-
作为一种新兴技术,空气取水技术能有效捕获空气中的水资源,可在全球范围内为人们提供洁净的饮用水[1]. 不同于雾收集技术[2]与空气冷却取水技术[3],吸附式空气取水技术是通过吸附材料捕获空气的水分子,在加热状态下蒸发解吸水分子,最后通过冷凝装置收集液态水[4],因此,吸附式空气取水技术[5 − 7]可克服气候条件的限制,低耗高效保障水资源供给.
硅胶[8]、分子筛[9]和以CaCl2[10]、LiCl[11]为主的吸湿盐等材料是吸附式空气取水装置的常用吸附剂,但实际应用中吸湿盐在水吸附过程易潮解形成液体,而硅胶、分子筛在解吸过程的能耗较高[12 − 13]. 金属有机框架材料(metal-organic framawork, MOF)材料具有稳定的框架结构保证材料的水稳定性能[14 − 18]。以MOF-801(Zr)[19]、MOF-303(Al)[20]等材料为例,由于其高比表面积和高孔隙率,材料能快速吸附空气中的水分子并具有较高的水吸附容量[21 − 22]. 将传统吸湿盐负载到MOF材料的框架结构中[23 − 24],能成功抑制吸湿盐遇水易潮解的问题,增加MOF材料的水吸附性能. 因此,MOF材料是种极具潜力的水吸附材料,在吸附式空气取水技术中具有较大应用前景[25].
在MOF材料制备过程,金属源和分散剂的选择多样,以铝基MIL-53[26]和富马酸铝[27]为例,氯化铝、硫酸铝和硝酸铝等铝盐都可作为铝源,在MOF-303(Al)制备过程中存在LiOH和NaOH两种分散剂的使用[20, 28]. 为推广MOF材料的实际应用,明确不同制备条件对材料性能的影响具有重要意义,原材料的易得性、制备方法的简单化和易放大也是重要的因素.
本研究基于水吸附性能优异的MOF-303(Al)材料,结合场发射扫描电子显微镜(Field Emission Scanning Electron Microscope, FESEM)、X-射线粉末衍射图谱(X-ray diffraction, XRD)、Brunauer-Emmett-Teller (BET)比表面积、孔容孔径等表征方法及水蒸气吸附性能测试,考察水溶剂、金属源、反应温度和分散剂类型等制备条件对材料水吸附性能和结构形貌的影响,以期为MOF-303(Al)及MOF材料的制备和推广提供支撑.
-
十八水合硫酸铝(Al2(SO4)3·18H2O,AR,99.0%)购于天津市福晨化学试剂厂,甲醇(CH3OH,纯度>99.5%)购自于北京国药集团化学试剂有限公司,无水氯化铝(AlCl3,AR,99%)、异丙醇铝(Al(O(CH3)2)3,AR)、碱式乙酸铝(Al(OH)(OOCCH3)2,AR)、3,5-吡唑二甲酸一水合物(C5H4O4N2,AR,97%)、氢氧化锂(LiOH,AR,98%)、氢氧化钠 (NaOH,AR,95%)和氢氧化钙(Ca(OH)2)均购自上海麦克林生化科技有限公司. 试验过程所用超纯水由美国Milli-Q纯水系统制备.
-
采用水热法,基于参考文献[20, 28]的方案并进行调整. 其中,有机配体(3,5-吡唑二羧酸一水合物):氢氧根离子(OH-,由分散剂提供):三价铝离子(Al3+)的摩尔比为1:2:1. 制备过程如下:先将有机配体和分散剂混合加入水溶剂,在超声状态下(KQ5200DE,昆山市超声仪器有限公司)得到澄清溶液;加入金属铝源充分搅拌,生成白色乳浊液前驱体;将前驱体溶液转移至水热反应釜(100 mL聚四氟乙烯内衬)中至60%容积,放入预热至100 ℃的烘箱中反应12 h;冷却至室温,离心收集产物,用甲醇和去离子水分别清洗数次后在150 ℃、0.01 bar真空干燥箱(DZF-6020,上海红华仪器有限公司)活化6 h得到MOF-303(Al)样品并保存备用.
试验主要考察水溶剂(去离子水、超纯水、自来水)、金属源(氯化铝、硫酸铝、碱式乙酸铝、异丙醇铝)、反应温度(25、85、100、150 ℃)和分散剂(氢氧化锂、氢氧化钠、氢氧化钙)对材料水吸附性能和形貌结构的影响,样品编号及制备条件见表1.
-
采用场发射扫描电子显微镜(FE-SEM,SU-8020,日本 Hitachi公司)和X射线衍射仪(XRD,D8 focus,德国 Bruker公司)进行形貌成分分析,采用EDX(Energy Dispersive X-Ray Spectroscopy) 进行面扫获得元素分布,扫描次数为5次. 采用比表面积及孔径分析仪(BET,ASAP 2460,美国 Micromeritics公司)测试材料的比表面积和孔容,同时得到材料的微孔比表面积和微孔孔体积. 阴、阳离子采用电感耦合度等离子体原子发射光谱仪(Agilent,ICP-OES730,美国安捷伦公司)和离子色谱(IC,ICS-1100,Thermo Dionex,美国赛默飞世尔Thermo公司)进行测试. 通过热重分析仪(STA 449 F5/F3 Jupiter,德国耐驰(NETZSCH)公司)测试材料的热稳定性能,温度范围为25—600℃,样品测试前在潮湿氛围下充分吸附水分子并基于失水峰通过公式1初步计算水吸附容量.
-
MOF材料的吸附容量采用可程式恒温恒湿试验箱(HK-80G,东莞市勤卓环境测试设备有限公司)进行测试,测试过程如下:首先记录铝箔称量皿的质量为m1,其次称取样品放于称量皿中并于预热105 ℃烘箱中烘干12 h得到质量m2,最后将烘干样品放置于恒温恒湿试验箱中,在25 ℃下,20%、40%、60%、80%和95%五种相对湿度条件下经过12 h的充分吸附后称量得到质量m3. 样品饱和吸附容量Wa计算公式如下:
-
吸附-解吸动力学测试采用多站重量法气体蒸汽吸附仪(BSD-VVS,北京贝世德计量检测中心),测试过程如下:在105 ℃条件下对样品进行3 h烘干预处理除去水分子,设置25 ℃和5%—95%RH之间多个相对湿度节点进行等温连续水吸附测试,节点吸附平衡时间上限为3 h,解吸过程选择保持恒温逐步降低相对湿度和设置85 ℃、0%RH两种条件完成解吸,吸附解吸过程中样品质量变化由仪器自动记录,最终得到样品吸附解吸动力学曲线.
-
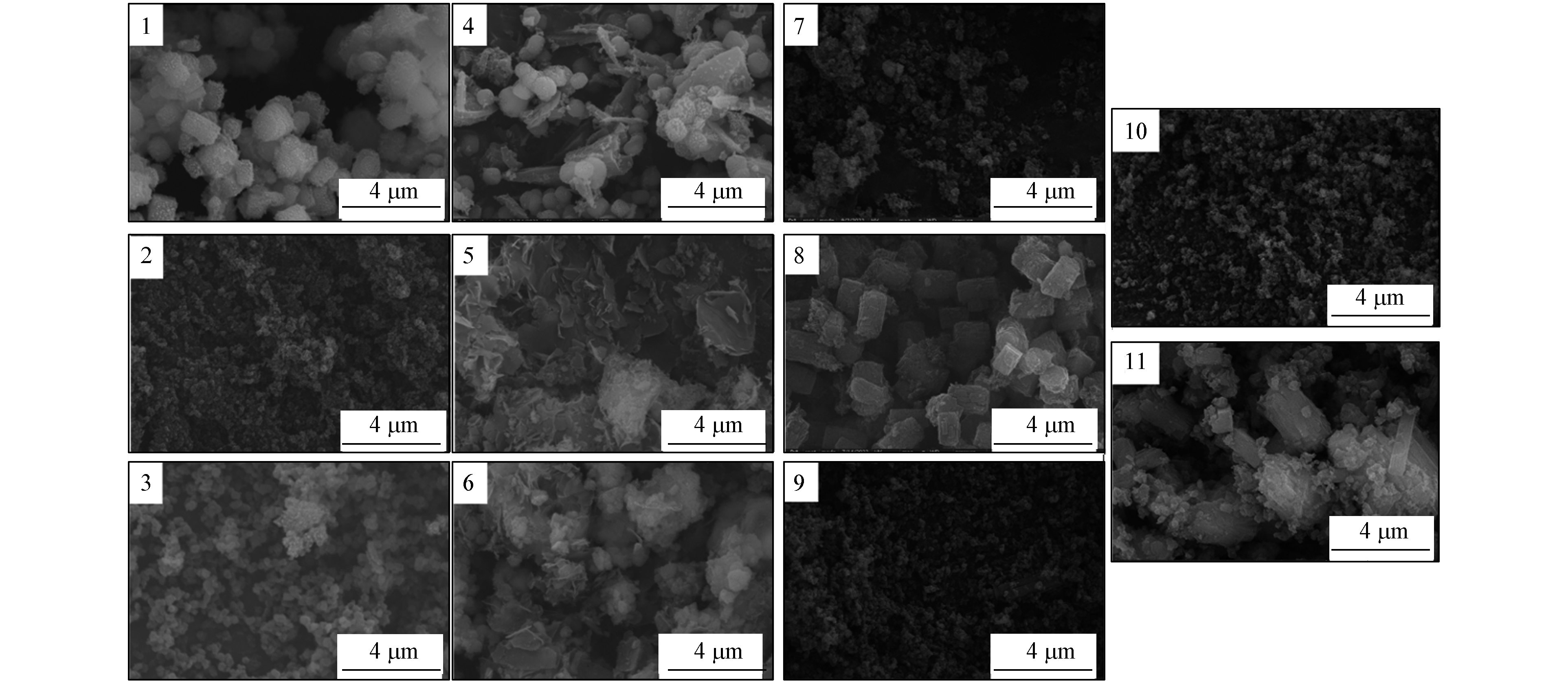
材料FESEM分析结果如图1所示,水溶剂中杂质离子增多会影响样品的规则性和颗粒大小(图1,编号1—3),无机铝源能保证样品的颗粒结构,但以硫酸铝作为铝源时材料显示多种形貌的混合形态(图1,编号4),有机铝源会显著影响样品颗粒结构呈现薄膜状(图1,编号5、6). 随着反应温度的升高,材料晶体结构规则化程度会呈现先增加后减弱的趋势(图1,编号7—9),Ca(OH)2分散剂由于溶解度较小,反应过程中部分未溶解分散剂会成为优先成核点,明显观察到大块长条块状物(图1,编号11).
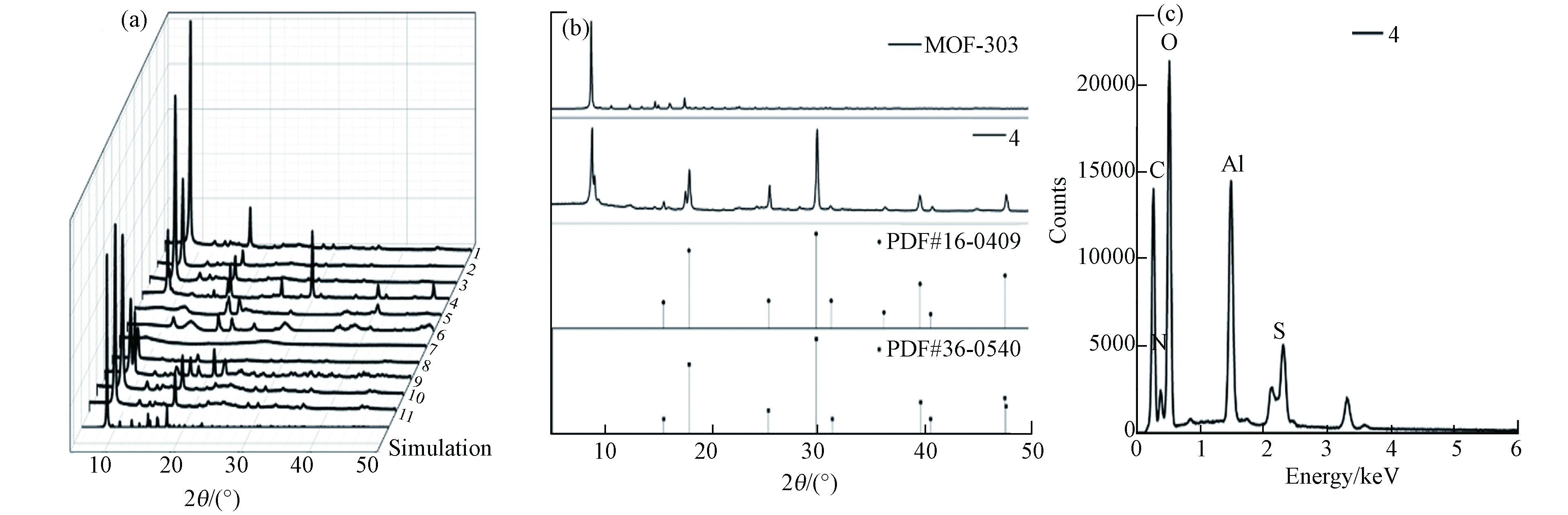
材料XRD分析结果如图2a所示,MOF-303(Al)的标准曲线三强峰2θ位于8.935°、14.787°和17.612°. 水溶剂和分散剂的改变并不影响材料的XRD图谱,三强峰均能在曲线中明显观察到,表明材料MOF-303(Al)的纯度极高. 在有机铝源作用下,材料XRD曲线特征峰拟合度较低. 以硫酸铝为铝源时,表征曲线会存在其他明显特征峰,且特征峰与PDF#36-0540水合硫酸铝卡片与PDF#16-0409 硫酸氧铝氢氧化物卡片的信息基本一致(图2b). 同时,EDX面扫结果表明,材料存在S元素 (图2c),离子色谱测试表明材料的硫酸根浓度仅为362.6321 μg·g−1,可忽略不计,材料中S元素的赋存可能是由于水热反应过程存在硫酸根与有机配体的化学反应. 反应温度25 ℃时的材料特征峰不明显,而85 ℃时材料与标准曲线(simulation)基本吻合,当温度达到150 ℃,材料在8.935°附近存在明显杂峰,而以NaOH分散剂在150 ℃制得的材料则无杂峰,表明150 ℃时下Li会影响材料结晶过程.
-
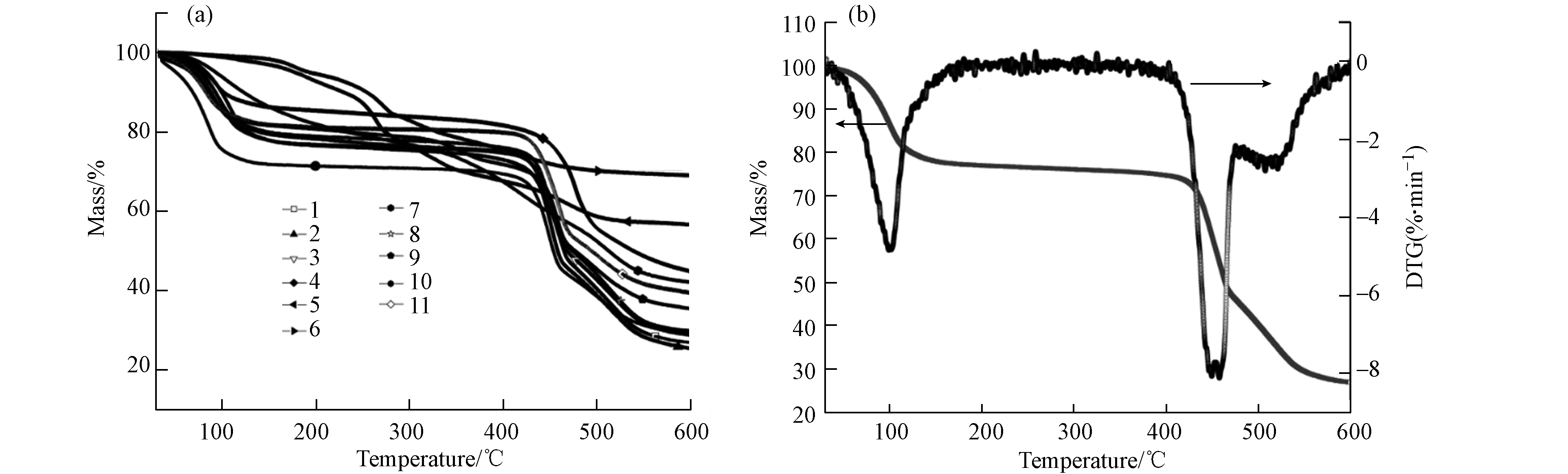
材料的热重曲线如图3a所示,当反应温度为25 ℃和有机铝盐为铝源(5、6、7)时,材料呈现多步失重且不存在明显的热稳定区间;其余材料在100—450 ℃间均存在明显的热稳定区间,而2号材料的TG-DTG曲线如图3b所示,由于水分子解吸和有机配体受热分解材料在100 ℃和450 ℃两个节点存在显著的失重峰,其余温度范围材料均保持稳定,显示出极好的热稳定性.
-
通过 N2-77 K等温吸附曲线计算得到材料的BET比表面积、孔容和孔容(表2). 当反应温度为25 ℃和有机铝盐为铝源时,基于材料N2吸附等温线和孔径分布曲线发现,6—8号材料属于典型的介孔材料,其余材料均表现出I型吸附曲线,属于典型的微孔材料[29]. 密度泛函理论(DFT)分析计算表明,材料吸附过程的平均孔径在2 nm附近波动,符合微孔材料的孔道特性. 如表2所示,微孔比表面积占总比表面积的85%—95%,微孔孔体积占总孔体积的25%—75%.
-
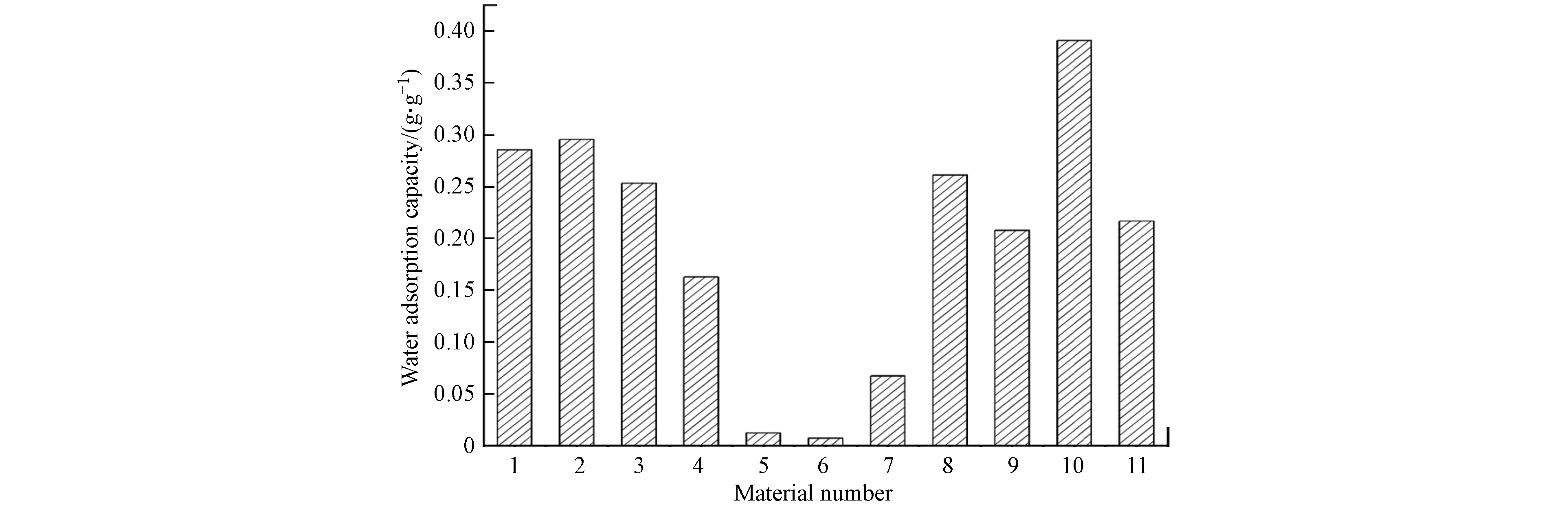
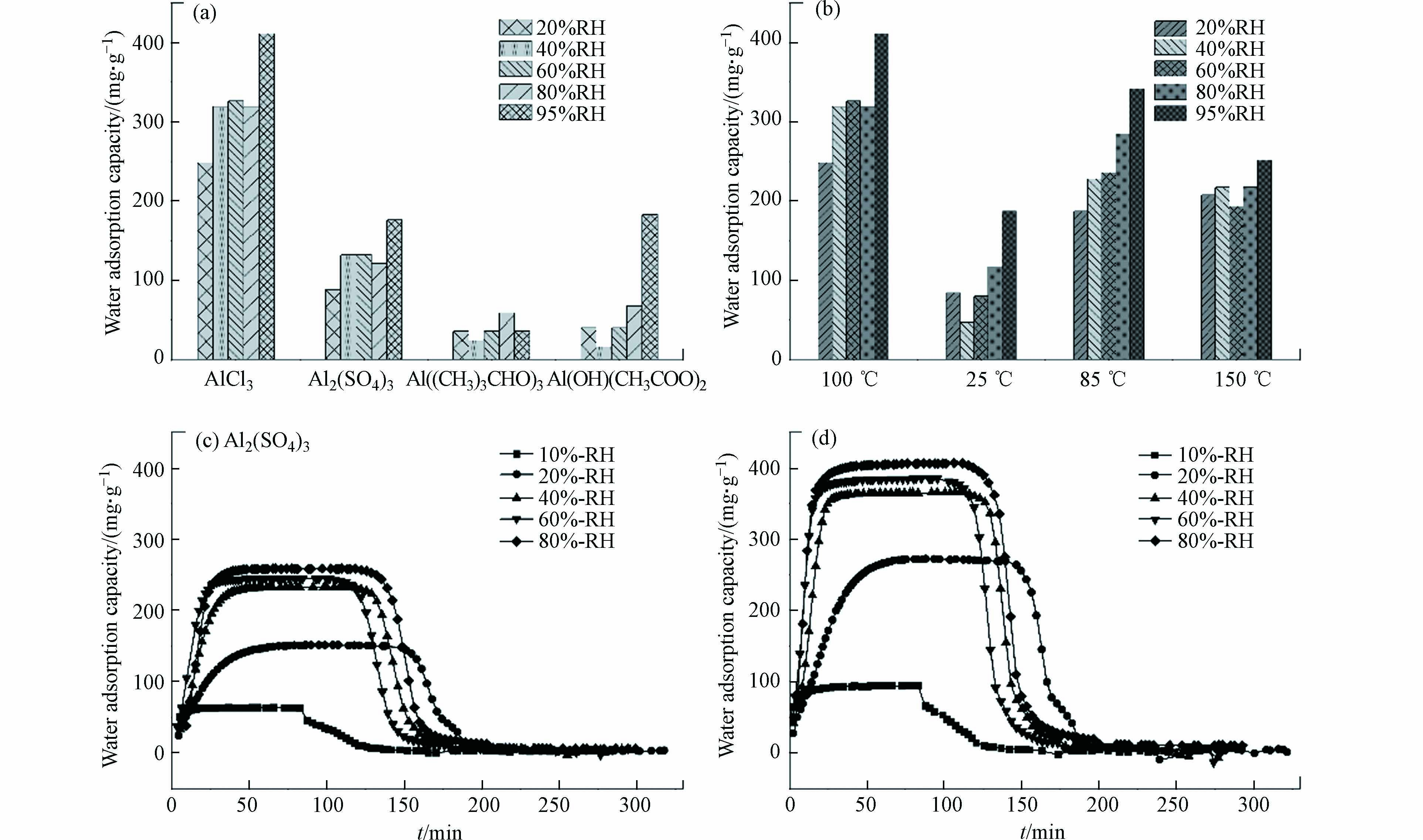
由于水分子解吸,材料热重曲线在100 ℃存在失重峰,经公式(1)计算得到材料水吸附容量如图4所示,其中铝源和反应温度对材料水吸附性能存在显著影响,材料水吸附容量存在大幅度的降低,当制备条件为氯化铝、去离子水、100 ℃和氢氧化钠分散剂时,10号材料的水吸附性能最优.
-
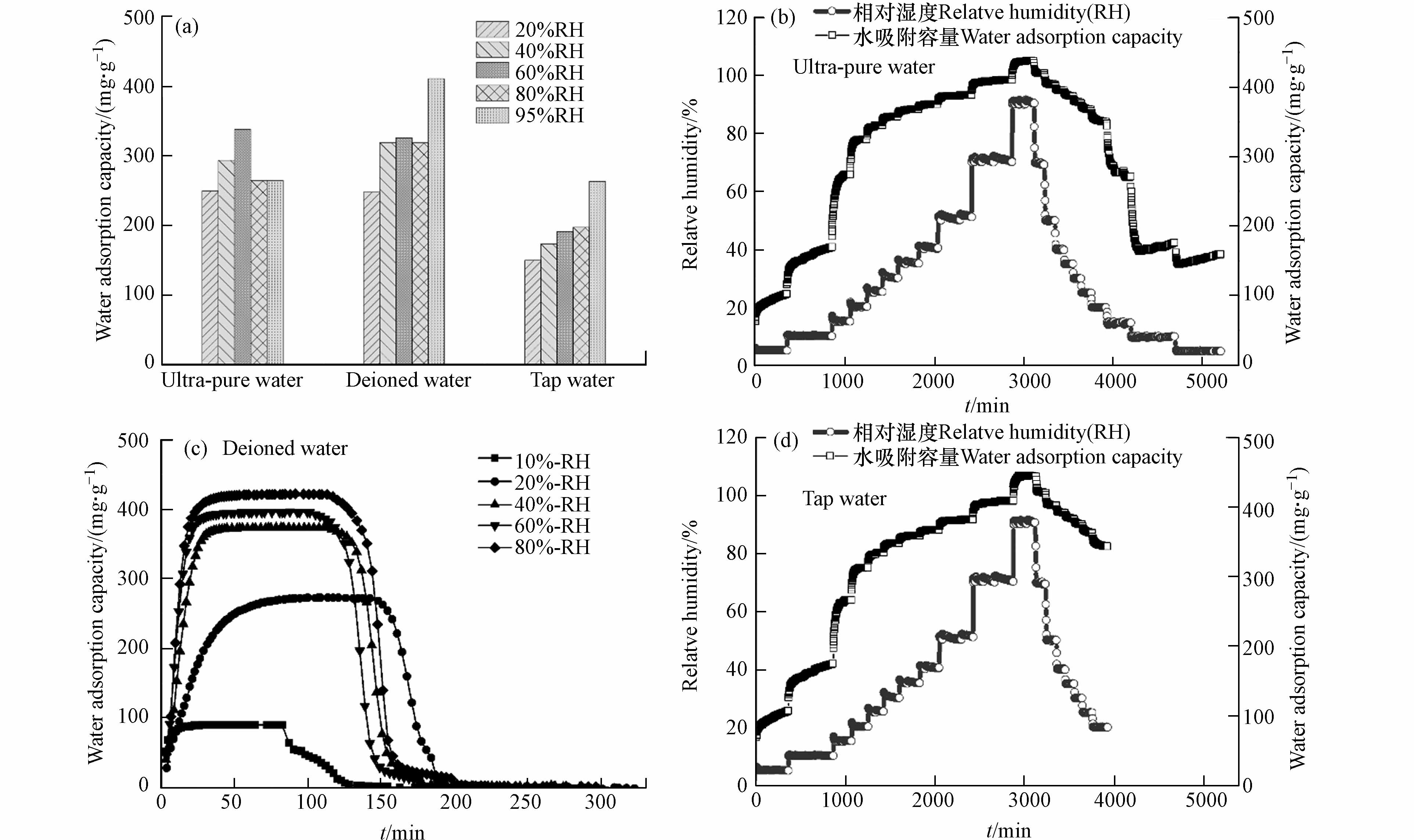
如图5a所示,基于恒温恒湿箱测得不同相对湿度条件下,以去离子水为溶剂时材料的水吸附容量高于其它水溶剂. 吸附动力学曲线表明,材料饱和吸附容量为0.4 g·g−1,且60 min内能快速完成饱和吸附,并在85 ℃条件下30 min内实现水分子的完全脱附(图5c). 以超纯水和自来水为溶剂时,在吸附阶段随着湿度的增加材料均能快速达到饱和吸附(图5b、d),且水吸附容量与图5c一致,均为0.4 g·g−1;在等温解吸过程,以超纯水为水溶剂时材料在5%RH条件时仅存150 mg·g−1的残余水分,水分基本已完全解吸,而以自来水为溶剂时材料在5%RH时仍存在350 mg·g−1的水分未能解吸,需在高温条件(85℃)下促进水分子的完全解吸. 同时基于恒温恒湿箱对3份样品进行5次吸附循环测试,材料均能保持稳定的水吸附容量. 因此,以去离子水为水溶剂时,材料水吸附容量最优;以自来水为水溶剂时具有更好的制备效益,能有效保证材料的水吸附容量但解吸过程需外部提供能量;随着水溶剂的纯度提高,材料吸附解吸的可逆性增强,可依靠湿度变化实现自动化空气取水.
-
如图6a所示,以氯化铝为铝源时材料水吸附性能最优,但以有机铝源合成的材料,其水吸附性能显著降低. 以硫酸铝为铝盐时材料吸附动力学曲线如图6c所示,材料吸附快速,但吸附容量显著降低. 结合FESEM、XRD分析与比表面积等表征结果,这是由于S元素在MOF上的赋存减少了水吸附位点减少、降低了比表面积孔体积,导致材料水吸附容量降低[21, 30]. 因此,氯化铝是制备MOF-303(Al)的最佳铝源,MOF材料在选择无机金属源时需结合表征结果判断材料的纯度以确保其水吸附性能.
-
如图6b所示,反应温度为100 ℃时,材料的水吸附容量高于其它温度,但随着反应温度升高,材料水吸附容量存在先增加后减少的趋势. 反应温度为85 ℃时材料的水吸附动力学曲线如图6d所示,材料的水吸附容量和吸附解吸速率均与100 ℃(图5c)时基本一致. 结合FESEM表征结果,随着反应温度升高,材料晶体规则性会先增加后减少,材料存在最适结晶温度,其制备过程最适反应温度区间为85—100 ℃.
-
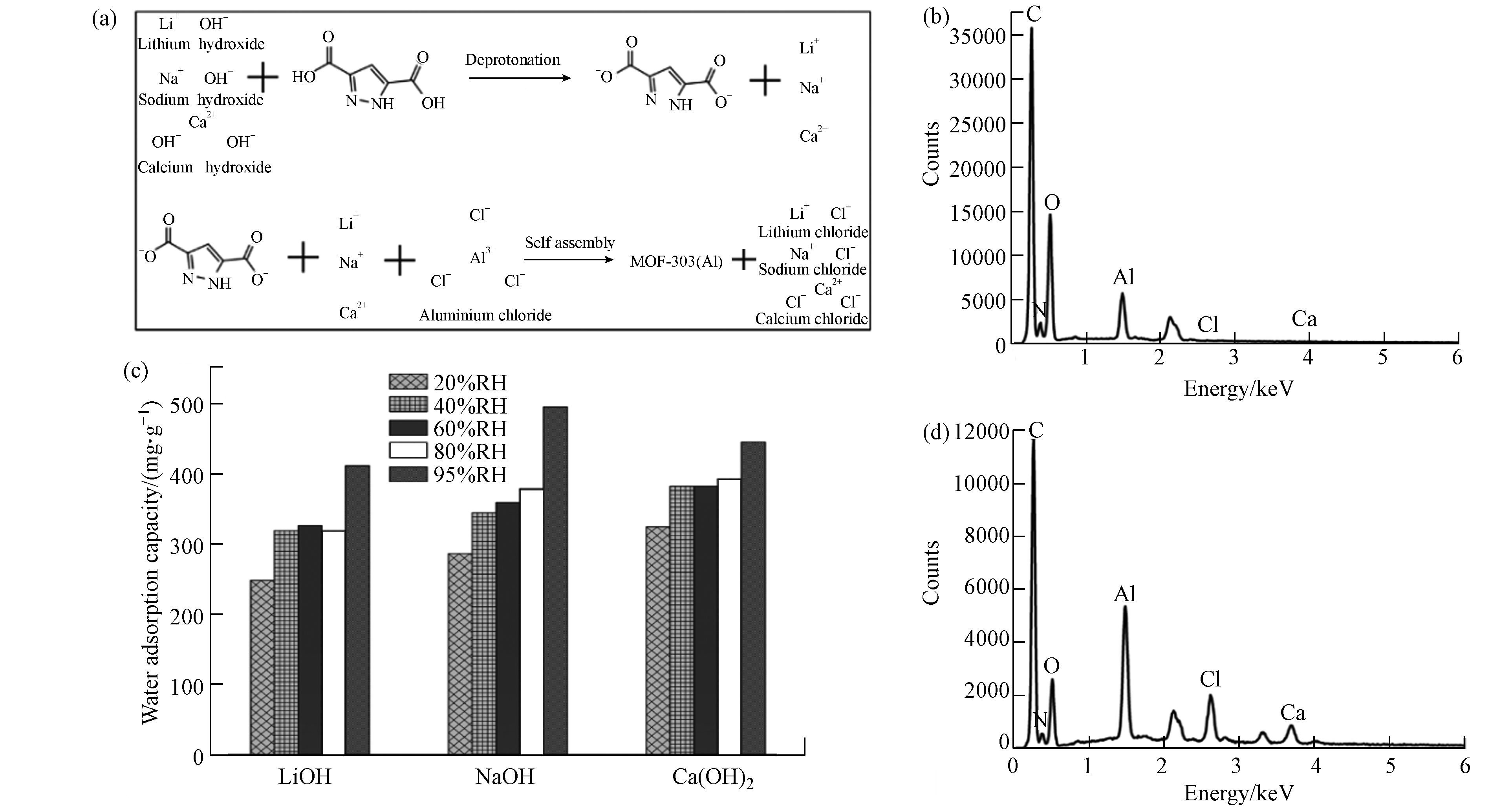
由于有机配体的酸属性特征,引入碱性试剂后,通过酸碱中和反应既可实现有机配体的去质子化,又可使有机配体在水溶剂中完全分散溶解(如图7a). 本研究选择LiOH、NaOH和Ca(OH)2等3种碱性试剂,当搅拌时间一致时,以LiOH和NaOH为分散剂的溶液显示为澄清状态;由于Ca(OH)2溶解度较低,选择其为分散剂时溶液存在一定浑浊. 从图7c及材料表征结果来看,分散剂选择对材料晶体结构和水吸附性能的影响较小,均能达到0.4 g·g−1的水吸附容量,但以氢氧化钙为分散剂的材料水吸附性能存在微弱优势,因此,分散剂的选择对材料的水吸附性能影响较小.
如图7a所示,在分散剂作用下,水热法制备MOF-303(Al)的前驱体溶液中存在额外的无机盐离子,如LiCl、CaCl2、NaCl,在水热反应结束后的冷却阶段,会随着MOF-303(Al)颗粒沉降至反应器底部,因此MOF-303(Al)颗粒内部会存在无机盐离子的赋存. 同时,为增强MOF材料的水吸附性能,将MOF粉末分散到吸湿盐溶液中得到MOF -吸湿盐复合材料也是一种常见的方法[31]. ICP-OES分析结果(表3)表明,经常规方法洗涤分离后,只有极微量无机盐离子仍存在MOF-303(Al)中,其金属摩尔比值(Al:X)保持在100左右,无机盐含量对MOF-303(Al)水吸附性能的影响可忽略不计. 同时,LiCl和CaCl2属于常见的吸湿性无机盐,能有效增强材料的水吸附性能. 通过减少洗涤步骤简易制备得到3份对照材料,以Ca(OH)2为分散剂的材料元素分布如图7b、d所示,钙元素与氯元素含量存在显著改变,表明减少洗涤步骤能有效达到无机盐与MOF-303(Al)的复合.
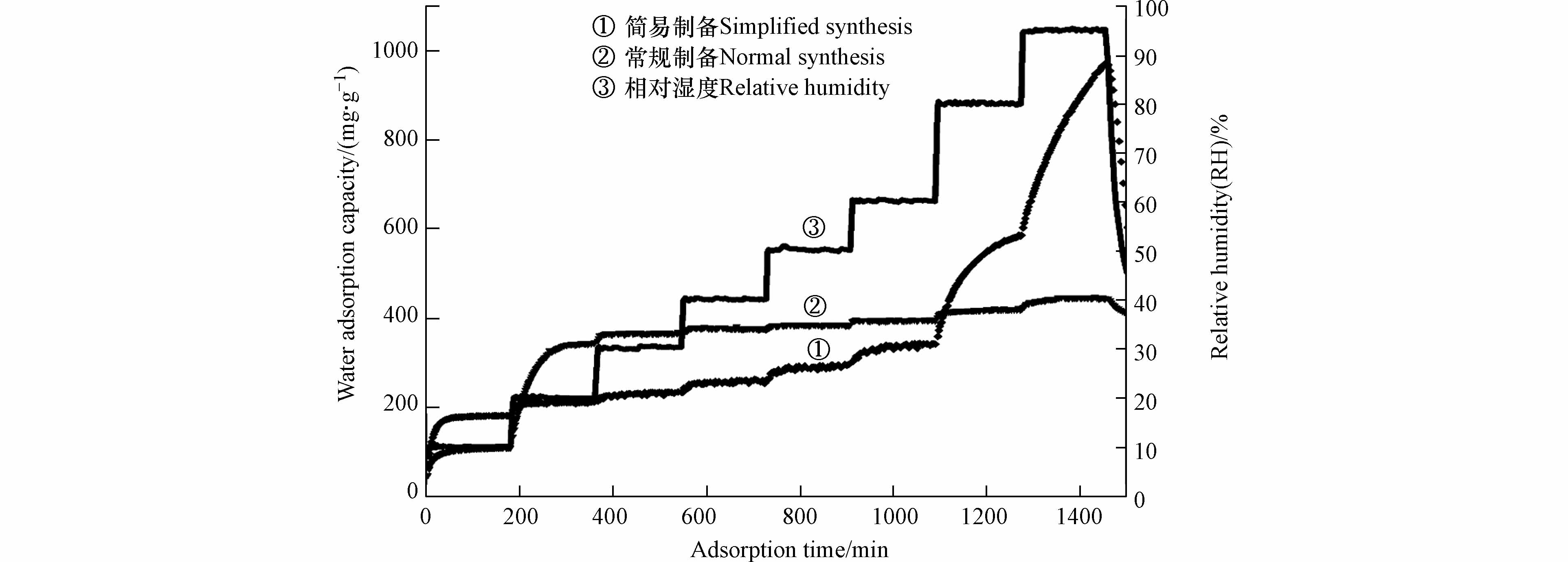
材料水吸附容量如表4所示,在低湿度条件时,减少洗涤步骤降低了材料的水吸附容量;在高湿度条件,当达到95%RH时材料的水吸附容量可达到1.834、2.055、1.567 g·g−1,明显高于常规吸湿盐[24]和MOF-303 (Al)粉末. 以氢氧化钙为分散剂时材料吸附动力学曲线如图8 所示,结果表明水分子的快速吸附解吸过程并未受到水热过程步骤简化的影响. 动力学曲线中的材料水吸附容量结果表明,在10%RH超低湿环境下,减少洗涤步骤能有效增强材料的水吸附容量. 因此,可通过简化水热制备过程增强材料在高湿度环境下的水吸附性能,拓宽材料的应用场景.
-
基于水热合成,本研究考察了水溶剂、金属源、反应温度和分散剂等因素对MOF-303(Al)材料水吸附性能和结构形貌的影响,主要结论如下:
1)有机铝源为铝源和常温条件(25℃)会破坏MOF-303(Al)材料的结构形貌和孔径结构,而且降低MOF-303(Al)材料的比表面积和孔隙率,影响其水吸附性能;
2)以自来水为溶剂,MOF-303(Al)材料的水吸附容量且循环性能较好;
3)分散剂对材料水吸附性能影响较小,简化水热制备步骤可有效对MOF-303(Al)和无机盐进行复合,增强了MOF的水吸附性能.
MOF-303(Al)的制备优化及水吸附性能
Preparation optimization and water adsorption performance of MOF-303(Al)
-
摘要: 吸附式空气取水技术可在全球范围内为人们提供清洁的饮用水. 本研究采用水热法制备MOF-303(Al)材料,考察水溶剂、金属铝源、反应温度和分散剂等条件对其水吸附性能和结构形貌的影响,并探究水热过程直接实现MOF-303(Al)与无机盐复合的可能性. 结果表明,水溶剂和分散剂对MOF-303(Al)的水吸附性能影响较小,其水吸附容量均为0.4 g·g−1,且能在0.5 h内达到饱和吸附;温度和铝源显著影响材料的水吸附性能,水吸附容量变化幅度为0.3 g·g−1;减少水热过程的洗涤步骤能直接完成无机盐与MOF-303(Al)的复合,且材料在95%RH时的吸附容量达2.055 g·g−1.
-
关键词:
- 水热制备 /
- 空气取水 /
- 水吸附性能 /
- MOF-303(Al).
Abstract: The adsorption water harvesting technology can provide clean drinking water to people worldwide. This paper prepares the MOF-303(Al) by hydrothermal, investigates the effects of preparation conditions such as aqueous solvent, metal-aluminum source, reaction temperature, and dispersant type on water adsorption performance and structure topography, and the possibility of directly achieving the composite of MOF-303(Al) with inorganic salts based on hydrothermal process was explored. The results show that the aqueous solvent and dispersant have little effect on the water adsorption performance; both can achieve a water adsorption capacity of 0.4 g·g−1 and the fast adsorption kinetics of saturation adsorption can be achieved in half hour. Temperature and aluminum source will significantly affect the water adsorption performance, with a floating change of 0.3 g·g−1. Reducing the washing step of hydrothermal process can directly realize the recombination of inorganic salt and MOF-303(Al), and achieve a high adsorption capacity of 2.055 g·g−1 at 95%RH.-
Key words:
- hydrothermal /
- atmosphere water harvesting /
- water adsorption performance /
- MOF-303(Al).
-

-
图 6 (a)不同铝源条件下制得材料的水吸附容量(样品编号:2,4—6);(b)不同温度条件下制得材料的水吸附容量(样品编号:2,7—9);(c)硫酸铝为铝源时材料吸附动力学曲线(样品编号:4);(d)85℃反应温度时材料吸附动力学曲线(样品编号:8)
Figure 6. (a)water adsorption capacity based on the influence of aluminum source (sample number:2,4—6);(b)water adsorption capacity based on the influence of temperature (sample number:2,7—9) (c)adsorption kinetic curves under aluminum sulfate as aluminum source(sample number:4) ;(d)adsorption kinetic curves under temperature of 85℃(sample number:8)
图 7 (a) MOF-303(Al)制备过程; (b) MOF材料元素分布图(氢氧化钙为分散剂,样品编号:11);(c) 分散剂影响下材料水吸附容量(样品编号:2,10-11); (d) MOF材料元素分布图(氢氧化钙为分散剂时,省略洗涤分离步骤)
Figure 7. (a) The synthesis process of MOF-303(Al); (b) Material element distribution map (calcium hydroxide as dispersant,sample number :11); (c) Water adsorption capacity based on the influence of dispersant (sample number :2,10-11);(d) Material element distribution map (calcium hydroxide as dispersant without the wash and separation step)
表 1 样品制备条件
Table 1. Synthesis conditions of samples
编号
Number水溶剂
Water solvent铝源
Aluminum source温度
Temperature分散剂
Dispersants1 超纯水 AlCl3 100 ℃ LiOH 2 去离子水 AlCl3 100 ℃ LiOH 3 自来水 AlCl3 100 ℃ LiOH 4 去离子水 Al2(SO4)3 100 ℃ LiOH 5 去离子水 Al((CH3)3CHO)3 100 ℃ LiOH 6 去离子水 Al(OH)(CH3COO)2 100 ℃ LiOH 7 去离子水 AlCl3 25 ℃ LiOH 8 去离子水 AlCl3 85 ℃ LiOH 9 去离子水 AlCl3 150 ℃ LiOH 10 去离子水 AlCl3 100 ℃ NaOH 11 去离子水 AlCl3 100 ℃ Ca(OH)2 表 2 样品比表面积、孔容与孔径
Table 2. Specific surface area, pore volume, and pore size of samples
编号
Number比表面积/(m2·g−1)
Specific surface area微孔比表面积/(m2·g−1)
Micropore specific
surface area孔体积/
(cm3·g−1)
Pore volume微孔孔体积/(cm3·g−1)
Micropore pore volume吸附平均孔径/ nm
Adsorption average
pore diameter1 1199.2469 1156.3449 0.5506 0.4321 1.8364 2 1407.1263 1294.6678 0.9886 0.4920 2.8101 3 596.2009 553.1305 0.3659 0.2090 2.4548 4 169.2528 154.8814 0.0915 0.0589 2.1635 5 51.0881 8.1074 0.1816 0.0032 14.2184 6 92.3021 — 0.2367 0.0006 10.2589 7 132.6209 21.7183 0.8211 0.0087 24.7664 8 734.9189 683.7707 0.4418 0.2570 2.4050 9 330.6443 294.8973 0.2747 0.1110 3.3234 10 582.2841 496.4246 0.7303 0.1895 5.0165 11 616.1999 580.6024 0.3045 0.2166 1.9769 “—”无数据 表 3 MOF材料ICP-OES结果
Table 3. ICP-OES results of MOF materials
分散剂
Dispersants是否洗涤
Wash or not元素
Element元素摩尔比
Mole ratioLiOH 是 Al:Li 96.6 否 1.16 NaOH 是 Al:Na 未检出Na 否 1.29 Ca(OH)2 是 Al:Ca 164 否 2.71 表 4 MOF材料吸附容量变化(g·g−1)
Table 4. The change of water adsorption capacity of the MOF material(g·g−1)
分散剂
Dispersants氢氧化锂
Lithium hydroxide容量变化
Capacity variation氢氧化钠
Sodium hydroxide容量变化
Capacity variation氢氧化钙
Calcium hydroxide容量变化
Capacity variation是否洗涤
Wash or not否
No是
Yes否
No是
Yes否
No是
Yes20%RH 0.243 0.262 −7% 0.194 0.320 −39% 0.209 0.325 −36% 40%RH 0.288 0.319 −10% 0.212 0.345 −39% 0.276 0.382 −28% 60%RH 0.384 0.326 18% 0.229 0.359 −36% 0.367 0.382 −4% 80%RH 0.734 0.319 130% 0.824 0.379 117% 0.684 0.393 74% 95%RH 1.834 0.411 346% 2.055 0.495 315% 1.567 0.445 252% -
[1] JARIMI H, POWELL R, RIFFAT S. Review of sustainable methods for atmospheric water harvesting [J]. International Journal of Low-Carbon Technologies, 2020, 15(2): 253-276. doi: 10.1093/ijlct/ctz072 [2] KLEMM O, SCHEMENAUER R S, LUMMERICH A, et al. Fog as a fresh-water resource: overview and perspectives [J]. Ambio, 2012, 41(3): 221-234. doi: 10.1007/s13280-012-0247-8 [3] KHALIL B, ADAMOWSKI J, SHABBIR A, et al. A review: dew water collection from radiative passive collectors to recent developments of active collectors [J]. Sustainable Water Resources Management, 2016, 2(1): 71-86. doi: 10.1007/s40899-015-0038-z [4] 王雯雯, 葛天舒, 代彦军, 等. 太阳能吸附式空气取水研究现状 [J]. 太阳能, 2020, 309(1): 33-46. WANG W W, GE T S, DAI Y J, et al. Status of solar-driven sorption-based atmosphere water harvesting[J]. Solar Energy, 2020, 309(1): 33-46 (in Chinese).
[5] TU R, HWANG Y H. Reviews of atmospheric water harvesting technologies [J]. Energy, 2020, 201: 117630. doi: 10.1016/j.energy.2020.117630 [6] TU Y D, WANG R Z, ZHANG Y N, et al. Progress and Expectation of Atmospheric Water Harvesting [J]. Joule, 2018, 2(8): 1452-1475. doi: 10.1016/j.joule.2018.07.015 [7] EJEIAN M, WANG R Z. Adsorption-based atmospheric water harvesting [J]. Joule, 2021, 5(7): 1678-1703. doi: 10.1016/j.joule.2021.04.005 [8] SLEITI A K, AL-KHAWAJA H, AL-KHAWAJA H, et al. Harvesting water from air using adsorption material - Prototype and experimental results [J]. Separation and Purification Technology, 2021, 257: 117921 doi: 10.1016/j.seppur.2020.117921 [9] GADO M G, NASSER M, HASSAN A A, et al. Adsorption-based atmospheric water harvesting powered by solar energy: Comprehensive review on desiccant materials and systems [J]. Process Safety and Environmental Protection, 2022, 160: 166-183. doi: 10.1016/j.psep.2022.01.061 [10] 张晶, 赵惠忠, 张真真, 等. CaCl2复合吸附剂在太阳能空气取水的吸附性能实验研究 [J]. 应用化工, 2022, 51(2): 395-400. ZHANG J, ZHAO H Z, ZHANG Z Z, et al. Experimental study on adsorption performance of CaCl2 composite adsorbent in solar air water intak[J]. Applied Chemical Industry, 2022, 51(2): 395-400(in Chinese).
[11] SHAN H, LI C, CHEN Z, et al. Exceptional water production yield enabled by batch-processed portable water harvester in semi-arid climate [J]. Nature Communication, 2022, 13(1): 5406. doi: 10.1038/s41467-022-33062-w [12] YANG K, PAN T, LEI Q, et al. A Roadmap to Sorption-Based Atmospheric Water Harvesting: From Molecular Sorption Mechanism to Sorbent Design and System Optimization [J]. Environment Science Technology, 2021, 55(10): 6542-6560. doi: 10.1021/acs.est.1c00257 [13] 耿浩清, 石成君, 苏亚欣, 等. 空气取水技术的研究进展 [J]. 化工进展, 2011, 30(8): 1664-1669. doi: 10.16085/j.issn.1000-6613.2011.08.004 Geng H Q, Shi C J, Su Y X, et al. Research progress of air water intake technology[J]. Chemical Industry Progress, 2011, 30(8): 1664-1669(in Chinese). doi: 10.16085/j.issn.1000-6613.2011.08.004
[14] LIU X, WANG X, KAPTEIJN F. Water and Metal-Organic Frameworks: From Interaction toward Utilization [J]. Chemical Reviews, 2020, 120(16): 8303-8377. doi: 10.1021/acs.chemrev.9b00746 [15] MOUCHAHAM G, CUI F S, NOUAR F, et al. Metal-Organic Frameworks and Water: 'From Old Enemies to Friends'? [J]. Trends in Chemistry, 2020, 2(11): 990-1003. doi: 10.1016/j.trechm.2020.09.004 [16] HU Y, YE Z, PENG X. Metal-organic frameworks for solar-driven atmosphere water harvesting [J]. Chemical Engineering Journal, 2023, 452(4): 139656. doi: 10.1016/j.cej.2022.139656 [17] KALMUTZKI M J, HANIKEL N, YAGHI O M. Secondary building units as the turning point in the development of the reticular chemistry of MOFs [J]. Science Advance, 2018, 4(10): eaat9180. doi: 10.1126/sciadv.aat9180 [18] 魏源送, 吴其洋, 郑利兵. 面向空气取水的金属有机框架 (MOFs) 材料研究进展[J]. 环境化学, 2024, 43(3): 1-14. doi: 10.7524/j.issn.0254-6108.2022082402 WEI Y S, WU Q Y, ZHENG L B. Research progress on metalorganic frameworks (MOFs) for atmosphere water harvesting [J]. Environmental Chemistry, 2023, 42 (X1): 1-14(in Chinese). doi: 10.7524/j.issn.0254-6108.2022082402
[19] KIM H, YANG S, RAO S R, et al. Water harvesting from air with metal-organic frameworks powered by natural sunlight [J]. Science, 2017, 356(6336): 430-434. doi: 10.1126/science.aam8743 [20] HANIKEL N, PREVOT M S, FATHIEH F, et al. Rapid Cycling and Exceptional Yield in a Metal-Organic Framework Water Harvester [J]. ACS Centeal Science, 2019, 5(10): 1699-1706. doi: 10.1021/acscentsci.9b00745 [21] FARHA O K, ERYAZICI I, JEONG N C, et al. Metal–Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? [J]. Journal of the American Chemical Society, 2012, 134(36): 15016-15021. doi: 10.1021/ja3055639 [22] YANAGITA K, HWANG J, SHAMIM J A, et al. Kinetics of Water Vapor Adsorption and Desorption in MIL-101 Metal–Organic Frameworks [J]. The Journal of Physical Chemistry C, 2018, 123(1): 387-398. doi: 10.1021/acs.jpcc.8b08211 [23] TOULOUMET Q, SILVESTER L, BOIS L, et al. Water sorption and heat storage in CaCl2 impregnated aluminium fumarate MOFs [J]. Solar Energy Materials and Solar Cells, 2021, 231: 111332 doi: 10.1016/j.solmat.2021.111332 [24] HU Y, FANG Z, MA X, et al. CaCl2 Nanocrystals decorated photothermal Fe-ferrocene MOFs hollow microspheres for atmospheric water harvesting [J]. Applied Materials Today, 2021, 23: 101076 doi: 10.1016/j.apmt.2021.101076 [25] RAVEESH G, GOYAL R, TYAGI S K. Advances in atmospheric water generation technologies [J]. Energy Conversion and Management, 2021, 239: 114226. doi: 10.1016/j.enconman.2021.114226 [26] AHNFELDT T, GUNZELMANN D, LOISEAU T, et al. Synthesis and modification of a functionalized 3D open-framework structure with MIL-53 topology [J]. Inorganic Chemistry, 2009, 48(7): 3057-3064. doi: 10.1021/ic8023265 [27] TAN B Q, LUO Y S, LIANG X H, et al. In Situ Synthesis and Performance of Aluminum Fumarate Metal-Organic Framework Monolithic Adsorbent for Water Adsorption [J]. Industrial & Engineering Chemistry Research, 2019, 58(34): 15712-15720 . doi: 10.1021/acs.iecr.9b03172 [28] HANIKEL N, PEI X, CHHEDA S, et al. Evolution of water structures in metal-organic frameworks for improved atmospheric water harvesting [J]. Science, 2021, 374(6566): 454-459. doi: 10.1126/science.abj0890 [29] THOMMES M, KANEKO K, NEIMARK A V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report) [J]. Pure and Applied Chemistry, 2015, 87(9-10): 1051-1069. doi: 10.1515/pac-2014-1117 [30] WANG M, YU F Q. High-throughput screening of metal-organic frameworks for water harvesting from air [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 624: 126746. doi: 10.1016/j.colsurfa.2021.126746 [31] XU J, LI T, CHAO J, et al. Efficient Solar-Driven Water Harvesting from Arid Air with Metal-Organic Frameworks Modified by Hygroscopic Salt [J]. Angewandte Chemie Internationnal Edition English, 2020, 59(13): 5202-5210. doi: 10.1002/anie.201915170 -



 下载:
下载: