT M, KLEBER M, et al. Redox properties of plant biomass-derived black carbon (biochar)[J]. Environmental Science & Technology, 2014, 48(10): 5601-5611. [33] 张月. 生物炭的氧化还原机制及其环境应用[D]. 上海: 上海交通大学, 2019. ZHANG Y. Redox mechanism of biochar and its environmental application[D]. Shanghai: Shanghai Jiao Tong University, 2019 (in Chinese).
[34] SCOTT D T, McKNIGHT D M, BLUNT-HARRIS E L, et al. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms[J]. Environmental Science & Technology, 1998, 32(19): 2984-2989. [35] CAI X L, THOMASARRIGO L K, FANG X, et al. Impact of organic matter on microbially-mediated reduction and mobilization of arsenic and iron in arsenic(Ⅴ)-bearing ferrihydrite[J]. Environmental Science & Technology, 2021, 55(2): 1319-1328. -



 下载:
下载: