-
四溴双酚A(tetrabromobisphenol A,TBBPA)是世界上生产和使用量最大的一种溴代阻燃剂,占溴代阻燃剂全球使用量的60%,被广泛用于塑料、纺织品和电子电气设备的生产[1]。研究发现TBBPA在表层土壤[2]、天然水体[3]、浮游动物和鱼类[4]、母乳[5]中广泛检出,在受到非单一点源污染的淡水水体(瑞典河流)中平均浓度为29 ng·L−1[6],而在污染严重的水体(中国巢湖)中浓度达到4870 ng·L−1[7]。已有研究发现TBBPA具有甲状腺激素[8]、性激素[9]等内分泌干扰作用和神经发育毒性[10],对水生生物的毒性效应尤为显著。正是由于TBBPA使用范围广、毒性作用强,致使人群暴露风险比较高,因此研究其环境行为和归趋,特别是在生物圈中的迁移、转化和代谢行为,有助于正确认识其在食物链中的行为以及对食物链造成的影响,从而正确评估与其相关的食品安全和环境与健康风险[11]。
植物是生物圈的重要组成部分,也是污染物从天然环境进入食物链,引起人类暴露风险的途径之一。单细胞藻类是研究污染物对水生生态系统影响的常见植物模型,其中普通小球藻(Chlorella vulgaris,C. vulgaris)作为一种典型的淡水微藻,已被用于2,4-二氯苯氧基乙酸[12]、氧化镍纳米颗粒[13]等污染物的风险评估。淡水藻对TBBPA(初始暴露浓度为0.8 μmol·L−1)生物转化的研究[14]表明月牙藻(Pseudokirchneriella subcapitata)、尖细栅藻(Scenedesmus acuminatus)、蛋白核小球藻(Chlorella pyrenoidosa)等6种淡水藻,在暴露7 d后使TBBPA发生明显生物转化,但TBBPA引起的藻类细胞生理变化的研究,特别是剂量-效应关系研究还比较欠缺。
鉴于微藻是含有光合色素的单细胞生物,因此是应用流式细胞检测技术开展研究的理想对象。本文将普通小球藻暴露于不同剂量的TBBPA后,测定了细胞增殖、色素合成等种群层面的指标变化,并利用流式细胞仪测定了酯酶活性[15]、线粒体膜电位[16]等细胞层面生理指标的时间变化,此外还通过定量测定母体化合物TBBPA,及其单糖基化产物TBBPA MG在培养液和藻细胞中的浓度变化考察了TBBPA的吸收和转化行为。通过探讨小球藻生理变化与其对TBBPA的吸收和转化行为之间的关联,研究了普通小球藻与TBBPA的交互作用,探究了小球藻经历TBBPA暴露后的解毒机制。
全文HTML
-
TBBPA(固体,0.25 g,99%)和进样内标D10-TBBPA(100 μg mL−1,乙腈,98.5%)购于Dr. Ehrenstorfer GmbH(德国),回收率内标13C12-TBBPA购于Cambridge Isotope Laboratories Inc.(美国)。单糖基化TBBPA(TBBPA mono-β-D-glucopyranoside,TBBPA MG,99%)标准品的合成路径、结构鉴定与纯化检测已在本课题组的其他工作中发表[17]。暴露实验中使用的TBBPA储备溶液浓度为2 mmol·L−1(甲醇)。Cleanert PEP 柱(500 mg/6 mL)购于 Bonna-Agela Technologies(中国)。乙酸和氨水溶液购于Sinopharm Chemical Reagent Co.(中国)。甲醇(HPLC级)购于J.T.Baker(美国)。实验中使用的超纯水(18.2 MΩ cm−1)由Milli-Q Advantage A10系统(美国)制备。
染色剂荧光素二乙酸酯(Fluorescein diacetate,FDA,固体,5 g,97%)和罗丹明123(Rhodamine 123,Rh123,固体,25 mg,98%)购自J&K Scientific Ltd(中国)。BG11培养基(固体,250 g)购自Hope Bio-Technology CO. Ltd(中国)。1×磷酸盐缓冲液(Phosphate Buffered Saline,PBS)购自Solarbio Science & Technology Co. Ltd(中国)。
-
本研究所使用的C. vulgaris(FACHB-8)从中国科学院淡水藻种库(中国科学院水生生物所)获得。将生长状况良好的普通小球藻与经高压蒸汽灭菌的新鲜BG11培养基溶液在250 mL锥形瓶中混合,置于光照培养箱(PGX-1000C,中国,光强22000 Lux,湿度80%)培养,光照强度为100%,光照和黑暗时长比为12 h∶12 h,恒温(25±1) ℃。当小球藻处于对数生长期时,开始暴露实验,暴露初始细胞浓度约为每毫升12×105个细胞。
有研究发现TBBPA对C. vulgaris暴露96 h后生长抑制的半效应浓度(concentration for 50% of maximal effect,EC50)为8.66 μmol·L−1[18],而在中国多个湖泊中检测发现TBBPA浓度≤ 1.91 ng·L−1(即3.51 μmol·L−1)[19],由于本文旨在研究藻细胞生长未受到抑制的情况下与TBBPA的交互作用,因此将初始暴露浓度设置为接近环境浓度的0.5、1.0、2.0 μmol·L−1 的3个实验组(实验组1—3)。此外还设置两组对照组,分别为空白对照组(正常藻液,无TBBPA添加,但加入等体积甲醇溶剂)和培养基对照组(培养基经高压蒸汽灭菌,不含小球藻但含1.0 μmol L−1 TBBPA)。空白对照组用以测定小球藻正常的生理状态并监控实验室TBBPA背景值对暴露体系的影响,培养基对照组用以监测TBBPA在无藻情况下可能发生的挥发、转化等行为。每个组别设置3个锥形瓶(250 mL)作为平行,每个锥形瓶中的暴露体系初始体积为200 mL,在暴露开始后的第0、1、3、4、5、7、10、12 d从暴露体系中移取10 mL藻液,完成生理指标和TBBPA以及TBBPA MG含量分析的测定。取样时充分摇匀藻液,以保证取样均质和体系稳定。暴露周期内每天按时摇晃锥形瓶3次以避免小球藻聚集贴壁,并随机改变锥形瓶在培养箱中的相对位置,以避免光照不均对实验结果的干扰。
-
检测的生理指标分别为小球藻的细胞增殖情况、色素含量、酯酶活性和线粒体膜电位,具体的操作如下。
-
根据文献报道,小球藻的细胞密度与藻液在680 nm下的吸光度值正相关[20]。因此,取200 μL藻液,用酶标仪(Thermo Scientific,VARIOSKAN FLASH)检测其在680 nm下的吸光度,与细胞计数仪检测所得细胞密度值作线性回归,所得吸光度与细胞密度的标准曲线如公式(1)所示(R2=0.9988),然后基于细胞密度计算藻细胞的比生长速率如公式(2)所示[21]。
其中,Nalgae为藻液单位体积内的细胞数量,即藻细胞密度,OD680为藻液在680 nm下的吸光度。μ为比生长速率,Nt和N0分别为时间t和t0的藻细胞密度。
-
取2 mL藻液进行离心分离(4000 r·min−1,10 min),弃去上清液,向沉淀的细胞中加入2 mL甲醇和水混合溶液(体积比9:1),60 ℃恒温加热20 min,再次离心后取200 μL上清液作为色素提取液,采用酶标仪检测其在665、652、470、750 nm的吸光度[21]。叶绿素a(chlorophyll a,Chl-a)、叶绿素b(chlorophyll b,Chl-b)、类胡萝卜素(carotenoids,Caro)和总色素含量(Total pigments,Total)按文献方法[22]计算,如公式(3)—(6)所示。由于暴露周期内细胞大量增殖使藻细胞密度增长较大,因此进一步计算了单位藻细胞数量的叶绿素生成量,即叶绿素单位生成量,用△表示,如公式(7)所示。
其中,CChl-a、CChl-b、CCaro、CTotal分别为单位体积藻液中叶绿素a、叶绿素b、类胡萝卜素和总色素的含量,OD665、OD652、OD470为色素提取液在665、652、470 nm的吸光度,并通过扣除750 nm的吸光度进行校正,以避免提取液中悬浮物质的干扰。
-
基于流式细胞仪单细胞荧光检测技术测定小球藻细胞的酯酶活性和线粒体膜电位。其中酯酶活性的测定需应用FDA染色剂,FDA本身不发荧光,通过被动运输进入细胞后与非特异性酯酶作用就会发出绿色荧光[23]。通过检测FDA染色后藻细胞的荧光强度可以获得细胞的酯酶活性,用来反映细胞活力[24-25]。具体操作如下:向1 mL待测藻液中加入25 μL FDA储备液(溶于丙酮,1 mmol·L−1),黑暗孵育8 min,利用流式细胞仪测定FL1通道(激发波长范围为515—545 nm)的平均荧光强度(mean fluorescence intensity,MFI)。为了保证充分染色,在暴露周期内不同取样时间获得的藻液均须预先稀释至每毫升106个细胞(稀释后体积为1 mL),以避免藻细胞密度差异导致的染色误差,进而保证藻细胞的荧光均匀性和可比性,线粒体膜电位的测定与此处理相同。
线粒体膜电位(mitochondrial membrane potential,MMP)的测定应用Rh123染色剂,它是一种阳离子荧光染料,能透过细胞膜与线粒体特异结合,根据染色后细胞的荧光强弱可以表征其线粒体的膜电位进而评估线粒体的生理状态,一般荧光增强说明线粒体发生超极化,反之则发生去极化[26]。具体操作为:向1 mL待测藻液中加入26 μL Rh123储备液(溶于超纯水,1 mmol·L−1),黑暗孵育30 min,检测FL 1荧光强度。
-
取5 mL藻液,以4000 r·min−1转速离心10 min,取适量上清液与甲醇等体积混合,加入进样内标10 ng D10-TBBPA后进行仪器分析,测定培养液中TBBPA及其代谢产物的浓度。对于藻细胞中的TBBPA和代谢产物的含量测定如下,离心分离后的藻细胞沉淀加入PBS重悬后再次离心以排除培养液残余量对细胞样品测定的干扰。向清洗后的细胞沉淀中加入1 mL甲醇、回收率内标10 ng 13C-TBBPA和2颗小钢珠(直径3 mm),剧烈振荡3—5 min使藻细胞完全破碎,离心后取上清液,萃取3次后合并上清液。纯化方法见本课题组之前的工作[17],简单来讲,细胞萃取液在氮气流动条件下至干燥状态,重新溶于5 mL含有5%甲醇的水,采用PEP固相萃取小柱进行纯化。PEP柱先后用5 mL甲醇和超纯水进行活化,加载样品后先后加入5 mL含有5%甲醇和2%乙酸的水和含有5%甲醇和2%氨的水进行除杂冲洗,最后用5 mL含有2%氨和8%水的甲醇洗脱。将洗脱液经氮吹溶剂置换为200 μL甲醇,加入进样内标10 ng D10-TBBPA进行仪器分析。
TBBPA及其代谢产物均采用液相色谱(Agilent 1290 Series Liquid chromatography system)与四重四极杆质谱(Agilent 6460 Triple Quadrupole MS/MS system)联用(LC-MS/MS)进行测定,质谱采用电子喷雾电离源(electrospray ionization,ESI)在负离子扫描模式下检测。色谱柱为ZORBAX SB-Phenyl柱(4.6 mm×250 mm×5 μm,Agilent Technologies)。流动相由超纯水和甲醇组成,采用梯度洗脱,条件设置如表1和表2所示。进样体积为10 μL,柱温为35 ℃。使用多反应监测(multiple reaction monitoring,MRM)模式,参数设置如表3所示。
含量分析过程中的质量保证和质量控制如下:玻璃器皿使用前均在马弗炉中以400 ℃加热4 h。仪器检测过程中每3个样品检测溶剂空白,未发现记忆效应。TBBPA在细胞样品中的平均回收率为83.1%,细胞样品的定量数据经回收率内标进行校正;培养液样品由于取样后直接与甲醇混合上样,不须回收率校正。
1.1. 药品和试剂
1.2. 培养和暴露实验
1.3. 小球藻的生理指标检测
1.3.1. 小球藻的生长速率
1.3.2. 小球藻细胞色素
1.3.3. 酯酶活性和线粒体膜电位
1.4. TBBPA及其代谢产物的分析
-
暴露过程中测定不同剂量暴露组和空白对照组的细胞密度以监测小球藻的生长情况,如图1(a)所示,暴露周期内(0—12 d)空白对照组小球藻细胞密度从每毫升(12 ± 0.17)×105个细胞增长至每毫升(92.67 ± 3.89)×105个细胞。为了排除由这种细胞正常增殖过程对细胞密度变化的干扰,从而更加直观地反映TBBPA对小球藻生长的作用,将3个浓度实验组分别与对应时间点的空白对照组作归一化处理,计算细胞密度的相对值。结果显示,在第1—10天,3个实验组(0.5、1.0、2.0 μmol·L−1 TBBPA)细胞密度均高于对照组,其中第1—5天,3个实验组的相对细胞密度均呈增加趋势,而到第7天后,实验组相对细胞密度下降,并于暴露12 d时趋近于对照组。而比较不同暴露浓度对小球藻细胞密度的影响可以发现,促进作用与暴露浓度呈正相关,即2.0 μmol·L−1 TBBPA促进作用最强,1.0 μmol·L−1次之,0.5 μmol·L−1最弱。
细胞密度是细胞增殖活动强弱变化的最终表现,而细胞增殖活动的强弱还可以通过计算细胞增殖速率来表征。选取第1天、第5天、第10天和第12天,分别计算0—1 、1—5 、5—10 、10—12 d的 4个区间内比生长速率。如图1(b)所示,不同剂量的暴露组在0—1 d和1—5 d的比生长速率μ均高于对照组,然而在5—10 d和10—12 d的比生长速率μ均低于对照组,表明在所选的浓度下,TBBPA在前5 天可以促进小球藻的细胞增殖,而5 d后开始使小球藻细胞增殖活动减弱,并最终导致细胞密度与对照组趋同。由此可见,TBBPA对小球藻的生长促进作用先增强后减弱,这种“先促进后恢复”的趋势,与氯氰菊酯对海洋卡盾藻的生长效应类似[27]。有研究者认为污染物对藻类生长的促进作用,与其脂质过氧化程度有关,一定程度的脂质过氧化可以刺激细胞生长,促进增殖[28]。低浓度丙溴磷(0.4—1.0 mg·L−1)可使藻细胞的脂质过氧化程度升高,从而对藻的生长具有刺激和促进作用,而加入抗氧化剂后这种生长促进作用有所降低,一定程度上证实了这一观点[29]。
将藻细胞叶绿素、类胡萝卜素和总色素在单位藻液中的含量与对照组作归一化处理,其时间变化趋势如图2(a)、(b)和(c)所示。结果显示,TBBPA暴露后,单位体积藻液的叶绿素、类胡萝卜素,以及总色素含量均高于对照组。
类似的,新型污染物如2,4-二氯苯氧基乙酸[12]或卡马西平[30]暴露后导致杜氏盐藻(Dunaliella tertiolecta)和C. vulgaris的叶绿素含量增加,而有的研究表明,在外界胁迫条件下微藻通过增加叶绿素含量,可以清除叶绿体内积累的活性氧(reactive oxygen species,ROS)以实现自我保护[30]。因此,小球藻细胞色素的变化也许是其经历污染物暴露后胞内发生氧化应激反应引发的响应之一。
考虑到在暴露实验周期内小球藻细胞密度发生近10倍增长,以及实验组与对照组在细胞密度上的差异,进一步计算了叶绿素单位生成量,与对照组作归一化,如图2(d)所示。与对照组相比,0.5、1.0、2.0 μmol·L−1 TBBPA 的3个实验组的叶绿素单位生成量多数时候高于对照组,效应与浓度呈正相关,即2.0 μmol·L−1 TBBPA影响最强,1.0 μmol·L−1次之,0.5 μmol·L−1最弱。此外,3个实验组对叶绿素合成的促进作用呈现先增强后减弱再增强的时间变化趋势,第5天最低,这种波动的变化趋势可能还与其他细胞活动有关。
-
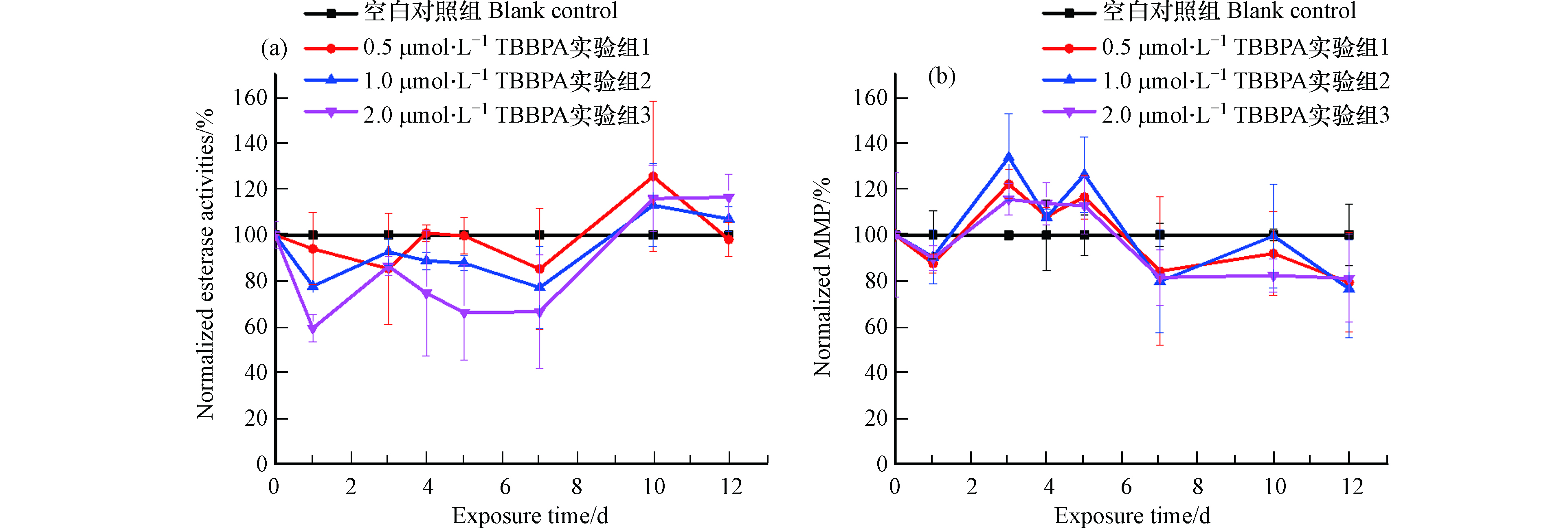
暴露组的藻细胞酯酶活性与对照组作归一化处理后,随时间的变化趋势如图3(a)所示。0.5、1.0、2.0 μmol·L−1实验组酯酶活性在暴露周期内呈现波动中缓慢上升的趋势,其中0—10 d实验组酯酶活性低于对照组,表明在这期间TBBPA抑制了藻细胞酯酶活性,并且这种抑制作用与暴露浓度呈正相关性,即2.0 μmol·L−1 TBBPA抑制作用最强,1.0 μmol·L−1次之,0.5 μmol·L−1最弱。第10天后酯酶活性高于对照组,酯酶活性升高说明TBBPA的抑制作用减弱。利用FDA测定的酯酶包括脂肪酶和酰基转移酶等,并不具有特异性[24],一般用来表征细胞活力和代谢活性,由此可见TBBPA暴露使小球藻细胞活性先受到抑制后回升。这与细胞增殖活动先促进后恢复的趋势有所不同,可能与所测定的非特异性酯酶并不是细胞增殖活动的专一酶有关,也可能与细胞增殖还受到其他非酶因素(脂质过氧化,如2.1节所述)调控有关。
Rh 123染色后测定MFI,与对照组作归一化观测线粒体膜电位的时间变化趋势,结果如图3(b)所示。线粒体膜电位呈现波动中缓慢下降的变化趋势,其中第3—5 天,实验组荧光强度高于对照组,说明线粒体膜电位发生超极化,这一结果与全氟辛烷磺酸(perfluorooctane sulfonate,PFOS)和全氟辛酸(perfluorooctanoic acid,PFOA)暴露3 d后S obliquus线粒体发生超极化的结果一致[26];第7 天后实验组荧光强度低于对照组,说明线粒体膜电位发生去极化。线粒体不仅是细胞内产生ATP为细胞生命活动供能的细胞器,而且是细胞新陈代谢和凋亡的控制中心[31]。MMP升高、线粒体发生超极化时,尽管线粒体内膜能量容量更高、能够合成更多ATP,但是通过线粒体呼吸链会产生更多ROS,而ROS的持续增加将对细胞有所损害[32],而有研究显示MMP的适当降低可以降低细胞内ROS水平[33]。暴露3—5 d,TBBPA导致线粒体异常,ROS在胞内积累,暴露第7 天后MMP的降低将有助于降低细胞内积累的ROS水平,进而表明线粒体生理状态有所恢复。由此可见,线粒体的生理状态呈现先异常后恢复的整体趋势。
-
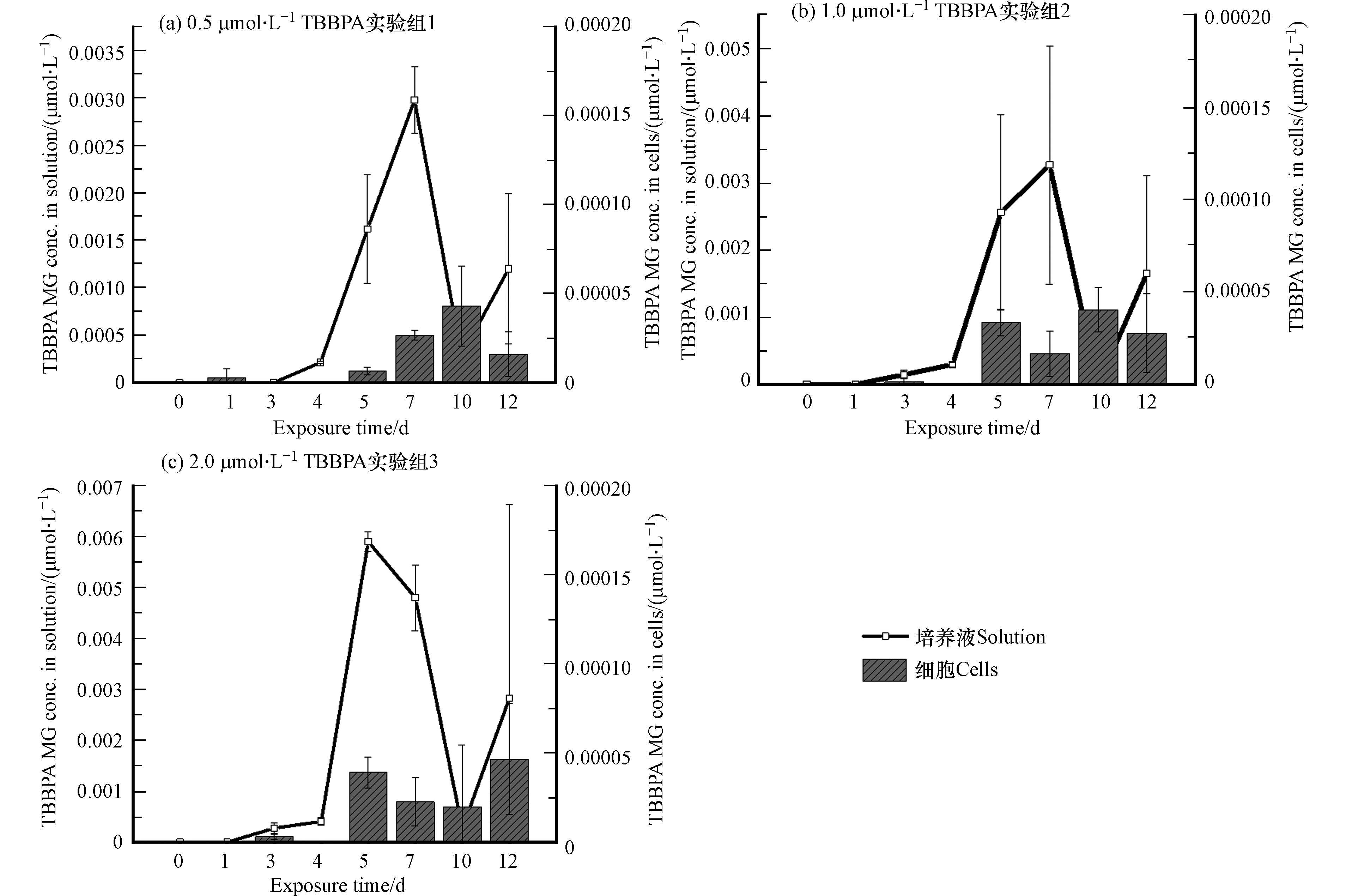
将培养液与藻细胞离心分离后,分别测定培养液和藻细胞中TBBPA的含量,如图4所示。第0—3天,3个实验组中TBBPA的含量主要分布在溶液中,与初始浓度相比基本没有变化,说明在暴露早期,藻细胞未能对体系中TBBPA发生显著的迁移或者转化作用,使其在溶液中能够保持稳定;但在第4—12天时,培养液中TBBPA含量开始降低,第7天能够在藻细胞中明显检测到TBBPA,说明TBBPA迁移进入藻细胞。而在整个暴露体系中,TBBPA的总量(溶液和藻细胞中的TBBPA含量之和)也在4 d后降低,说明其在培养液或/和藻细胞中发生了转化。通过对照组的研究,进一步证实了这一结果,空白对照组中未检测到TBBPA,而培养基对照组中TBBPA含量未发生明显变化,表明TBBPA在暴露体系中不存在明显的挥发损失,也没有交叉干扰,即暴露实验组中TBBPA的减少,确实是由于藻的转化作用造成的。暴露后的第12天,3个实验组TBBPA转化率分别达到67.95%±6.58%、68.17%±8.05%和68.05%±2.90%。这也与其他研究结论一致,暴露10 d后淡水藻处理的TBBPA含量降低60%—90%[14]。而在不同的暴露剂量下,TBBPA的转化率相当,表明在研究设置的浓度范围内,即小球藻通过自身代谢进行解毒的调控范围内,TBBPA的转化率与其初始浓度无关。
为进一步验证藻细胞对TBBPA的转化作用,本研究针对TBBPA的一种转化产物进行了定量分析。本课题组前期的研究已经发现,糖基化过程是TBBPA在南瓜植株内重要的代谢途径,TBBPA暴露南瓜幼苗后,约86%的母体化合物转化为糖基化产物[17],而在月牙藻和蛋白核小球藻对TBBPA的生物转化研究中,也发现了糖基化产物[14]。而对污染物的糖基化过程则被认为是植物应对毒物暴露的有效解毒机制。因此,采用实验室合成标准品对TBBPA的单糖基化产物TBBPA MG进行了定量分析,将其作为典型产物评估TBBPA的转化行为。如图5所示,体系中TBBPA MG的含量,主要分布在培养液中,藻细胞中基本未检出,而3个实验组中TBBPA MG的生成量随时间的变化呈现非常类似的先升高后降低的趋势。暴露初期,在培养液和藻细胞样品中均未检测到TBBPA MG,这与母体化合物检测结果(TBBPA初期未发生明显转化)一致;第4—7天,TBBPA MG浓度逐渐增加,结合此时培养液和藻细胞中的TBBPA总量逐渐降低的趋势,表明TBBPA逐渐发生转化;但在第10天,TBBPA MG浓度降低,这表明TBBPA MG可能会进一步发生转化反应生成其他物质,从而使其含量在体系中消减,例如,有研究发现污染物糖基化产物的糖基基团可以进一步发生丙二酰化反应[34],而糖基上活泼的羟基基团还可进一步被磺酸基等基团取代[35]。第12天,TBBPA MG浓度再次升高,这可能是由于培养体系内发生的其他反应产生了TBBPA MG,使TBBPA MG的生成速度高于它的去除速度,使体系中的TBBPA MG含量上升。有研究表明TBBPA单边取代衍生物可能来源于双边取代衍生物的微生物转化,比如TBBPA烯丙基醚(TBBPA allyl ether,TBBPA AE)可能来源于TBBPA二烯丙基醚(TBBPA bis(allyl) ether,TBBPA BAE),TBBPA 2,3-二溴丙基醚(TBBPA 2,3-dibromopropyl ether,TBBPA DBPE)可能来源于TBBPA二(2,3-二溴丙基)醚(TBBPA bis(2,3-dibromopropyl) ether,TBBPA BDBPE)[36],因此推测暴露第12天,TBBPA MG浓度升高可能源于双糖基化TBBPA(TBBPA di-β-D-glucopyranosid,TBBPA DG)的转化。
在培养基对照组中并未发现TBBPA MG,说明在无藻类的纯培养基体系中不能发生类似的转化反应,进而确认3个实验组培养液中的TBBPA MG是在小球藻参与的条件下转化产生的。
-
综合上述实验结果,不同暴露时间和不同暴露浓度条件下TBBPA与普通小球藻的交互作用表明,0—3 d,TBBPA未发生吸收或转化,TBBPA对小球藻的作用是“外部干扰阶段”,此时小球藻的酯酶活性受到抑制,线粒体处于超极化状态,同时细胞加快增殖和增加叶绿素单位生成量以抵御干扰;4—12 d,培养液和藻细胞中的TBBPA总量降低,是在小球藻体内和体外酶的作用下发生转化,可称之为“细胞解毒阶段”,此时小球藻的酯酶活性从抑制状态逐渐回升至与对照组相近,线粒体也逐步发生去极化,表明细胞通过转化TBBPA生成其糖基化产物的方式应对TBBPA的干扰,以这样的解毒方式使周围和内部环境中的TBBPA浓度降低,从而使藻细胞从被干扰状态逐渐恢复,与此同时细胞增殖活动逐渐放缓,细胞密度最终与对照组相近,叶绿素的单位生成量先降低后升高,其中第5天和第7天叶绿素单位生成量最低,对应此时TBBPA MG生成量也较高,推测叶绿素合成与细胞发生糖基化反应的解毒过程有关。
2.1. TBBPA对小球藻增殖和生长的影响
2.2. TBBPA对小球藻酯酶活性和线粒体膜电位的影响
2.3. 小球藻细胞对TBBPA的吸收和转化
2.4. 小球藻细胞与TBBPA的相互作用
-
本研究从TBBPA与小球藻的交互作用入手,研究了小球藻对TBBPA的吸收和转化行为,以及TBBPA对小球藻细胞和种群生理指标的影响,评估了小球藻受到干扰后的应激反应,以及进而发生解毒作用后细胞指标的变化,发现细胞解毒前小球藻细胞增殖和色素合成受到异常促进,酯酶活性受到抑制、线粒体膜电位发生超极化,而细胞解毒后以上指标均恢复至与空白对照组相近。本文的时间、剂量效应研究为进一步认识TBBPA的水生毒理效应及其机制提供了基础数据。




 下载:
下载: