-
硝酸盐污染是一种普遍存在于自然界的现象[1]. 据报道,我国多地的地下水[2]、饮用水[3]等水体中硝酸盐含量超标,可能导致铁血红蛋白症、消化系统疾病等危害公众健康[1, 4]. 目前常用的去除方法包括反渗透、离子交换、吸附法、化学还原[5]、电还原[6]和生物法[7],但这些方法存在着能耗高、去除效率低和氮气转化率低等问题. 高级还原技术(ARPs,advanced reduction processes)是一种新型的处理方法,利用激发生成活性自由基(CO2·−和ecb−),高效高选择性地去除水体中的硝酸盐[8]. 然而,同相技术需要投加大量甲酸、乙酸等小分子有机酸和重金属离子[9 − 11],影响水体pH(酸性条件),因金属超标和副产物生成,增加二次污染风险,在实际中应用存在困难. 相比较,紫外光催化结合异相技术具有药剂投量低、易循环使用等优点,因此成为当前的研究热点.
目前,钛基材料作为常见的催化剂,存在还原效率和氮气转化率低等问题. 在紫外/乙醇体系中,钛基材料将硝酸盐还原为NH4+[12],在可见光下氮气转化率仅为32%[13]. 为提高硝酸盐去除率和氮气转化率,通常采用负载贵金属(如Pd[12]、Pt[14]、Au和Ag[15])或耦合半导体材料(如H3PW12O40/TiO2[16]、g-C3N4/TiO2[17]、Ti3C2/TiO2[18])等方法. 然而,这些方法存在成本高、循环性差等问题. 因此,仍需合适的钛基催化剂改性方法,以提高硝酸盐还原效能.
研究表明,钙钛矿、尖晶石等多金属氧化物具有窄带隙且合适的EVBM,具有较高的硝酸盐还原效能,如LiNbO3(二次谐波效应)[19]、Pd/GdCrO3[20]和天然钛铁矿[21]的氮气转化率超90%,但普遍存在材料的回收性能差问题. 其中,铁基材料具有易循环回用的铁磁性,且载流迁移率高和带隙能低[22]. 铜铁复合材料中Cu(Ⅲ)/Cu(Ⅱ)和Fe(Ⅲ)/Fe(Ⅱ)的内置电场促进硝酸盐降解,如AgBr(Ag)/MIL-101(Cr)/CuFe2O4[23]. 而铜铁改性的钛基材料可提高催化性能[24],如Ag/CuFe2O4/TiO2[25],及Cu/Fe/TiO2还原硝酸盐效能提升4.7倍(但氮气转化率低)[24],受到研究者关注. 综上铜铁钛催化剂具有高效去除硝酸盐潜能,但在光催化还原硝酸盐为氮气的领域缺乏相应研究. 因此,开发更为高效、高氮气转化率、易回收且循环性能好的催化材料,将有重要现实意义.
本研究采用改性的溶剂热法和化学沉淀法成功制备了AgCl(Ag)/CuFexTix+1Oy光催化材料. 通过调控不同AgCl(Ag)掺杂量和铜铁钛配比,制备了一系列复合材料,并利用SEM、XRD、XPS和UV-vis等方法进行表征,以获得更高硝酸盐去除率和氮气转换率的催化材料,并探究了复合材料在实际水处理中光催化还原硝酸盐的效能.
-
试剂采用分析纯,硝酸钾、无水乙醇、钛酸四丁酯等购自国药集团,硝酸银、硝酸铜、硝酸铁等购自阿拉丁试剂有限公司.
-
参考河西走廊和东营市地下水水质特征[11, 26],配制模拟地下水,水质指标见表1.
-
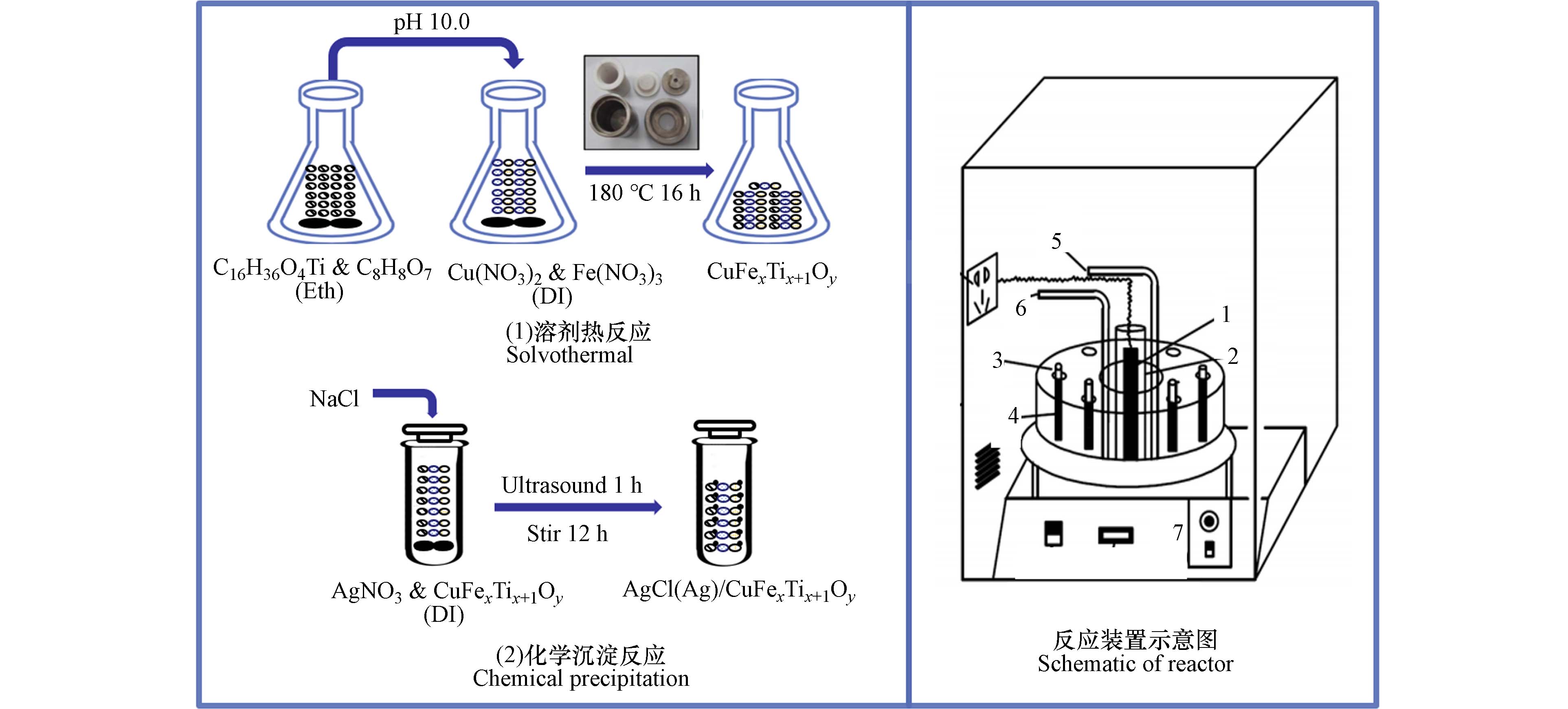
复合材料的制备方法如图1. CuFexTix+1Oy采用改性溶剂热法制备. 首先,在15 mL乙醇中溶解1.38 g柠檬酸并超声,滴加1.40 mL钛酸四丁酯并再次超声,得混合液A. 同时,在75 mL去离子水中溶解0.375 g硝酸铜和0.967 g硝酸铁,搅拌得混液B. 将混液A滴加到混液B中,搅拌均匀并调节pH至10.0. 将上述混合物置于有效容积为110 mL的聚四氟乙烯反应釜中,在(180 ± 2) ℃的条件下进行溶剂热反应16 h. 将产物洗涤干燥,得到CuFexTix+1Oy. 根据不同的铜铁钛摩尔比(3:1:4、2:1:3、1:1:2、1:2:3和1:3:4),分别记所得材料为C3FT4、C2FT3、CFT2、CF2T3和CF3T4.
AgCl(Ag)/CuFexTix+1Oy采用化学沉法制备[27]. 将0.2 g的CuFexTix+1Oy加入50 mL去离子水中并搅拌均匀,然后滴加适量AgNO3和NaCl溶液,超声1 h并搅拌12 h. 随后将混合物洗涤干燥,得到AgCl(Ag)/CuFexTix+1Oy. 根据AgCl(Ag)的掺杂量(按质量算,30%和40%),将复合材料记为30AC和40AC.
-
实验装置为XPA-7光化学反应器(图1). 实验条件:室温(25 ± 0.2) ℃,初始pH为(7.1 ± 0.1),溶解氧浓度为(8.2 ± 0.1) mg·L−1. 硝酸盐、甲酸(FA)和复合材料投加量分别为62.0 mg·L−1 (1 mmol·L−1)、3 mmol·L−1和0.2 g·L−1. 中压汞灯(UV-L, 500 W)预热5 min,此时反应管(容积1.6 L)中辐照强度为9.8 mW·(cm−2),暗处搅拌15 min后开始反应[11, 28]. 样品经离心、过滤(0.22 μm)处理. 采用离子色谱(Aquion)检测NO3−和NO2−浓度,紫外分光光度计(UV-2550)检测NH4+浓度,总有机碳分析仪(TOC-L CPH)检测总氮(TN). 假设气态氮为N2[9],计算得N2含量. 所有实验至少独立重复3次,计算平均值和标准化偏差.
总氮去除率、硝酸盐去除率和氮气转化率分别用式(1)—(3)计算[9]:
其中,
$ {\left[\mathrm{T}\mathrm{N}\right]}_{0} $ 和$ {\left[\mathrm{T}\mathrm{N}\right]}_{t} $ 为初始和反应t时刻总氮浓度;$ {\left[{\mathrm{N}\mathrm{O}}_{3}^{-}\right]}_{0} $ 和$ {\left[{\mathrm{N}\mathrm{O}}_{3}^{-}\right]}_{t} $ 为初始和反应t时刻硝酸盐浓度;$ {\left[{\mathrm{N}\mathrm{O}}_{2}^{-}\right]}_{t} $ 为反应t时刻亚硝酸盐浓度;$ {\left[{\mathrm{N}\mathrm{H}}_{4}^{+}\right]}_{t} $ 为反应t时刻氨氮浓度. -
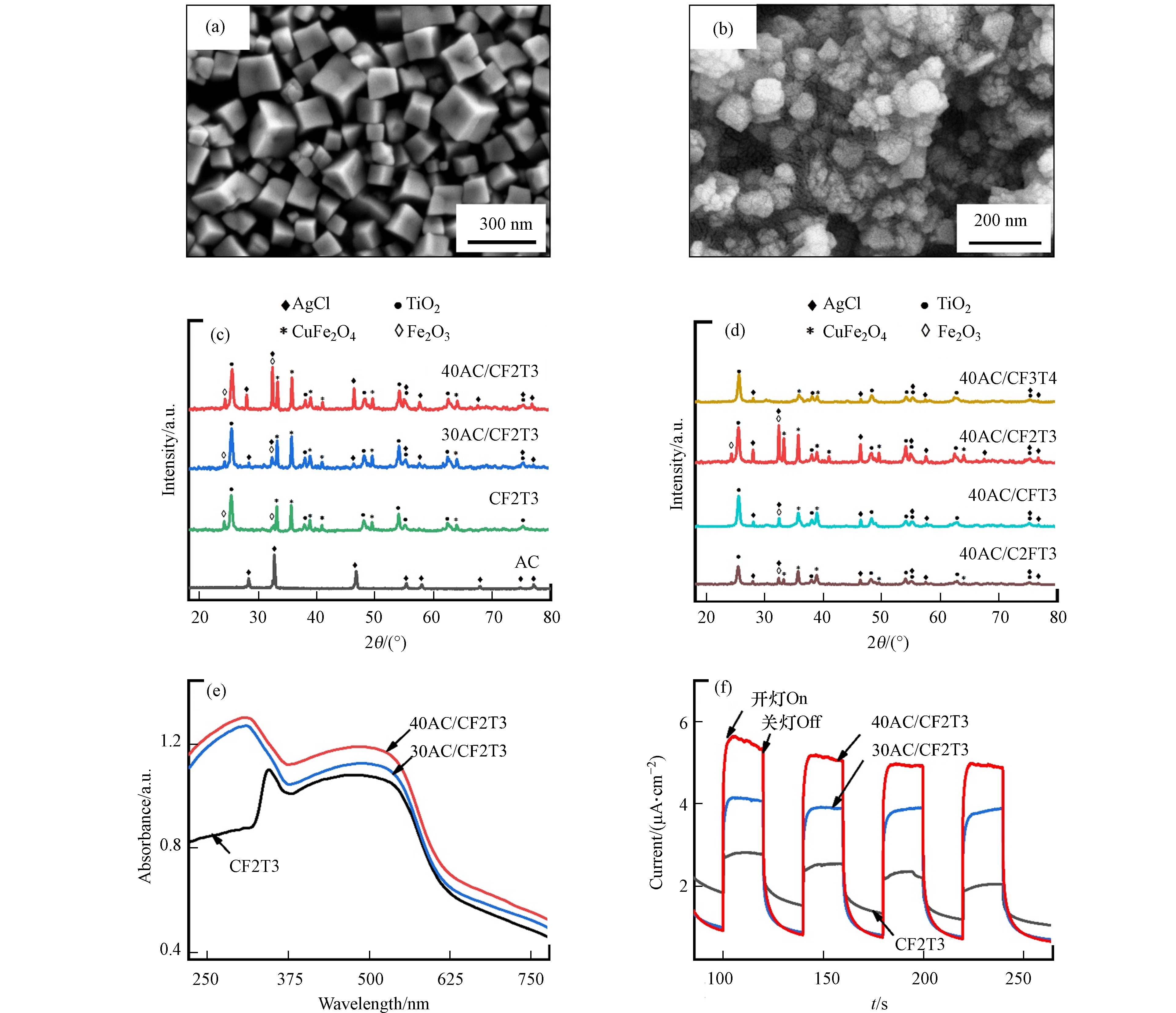
通过SEM表征催化材料表面形貌. 图2(a)中,合成的CF2T3颗粒均匀,多为六面体立方型,具有较好的晶型,粒径范围50—185 nm. 图2(b)中40AC/CF2T3形成更多方形颗粒,颗粒边缘模糊,发生了团聚现象,可能由于超声操作导致,但复合材料仍保持纳米级颗粒骨架,说明复合过程对CF2T3本身基本不造成影响. 此外,BET数据显示AC、CF2T3和40AC/CF2T3的比表面积分别为4.1、91.1、67.3 m2·g−1,说明制备所得的CF2T3基催化材料有较高的污染物吸附潜力.
图2(c)和(d)采用XRD谱图表征催化材料晶体结构和组成. 其中,AC/CF2T3中包含尖晶石CuFe2O4(JCPDS:77—0010)、锐钛矿TiO2(JCPDS:21—1272)和AgCl(JCPDS:31—1238)等结构. CF2T3峰尖锐,说明制得纯度较高,其衍射峰几乎是尖晶石CuFe2O4和锐钛矿TiO2的衍射峰叠加,其中峰24.1°和32.3°为Fe2O3(JCPDS:39—1346)等铁氧化物结构. 图2(c)显示,AgCl掺杂浓度不影响CF2T3主体晶体结构,30AC/CF2T3和40AC/CF2T3晶型相似,且40AC/CF2T3晶型更为明显. 图3(d)中,AgCl掺杂量为40%(按质量算)的几种不同复合催化材料包含CuFe2O4、锐钛矿TiO2和AgCl等结构. 其中,40AC/CF2T3衍射峰更尖锐,具有较高纯度.
-
根据图2(e)UV-Vis DRS图谱分析复合材料光吸收能力. 可见,随AgCl掺杂量升高,复合材料在紫外区(245—365 nm)的吸光能力增强,与锐钛矿TiO2相对应[29]. 40AC/CF2T3在309 nm处达到峰值. 复合材料在400—550 nm范围内表现出良好的光吸收性能,与CuFe2O4相对应[30],在497 nm达到峰值. 使用Kubbelka-Munk公式估算CF2T3禁带宽度(Eg)为1.70—2.21 eV,CF2T3及其AgCl掺杂材料均具有可见光催化潜力. 其中,40AC/CF2T3表现最强吸光性能.
通过图3(f)光电流响应(i-t)测试复合材料的光生电子-空穴对的分离性能. 由0.5 V光照产生的光电流数据,AgCl的掺杂提高了CF2T3材料的瞬态光电流,光生电子-空穴对的分离效率40AC/CF2T3 > 30AC/CF2T3 > CF2T3. 其中,40AC/CF2T3的光电流响应信号有明显提高,是CF2T3的1.9—2.4倍,后续实验中选择40AC/CF2T3进行研究.
-
通过图3(a)XPS谱图,分析复合材料表面化学组成及状态. CF2T3包含5种元素(C 1s、O 1s、Cu 2p、Fe 2p和Ti 2p),40AC/CF2T3含7种元素(增加Ag 3d和Cl 2p),说明CF2T3和40AC/CF2T3复合材料的成功合成. 图3(b)中,C 1s谱图显示了制备原料和环境残留中的碳氢化合物[31]. O 1s谱图(图3(c))中,40AC/CF2T3中532.2 eV和530.0 eV分别对应化学结合氧和物理吸附氧[32]. Cu 2p谱图(图3(d))中,40AC/CF2T3中的952.9 eV对应Cu 2p1/2,而Cu 2p3/2分裂为932.6 eV(Cu+)和934.3 eV(Cu2+),表明存在两种价态Cu[33]. Fe 2p谱图(图3(e))中的两个峰对应Fe 2p1/2和Fe 2p3/2,如40AC/CF2T3中分裂为710.8 eV(Fe2+)和714.8 eV(Fe3+),723.9 eV(Fe2+)和728.2 eV(Fe3+),表明存在两种价态Fe[34]. Ti 2p谱图(图3(f))中有Ti 2p1/2(464.4 eV和463.8 eV)和Ti 2p3/2(458.8 eV和458.0 eV)两个峰,表明存在Ti4+ [32]. 在图3(g)中,40AC/CF2T3的Ag 3d谱图中两个峰对应Ag 3d5/2和Ag 3d3/2,分裂为367.7 eV(Ag+)、368.2 eV(Ag0)和373.7 eV(Ag+)、374.3 eV(Ag0)[35],表明存在单质Ag.
-
将不同配比的铜铁钛氧化物与40% AgCl(Ag)进行复合,用于去除62.0 mg·L−1硝酸盐实验,以获得更高还原效能的催化材料. 如图4,150 min后硝酸盐去除率分别为40AC/CF2T3(94.6%)、40AC/CFT2(77.3%)、40AC/CF3T4(72.1%)、40AC/C2FT3(57.4%)和40AC/C3FT4(42.5%). 其中,铜铁钛配比范围为(2:1:3)—(1:3:4)的复合材料,氮气转化率均在80%以上,有效控制了氮气选择的反应路径. 此外,复合材料的光催化还原硝酸盐性能与AgCl(Ag)掺杂量成正比(图4(b)),40AC/CF2T3的硝酸盐去除率相较于30AC/CF2T3和CF2T3分别提高了18.3%和48.4%. 综上所述,选择还原性能最佳的40AC/CF2T3进行后续实验.
-
40AC/CF2T3(0.2 g·L−1)对不同浓度硝酸盐的还原性能(如图5). 经150 min后,31.0、62.0、93.0、124.0 mg·L−1的硝酸盐去除率分别为99.6%、95.6%、71.3%和43.9%,符合准一级动力学模型,对应的反应速率常数由0.0300 min−1降至0.0047 min−1. 其中,硝酸盐浓度低于62.0 mg·L−1时,去除率超过95%,此时体系生成的CO2·−浓度处于饱和状态. 然而,硝酸盐浓度从62.0 mg·L−1增至124.0 mg·L−1时,还原效果显著降低(去除率和反应速率分别降低50.8%和74.5%). 分析认为,可能由于体系生成有限浓度的CO2·−,且高浓度污染物的光解受限,导致还原过程被抑制.
-
通过40AC/CF2T3催化去除62.0 mg·L−1硝酸盐实验和元素平衡分析,研究了复合材料还原硝酸盐机理. 据报道,UV/FA体系中主要活性物质为受激发产生的CO2·−(E°(CO2/CO2·−)=−2.3 eV)和光生电子[8]. 如图6(a),150 min后UV和UV/FA体系中硝酸盐去除率仅为20.0%—32.2%. 投加0.2 g·L−1 CF2T3后,硝酸盐去除率增至47.2%,而投加40AC/CF2T3时硝酸盐去除率高达95.6%,分析40AC/CF2T3能产生更多的CO2·−,使还原效率显著提高. 在AC/CF2T3中,Ag可以捕捉光生电子、减少光生电子-空穴对的复合[15],增强材料光吸收性能(图2(c)). 参考CuFe2O4和TiO2的电化学数据[36],CF2T3受紫外光激发后快速产生ecb−,可以直接还原NO3−和NO2−. 由AgCl导带电势为−0.046 eV[35],接近CF2T3的价带,AgCl(Ag)表面的ecb−传递到CF2T3,形成AgCl(Eg= 3.2 eV)[35]和CF2T3(Eg= 1.70—2.21 eV)的异质结构,提高光量子产率,有效提高了硝酸盐去除效率.
此外,硝酸盐还原产物为N2、NO2−和NH4+,提高无害产物(即氮气转化率)是材料改性的主要目标. 如图6(b),在40AC/CF2T3催化还原硝酸盐中,总氮和硝酸根的去除符合准一级动力学模型,150 min后去除率分别达95.5%和94.6%,对应反应速率常数分别为(0.0178 ± 0.0021) min−1和(0.0188 ± 0.0017) min−1. 还原产物N2浓度迅速上升,90 min后氮气转化率超过91.2%. NO2−和NH4+的生成量较低,最高浓度仅为15.7 μmol·L−1和74.0 μmol·L−1. 生成的NO2−会被快速还原为N2,在150 min时,NO2−未被检出到,NH4+浓度降为为7.6 μmol·L−1.
-
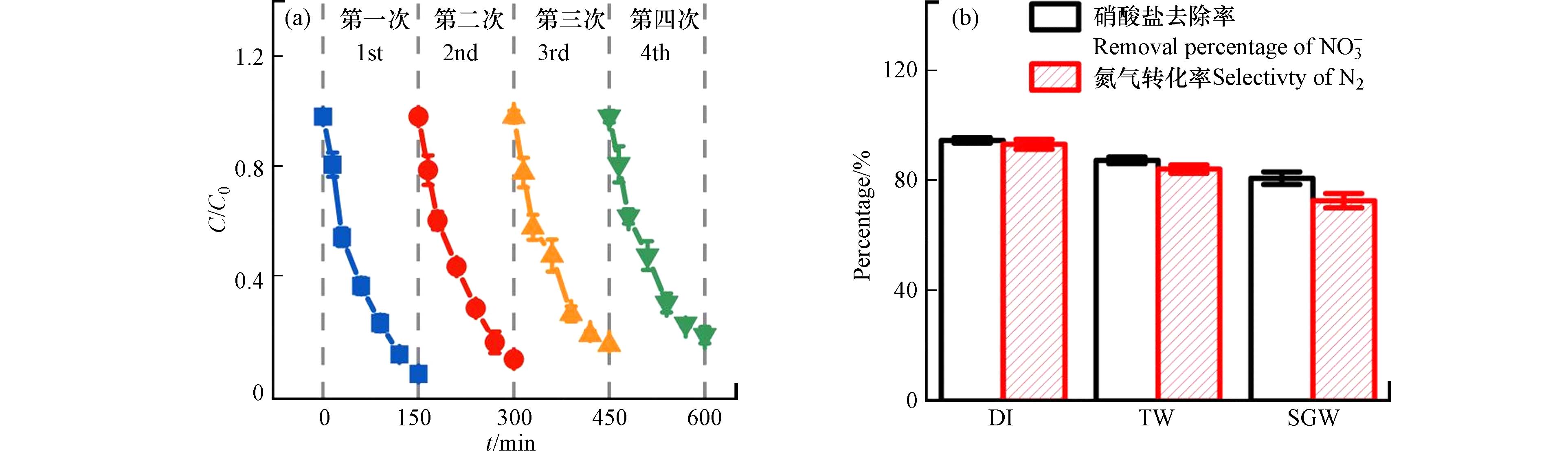
经过4次循环,40AC/CF2T3对硝酸盐的去除性能保持稳定,硝酸盐去除率为95.6%—81.6%,表现出良好的循环回用性能(见图7(a)).
将40AC/CF2T3应用于去除实际水体中的硝酸盐污染,水质采用自来水和模拟西北地下水(水质参数见表1). 根据图7(b)显示,当40AC/CF2T3和甲酸投加量为0.4 g·L−1和6 mmol·L−1时,150 min后,自来水和模拟地下水中的硝酸盐去除率相对于去离子水(124.0 mg·L−1 NO3−)分别降低了7.2%和13.2%,但仍保持在80%以上,氮气转化率降至93.2%—72.7%. 分析表明,实际水环境中存在的HCO3−(122 mg·L−1)和有机物会竞争硝酸盐与CO2·−的反应,导致活性还原自由基浓度降低,从而使硝酸盐去除性能下降.
-
(1)通过改进溶剂热法和化学沉淀法成功合成了AgCl(Ag)/CuFexTix+1Oy复合催化材料. 采用SEM、XRD、XPS和UV-vis等表征,当AgCl(Ag)掺杂量为40%(按质量算)时,CF2T3的光催化性能得到显著增强. 同时,当铜铁钛配比为1:2:3时,对硝酸盐的还原效果最为显著.
(2)采用40AC/C3FT4进行光催化去除硝酸盐实验,当投量为0.2 g·L−1时,对31.0—124.0 mg·L−1的硝酸盐去除率为99.6%—43.9%,当硝酸盐浓度低于62.0 mg·L−1时,去除率高于95%.
(3)40AC/C3FT4中AgCl(Ag)和CuFe2Ti3Oy组成复合材料,表现出高效的硝酸盐光催化还原性能,主要还原产物为N2. 经过4次循环实验,复合材料的硝酸盐还原性能保持稳定,150 min后污染物去除率仍高于81.6%.
(4)40AC/C3FT4在去除自来水和模拟西北地下水中的硝酸盐时表现出稳定的催化还原性能,150 min后硝酸盐去除率超过80%,氮气转化率高于72%,在水处理领域中具有广阔的应用前景.
氯化银(银)-铜铁钛氧化物光催化材料制备及还原硝酸盐性能
AgCl(Ag)/CuFexTix+1Oy for photocatalytic reduction of nitrate
-
摘要: 本研究采用改性的溶剂热法和化学沉淀法制备氯化银(银)-铜铁钛氧化物(AgCl(Ag)/CuFexTix+1Oy)复合光催化材料,用于去除水中的硝酸盐. 分析表明,AgCl(Ag)促进CuFexTix+1Oy产生光生电子,并促进光生电子-空穴对的分离,从而提高复合材料光催化还原硝酸盐性能. 研究重点探究了复合材料在紫外/甲酸体系中的光还原性能,并将其应用于实际水处理中. 实验结果表明:当AgCl掺杂量为40%(按质量算),且铜铁钛的配比为1:2:3时,光催化还原硝酸盐的效果最佳. 在150 min时,硝酸盐去除率达94.6%,去除速率为(0.0188 ± 0.0017) min−1,氮气转化率达99.0%. 在实际水处理中,该体系对自来水和模拟地下水的硝酸盐去除率分别为87.4%和80.9%,氮气转化率均大于70%,显示出良好的应用潜力.
-
关键词:
- 氯化银(银)-铜铁钛氧化物 /
- 高级还原 /
- 硝酸盐 /
- 氮气转化率
Abstract: A photocatalyst AgCl(Ag)/CuFexTix+1Oy were prepared by synthetic methods including solvothermal and chemical precipitation methods. The analysis revealed that the presence of AgCl(Ag) facilitated the generation of photogenerated electrons in CuFexTix+1Oy, while inhibiting the recombination of electrons and holes, consequently enhancing photocatalytic efficiency. This article took nitrate as the removal object and investigated the photoreduction capabilities of the composite materials in the UV/formic acid system as well as their potential application in realistic water. The experimental results indicated that the optimal outcome was achieved when the AgCl doping concentration was 40% by massand the ratio of copper, iron, and titanium was 1:2:3. After150 minutes of reaction, the nitrate removal rate was 94.6%, the degradation rate reached (0.0188 ± 0.0017) min−1, and the nitrogen conversion rate reached 99.0%. Additionally, in implementation in realistic water treatments, the system exhibited nitrate removal rates of 87.4% for tap water and 80.9% for simulated groundwater, with a nitrogen conversion rate exceeding 70%. These results suggesting a promising potential for application.-
Key words:
- AgCl(Ag)/CuFexTix+1Oy /
- APRs /
- nitrate /
- nitrogen conversion rate
-

-
表 1 水质指标
Table 1. Water quality indicators
指标
Indicators自来水
Tap water模拟地下水
Simulated groundwaterpH 7.1 7.3 TOC/(mg·L−1) 1.92 3.00 DO/(mg·L−1) 8.21 8.50 硫酸盐/(mg·L−1) 55.2 384.0 氯化物/(mg·L−1) 21.1 142.0 碳酸氢盐/(mg·L−1) 41.0 122.0 硝酸盐/(mg ·L−1 N) 5.5 27.1 -
[1] DOUDRICK K, YANG T, HRISTOVSKI K, et al. Photocatalytic nitrate reduction in water: Managing the hole scavenger and reaction by-product selectivity[J]. Applied Catalysis B: Environmental, 2013, 136/137: 40-47. doi: 10.1016/j.apcatb.2013.01.042 [2] 王开然, 陈华伟, 吴振, 等. 济南泉域岩溶水系统硝酸盐空间分布及溯源解析[J]. 环境化学, 2024, 43(3): 961-973. doi: 10.7524/j.issn.0254-6108.2022080903 WANG K R, CHEN H W, WU Z, et al. Spatial distribution and traceability analysis of nitrate in Karst water system in Jinan spring basin[J]. Environmental Chemistry, 2024, 43(3): 961-973 (in Chinese). doi: 10.7524/j.issn.0254-6108.2022080903
[3] 田寒梅, 盛欣, 王冰, 等. 2014—2019年某市农村地区自备井水中硝酸盐含量及影响因素分析[J]. 环境卫生学杂志, 2021, 11(5): 415-419. TIAN H M, SHENG X, WANG B, et al. Content of nitrates and its influencing factors in well water in rural areas of a city, 2014—2019[J]. Journal of Environmental Hygiene, 2021, 11(5): 415-419 (in Chinese).
[4] MAHESHWARI R K, CHAUHAN A K, LAL B, et al. Nitrate toxicity in groundwater: Its clinical manifestations, preventive measures and mitigation strategies[J]. Octa Journal of Environmental Research, 2013, 1(3): 217-230. [5] 陈西亮, 刘国, 高阳阳, 等. 零价纳米铁炭微电解体系去除水中硝酸盐[J]. 环境化学, 2016, 35(8): 1670-1675. doi: 10.7524/j.issn.0254-6108.2016.08.2015123003 CHEN X L, LIU G, GAO Y Y, et al. Removal of nitrate from water by nano-zero-valent iron-carbon microelectrolysis system[J]. Environmental Chemistry, 2016, 35(8): 1670-1675 (in Chinese). doi: 10.7524/j.issn.0254-6108.2016.08.2015123003
[6] 郭睿, 秦侠, 郭城睿, 等. Ni foam/Cu电极电催化还原硝酸盐氮[J]. 环境化学, 2022, 41(6): 2103-2111. doi: 10.7524/j.issn.0254-6108.2021022601 GUO R, QIN X, GUO C R, et al. Electrocatalytic reduction of nitrate nitrogen by Ni foam/Cu electrode[J]. Environmental Chemistry, 2022, 41(6): 2103-2111 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021022601
[7] REZVANI F, SARRAFZADEH M H, EBRAHIMI S, et al. Nitrate removal from drinking water with a focus on biological methods: A review[J]. Environmental Science and Pollution Research, 2019, 26(2): 1124-1141. doi: 10.1007/s11356-017-9185-0 [8] ALOWITZ M J, SCHERER M M. Kinetics of nitrate, nitrite, and Cr(VI) reduction by iron metal[J]. Environmental Science & Technology, 2002, 36(3): 299-306. [9] TUGAOEN H O, GARCIA-SEGURA S, HRISTOVSKI K, et al. Challenges in photocatalytic reduction of nitrate as a water treatment technology[J]. The Science of the Total Environment, 2017, 599/600: 1524-1551. doi: 10.1016/j.scitotenv.2017.04.238 [10] CHEN G D, HANUKOVICH S, CHEBEIR M, et al. Nitrate removal via a formate radical-induced photochemical process[J]. Environmental Science & Technology, 2019, 53(1): 316-324. [11] SHI Z Y, WANG F L, XIAO Q, et al. Selective and efficient reduction of nitrate to gaseous nitrogen from drinking water source by UV/oxalic acid/ferric iron systems: Effectiveness and mechanisms[J]. Catalysts, 2022, 12(3): 348. doi: 10.3390/catal12030348 [12] KUDO A, DOMEN K, MARUYA K I, et al. Photocatalytic reduction of NO3− to form NH3 over Pt–TiO2[J]. Chemistry Letters, 1987, 16(6): 1019-1022. doi: 10.1246/cl.1987.1019 [13] BEMS B, JENTOFT F C, SCHLÖGL R. Photoinduced decomposition of nitrate in drinking water in the presence of titania and humic acids[J]. Applied Catalysis B: Environmental, 1999, 20(2): 155-163. doi: 10.1016/S0926-3373(98)00105-2 [14] KUDO A, DOMEN K, MARUYA K, et al. Reduction of nitrate ions into nitrite and ammonia over some photocatalysts[J]. Journal of Catalysis, 1992, 135(1): 300-303. doi: 10.1016/0021-9517(92)90287-R [15] ZHANG F X, JIN R C, CHEN J X, et al. High photocatalytic activity and selectivity for nitrogen in nitrate reduction on Ag/TiO2 catalyst with fine silver clusters[J]. Journal of Catalysis, 2005, 232(2): 424-431. doi: 10.1016/j.jcat.2005.04.014 [16] GE X H, FU W Z, WANG Y J, et al. Removal of nitrate nitrogen from water by phosphotungstate-supported TiO2 photocatalytic method[J]. Environmental Science and Pollution Research, 2020, 27(32): 40475-40482 doi: 10.1007/s11356-020-09947-y [17] ZHANG H J, LIU Z H, LI Y, et al. Intimately coupled TiO2/g-C3N4 photocatalysts and in-situ cultivated biofilms enhanced nitrate reduction in water[J]. Applied Surface Science, 2020, 503: 144092. doi: 10.1016/j.apsusc.2019.144092 [18] ZHENG R, LI C H, HUANG K L, et al. In situ synthesis of N-doped TiO2 on Ti3C2 MXene with enhanced photocatalytic activity in the selective reduction of nitrate to N2[J]. Inorganic Chemistry Frontiers, 2022, 9(6): 1195-1207. doi: 10.1039/D1QI01614H [19] LI X, WANG S, AN H Z, et al. Enhanced photocatalytic reduction of nitrate enabled by Fe-doped LiNbO3 materials in water: Performance and mechanism[J]. Applied Surface Science, 2021, 539: 148257. doi: 10.1016/j.apsusc.2020.148257 [20] HOU Z A, CHEN F F, WANG J N, et al. Novel Pd/GdCrO3 composite for photo-catalytic reduction of nitrate to N2 with high selectivity and activity[J]. Applied Catalysis B: Environmental, 2018, 232: 124-134. doi: 10.1016/j.apcatb.2018.03.055 [21] SILVEIRA J E, RIBEIRO A R, CARBAJO J, et al. The photocatalytic reduction of NO3− to N2 with ilmenite (FeTiO3): Effects of groundwater matrix[J]. Water Research, 2021, 200: 117250. doi: 10.1016/j.watres.2021.117250 [22] 官海汕, 李帅, 许伟城, 等. 铁基双金属催化剂耦合过一硫酸盐去除污染物的研究进展[J]. 环境化学, 2024, 43(11): 1-13. doi: 10.7524/j.issn.0254-6108.2023052202 GUAN H S, LI S, XU W C, et al. Research progress on the coupling of iron based bimetallic catalysts with peroxymonosulfate for pollutants removal[J]. Environmental Chemistry, 2024, 43(11): 1-13 (in Chinese). doi: 10.7524/j.issn.0254-6108.2023052202
[23] LI Z Y, ZHAO Y J, GUAN Q, et al. Novel direct dual Z-scheme AgBr(Ag)/MIL-101(Cr)/CuFe2O4 for efficient conversion of nitrate to nitrogen[J]. Applied Surface Science, 2020, 508: 145225. doi: 10.1016/j.apsusc.2019.145225 [24] YANG X H, WANG R, WANG S, et al. Sequential active-site switches in integrated Cu/Fe-TiO2 for efficient electroreduction from nitrate into ammonia[J]. Applied Catalysis B: Environmental, 2023, 325: 122360. doi: 10.1016/j.apcatb.2023.122360 [25] WANG N, WANG J, LIU M N, et al. Preparation of Ag@CuFe2O4@TiO2 nanocomposite films and its performance of photoelectrochemical cathodic protection[J]. Journal of Materials Science & Technology, 2022, 100: 12-19. [26] 韩术鑫, 王利红, 李剑, 等. 东营市化工聚集区地下水TOC污染空间分布特性[J]. 中国环境监测, 2018, 34(4): 85-94. HAN S X, WANG L H, LI J, et al. Study on the spatial distribution characteristics of TOC pollution for groundwater in Dongying chemical industry gathering area[J]. Environmental Monitoring in China, 2018, 34(4): 85-94 (in Chinese).
[27] GAO S T, LIU W H, SHANG N Z, et al. Integration of a plasmonic semiconductor with a metal–organic framework: A case of Ag/AgCl@ZIF-8 with enhanced visible light photocatalytic activity[J]. RSC Advances, 2014, 4(106): 61736-61742. doi: 10.1039/C4RA11364K [28] XIAO Q, WANG T, YU S L, et al. Influence of UV lamp, sulfur(IV) concentration, and pH on bromate degradation in UV/sulfite systems: Mechanisms and applications[J]. Water Research, 2017, 111: 288-296. doi: 10.1016/j.watres.2017.01.018 [29] SOLANO R, MAESTRE D, MUESES M, et al. TiO2-CuO heterojunction nanoparticles synthesized by green chemistry supported on beach sand granules: Optical, morphological and structural characterization[J]. Nano-Structures & Nano-Objects, 2023, 35: 101024. [30] ZHANG H, ZHOU Y, LIU S Q, et al. Molecular-level understanding of selectively photocatalytic degradation of ammonia via copper ferrite/N-doped graphene catalyst under visible near-infrared irradiation[J]. Catalysts, 2018, 8(10): 405. doi: 10.3390/catal8100405 [31] ZHANG X Y, DING Y B, TANG H Q, et al. Degradation of bisphenol A by hydrogen peroxide activated with CuFeO2 microparticles as a heterogeneous Fenton-like catalyst: Efficiency, stability and mechanism[J]. Chemical Engineering Journal, 2014, 236: 251-262. doi: 10.1016/j.cej.2013.09.051 [32] YU J G, RAN J R. Facile preparation and enhanced photocatalytic H2-production activity of Cu(OH)2 cluster modified TiO2[J]. Energy & Environmental Science, 2011, 4(4): 1364-1371. [33] IVANOVA T M, MASLAKOV K I, SIDOROV A A, et al. XPS detection of unusual Cu(Ⅱ) to Cu(I) transition on the surface of complexes with redox-active ligands[J]. Journal of Electron Spectroscopy and Related Phenomena, 2020, 238: 146878. doi: 10.1016/j.elspec.2019.06.010 [34] LU L R, AI Z H, LI J P, et al. Synthesis and characterization of Fe-Fe2O3 core-shell nanowires and nanonecklaces[J]. Crystal Growth & Design, 2007, 7(2): 459-464. [35] FAN G D, ZHENG X M, LUO J, et al. Rapid synthesis of Ag/AgCl@ZIF-8 as a highly efficient photocatalyst for degradation of acetaminophen under visible light[J]. Chemical Engineering Journal, 2018, 351: 782-790. doi: 10.1016/j.cej.2018.06.119 [36] UDDIN M R, KHAN M R, RAHMAN M W, et al. Photocatalytic reduction of CO2 into methanol over CuFe2O4/TiO2 under visible light irradiation[J]. Reaction Kinetics, Mechanisms and Catalysis, 2015, 116(2): 589-604. doi: 10.1007/s11144-015-0911-7 -



 下载:
下载: