-
大气中 CO2、CH4和N2O是重要的温室气体[1],在农牧生产系统中,养殖场粪肥管理设施及其农田利用过程是温室气体及氨等有害气体排放的主要来源[2 − 5]. 固液分离是养殖场畜禽粪便最常见的处理技术[6],这是由于粪肥固液分离不仅可以降低粪肥运输成本[7],而且有助于降低粪肥储存期间温室气体排放[5]. 在我国,大多数养殖场先用固液分离机对粪肥进行分离,分离后液态组分既可以通过沼气工程进行发酵处理,也可通过泵传输灌溉施入农田[8],且由于液态组分可以快速渗入土壤故而可降低土壤氮素损失[9].
研究发现,液态粪肥中大多数固体(大部分为有机物)和营养物质通常存在于小于125 μm的细颗粒中[10],不易通过普通机械筛(筛孔尺寸为0.5 mm)或压机分离[11],因此导致分离的液态组分中固体物质含量较高. 添加絮凝剂是提高粪肥固液分离效率的有效措施之一[12-13],但传统的絮凝剂材料主要是Fe3+和Al3+等金属离子[14],除了对公众健康具有潜在的影响外,还会给环境带来一定的负担,并且阳离子聚合物不能去除溶解的有机物[15]. 因此当前研究特别关注自然资源的循环利用,希望从中可以找到环境友好的絮凝剂[16].
单宁酸是一种天然高分子絮凝剂,主要是通过沉淀作用将污水中细小颗粒絮凝成较大的团聚体[17],可有效降低污水浊度[18],因此具有絮凝性好、无二次污染等优势[19]. 有研究表明,单宁酸进入土壤后可以抑制粪便的微生物矿化,增加N的保留,同时单宁酸也能起到短暂碳源的作用[20- 21]. 然而,目前关于单宁酸作为絮凝剂添加到粪肥以提高固液分离效率的研究很少,对于添加单宁酸固液分离后,液态粪肥对土壤温室气体排放的影响研究还是空白,本研究以牛粪作为试验材料,对比了添加和不添加单宁酸的固液分离技术的液态组分施用后的气体排放特征,探明不同固液分离技术的实施对土壤温室气体排放的影响,这对我国养殖业绿色健康发展具有重要意义.
-
本试验以牛粪为试验材料,试验设置4个处理,不施肥(CK)处理、未分离牛粪(RM)处理、常规螺旋挤压(0.5 mm筛网)固液分离的液态粪肥(LF)处理、添加单宁酸絮凝后螺旋挤压(0.5 mm筛网)固液分离的液态粪肥(LFT)处理. 单宁酸浓度为5%,添加量为3 g·L−1粪肥.
粪肥固液分离后用激光粒度仪(型号:S3500&Bluewave)测定各处理粪肥粒径分布,如图1所示,小于100 μm粒径范围内表现为LFT>LF>RM,大于100 μm粒径范围内表现为RM>LF>LFT.
试验所用土壤:潮褐土取于河北农业大学三分场,取 0—20 cm表层土壤,经自然风干,过 2 mm筛备用. 土壤容重为1.35 g·cm−3,pH 7.9,有机质含量11.21 g·kg−1,全氮含量0.89 g·kg−1,NH4+-N和NO3−-N含量分别为1.86 mg·kg−1和0.63 mg·kg−1.
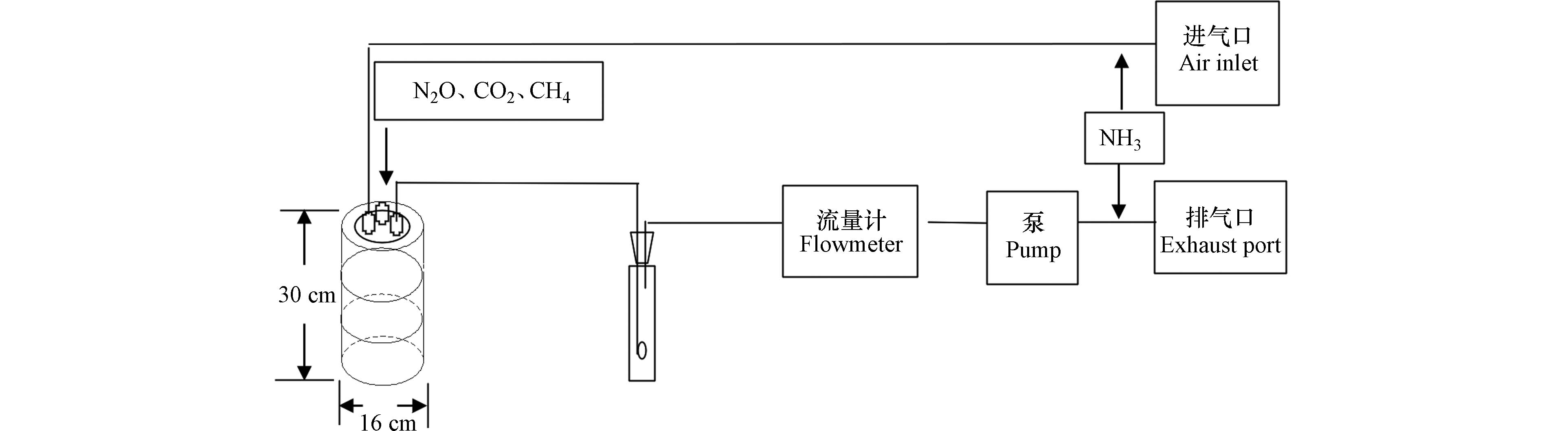
采集气体试验装置为半径8 cm、高30 cm的PVC管,每个培养装置土壤填入量为5.5 kg,填土高度为20 cm. 土壤采集装置为直径 5.7 cm、高 30 cm的圆柱形装置,填土高度为20 cm. 土柱装填后,经干湿交替至土壤容重稳定后进行试验. 施氮量为150 kg·hm−2[22],各处理的液态粪肥施用均依据等氮、等水的原则灌入土柱内. 每个处理粪肥养分投入量如表1.
-
N2O、CO2和CH4采集与测定方法(图2):灌入粪肥后进行气体采集,培养装置由内半径8 cm、高度为30 cm、底部封底的PVC管组成. 密封盖上有3个阀门,分别为测定室压力平衡口,N2O、CO2和CH4采集口和NH3采集口. 测定N2O和CH4排放时,用密封盖盖在箱体上,关闭室外进气口和室内排气口的阀门,顶部形成一个密闭的静态箱,密闭30 min,分别在第0和30 min时用50 mL注射器采集气体30 mL. 利用气相色谱仪(Agilent6820)在24 h内测定气体样品的N2O和CH4浓度. 通过Innova测定箱体CO2浓度. 采集NH3时,关闭中部的N2O采集阀门,打开室外进气口和室内排气口的阀门,箱体顶部形成一个动态箱,打开抽气泵的开关,让各处理的气体流量保持一致,用1个酸吸收瓶(每个酸吸收瓶含0.05 mol·L−1稀硫酸200 mL)吸收室外流动空气中的NH3,气体流量控制在20 L·min−1,抽气1 h后收集酸吸收液[23]. 在采集各处理NH3排放时增加3个背景酸吸收瓶,保证和其他处理相同的时间和抽气流量,排除空气中的NH3对本试验的影响. 使用化学分析仪(SmartChem200)测定酸吸收液中NH4+的浓度.
-
灌溉后每隔1天取10 cm表层土壤样品,测定土壤NO3−-N、NH4+-N和DOC等养分指标. 土壤NH4+-N和NO3−-N用2 mol·L−1的KCl溶液以1:5的土液比将土壤和KCl溶液混合,振荡1 h后过滤,收集滤液,使用化学分析仪(Smart Chem200)测定NH4+和NO3−浓度. 土壤DOC通过水浸提比色法测定.
-
土柱试验 N2O、CH4和CO2排放速率:
式中:F为N2O、CH4和CO2排放速率(mg·m−2·h−1);dCt /dt为箱内气体浓度随时间变化的直线斜率(mg·m−3·h−1);H为土壤表面到培养箱顶盖的高度(m);T0为标准状态下空气的绝对温度(K);T为实际空气温度(℃).
土柱模拟试验NH3-N排放速率:
式中:F为NH3-N排放速率(mg·m−2·h−1);C为硫酸吸收的 NH4 +的浓度(mg·L−1);V为酸吸收液的体积(L);t为抽气时间(h);S为箱体的表面积(m2).
N2O、CO2、CH4和NH3累积排放量:
式中:Q为试验期间 N2O-N、CO2、CH4和NH3-N总累积排放量(mg·m−2);n为试验期间测定总次数;i为采样次数;F为N2O-N、CO2、CH4和NH3-N排放速率(mg·m−2·h−1);t为测定的时间(h).
在100年内,N2O、NH3和CH4的增温潜势[24]:
式中:GHG为N2O、NH3和CH4增温潜势(计CO2-e),A为N2O直接排放量(mg·m−2);B为NH3排放引起的氮损失(mg·m−2),该项的计算结果为挥发氨沉降后引起的N2O间接排放;C为CH4排放量(mg·m−2).
-
采用 Excel 对试验数据进行处理,SPSS24.0进行数据统计分析,采用LSD法(α=0.05)比较处理间差异的显著性. 运用Origin 2021作图软件作图.
-
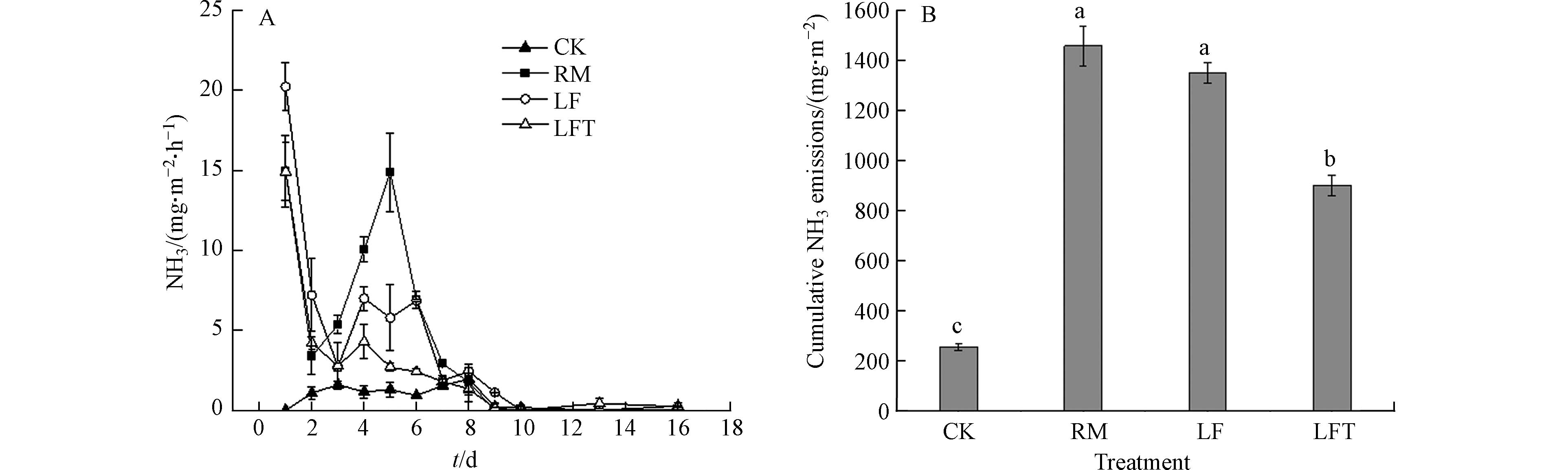
试验期间各处理土壤NH3排放特征如图3A所示. 与CK处理相比,施用粪肥明显增加了土壤NH3排放,且各施肥处理的NH3排放速率和动态特征也存在较大差异. 在第1—3天,各施肥处理NH3排放速率均随时间逐渐下降,但在第4—5天,RM和LF处理呈现出明显的NH3排放峰,且RM处理的NH3排放峰值达14.86 mg·m−2·h−1,显著高于其它处理. 监测期间,各处理NH3排放速率顺序基本为RM>LF>LFT> CK.
图3B表示的是各处理土壤NH3累积排放量,整个监测期内RM处理NH3累积排放量为
1456.18 mg·m−2,显著高于其他处理;LF处理次之,为1323.90 mg·m−2;LFT处理显著低于LF处理,因此添加单宁酸的LFT处理显著降低了土壤NH3排放,减排率为35.62%. LF处理NH3排放量低于RM处理,这与Amon等[5] 的研究结果一致,这可能是由于固液分离后液态粪肥中干物质含量较低,能较好地渗入到土壤中,因此不易发生NH3损失;RM处理NH3的第二个排放峰,远高于其他处理,可能是其中大量的有机氮矿化导致土壤铵根离子浓度升高所致,而LF处理排放较低可能是固液分离过程使得这部分有机氮含量大幅下降所致[25]. 测定LF和LFT处理土壤平均pH分别为7.83和7.87,两者相差较小,因此排除pH对NH3排放影响的可能. -
不同处理土壤N2O排放特征如图4A所示. 与CK相比,RM、LF、LFT处理均存在明显的N2O排放峰。相较而言,RM处理N2O排放上升期略晚于LF和LFT处理,但峰值明显高于这两个处理;LFT处理的N2O的上升期与LF处理相同,但后期排放速率的下降明显早于LF处理.
整个监测期内各处理N2O累积排放量如图4B所示,RM处理N2O累积排放量为475.61 mg·m−2,而LF处理累积排放量达到565.45 mg·m−2,显著高于RM处理,而添加单宁酸处理LFT的N2O排放比LF处理排放量显著降低,减排率为36.00%. 综上所述,与不分离粪肥RM相比,常规固液分离的液态粪肥LF可能会增加土壤N2O排放,常规固液分离与单宁酸絮凝相结合,显著降低了施用液态粪肥土壤N2O排放. LF处理N2O排放量比RM处理高,与Fangueiro等[26] 研究结果一致. 这可能是由于液态分离组分中水溶性碳和氨氮浓度较高,施入土壤后不仅改变了真菌的α多样性[27],还通过增加硝化过程中氨氧化细菌(AOB)、反硝化过程中nirS的丰度并降低nosZ的丰度,从而引起N2O排放增加[28 − 31]. 与LF处理相比,LFT处理N2O排放显著降低,这可能是因为单宁与有机氮络合使得有机氮矿化速率降低,引起土壤矿物质氮NH4+-N和NO3−-N含量下降[32],最终导致土壤N2O排放降低[33]
-
表2为施肥后土壤各处理NH3-N和N2O-N损失占氮素投入量的系数. 其中,RM处理NH3-N损失系数为7.96%,LF处理比RM处理降低了0.83个百分点,LFT处理NH3-N失系数比LF处理降低了2.83个百分点,差异显著;RM处理N2O-N损失系数为3.08%,LF处理的N2O-N损失系数比RM处理增加了0.60个百分点,LFT处理N2O-N损失系数比LF处理显著降低了1.14个百分点;RM处理和LF处理(NH3+N2O)-N损失系数相近,LF和LFT处理(NH3+N2O)-N损失量分别为10.81%和6.84%. 综上所述,常规机械固液分离对(NH3+N2O)-N损失系数影响不大,添加单宁酸显著降低了(NH3+N2O)-N损失系数.
-
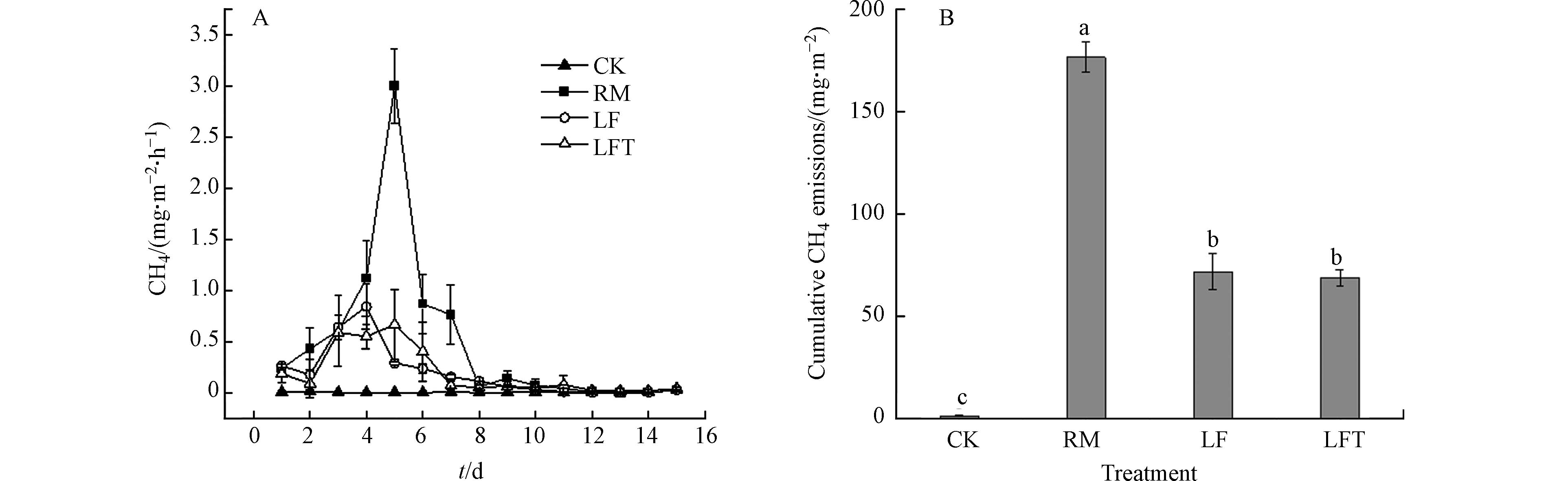
土壤CH4速率排放特性如图5A所示. 各处理在监测第4—5天出现排放峰,表现为RM>LF>LFT>CK,RM处理排放峰值为3.00 mg·m−2·h−1,明显高于其他处理,LF和LFT处理排放峰值大小相近,CK处理在整个监测期内排放速率最低.
监测期内RM处理土壤CH4累积排放量为176.53 mg·m−2显著高于其它处理图5B;LF处理为71.75 mg·m−2,较RM处理降低59.36%;CK处理最低,仅有1.18 mg·m−2. 综上所述,固液分离有利于降低土壤CH4排放量,而添加单宁酸的LFT处理土壤CH4排放影响不大. RM处理土壤CH4累积排放量均高于LF处理,这是由于不分离粪肥有机质含量比LF处理高所致(表1).
-
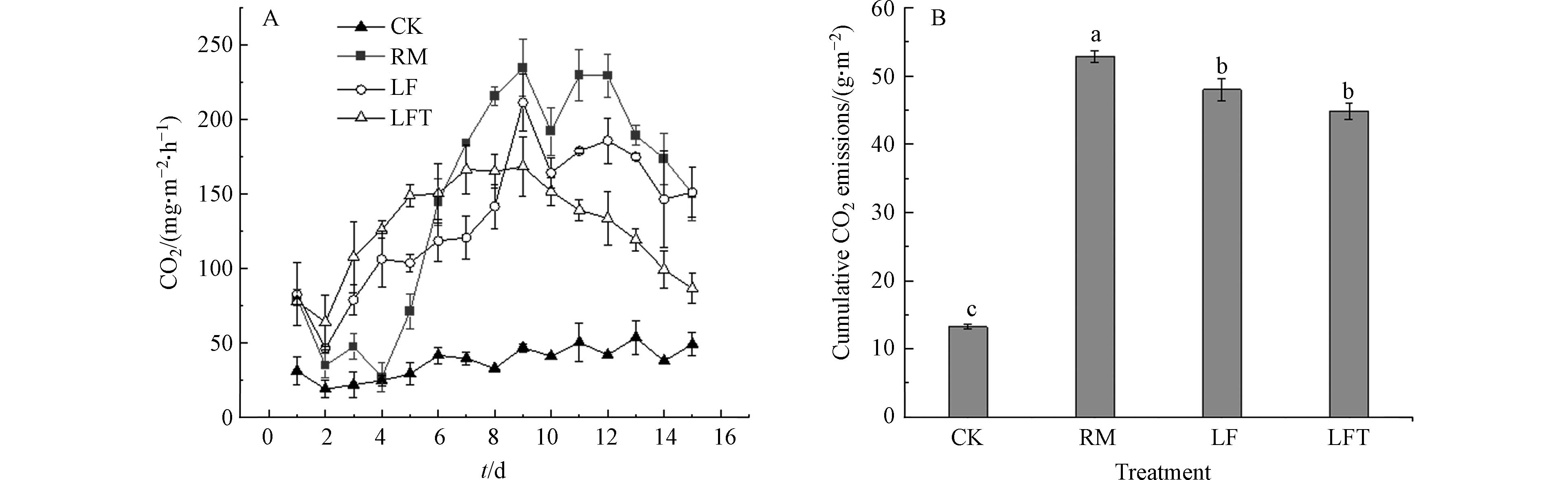
图6A为CO2排放速率特征曲线,在整个监测期内,前6天各处理CO2排放速率表现为LFT>LF>RM,监测第8天后,各处理排放特征表现为RM>LF>LFT,CK处理在整个监测期内排放速率最低. Fangueiro等将粪肥粒径分级后研究其对土壤CO2排放结果表明,在试验前期发现粒径较小的处理CO2排放量较高,而后期粗粒级处理的CO2排放量最高[34],这与本研究结果高度一致. 推断这可能是由于粪肥的生物和物理化学降解效率受其固体含量和粒度分布等因素的影响,而固液分离技术有效去除了粪肥中粒径较大、难降解的有机颗粒,因此导致CO2排放呈现出前期高、后期低的特征[35].
各处理CO2累积排放量如图6B所示,RM处理土壤CO2累积排放量最高为52.80 g·m−2,显著高于LF处理;LF和LFT处理CO2累积排放量相近,分别为47.98 g·m−2和44.84 g·m−2,CK处理最低,仅13.25 g·m−2. 综上所述,固液分离显著降低了土壤CO2排放,这是由于不分离粪肥有机质含量比LF处理高所致(表1),添加单宁酸对土壤CO2排放影响不大.
-
图7A为液态粪肥对土壤NH4+-N的影响. 第5天,各处理土壤NH4+-N含量最高,LF处理土壤NH4+-N含量为219.89 mg·kg−1显著高于RM处理;5d后,LFT处理土壤NH4+-N含量显著低于LF处理.
土壤NO3−-N含量呈逐渐升高趋势图7B,表现为LF>RM>LFT,LF处理土壤NO3−-N含量最高为120.54 mg·kg−1,比RM处理较高;LFT处理土壤NO3−-N含量显著低于LF处理.
由图7C可知,在试验第5天LFT处理土壤DOC含量达到最高水平,为8.97 mg·kg−1,显著高于LF处理;LF处理为3.89 mg·kg−1显著高于RM处理. 综上所述,传统机械固液分离增加了土壤NO3−-N和NH4+-N含量,添加单宁酸,降低了土壤NO3−-N和NH4+-N含量. 产生该现象的原因可能是:单宁酸能显著抑制硝化作用[36],这可能与单宁酸对蛋白质的络合作用有关,即液态分离组分中残留的单宁酸可通过抑制土壤酶活性等途径降低微生物矿化和反硝化作用,进而引起土壤NH4+-N和NO3−-N的浓度下降. 添加单宁酸明显增加了土壤DOC含量,可能由于单宁充当了微生物碳源[37]
-
使用气体的全球升温潜势值(GWP)将不同气体排放量转换为CO2-e. 由表3可知,各处理N2O排放对CO2-e排放量贡献最大,其次为CH4,NH3最低. 各处理CO2-e表现为LF>RM>LFT>CK,LF处理CO2-e排放量为157.36 g·m−2,显著高于RM处理,LFT处理与LF处理相比显著降低了土壤CO2-e排放量,减排率为30.00%,差异显著. 综上所述,固液分离后液态粪肥还田增加了土壤CO2-e,应用单宁酸作为絮凝剂有利于降低施液态粪肥土壤CO2-e排放. 整体看来,LF处理CO2-e排放量显著高于RM和LFT处理,这主要是由于该处理N2O排放高于RM和LFT处理所致. 此外,Dinuccio等研究发现,液态和固态分离后在储存期间和土壤施用后CO2-e排放量比未分离粪肥高11%[38],这在一定程度上抑制了固液分离技术在规模化养殖场的应用前景. 因此当采用单宁酸作为絮凝剂后,使得液态分离组分农田施用过程中CO2-e排放显著下降,很大程度弥补了当前常规固液分离技术的缺陷,因此具有较大的应用空间和前景. 然而,还需要针对基于单宁酸絮凝剂添加的强化处理开展深入研究,探索强化分离液态组分土壤N2O、CH4微生物学产排机制,深入揭示粪肥固液分离后液态粪肥对土壤温室气体的减排效果和潜力.
-
本研究探讨了常规和强化固液分离液态组分施入土壤后的温室气体排放. 主要结论如下:
1)与未分离粪水相比,常规固液分离技术对土壤NH3影响较小,显著降低了土壤CO2和CH4排放,但却显著增加了土壤N2O排放,并导致土壤CO2-e排放增加.
2)与常规分离技术相比,添加单宁酸的强化分离技术对CO2和CH4排放影响较小,但显著降低了土壤NH3和N2O排放,最终导致土壤CO2-e排放显著下降.
简单的机械固液分离技术可能存在导致液态粪肥农田施用阶段氮素损失和温室气体排放增加的风险,而添加絮凝剂单宁酸的强化分离技术可以一定程度上避免该风险,但仍需要深入研究揭示其减排机制并验证田间应用效果.
单宁酸强化固液分离液态组分对土壤NH3和温室气体排放的影响
Ammonia and greenhouse gas emissions from soil applied with liquid fractions by enhanced solid-liquid separation technology with tannic acid
-
摘要: 固液分离技术是当前规模化养殖场液态粪便主要处理技术之一,提高分离效率是推动液态粪便循环利用的有效途径. 为探明不同固液分离技术下液态粪肥对土壤温室气体排放的影响,本文以牛粪为试验材料,将固液分离后的液态粪肥进行室内土柱培养试验. 试验设置4个处理,分别为不施肥(CK)处理、未分离(RM)处理、常规螺旋挤压(LF)处理、添加单宁酸絮凝后螺旋挤压(LFT)处理. 运用静态箱法监测培养期内土壤N2O、CO2和CH4的排放情况,同时采用动态箱法监测土壤NH3的排放情况. 结果表明:整个监测期内各处理NH3排放量表现为RM>LF>LFT>CK;N2O排放顺序为LF>RM>LFT>CK. 各处理氮素气态损失均表现为NH3-N >N2O-N,LF和LFT处理(NH3+N2O)-N损失分别占施氮量10.81%和6.84%,且后者显著降低了(NH3+N2O)-N损失. 与RM处理相比,LF和LFT处理均显著降低了土壤CH4和CO2排放. 与RM处理相比,LF处理显著增加了土壤CO2当量(CO2-e)排放,而LFT处理的土壤CO2-e排放与LF处理相比显著降低了30.00%. 常规机械固液分离技术可能有增加液态粪肥农田施用阶段氮素损失和温室气体排放的风险,而添加絮凝剂单宁酸的强化分离技术可以在一定程度上避免该风险,但仍需要深入研究揭示其减排机制并验证田间应用效果.Abstract: Solid-liquid separation technology is a major practice for the liquid manure management on intensive animal farms, and improving the separation efficiencies of different separation technologies is of importance for enhancing the recycling of liquid animal manure. To investigate the impacts of separated liquid fractions of dairy slurry by different solid-liquid separation technologies on soil greenhouse gas emissions, an indoor incubation of soil column culture experiment was conducted. Four treatments including no fertilization (CK), raw slurry application (RM), application of liquid fraction by screw-press separation (LF), and application of liquid fraction by screw press+tannic acid flocculant (0.5mm screen) (LFT) were set up. The nitrous oxide (N2O), carbon dioxide (CO2) and methane (CH4) emissions of the incubation setup were measured using static chamber technique, and the NH3 emissions were measured using a dynamic chamber technique. The results showed that: (1) the NH3 emission of different treatments was in an order of RM>LF>LFT>CK, and the N2O emission was in an order of LF>RM>LFT>CK during the whole monitoring period. (2) The gaseous nitrogen losses of each treatment were NH3-N >N2O-N; and the (NH3+N2O)-N of the LF and LFT treatment accounted for 10.81% and 6.84% of the input N, respectively, where the latter significantly reduced (NH3+N2O)-N loss. (3) Compared to the RM treatment, LF and LFT treatments significantly reduced CH4 and CO2 emissions. (4) Compared to the RM treatment, LF treatment significantly increased soil CO2 equivalent (CO2-e) emission, while LFT treatment significantly reduced soil CO2-e emission by 30.00%. In conclusion, conventional mechanical solid-liquid separation technology may increase the risk of nitrogen loss and greenhouse gas emissions during liquid manure application, while such can be avoided to some extent when the conventional separation technology is combined with the use of tannic acid as flocculant. However, further research is needed to reveal its emission reduction mechanism of the screw press+tannic acid flocculent technology and to verify the mitigation in fields.
-
Key words:
- liquid manure /
- solid-liquid separation technology /
- tannic acid /
- ammonia /
- greenhouse gas
-

-
表 1 各处理养分投入量
Table 1. Nutrient inputs in each treatment
处理
Treatment有机碳/(g·m−2)
Organic carbon总氮/(g·m−2)
TN氨氮N /(g·m−2)
TARM 181.86 15 4.56 LF 116.36 15 5.29 LFT 146.86 15 5.41 表 2 不同施肥处理NH3-N和N2O-N损失系数
Table 2. Proportions of NH3-N and N2O-N losses to the N inputs in different treatments
处理
TreatmentNH3-N/% N2O-N/% (NH3+N2O)-N /% RM 7.96a 3.08b 11.04a LF 7.13a 3.68a 10.81a LFT 4.30b 2.54c 6.84b 注:同列数据后小写字母表示系统内对比差异显著(P<0.05).
Lowercase letters after data in the same column indicate significant comparison differences within the system(P<0.05)表 3 各处理CO2-e排放量
Table 3. Emissions of CO2-e from different treatments
处理
TreatmentCH4 /(g·m−2) N2O /(g·m−2) NH3 /(g·m−2) 合计/(g·m−2)
TotalCK 0.03c 3.47d 1.06c 4.56d RM 4.94a 126.04b 6.02a 137.00b LF 2.01b 149.85a 5.51a 157.36a LFT 1.93b 104.49c 3.74b 110.15c 注:同列数据后小写字母表示系统内对比差异显著(P<0.05).
Lowercase letters after data in the same column indicate significant comparison differences within the system(P<0.05). -
[1] 李晓密, 伦小秀, 陈琪, 等. 不同施肥处理下冬小麦-夏玉米轮作农田温室气体的排放[J]. 环境化学, 2014, 33(4): 591-596. doi: 10.7524/j.issn.0254-6108.2014.04.019 LI X M, LUN X X, CHEN Q, et al. Greenhouse gas emission from a winter wheat-summer maize crop rotation farmland under different fertilization treatments[J]. Environmental Chemistry, 2014, 33(4): 591-596 (in Chinese). doi: 10.7524/j.issn.0254-6108.2014.04.019
[2] 闫昭明, 李文睿, 陈清华. 畜禽规模化养殖发展对我国生态环境的影响及保护措施[J]. 畜牧业环境, 2019(11): 10-11. YAN Z M, LI W R, CHEN Q H. Influence of large-scale livestock and poultry breeding development on China’s ecological environment and its protection measures[J]. Animal Industry and Environment, 2019(11): 10-11 (in Chinese).
[3] VELTMAN K, ROTZ C A, CHASE L, et al. A quantitative assessment of Beneficial Management Practices to reduce carbon and reactive nitrogen footprints and phosphorus losses on dairy farms in theUS Great lakes region[J]. Agricultural Systems, 2018, 166: 10-25. doi: 10.1016/j.agsy.2018.07.005 [4] HUTCHINGS N, AMON B, DAMMGEN U. Animal husbandry and manure management[J]. EMEP/EEA air pollutant emission inventory guidebook, 2009: 1-73. [5] AMON B, KRYVORUCHKO V, AMON T, et al. Methane, nitrous oxide and ammonia emissions during storage and after application of dairy cattle slurry and influence of slurry treatment[J]. Agriculture, Ecosystems & Environment, 2006, 112(2/3): 153-162. [6] AGUIRRE-VILLEGAS H A, LARSON R A. Evaluating greenhouse gas emissions from dairy manure management practices using survey data and lifecycle tools[J]. Journal of Cleaner Production, 2017, 143: 169-179. doi: 10.1016/j.jclepro.2016.12.133 [7] BALSARI P, DINUCCIO E, SANTORO E, et al. Ammonia emissions from rough cattle slurry and from derived solid and liquid fractions applied to alfalfa pasture[J]. Australian Journal of Experimental Agriculture, 2008, 48(2): 198. doi: 10.1071/EA07234 [8] 王浚峰, 高继伟, 冯英, 等. 现代化牧场的粪污处理[J]. 中国奶牛, 2011(2): 60-63. WANG J F, GAO J W, FENG Y, et al. Treatment of manure in modern pasture[J]. China Dairy Cattle, 2011(2): 60-63. (in Chinese)
[9] GUILAYN F, JIMENEZ J, ROUEZ M, et al. Digestate mechanical separation: Efficiency profiles based on anaerobic digestion feedstock and equipment choice[J]. Bioresource Technology, 2019, 274: 180-189. doi: 10.1016/j.biortech.2018.11.090 [10] MEYER D, RISTOW PL, LIE M. Particle size and nutrient distribution in fresh dairy manure[J]. Applied Engineering in Agriculture, 2007, 23(1): 113-118. doi: 10.13031/2013.22318 [11] PETERS K, HJORTH M, JENSEN L S, et al. Carbon, nitrogen, and phosphorus distribution in particle size-fractionated separated pig and cattle slurry[J]. Journal of Environmental Quality, 2011, 40(1): 224-232. doi: 10.2134/jeq2010.0217 [12] AMON B, AMON T, BOXBERGER J, et al. Emissions of NH3, N2O and CH4 from dairy cows housed in a farmyard manure tying stall (housing, manure storage, manure spreading)[J]. Nutrient Cycling in Agroecosystems, 2001, 60(1): 103-113. [13] VANOTTI M B, RASHASH D M C, HUNT P G. Solid–liquid separation of flushed swine manure with pam: Effect of wastewater strength[J]. Transactions of the ASAE, 2002, 45(6): 1959. [14] ZHANG Z G, WANG J, LIU D, et al. Hydrolysis of polyaluminum chloride prior to coagulation: Effects on coagulation behavior and implications for improving coagulation performance[J]. Journal of Environmental Sciences, 2017, 57: 162-169. doi: 10.1016/j.jes.2016.10.014 [15] LIU Z, CARROLL Z S, LONG S C, et al. Use of cationic polymers to reduce pathogen levels during dairy manure separation[J]. Journal of Environmental Management, 2016, 166: 260-266. [16] ALJUBOORI A H R, IDRIS A, ABDULLAH N, et al. Production and characterization of a bioflocculant produced by Aspergillus flavus[J]. Bioresource Technology, 2013, 127: 489-493. doi: 10.1016/j.biortech.2012.09.016 [17] SÁNCHEZ-MARTÍN J, GONZÁLEZ-VELASCO M, BELTRÁN-HEREDIA J. Surface water treatment with tannin-based coagulants from Quebracho (Schinopsis balansae)[J]. Chemical Engineering Journal, 2010, 165(3): 851-858. doi: 10.1016/j.cej.2010.10.030 [18] SINGH R, KUMAR S, GARG M. Domestic wastewater treatment using tanfloc: A tannin based coagulant[C]//Geostatistical and Geospatial Approaches for the Characterization of Natural Resources in the Environment. Cham: Springer, 2016: 349-354. [19] 葛冬冬. 基于单宁酸调理改善污泥脱水性能的技术及机理研究[D]. 上海:上海交通大学, 2020. GE D D. Tannic acidcid-based conditioning technology for improving sludge dewaterability and mechanism insight[D]. Shanghai: Shanghai Jiao Tong University, 2020 (in Chinese).
[20] NIEROP K G J, VERSTRATEN J M, TIETEMA A, et al. Short-and long-term tannin induced carbon, nitrogen and phosphorus dynamics in corsican pine litter[J]. Biogeochemistry, 2006, 79(3): 275-296. doi: 10.1007/s10533-005-5274-0 [21] NIEROP K G J, PRESTON C M, VERSTRATEN J M. Linking the B ring hydroxylation pattern of condensed tannins to C, N and P mineralization. A case study using four tannins[J]. Soil Biology and Biochemistry, 2006, 38(9): 2794-2802. doi: 10.1016/j.soilbio.2006.04.049 [22] 孟祥海, 魏丹, 王玉峰, 等. 氮素水平与施氮方式对稻田氨挥发影响[J]. 黑龙江农业科学, 2011(12): 38-42. MENG X H, WEI D, WANG Y F, et al Nitrogen levels and nitrogen application modes on ammonia volatilization effect[J]. Heilongjiang Agricultural Sciences, 2011(12): 38-42 (in Chinese).
[23] CABRERA M L, KISSEL D E, DAVIS R C, et al. Design and ammonia-recovery evaluation of a wind speed-sensitive chamber system[J]. Soil Science Society of America Journal, 2001, 65(4): 1302-1306. doi: 10.2136/sssaj2001.6541302x [24] CHANGE I C. Synthesis report. Contribution of working groups Ⅰ. Ⅱ and III to the fifth assessment report of the intergovernmental panel on climate change, 2014,151(10.1017)[C]. [25] GRELL T, MARCHUK S, WILLIAMS I, et al. Resource recovery for environmental management of dilute livestock manure using a solid-liquid separation approach[J]. Journal of Environmental Management, 2023, 325(Pt A): 116254. [26] FANGUEIRO D, SENBAYRAN M, TRINDADE H, et al. Cattle slurry treatment by screw press separation and chemically enhanced settling: Effect on greenhouse gas emissions after land spreading and grass yield[J]. Bioresource Technology, 2008, 99(15): 7132-7142. doi: 10.1016/j.biortech.2007.12.069 [27] WEN Y C, LI H Y, LIN Z A, et al. Long-term fertilization alters soil properties and fungal community composition in fluvo-aquic soil of the North China Plain[J]. Scientific Reports, 2020, 10(1): 7198. doi: 10.1038/s41598-020-64227-6 [28] DOMEIGNOZ-HORTA L A, PHILIPPOT L, PEYRARD C, et al. Peaks of in situ N2O emissions are influenced by N2O-producing and reducing microbial communities across arable soils[J]. Global Change Biology, 2018, 24(1): 360-370. doi: 10.1111/gcb.13853 [29] BLAGODATSKAYA Е, ZHENG X, BLAGODATSKY S, et al. Oxygen and substrate availability interactively control the temperature sensitivity of CO2 and N2O emission from soil[J]. Biology and Fertility of Soils, 2014, 50: 775-783. doi: 10.1007/s00374-014-0899-6 [30] CHARLES A, ROCHETTE P, WHALEN J K, et al. Global nitrous oxide emission factors from agricultural soils after addition of organic amendments: A meta-analysis[J]. Agriculture, Ecosystems & Environment, 2017, 236: 88-98. [31] XU X Y, LIU Y W, SINGH B P, et al. NosZ clade II rather than clade i determine in situ N2O emissions with different fertilizer types under simulated climate change and its legacy[J]. Soil Biology and Biochemistry, 2020, 150: 107974. doi: 10.1016/j.soilbio.2020.107974 [32] HÄTTENSCHWILER S, VITOUSEK P M. The role of polyphenols in terrestrial ecosystem nutrient cycling[J]. Trends in Ecology & Evolution, 2000, 15(6): 238-243. [33] SAHINER N, SAGBAS S, SAHINER M, et al. Biocompatible and biodegradable poly(tannic acid) hydrogel with antimicrobial and antioxidant properties[J]. International Journal of Biological Macromolecules, 2016, 82: 150-159. doi: 10.1016/j.ijbiomac.2015.10.057 [34] FANGUEIRO D, CHADWICK D, DIXON L, et al. Quantification of priming and CO2 emission sources following the application of different slurry particle size fractions to a grassland soil[J]. Soil Biology and Biochemistry, 2007, 39(10): 2608-2620. doi: 10.1016/j.soilbio.2007.05.012 [35] MASSE L, MASSÉ D I, BEAUDETTE V, et al. Size distribution and composition of particles in raw and anaerobically digested swine manure[J]. Transactions of the ASAE, 2005, 48(5): 1943-1949. doi: 10.13031/2013.20003 [36] ADAMCZYK S, KIIKKILÄ O, KITUNEN V, et al. Potential response of soil processes to diterpenes, triterpenes and tannins: Nitrification, growth of microorganisms and precipitation of proteins[J]. Applied Soil Ecology, 2013, 67: 47-52. doi: 10.1016/j.apsoil.2013.02.009 [37] KRAUS T E C, ZASOSKI R J, DAHLGREN R A, et al. Carbon and nitrogen dynamics in a forest soil amended with purified tannins from different plant species[J]. Soil Biology and Biochemistry, 2004, 36(2): 309-321. doi: 10.1016/j.soilbio.2003.10.006 [38] DINUCCIO E, BERG W, BALSARI P. Effects of mechanical separation on GHG and ammonia emissions from cattle slurry under winter conditions[J]. Animal Feed Science and Technology, 2011, 166/167: 532-538. doi: 10.1016/j.anifeedsci.2011.04.037 -




 下载:
下载: