-
消化系统疾病是我国的常见病、多发病,也是严重危害人类健康的全球性疾病. 近年来,消化道恶性肿瘤的发病率明显上升,并且有年轻化趋势[1]. 环境暴露与生活方式相关的危险因素对消化系统肿瘤的发生发展有着显著影响[2 − 3]. 有害化学物质的环境暴露是增加罹患癌症风险的重要诱发因素[4]. 双酚类物质(Bisphenols, BPs)是一类人工合成的化学品,广泛应用于塑料、食品接触材料等领域. 然而,BPs也是一类典型的环境内分泌干扰物,与生殖系统损伤、神经发育迟缓和免疫毒性等多种毒性效应有关. 膳食摄入是BPs暴露的主要途径之一[5],伴随外卖餐饮带动的塑料制品消费需求增长,暴露于双酚类污染物的胃肠道健康风险不容忽视[6].
双酚类污染物作为极具代表性的环境内分泌干扰物,被认为是导致近年来全球肥胖、糖尿病、乳腺癌、甲状腺癌和生殖发育障碍发病率上升的环境健康风险因素之一. 然而,双酚类污染物对人体不同组织器官生理功能的扰动机制尚不完全清楚,其体内代谢转化与生物分子相互作用的动态过程仍是一个科学难题.
-
双酚A (Bisphenol A, BPA)是世界上产量最高的工业化学品之一,主要用于合成聚碳酸酯、环氧树脂和聚丙烯酸酯等聚合物,这些高分子聚合物被广泛用于各种日常消费品中,包括食品容器、厨具、热敏纸、水管、塑料瓶、牙科材料等,从而导致人们可通过不同途径的饮食和非饮食来源接触到这种化学物质[7]. BPA作为一种典型的环境内分泌干扰物(EDCs),多项研究已证实其对内分泌系统、神经系统、免疫系统以及生殖系统可产生一系列不良影响[8 − 11].
鉴于BPA存在的健康风险,从2010年起,加拿大、中国、美国等一些国家和地区相继出台了限制BPA生产和应用的相关政策法规[12 − 13]. 随着公众对BPA的关注和政府对BPA的监管,促使大量BPA替代物质的开发和生产,以取代BPA在众多日常消费品中的应用. 目前,许多结构和性能上与双酚A相似的新型替代化合物,如双酚F(BPF)、双酚S(BPS)、双酚AF(BPAF)、双酚B(BPB)等在市场上不断涌现,使用量不断增加,逐渐成为新型环境污染物. BPF一般广泛应用于塑料、水管、牙科密封剂、口腔修复材料和食品包装材料;BPS主要用于饮料罐内部涂层和收据纸,同时也用作一些染料的添加剂;BPAF最常用作氟弹性体,如食品加工设备中的垫圈、聚碳酸酯塑料等,此外,还用做光纤和电子材料中的交联剂[14 − 16]. 随着这些双酚类似物在工业中的大量生产、日常消费中的广泛使用及其废弃、老化,BPs很容易从这些产品中被释放到环境[17],使得其在地表水、沉积物、污水等环境介质中被广泛检测到,曾在中国太湖中检测到BPAF的浓度高达140 ng·L−1,甚至高于被检测到的BPA的浓度[18]. 由于BPs特殊的化学结构及其理化性质,导致这些高分子聚合物表现出高环境持久性,数据显示,其在水中的半衰期约为15—180 d,在土壤中的半衰期约为30—360 d,在沉积物中的半衰期高达135—
1621 d[18]. 因此,我们不得不重视BPs对生态环境的潜在风险和对人类健康构成的风险.由于人体接触BPA的途径是多种多样的,根据BPA暴露的食物来源(塑料瓶、食品包装材料等)和非食物来源(热敏纸、牙科材料等)数据比较显示,食物来源的双酚A暴露量通常比非食物来源的暴露量高出至少一个数量级[19]. 在对美国纽约州的各种食品,包括饮料、乳制品、油脂、鱼和海鲜、谷物、肉类和肉制品、水果、蔬菜和“其他”九大类食物样本调查中发现,约75%的食物中含有BPs,主要为BPA和BPF,平均浓度分别为3 ng·g−1(湿重)和0.93 ng·g−1(湿重),其中BPs浓度最高的是“其他”类别,主要为腌制的袋装即食食品;而且,数据还显示罐装食品比装在玻璃、纸或塑料容器内的食品含有更高浓度的BPs[20]. 与此同时,在一些欧洲国家的罐装食品和饮料中也发现了BPs,包括BPA、BPF、BPS及BPB[21 − 23]. 同样地,对中国9个城市的13类食品样本(谷类及谷类制品、肉类及肉制品、鱼类及海鲜、蛋类、奶制品、豆制品、水果、蔬菜、零食、饮料、食用油、调味品等)也进行了BPs的浓度分析,数据显示各种食品样本中BPs的平均浓度为9.35 ng·g−1,其中,中国成年男性和女性通过食物平均每日摄入BPs(EDI)分别为646 ng·kg−1 ·d−1和664 ng·kg−1 ·d−1,远高于美国成年人的平均每日摄入量(EDI)54.6 ng·kg−1 ·d-1[24]. 此外,在第五次中国总膳食研究(2009—2013年)中也发现了BPs的暴露,在谷物(33.7%—41.3%)和土豆(33.7%—14.3%)中都检测到BPAF的存在[25]. 尽管BPA被大量限制使用,但BPA类似物的种类及其应用途径在市场上仍然层出不穷,人类通过饮食或饮用水等途径暴露于BPs的风险与日俱增,因此,需要全面评估BPs暴露与胃肠道健康风险之间的关系.
-
胃肠道是外源化学物质进入人体的主要途径,经常暴露于各类有害物质,与食品接触材料相关的双酚类污染物尤为常见[26]. 目前,双酚类污染物的健康危害机制主要集中在甲状腺[27]、雌激素内分泌干扰效应[28]和疑似致癌性研究[29 − 30],对双酚类污染物造成消化道损伤效应的研究较少. 开展非雌雄激素受体介导的肠道功能损伤研究,是科学解析双酚类物质长期环境暴露隐形健康风险的迫切需求[31].
肠黏膜屏障是隔离体内环境与各种食物、潜在有害物质及微生物的生物界面,在维持肠道上皮完整性和机体免疫稳态中发挥重要作用. 已有证据表明,暴露于BPA会改变小鼠免疫系统中Th1/Th2极化,从而增加Th1免疫反应[32 − 34]. 围产期口服暴露于BPs可通过干扰细胞因子谱增加对肠道感染的易感性,并诱导后代小鼠的肠道和全身免疫调节失衡[35 − 39]. 参考剂量水平的BPA经口暴露能影响细胞旁通透性,并加重雌性子代肠道炎症反应[40]. BPA膳食摄入显著降低小鼠结肠上皮中紧密连接蛋白的表达,改变肠道微生物的多样性和结构组成,增加结肠通透性,致肠道损伤的影响与肠屏障功能的破坏密切相关[41]. 近期有研究报道,无明显损害作用剂量下的双酚类物质(BPA和BPF)能通过Notch/Wnt信号通路干扰肠道稳态,诱导肠黏膜屏障功能障碍和炎症反应,造成小鼠肠道损伤[42]. 由此可见,低浓度水平的双酚类污染物长期暴露会造成肠屏障功能受损和慢性炎症,可能是其诱发肠道病变的起始性环节.
一个完整的肠道屏障,对于维持机体正常的生理功能和预防疾病至关重要. 肠道菌群作为寄居于人体最大的微生态系统,其微生物群落多样性组成或代谢产物影响肠道黏膜屏障结构和功能的完整性,并与宿主的生理病理状态密切相关[43]. 目前,在人群水平有报道结肠炎活动期的患者血清双酚A水平显著升高,增强的系统性炎症反应与肠道屏障功能障碍及细菌内毒素易位有关[44]. 另有小鼠模型证实,双酚A暴露能直接影响肠道微生物群落组成,朝着代谢综合征模式转变[45];改变肠道菌群色氨酸代谢而导致结肠炎的加重恶化[46]. 综上所述,双酚类污染物对肠道健康的影响可能主要通过扰乱肠道微生态系统、破坏肠上皮屏障功能,尤其是干扰宿主-微生物共代谢而发挥作用.
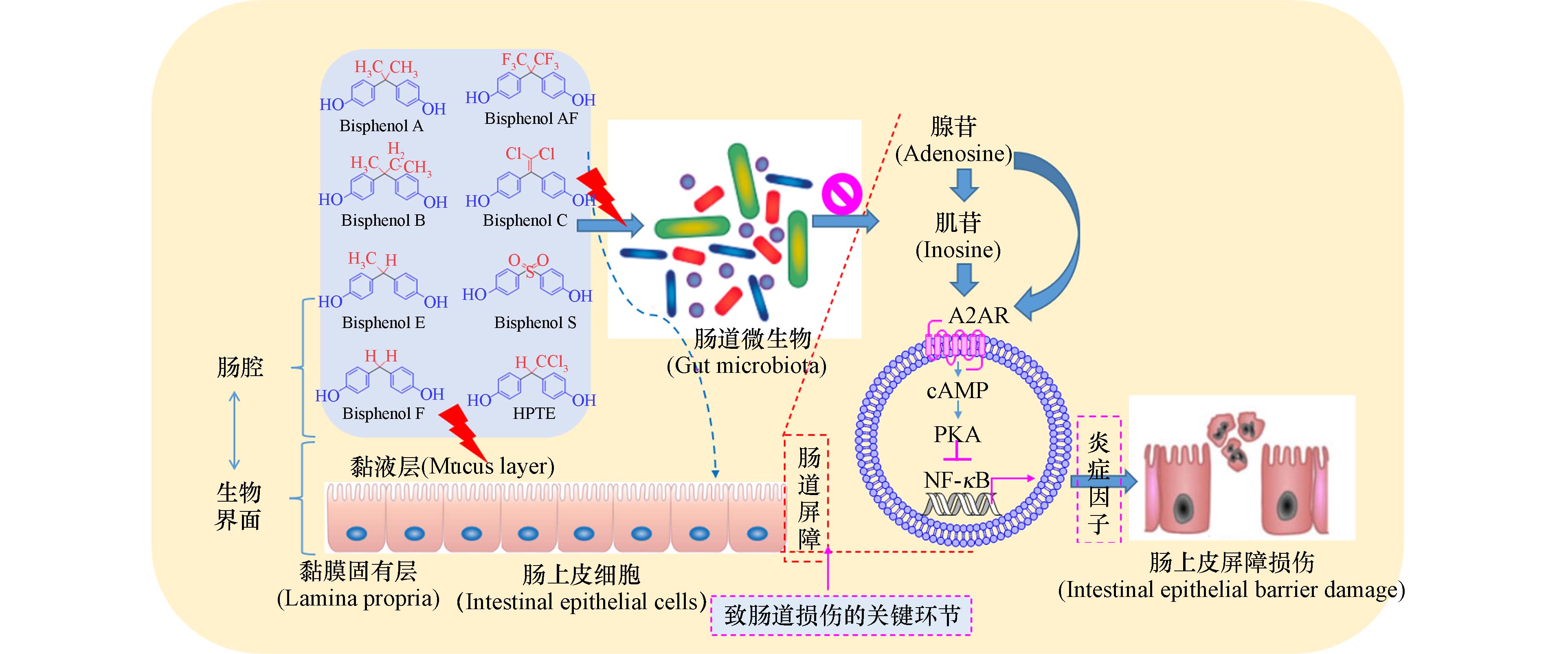
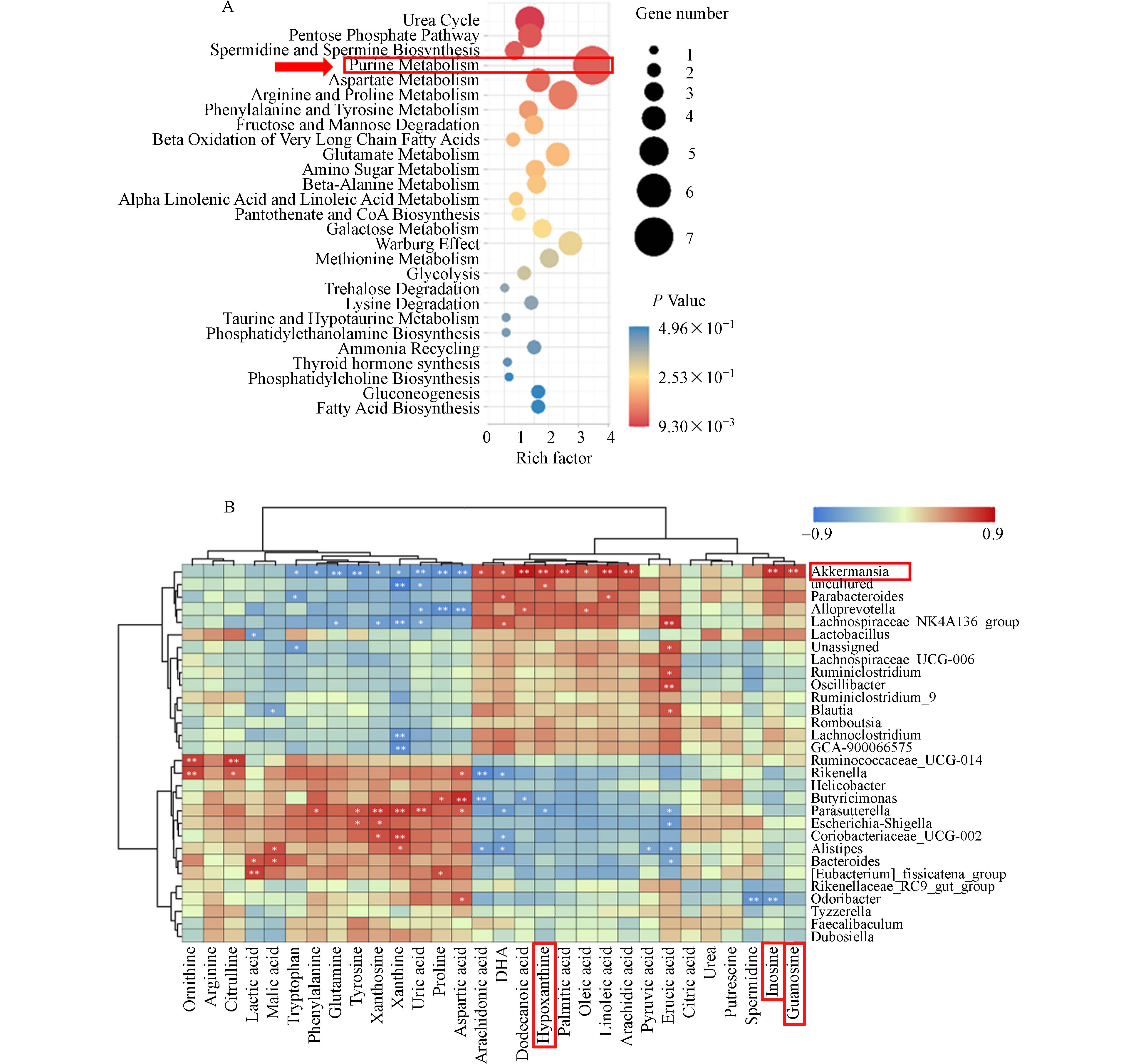
在课题组前期研究中发现:BPA在环境剂量下低水平持续暴露五个月,会显著改变小鼠肠道微生物组成和扰动腺苷信号(嘌呤代谢),如图1所示. 需要指出的是,腺苷是串联机体物质(能量)代谢和信号转导两大重要功能的关键枢纽分子,在生物合成、代谢信息传递和细胞间通讯中扮演重要角色[47].
基于剂量反应关系的代谢组学和通路敏感性分析表明,腺苷途径相关通路(嘌呤代谢)可能是BPA暴露的潜在内源性生物标志物[48]. 利用分子对接的计算毒理学方法鉴定到BPA与腺苷受体A2AR有良好的理论亲和力[49]. 单细胞转录组测序发现,双酚类污染物(BPA、BPF和BPAF)可通过诱导肠上皮不同细胞的异质性反应而致肠损伤和代谢异常[50]. 由此可见,扰动腺苷代谢信号可能是双酚类污染物暴露致肠道上皮屏障功能受损的内在机制.
-
由于扰动腺苷代谢信号可能是双酚类污染物暴露致肠道上皮屏障功能受损的内在机制,因此,在环境剂量水平下,双酚类污染物暴露将很有可能通过干扰腺苷代谢信号引发肠上皮屏障功能障碍,进而对肠道疾病的发生发展和转归有重要影响(如图2所示).
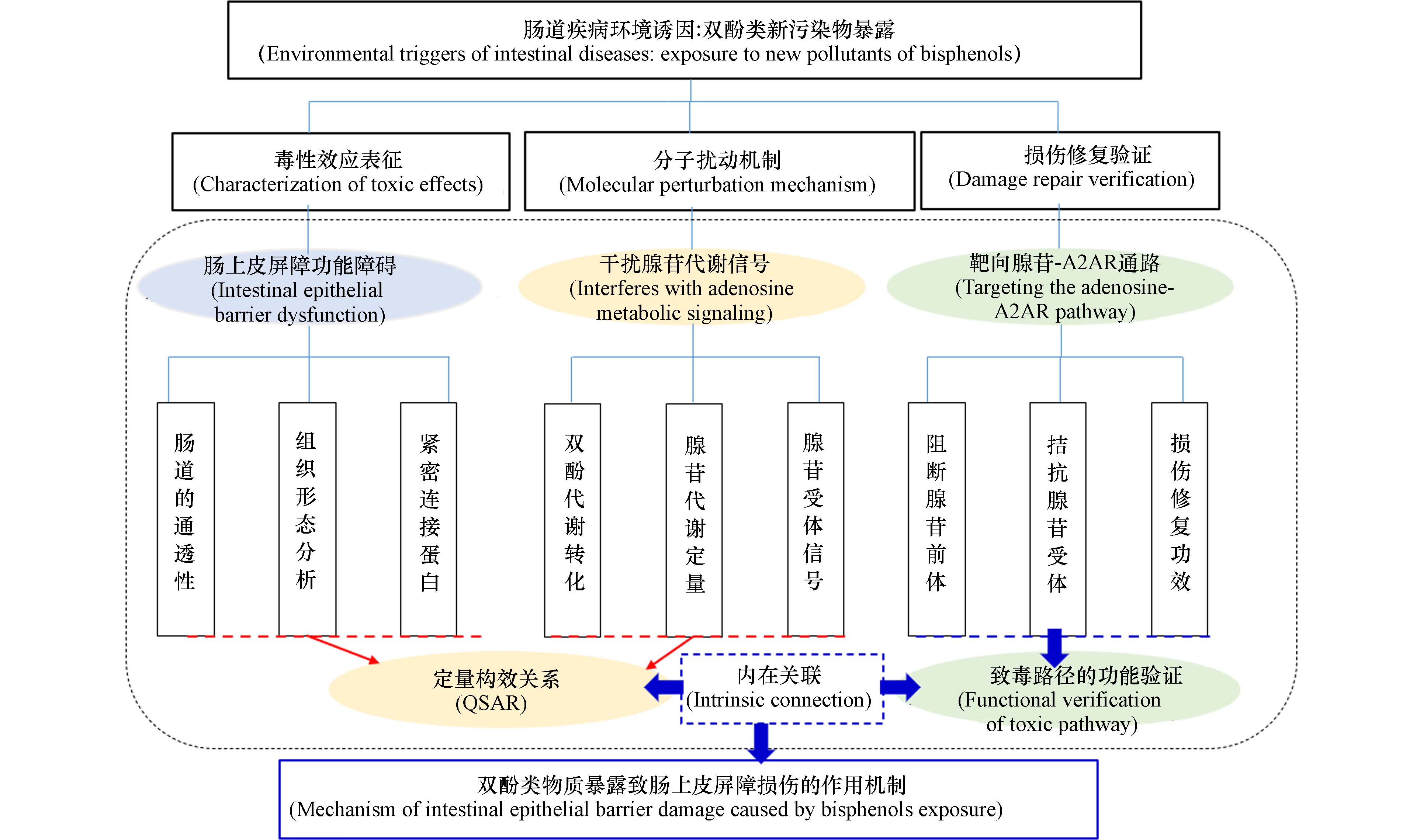
课题组将聚焦于双酚类污染物(BPA、BPB、双酚E(BPE)、BPF、BPAF、双酚C(BPC)和BPS,HPTE作为对照),在环境暴露剂量水平下,对肠道上皮屏障功能的影响及作用机制研究,拟从如下三个方面开展:双酚类污染物暴露致肠道屏障功能损伤的毒性效应表征、干扰腺苷代谢信号的分子机制、肠屏障损伤修复的功能验证实验.
-
总体技术路线如图3所示.
-
肠上皮屏障功能损伤是肠道病变的关键环节. 整合利用整体动物和细胞模型,开展环境污染物体内外暴露实验,能全面细致地评估待测物质的毒性效应. 因此,拟采用整体动物暴露和体外细胞处理相结合的方法,研究双酚类污染物环境剂量水平下暴露对肠道屏障功能的影响.
(1)肠屏障损伤动物模型和双酚类污染物暴露
肠道屏障损伤小鼠模型:按照文献[51],利用C57BL/6J小鼠,通过葡聚糖硫酸钠(DSS)化学诱导法制备肠道屏障功能损伤动物模型. 以肠道组织病理形态学指标确认肠屏障损伤模型是否成功. 通过优化DSS用药剂量和持续时间而诱导呈现典型的肠屏障损伤病理特征,此为阳性对照试验.
双酚类污染物染毒方式:鉴于膳食暴露是普通人群双酚类物质最常见的暴露途径[5],动物实验染毒方式确定为灌胃,将小鼠随机分为空白对照组和不同剂量的双酚类物质暴露组(每组小鼠各10只);双酚类物质用橄榄油溶解配制,空白对照组为不含双酚的橄榄油,连续暴露1—2周或2—4月(急性和慢性染毒). 申请入已注意到,由于皮肤接触是日常生活中双酚类物质人体暴露不可避免的途径,本研究也考虑小样本皮肤暴露预试验,根据预实验来判断是否调整染毒方式.
双酚类污染物暴露浓度:双酚类污染物的染毒剂量,根据我国一般人群中双酚类物质的内暴露水平来设定[52],包括低、中和高的3个剂量水平. 由于不同结构类型的双酚类物质人体负荷水平和生物活性有较大差异,本研究将在覆盖机体负荷范围(0—100 ng·mL−1)的剂量条件下, 并通过液质联用仪定量检测双酚类物质小鼠体内内暴露水平,考察双酚类物质致肠道屏障功能损伤的毒性效应.
(2)双酚类物质影响小鼠肠道屏障功能的量化表征
肠道通透性的测定:肠黏膜通透性的变化能准确反映肠黏膜的损伤程度,是监测肠道屏障功能完整性的重要指标. 对双酚类物质暴露不同时间后的各组小鼠灌胃FITC-葡聚糖(FITC-dextran,FD 4),4 h后取外周血,紫外可见分光光度计(490 nm)检测血液中FITC含量,定量表征双酚类物质暴露后小鼠肠道通透性的动态变化,评估肠道屏障功能的完整性.
肠道组织形态学分析:肠黏膜组织学观察,能直接反映肠上皮形态结构的变化,是一种公认的评价肠道屏障功能的常用方法. 通过光学和电子显微镜观察肠道上皮细胞形态、绒毛结构、排列及亚细胞器变化情况. 本研究将采用扫描电镜和透射电镜观测双酚类污染物暴露对肠黏膜机械屏障的影响.
紧密连接蛋白的测定:紧密连接是肠上皮细胞间的主要连接方式,在维持肠道屏障完整性中发挥重要作用. 紧密连接蛋白是构成肠道黏膜屏障、决定肠道通透性的重要效应分子. 采用qRT-PCR检测肠道紧密连接蛋白(ZO-1)、密封蛋白(Claudin-1)、连接黏附分子(JAM)、黏蛋白-2(MUC-2)和闭锁蛋白(Occludin)的表达情况,分子水平上表征双酚类物质暴露对肠上皮屏障功能的影响.
(3)双酚类物质致肠屏障功能损伤的定量构效关系
体外肠道屏障模型:采用肠上皮细胞Caco-2体外培养,分别用肿瘤坏死因子-α(TNF- α)和脂多糖(LPS)处理细胞,建立肠上皮屏障功能研究的体外模型.
跨膜电阻抗(TEER)的测定:经双酚类物质暴露处理后,显微镜下观察Caco-2单层细胞的形态、测量跨膜电阻抗(TEER)和荧光黄透过率,以体外评估双酚类物质对肠上皮屏障功能的影响.
定量构效关系(QSAR)的研究:采用分子对接的方法模拟双酚类物质和腺苷受体(A2AR)的选择性结合关系,将其剂量反应关系曲线(响应值采用肠上皮细胞的跨膜电阻值TEER)分别和化合物与腺苷受体及雌激素受体的结合自由能进行拟合,以预测双酚类物质的肠上皮屏障损伤效应可能与腺苷受体的选择性有关.
-
利用第一部分研究所建立的动物模型和细胞模型,对双酚类污染物的体内外代谢转化和腺苷信号干扰效应进行定量分析,以明确双酚类物质暴露致肠上皮屏障功能损伤的效应标志物和生理扰动分子机制.
(1)双酚类污染物在小鼠体内的代谢转化规律
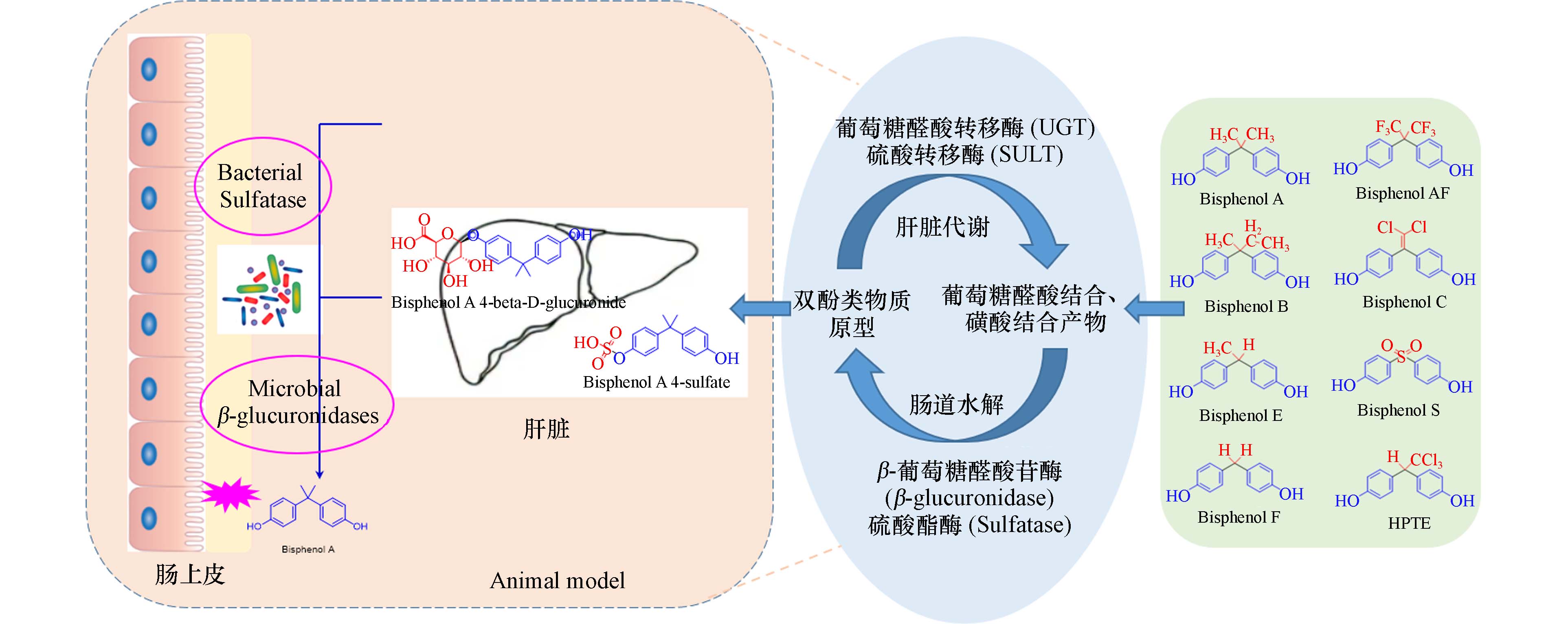
采用超高效液相色谱-三重四极杆串联质谱(UPLC-MS/MS)研究双酚类物质在C57BL/6J小鼠和Caco-2细胞中的代谢转化规律,定量分析双酚类化合物原型、羟基化代谢产物和葡萄糖醛酸结合及磺酸结合产物,总结双酚类化合物体内外生物转化规律;重点考察双酚类物质原型及其代谢产物在肝肠回路中的循环和分配情况(见图4),并与其暴露所致肠上皮屏障功能损伤毒性效应建立联系.
(2)双酚类污染物暴露扰动肠道腺苷代谢通路
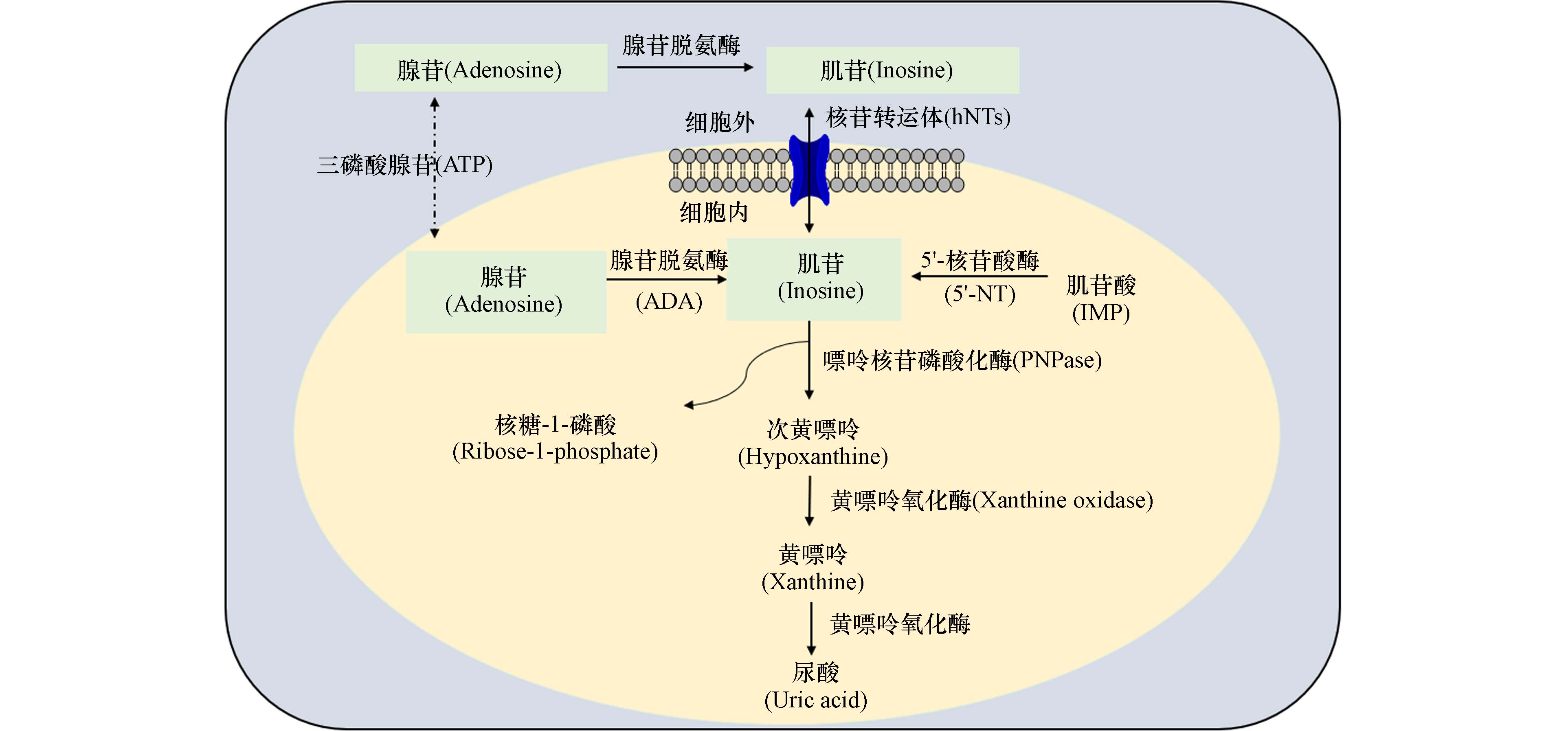
利用基于质谱技术的靶向定量代谢组学策略,对双酚类物质暴露后小鼠肠道、肠道内容物、粪便和Caco-2细胞及其培养液等生物样本,定量分析腺苷代谢相关的通路,包括嘌呤核苷酸的从头合成与补救途径、腺苷降解途径、ATP再生途径和细胞腺苷摄取途径(图5),以全面绘制双酚类污染物暴露对腺苷代谢通路干扰的全过程.
(3)双酚类污染物暴露干扰腺苷信号传导
腺苷代谢通路相关的小分子代谢物,不仅是体内外生化代谢反应的底物,而且作为信号分子,对维持机体生理功能发挥重要作用. 鉴于2A型腺苷受体亚型(A2AR)对腺苷的高亲和性,在腺苷信号通路中将被重点研究. 具体而言,采用UPLC-MS/MS精确定量环磷酸腺苷cAMP、腺苷和肌苷等关键信号分子,同时采用qRT-PCR、免疫印迹和免疫组化实验,对2A型腺苷受体(A2AR)-cAMP-PKA信号通路进行分析(图6),以更进一步证实双酚类物质致肠上皮屏障功能损伤的毒性通路.
-
靶向干预腺苷-A2AR通路以修复双酚类物质所致肠道损伤,此为功能验证实验. 分别从腺苷底物来源阻断和增强、腺苷受体拮抗和活化等正反两个方面,验证腺苷-A2AR通路在双酚类污染物暴露致肠上皮屏障损伤的作用机制和毒性贡献.
(1)验证双酚类物质扰动腺苷代谢何种途径而致肠屏障功能障碍
腺苷既是腺苷酸的前体,又是其代谢产物. 分别从腺苷的胞内外来源与合成分解代谢的角度,通过增强或阻断腺苷底物来源,确证具体何种腺苷代谢途径驱动双酚类物质暴露致肠上皮屏障功能损伤. 包括肠道微生物来源的腺苷、胞外ATP逐级水解产生的腺苷和细胞内能量代谢生成的腺苷等三条主要途径,分别用四联抗生素清除肠道菌群、化学抑制剂阻断相应的代谢通路来佐证.
(2)验证双酚类化合物竞争结合腺苷受体而致肠屏障功能障碍
双酚类化合物可能通过竞争性干扰腺苷或肌苷与其受体的结合而达到影响肠上皮屏障功能的效果. 分别采用A2AR激动剂CGS21680和拮抗剂ZM241385[53]联合双酚类物质暴露,结合第一和第二部分的方法,检测小鼠肠上皮屏障功能指标的变化,从生理功能上验证腺苷-A2AR通路在双酚类污染物致肠上皮屏障功能损伤中的作用.
-
BPA作为典型的环境内分泌干扰物,通过多种途径广泛地暴露于环境和日常生活中,随着BPA所造成的健康风险被越来越多的关注以及被有关政策的限制使用,BPS、BPF和BPAF等BPA类似物越来越多地用于消费品的制造中,塑料食品容器、罐装食品、厨具、玩具、塑料水瓶等日常消费品随处可见,环境中BPs的蓄积也在不断增长,人类通过饮食或饮用水暴露于BPs的剂量和健康风险不容忽视,当前针对BPs的研究大多集中于生殖发育、内分泌干扰、神经毒性及免疫毒性,有关BPs对胃肠道健康的影响及其具体作用机制仍然知之甚少.
本课题组拟通过质谱组学技术,对双酚类物质的代谢转化过程与肠上皮屏障界面的完整性进行同步分析,直观解析双酚类物质原型及代谢产物与肠道组织微环境相互作用的动态过程,着眼于双酚类物质对肠上皮屏障生理功能的影响及作用机制,聚焦研究双酚类污染物体内代谢转化过程与肠道功能性损伤的内在关联,探寻民众关注的胃肠道健康问题背后的环境风险因素,明确其对肠道屏障生理功能扰动的分子机制,为双酚类污染物的肠道健康风险评价提供技术支持,为双酚类物质暴露引发肠上皮屏障功能障碍而影响肠道疾病发生发展的作用机制提供理论依据.
双酚类污染物对肠道损伤的作用及机制研究
Studies on the role and mechanism of intestinal damage by bisphenol pollutants
-
摘要: 双酚类物质作为一类常用的工业原料,在许多行业中被广泛用作添加剂,特别是在食品包装、罐头食品、玩具、塑料和牙科密封剂等一些日常消费品的生产中,但与此同时,人类经口暴露于双酚类物质的风险也在与日俱增. 由于目前针对双酚类污染物的研究大多集中在内分泌系统、神经系统、生殖系统及免疫系统,有关其对消化系统损伤效应的研究仍十分匮乏,因此,开展非雌激素受体介导的肠道功能损伤研究,是探究环境中双酚类物质暴露对肠道疾病发病潜在影响的迫切需求. 本课题组将关键解决环境剂量水平下的双酚类污染物体内代谢全过程与肠上皮屏障功能损伤的关联机制,进一步重点阐明双酚类污染物是否通过扰动腺苷代谢信号、引发肠道屏障功能障碍作为起始性的关键步骤,进而影响肠道疾病的发病风险.Abstract: Bisphenols, as common industrial raw materials, are widely used as additives in many industries, especially in the production of a number of daily consumer products such as food packaging, canned foods, toys, plastics, and dental sealants. However, the risk of oral exposure of human beings to bisphenols is increasing. Since most of the current research on bisphenol pollutants focuses on the endocrine, nervous, reproductive, and immune systems, there is still a lack of research on their damaging effects on the digestive system. Therefore, studies on non-estrogen receptor-mediated impairments of intestinal function are urgently needed to investigate the potential effects on the pathogenesis of intestinal diseases. This work will critically address the mechanisms linking in vivo metabolism of bisphenol pollutants to the impairment of intestinal epithelial barrier at environmental exposure levels. And further focus was placed on elucidating whether bisphenol pollutants affect the risk of intestinal diseases by perturbing adenosine metabolic signaling and triggering intestinal barrier dysfunction as an initiating critical step, which in turn affects the risk of intestinal diseases.
-
Key words:
- bisphenols /
- adenosine metabolism /
- intestinal barrier /
- metabolomics
-

-
-
[1] BEN-AHARON I, van LAARHOVEN H W M, FONTANA E, et al. Early-onset cancer in the gastrointestinal tract is on the rise-evidence and implications[J]. Cancer Discovery, 2023, 13(3): 538-551. doi: 10.1158/2159-8290.CD-22-1038 [2] SANMARCO L M, CHAO C C, WANG Y C, et al. Identification of environmental factors that promote intestinal inflammation[J]. Nature, 2022, 611: 801-809. doi: 10.1038/s41586-022-05308-6 [3] WU Y, LI Y P, GIOVANNUCCI E. Potential impact of time trend of lifestyle risk factors on burden of major gastrointestinal cancers in China[J]. Gastroenterology, 2021, 161(6): 1830-1841. e8. [4] PETERS A, NAWROT T S, BACCARELLI A A. Hallmarks of environmental insults[J]. Cell, 2021, 184(6): 1455-1468. doi: 10.1016/j.cell.2021.01.043 [5] ZHANG J, YAO K, YIN J, et al. Exposure to bisphenolic analogues in the sixth total diet study - China, 2016-2019[J]. China CDC Weekly, 2022, 4(9): 180-184. doi: 10.46234/ccdcw2022.044 [6] 生吉萍, 宿文凡, 张靖宇. 食品接触材料中双酚A暴露风险及风险管理[J]. 食品科学技术学报, 2022, 40(1): 167-174. doi: 10.12301/spxb202100147 SHENG J P, SU W F, ZHANG J Y. Risk analysis and management of dietary exposure to bisphenol A from food contact materials[J]. Journal of Food Science and Technology, 2022, 40(1): 167-174 (in Chinese). doi: 10.12301/spxb202100147
[7] VANDENBERG L N, HAUSER R, MARCUS M, et al. Human exposure to bisphenol A (BPA)[J]. Reproductive Toxicology, 2007, 24(2): 139-177. doi: 10.1016/j.reprotox.2007.07.010 [8] CHEN D, KANNAN K, TAN H L, et al. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity-a review[J]. Environmental Science & Technology, 2016, 50(11): 5438-5453. [9] PARK J C, LEE M C, YOON D S, et al. Effects of bisphenol A and its analogs bisphenol F and S on life parameters, antioxidant system, and response of defensome in the marine rotifer Brachionus koreanus[J]. Aquatic Toxicology, 2018, 199: 21-29. doi: 10.1016/j.aquatox.2018.03.024 [10] XIA Z N, LV C, ZHANG Y, et al. Associations of exposure to bisphenol A and its substitutes with neurodevelopmental outcomes among infants at 12 months of age: A cross-sectional study[J]. Chemosphere, 2023, 341: 139973. doi: 10.1016/j.chemosphere.2023.139973 [11] BIGAMBO F M, WANG D D, SUN J, et al. Association between urinary BPA substitutes and precocious puberty among girls: A single-exposure and mixed exposure approach from a Chinese case-control study[J]. Toxics, 2023, 11(11): 905. doi: 10.3390/toxics11110905 [12] YUAN S F, LIU Z H, LIAN H X, et al. Simultaneous determination of estrogenic odorant alkylphenols, chlorophenols, and their derivatives in water using online headspace solid phase microextraction coupled with gas chromatography-mass spectrometry[J]. Environmental Science and Pollution Research, 2016, 23(19): 19116-19125. doi: 10.1007/s11356-016-7107-1 [13] HUANG R P, LIU Z H, YUAN S F, et al. Worldwide human daily intakes of bisphenol A (BPA) estimated from global urinary concentration data (2000-2016) and its risk analysis[J]. Environmental Pollution, 2017, 230: 143-152. doi: 10.1016/j.envpol.2017.06.026 [14] NI L, ZHONG J, CHI H, et al. Recent advances in sources, migration, public health, and surveillance of bisphenol A and its structural analogs in canned foods[J]. Foods, 2023, 12(10): 1989. doi: 10.3390/foods12101989 [15] NADERI M, WONG M Y L, GHOLAMI F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults[J]. Aquatic Toxicology, 2014, 148: 195-203. doi: 10.1016/j.aquatox.2014.01.009 [16] SHI J C, YANG Y J, ZHANG J, et al. Uptake, depuration and bioconcentration of bisphenol AF (BPAF) in whole-body and tissues of zebrafish (Danio rerio)[J]. Ecotoxicology and Environmental Safety, 2016, 132: 339-344. doi: 10.1016/j.ecoenv.2016.05.025 [17] CAO X L, KOSARAC I, POPOVIC S, et al. LC-MS/MS analysis of bisphenol S and five other bisphenols in total diet food samples[J]. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 2019, 36(11): 1740-1747. [18] LIU J C, ZHANG L Y, LU G H, et al. Occurrence, toxicity and ecological risk of Bisphenol A analogues in aquatic environment - A review[J]. Ecotoxicology and Environmental Safety, 2021, 208: 111481. doi: 10.1016/j.ecoenv.2020.111481 [19] GEENS T, AERTS D, BERTHOT C, et al. A review of dietary and non-dietary exposure to bisphenol-A[J]. Food and Chemical Toxicology: an International Journal Published for the British Industrial Biological Research Association, 2012, 50(10): 3725-3740. doi: 10.1016/j.fct.2012.07.059 [20] LIAO C Y, KANNAN K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure[J]. Journal of Agricultural and Food Chemistry, 2013, 61(19): 4655-4662. doi: 10.1021/jf400445n [21] ALABI A, CABALLERO-CASERO N, RUBIO S. Quick and simple sample treatment for multiresidue analysis of bisphenols, bisphenol diglycidyl ethers and their derivatives in canned food prior to liquid chromatography and fluorescence detection[J]. Journal of Chromatography. A, 2014, 1336: 23-33. doi: 10.1016/j.chroma.2014.02.008 [22] CACHO J I, CAMPILLO N, VIÑAS P, et al. Stir bar sorptive extraction coupled to gas chromatography-mass spectrometry for the determination of bisphenols in canned beverages and filling liquids of canned vegetables[J]. Journal of Chromatography. A, 2012, 1247: 146-153. doi: 10.1016/j.chroma.2012.05.064 [23] CUNHA S C, ALMEIDA C, MENDES E, et al. Simultaneous determination of bisphenol A and bisphenol B in beverages and powdered infant formula by dispersive liquid-liquid micro-extraction and heart-cutting multidimensional gas chromatography-mass spectrometry[J]. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 2011, 28(4): 513-526. [24] LIAO C Y, KANNAN K. A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China[J]. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 2014, 31(2): 319-329. [25] YAO K, ZHANG J, YIN J, et al. Bisphenol A and its analogues in Chinese total diets: Contaminated levels and risk assessment[J]. Oxidative Medicine and Cellular Longevity, 2020, 2020: 8822321. [26] GEUEKE B, GROH K J, MAFFINI M V, et al. Systematic evidence on migrating and extractable food contact chemicals: Most chemicals detected in food contact materials are not listed for use[J]. Critical Reviews in Food Science and Nutrition, 2023, 63(28): 9425-9435. doi: 10.1080/10408398.2022.2067828 [27] LU L P, ZHAN T J, MA M, et al. Thyroid disruption by bisphenol S analogues via thyroid hormone receptor β: in vitro , in vivo , and molecular dynamics simulation study[J]. Environmental Science & Technology, 2018, 52(11): 6617-6625. [28] CAO L Y, REN X M, LI C H, et al. Bisphenol AF and bisphenol B exert higher estrogenic effects than bisphenol A via G protein-coupled estrogen receptor pathway[J]. Environmental Science & Technology, 2017, 51(19): 11423-11430. [29] WANG Z, LIU H Y, LIU S J. Low-dose bisphenol A exposure: A seemingly instigating carcinogenic effect on breast cancer[J]. Advanced Science, 2016, 4(2): 1600248. [30] WINKLER J, LIU P Y, PHONG K, et al. Bisphenol A replacement chemicals, BPF and BPS, induce protumorigenic changes in human mammary gland organoid morphology and proteome[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(11): e2115308119. [31] 任志华, 杨晓溪, 孙振东, 等. 环境内分泌干扰物对雌激素受体表达与转录激活的调控效应及分析技术[J]. 化学进展, 2022, 34(10): 2121-2133. doi: 10.7536/PC220215 REN Z H, YANG X X, SUN Z D, et al. Regulation of environmental endocrine disrupting chemicals on the expressions and transactivation of estrogen receptors and the related analytical techniques[J]. Progress in Chemistry, 2022, 34(10): 2121-2133 (in Chinese). doi: 10.7536/PC220215
[32] YANAGISAWA R, KOIKE E, WIN-SHWE T T, et al. Effects of oral exposure to low-dose bisphenol S on allergic asthma in mice[J]. International Journal of Molecular Sciences, 2022, 23(18): 10790. doi: 10.3390/ijms231810790 [33] YANAGISAWA R, KOIKE E, WIN-SHWE T T, et al. Oral exposure to low dose bisphenol A aggravates allergic airway inflammation in mice[J]. Toxicology Reports, 2019, 6: 1253-1262. doi: 10.1016/j.toxrep.2019.11.012 [34] GEBRU Y A, PANG M G. Modulatory effects of bisphenol A on the hepatic immune response[J]. Environmental Pollution, 2023, 336: 122430. doi: 10.1016/j.envpol.2023.122430 [35] MENARD S, GUZYLACK-PIRIOU L, LEVEQUE M, et al. Food intolerance at adulthood after perinatal exposure to the endocrine disruptor bisphenol A[J]. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 2014, 28(11): 4893-4900. doi: 10.1096/fj.14-255380 [36] MÉNARD S, GUZYLACK-PIRIOU L, LENCINA C, et al. Perinatal exposure to a low dose of bisphenol A impaired systemic cellular immune response and predisposes young rats to intestinal parasitic infection[J]. PLoS One, 2014, 9(11): e112752. doi: 10.1371/journal.pone.0112752 [37] MALAISÉ Y, MENARD S, CARTIER C, et al. Gut dysbiosis and impairment of immune system homeostasis in perinatally-exposed mice to Bisphenol A precede obese phenotype development[J]. Scientific Reports, 2017, 7: 14472. doi: 10.1038/s41598-017-15196-w [38] MALAISÉ Y, MÉNARD S, CARTIER C, et al. Consequences of bisphenol a perinatal exposure on immune responses and gut barrier function in mice[J]. Archives of Toxicology, 2018, 92(1): 347-358. doi: 10.1007/s00204-017-2038-2 [39] MALAISÉ Y, LENCINA C, CARTIER C, et al. Perinatal oral exposure to low doses of bisphenol A, S or F impairs immune functions at intestinal and systemic levels in female offspring mice[J]. Environmental Health: a Global Access Science Source, 2020, 19(1): 93. [40] BRANISTE V, JOUAULT A, GAULTIER E, et al. Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(1): 448-453. [41] FENG L, CHEN S J, ZHANG L J, et al. Bisphenol A increases intestinal permeability through disrupting intestinal barrier function in mice[J]. Environmental Pollution, 2019, 254(Pt A): 112960. [42] ZHU M, WEI R G, LI Y Y, et al. Bisphenol chemicals disturb intestinal homeostasis via Notch/Wnt signaling and induce mucosal barrier dysregulation and inflammation[J]. The Science of the Total Environment, 2022, 828: 154444. doi: 10.1016/j.scitotenv.2022.154444 [43] MARTEL J, CHANG S H, KO Y F, et al. Gut barrier disruption and chronic disease[J]. Trends in Endocrinology and Metabolism: TEM, 2022, 33(4): 247-265. doi: 10.1016/j.tem.2022.01.002 [44] LINARES R, FERNÁNDEZ M F, GUTIÉRREZ A, et al. Endocrine disruption in Crohn’s disease: Bisphenol A enhances systemic inflammatory response in patients with gut barrier translocation of dysbiotic microbiota products[J]. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 2021, 35(7): e21697. [45] LAI K P, CHUNG Y T, LI R, et al. Bisphenol A alters gut microbiome: Comparative metagenomics analysis[J]. Environmental Pollution, 2016, 218: 923-930. doi: 10.1016/j.envpol.2016.08.039 [46] DeLUCA J A, ALLRED K F, MENON R, et al. Bisphenol-a alters microbiota metabolites derived from aromatic amino acids and worsens disease activity during colitis[J]. Experimental Biology and Medicine, 2018, 243(10): 864-875. doi: 10.1177/1535370218782139 [47] ESTRELA A B, ABRAHAM W R. Adenosine in the inflamed gut: A Janus faced compound[J]. Current Medicinal Chemistry, 2011, 18(18): 2791-2815. doi: 10.2174/092986711796011274 [48] ZHAO H D, LIU M, LV Y B, et al. Dose-response metabolomics and pathway sensitivity to map molecular cartography of bisphenol A exposure[J]. Environment International, 2022, 158: 106893. doi: 10.1016/j.envint.2021.106893 [49] MONTES-GRAJALES D, OLIVERO-VERBEL J. Computer-aided identification of novel protein targets of bisphenol A[J]. Toxicology Letters, 2013, 222(3): 312-320. doi: 10.1016/j.toxlet.2013.08.010 [50] MU X Y, QI S Z, WANG H, et al. Bisphenol analogues induced metabolic effects through eliciting intestinal cell heterogeneous response[J]. Environment International, 2022, 165: 107287. doi: 10.1016/j.envint.2022.107287 [51] CHASSAING B, AITKEN J D, MALLESHAPPA M, et al. Dextran sulfate sodium (DSS)-induced colitis in mice[J]. Current Protocols in Immunology, 2014, 104: 15.25. 1-15.2515. 25.14. [52] JIN H B, ZHU J, CHEN Z J, et al. Occurrence and partitioning of bisphenol analogues in adults' blood from China[J]. Environmental Science & Technology, 2018, 52(2): 812-820. [53] WELIHINDA AA, KAUR M, GREENE K, et al. The adenosine metabolite inosine is a functional agonist of the adenosine A2A receptor with a unique signaling bias[J]. Cell Signal, 2016, 28(6): 552-560. doi: 10.1016/j.cellsig.2016.02.010 -



 下载:
下载: