-
Cr(Ⅵ)作为水生态环境中重金属污染之一,在水中的阴离子主要有Cr2O72−、CrO42−和HCrO4−,其稳定性强,废水排放标准为Cr(Ⅵ)浓度低于0.5 mg·L−1。不同的氧化态中Cr(Ⅵ)毒性最高的,是一种被证实的诱变剂和致癌物[1]。相反,Cr(Ⅲ)毒性就小很多,在环境中迁移性较差,容易在水中沉淀。Cr(Ⅵ)的化合物为分析工作的主要标准物,在镀铬工业和制革工业中用作鞣剂,因此工业废水中含有大量高毒性的Cr(Ⅵ),严重污染水和土壤,影响人类的健康。Ghorab等[2]利用半导体TiO2来对Cr(Ⅵ)进行光催化还原,将Cr(Ⅵ)还原为Cr(Ⅲ)。人们还对多种催化剂和还原剂进行了研究,如Cu基超疏水性的针铁矿[3],Pd纳米粒子[4],微生物燃料电池的电解还原[5],Mn(Ⅱ)[6],Pd纳米粒子负载胺类功能化SiO2[7],MOFs诱导超细Ni纳米粒子等[8]。
液相催化加氢是去除水中污染物最常用的方法,贵金属催化剂的失活也是催化中常见的现象,因此大量学者对此进行了研究。Lin等[9]通过研究确定了Pd/Al2O3催化剂的失活是因为催化剂使用后,Pd含量降低了9.8%。Choong等[10]通过在Rh的表面包裹Fe的氧化物来增加Rh与载体之间的作用力,有效避免了Rh的流失,从而有效避免了催化剂的失活。
本研究通过在im-Pd/Al2O3催化剂的表面有效包裹上C,增加金属Pd与载体Al2O3之间的相互作用力,来避免金属Pd的流失,从而能够使催化剂具有高活性和高稳定性。
全文HTML
-
采用工业级Al2O3,于马弗炉中600 ℃焙烧5 h制得γ-Al2O3载体。取Al2O3与PdCl2溶液于烧杯中,磁力搅拌剧烈4 h后将其在80 ℃水浴蒸干。然后放入烘箱中105 ℃的烘干12 h后取出,过筛(200目)后放入坩埚,再入马弗炉中在300 ℃下焙烧4 h。然后将该粉末在30 mL·min−1的H2氛围中还原2 h,即得到im-Pd/Al2O3催化剂。
将适量的im-Pd/Al2O3催化剂与葡萄糖按照适当比例悬浮于加水的烧杯中,在100 Hz的条件下超声1 h。将该悬浮液放入高压反应釜并加固,于200 ℃下水热处理24 h。待温度降至室温后取出悬浮液,用大量去离子水冲洗干净后,于真空干燥箱中在60 ℃下烘干10 h。然后过筛后并将该粉末放在瓷舟中,在50 mL·min−1的N2氛围中,分别在400、600、800 ℃的温度下炭化4 h,制备催化剂im-Pd/Al2O3@C-400、im-Pd/Al2O3@C-600、im-Pd/Al2O3@C-800。
取1 g的im-Pd/Al2O3催化剂悬浮于200 mL的去离子水中,在80 ℃水浴锅中,同时通50 mL·min−1的N2流到悬浮液底部。将1 mol·L-1 Na2SiO3溶液逐滴加入到悬浮液中,共加入20 mL,在2 h内滴加完毕。然后用2 mol·L−1 HCl调节悬浮液到pH 6.0。保持在80 ℃搅拌3 h。取出该悬浮液过滤,用大量去离子水冲洗滤饼,于真空干燥箱中60 ℃烘干10 h,制备im-Pd/Al2O3@SiO2催化剂。
-
催化剂的活性通过其对溶液中的催化还原能力评价。在250 mL的四口烧瓶中,加入20 mg的催化剂和1 mmol·L−1的Cr(Ⅵ)溶液200 mL,Cr(Ⅵ)来源于K2Cr2O7。该溶液事先用1 mol·L−1 H2SO4将pH值调到2.0。将100 mL·min−1的N2通入该悬浮液底部,并保持剧烈搅拌30 min,切换H2开始反应。反应过程中以一定的时间间隔定时取样分析。取出的样品经过滤出催化剂后用紫外分光光度计对样品中Cr(Ⅵ)的浓度进行分析,以1,5-二苯基卡巴肼(俗称二苯氨基脲)为显色剂,测定Cr(Ⅵ)的浓度。
在转化率低于25%的情况下,反应物的浓度与时间关系做图,反应符合准一级动力学模型。
1.1. 催化剂的制备
1.2. 催化剂的活性评价
-
不同催化剂的Pd含量和BET比表面见表1,Pd含量由ICP表征得到。ICP结果表明,包裹碳层后催化剂中Pd含量有所降低,碳层有效的包裹在了im-Pd/Al2O3催化剂上。随着碳化温度的增加,催化剂中的Pd含量提高,这是因为随着碳化温度增加,催化剂中的碳层逐渐变薄。通过比较5次循环后im-Pd/Al2O3和im-Pd/Al2O3-600中Pd含量的变化可以发现,im-Pd/Al2O3中Pd出现显著流失,而im-Pd/Al2O3-600中的Pd几乎没有发生流失。BET的结果显示,包裹碳层后随着碳化温度的增加催化剂的比表面积先降低后增高。
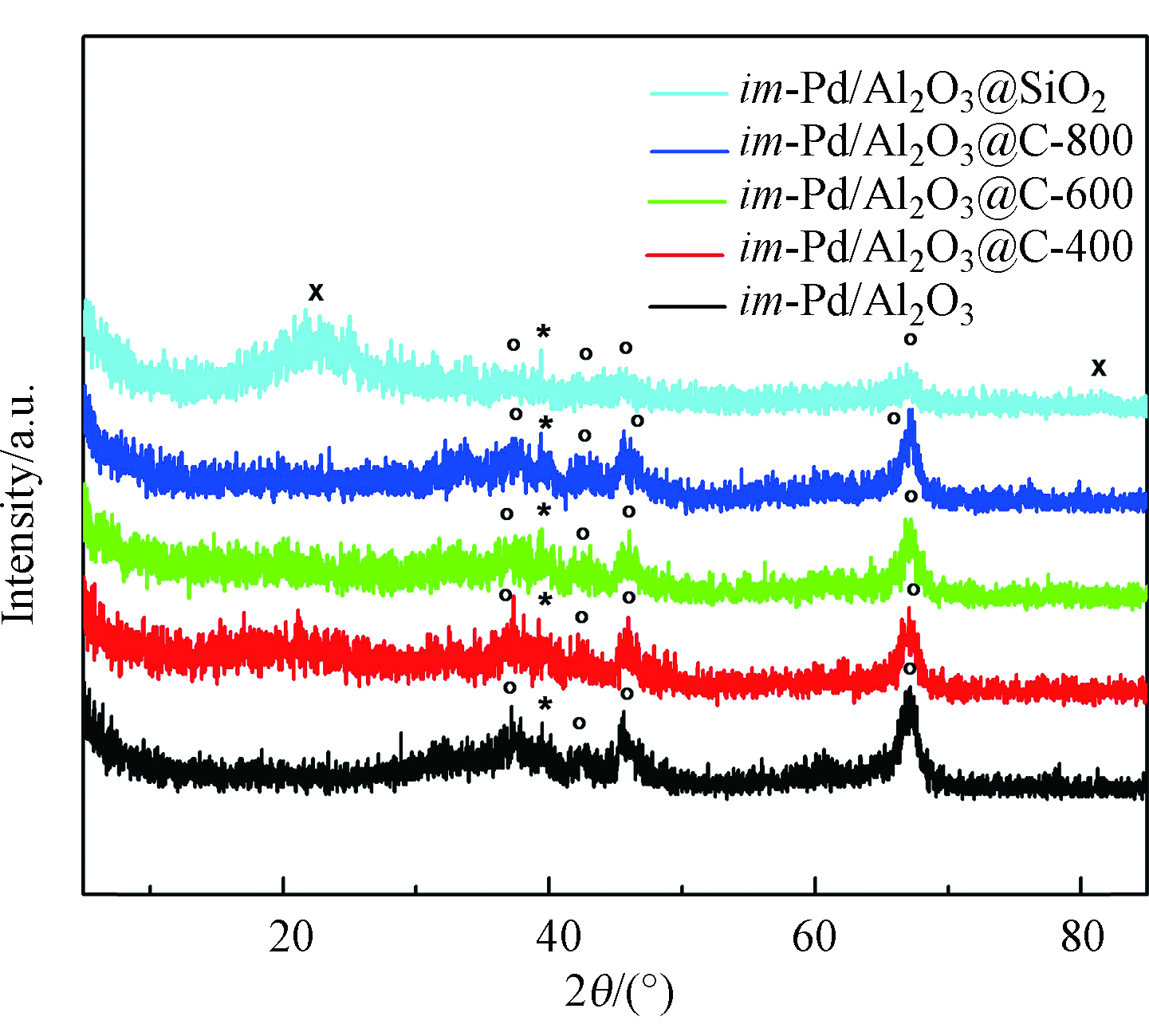
不同催化剂的XRD图谱见图1,im-Pd/Al2O3、im-Pd/Al2O3@C-400、im-Pd/Al2O3@C-600和im-Pd/Al2O3@C-800催化剂,在衍射角为39.4°有Pd的特征衍射峰,在37.3°、42.5°、45.7°、67.1°有Al2O3的特征衍射峰,im-Pd/Al2O3@SiO2催化剂在22.3°和81.5°有SiO2的特征衍射峰。但是并没有观察到C的特征衍射峰。这可能是因为C的粒子太小,在XRD的检测极限之下,也有可能这里的C是以无定形的形态存在的。
催化剂的SEM表征中,没有包裹C的im-Pd/Al2O3催化剂的表面有直径约50 μm的不规则形状Pd粒子,而包裹了C后的im-Pd/Al2O3@C-400、im-Pd/Al2O3@C-600和im-Pd/Al2O3@C-800催化剂,在300倍的扫描电镜中,可以观察到呈葡萄状的聚集颗粒,聚集团簇大小以50—150 μm不等;在5000倍的扫描电镜中,可以观察到im-Pd/Al2O3@C-400、im-Pd/Al2O3@C-600和im-Pd/Al2O3@C-800催化剂的内表面有直径约5 μm的球状颗粒,归因于C粒子覆盖在了Pd粒子的表面。同时在im-Pd/Al2O3@SiO2催化剂的表面没有观察到相应的球状粒子,说明球状粒子的生成与C的负载有关。
不同催化剂的拉曼光谱中,单金属的im-Pd/Al2O3催化剂只在637 cm−1看到一个峰带,而im-Pd/Al2O3@C-400、im-Pd/Al2O3@C-600和im-Pd/Al2O3@C-800催化剂分别在1348 cm−1和1590 cm−1看到两个明显的峰带,即分别为im-Pd/Al2O3@C催化剂的D带和G带,分别为C的缺陷位贡献[11]。说明C成功地将催化剂中的Pd颗粒包覆。
-
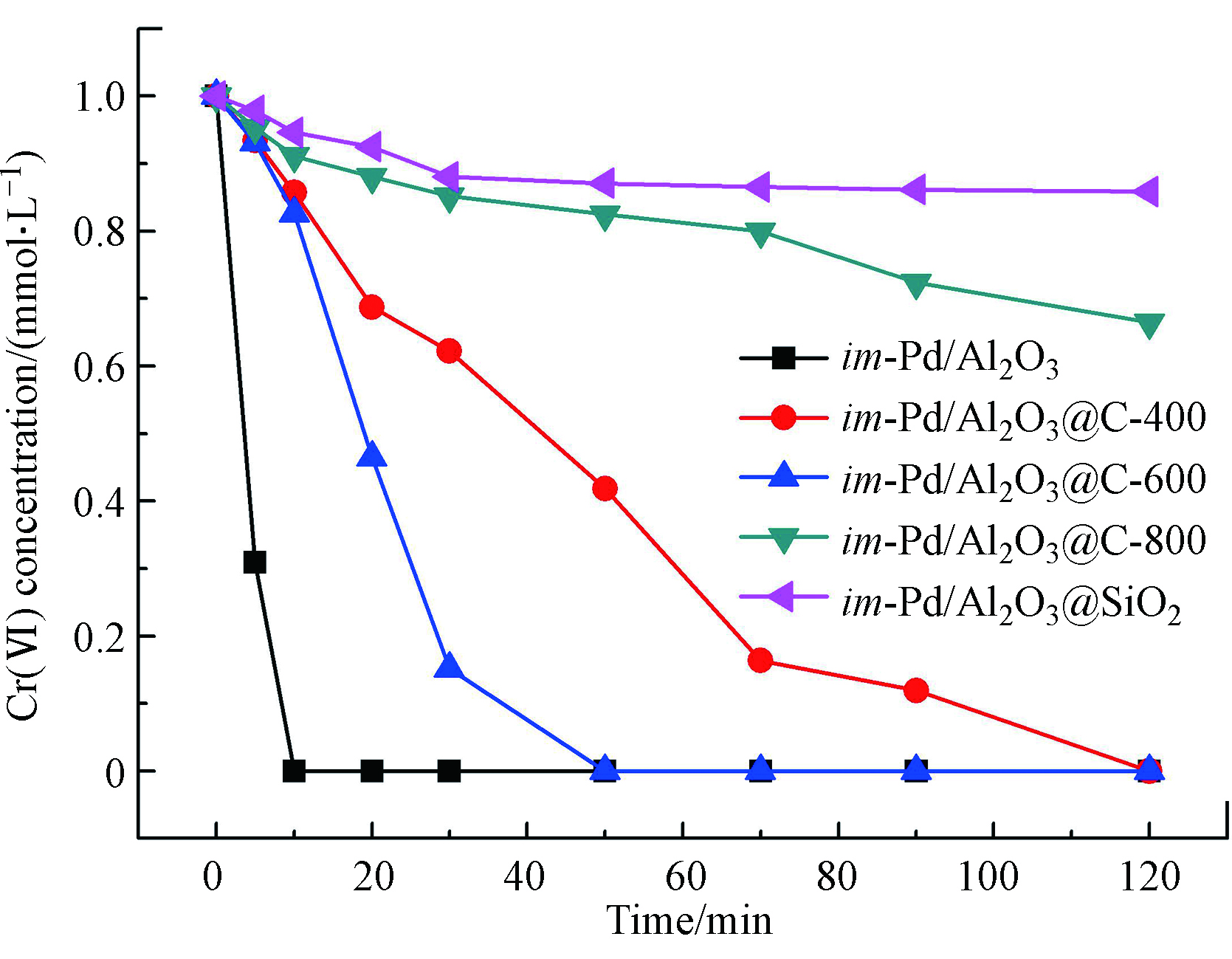
不同的催化剂上Cr(Ⅵ)催化加氢活性见图2。反应在常温常压下进行。催化剂的用量为20 mg,Cr(Ⅵ)的初始浓度为1 mmol·L−1,溶液的pH值为2.0。从图2中可以看出,在反应最初的20 min里,催化反应的速率最高,随时间推移逐渐减缓,按照催化反应原理,在第一阶段符合动力学一级定律,也就是随着反应的进行,反应速率下降。单金属im-Pd/Al2O3催化剂对Cr(Ⅵ)的催化加氢活性最高,反应15 min后Cr(Ⅵ)的浓度已经降低到了0 mmol·L−1,其次活性较高的是im-Pd/Al2O3@C-600,50 min时反应完毕。im-Pd/Al2O3@C-800的活性很低,反应120 min时Cr(Ⅵ)的浓度还有最初的70%。相比之下,im-Pd/Al2O3@SiO2对Cr(Ⅵ)的催化活性比所有的催化剂的催化活性都要低,这是因为包裹了C的im-Pd/Al2O3@C催化剂能够通过C来吸附和活化H2,从而给出有活性的电子,这是通过金属和C之间的相互作用来实现的[12],而SiO2则不具备这一转移电子的能力,因此im-Pd/Al2O3@SiO2的活性最差,反应进行120 min时,Cr(Ⅵ)的浓度还有最初的90%。同时从图2中也可以看出来,不同的炭化温度对催化剂的活性影响也比较大。当温度从400 ℃升高到600 ℃时,im-Pd/Al2O3@C催化剂的活性明显随炭化温度的升高而提高,从600 ℃升高到800 ℃的时候,im-Pd/Al2O3@C催化剂的活性明显随炭化温度的升高而降低。在温度低于600 ℃时,提高炭化温度可能通过降低电阻率来提高C的导电率,因此,im-Pd/Al2O3@C催化剂的高活性归因于高的导电率。但是当炭化温度从600 ℃提高到800 ℃,im-Pd/Al2O3@C催化剂的活性反而下降,这也印证了im-Pd/Al2O3@C催化剂对Cr(Ⅵ)的催化加氢活性源于催化剂的电导率和C材料的疏水性。
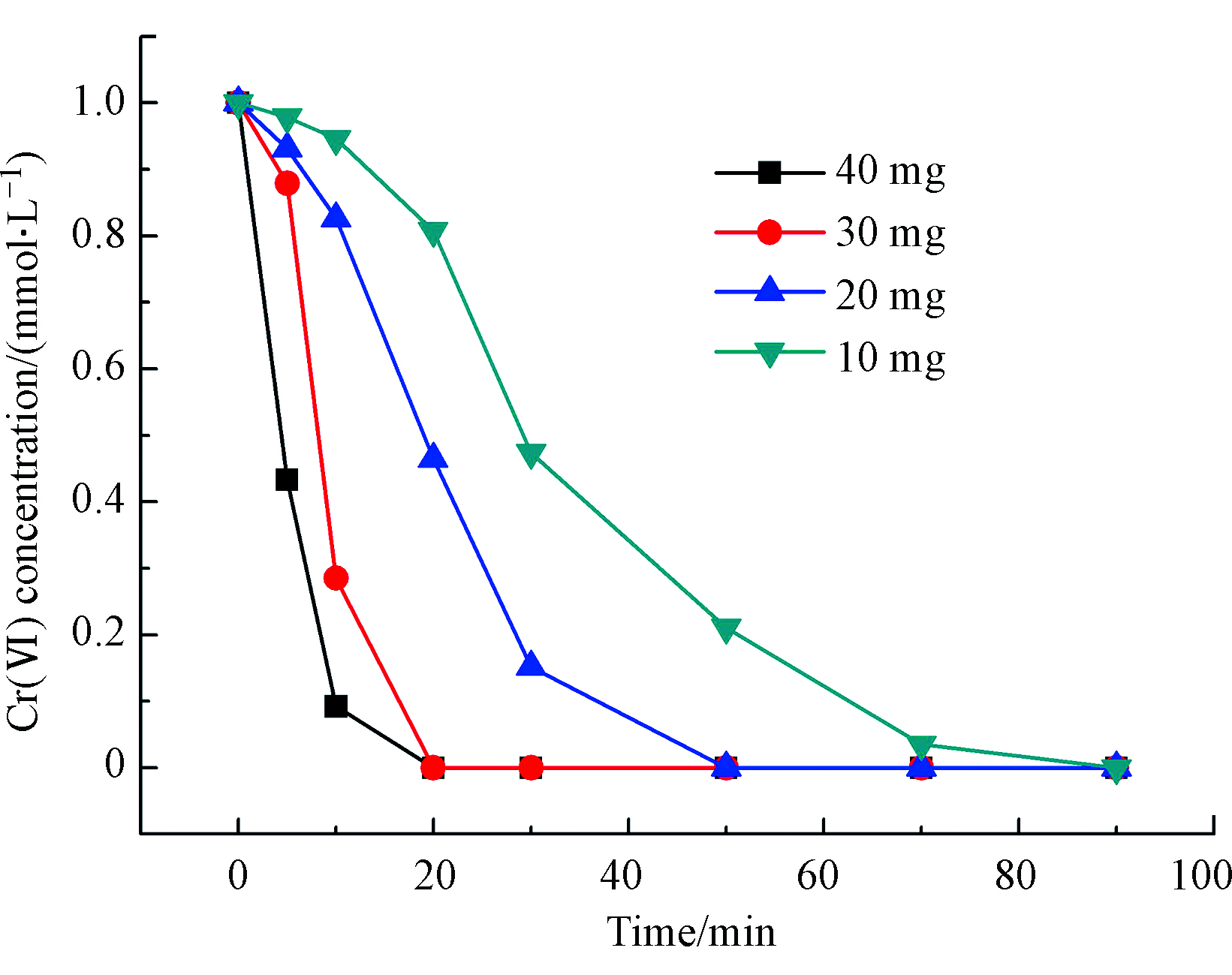
不同投加量下的催化加氢还原活性见图3。im-Pd/Al2O3@C-600催化剂的投加量分别是10 mg (34.0% wt)、20 mg (68.0% wt)、30 mg (102.0% wt)和40 mg (146.0% wt)。从图3中可以看出,随着催化剂投加量的增加,催化剂的初活性也明显提高,Cr(Ⅵ)催化还原为Cr(Ⅲ)的速率明显加快,且反应速率与催化剂投加量的立方呈正比。这是因为在反应物浓度一定的情况下,增加催化剂的投加量,也就增加了反应液中Pd的活性位数量,从而有效促进了Cr(Ⅵ)的催化加氢还原速率。
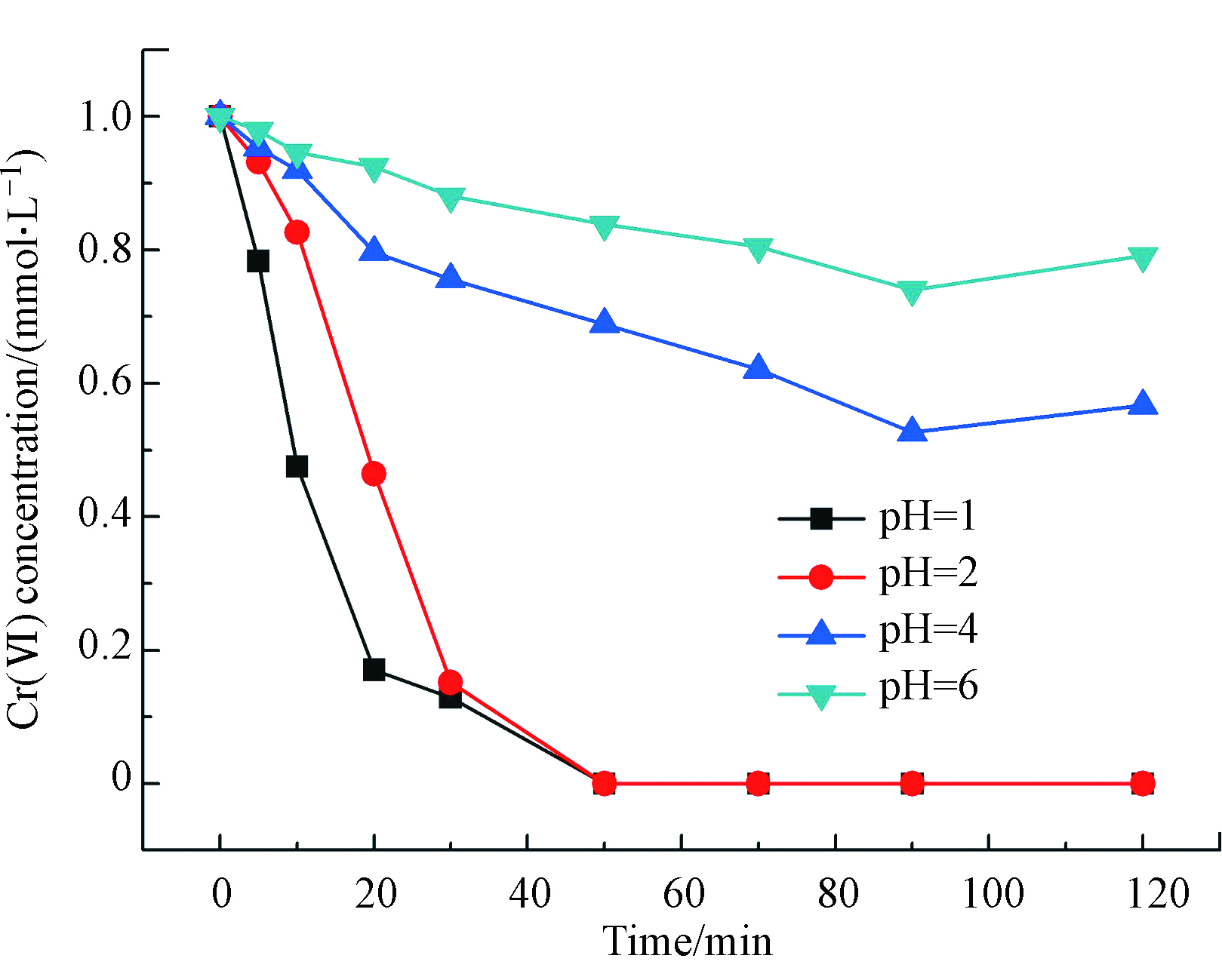
不同Cr(Ⅵ)溶液pH值环境下Cr(Ⅵ)的液相催化加氢还原反应行为如图4所示。催化剂为im-Pd/Al2O3@C-600,溶液的pH值分别为1、2、4和6。从图4可以看出来,酸性越强,催化反应越剧烈,且反应速率与H+浓度呈正比关系。这是因为随着pH值的增加,作为质子的H+逐渐减少,催化剂表面的正电荷减少,从而导致Cr(Ⅵ)的吸附和氧化还原能力减弱[13]。Cr(Ⅵ)在水中是以阴离子的形态存在的,因此pH值的升高也就抑制了Cr(Ⅵ)的吸附。Cr(Ⅵ)要被还原成为Cr(Ⅲ),那么首先得在催化剂的表面上发生吸附。Cr(Ⅵ)还原成为Cr(Ⅲ)与溶液pH值的减少是呈线性关系的,这也就说明了Cr(Ⅵ)更易于在酸性的环境中被还原成Cr(Ⅲ)[14]。
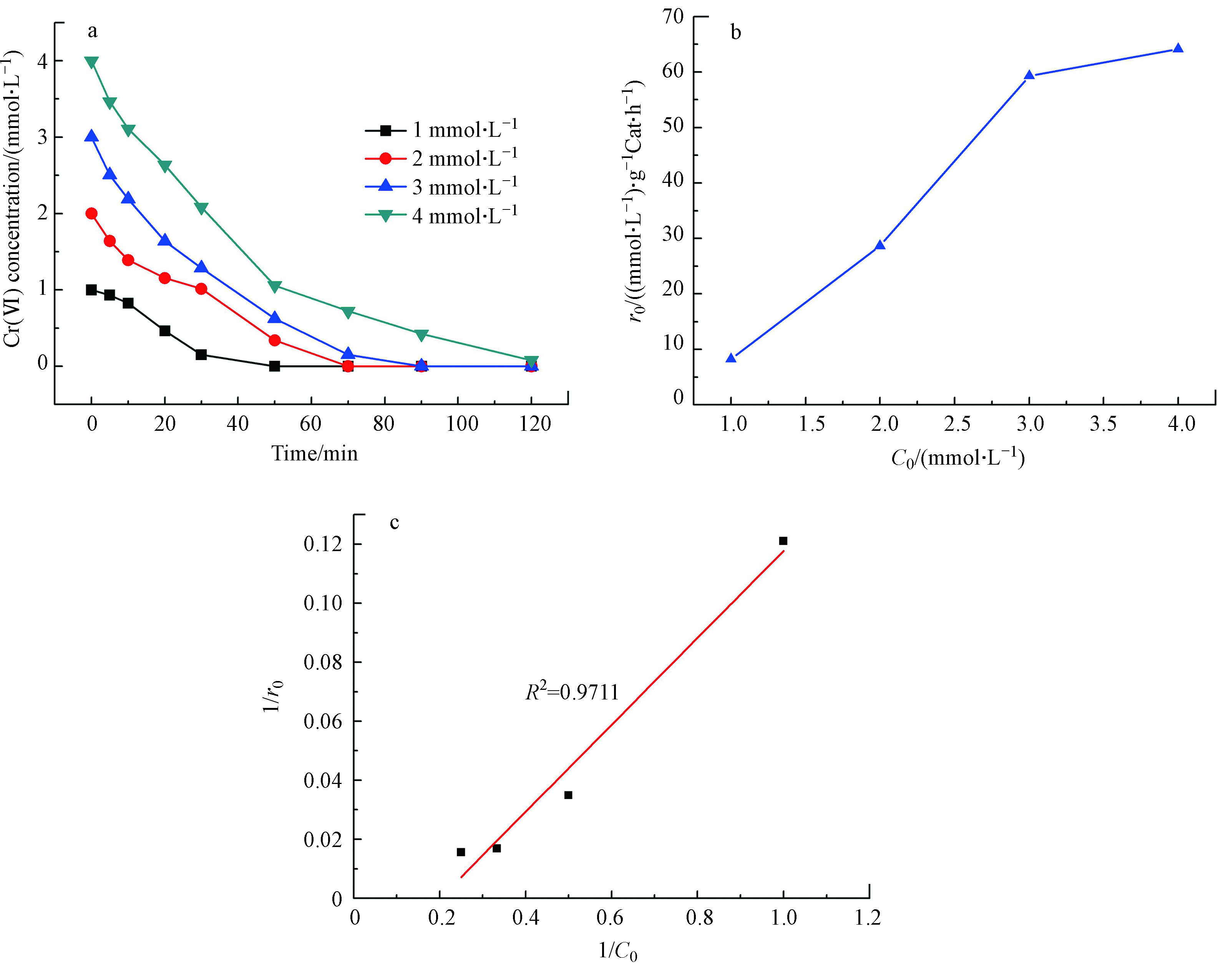
不同初始Cr(Ⅵ)浓度下的催化加氢还原反应见图5。由于选取的反应为非均相的反应,对于非均相的Cr(Ⅵ)的催化加氢还原反应而言,反应物Cr(Ⅵ)在催化剂表面的吸附是影响催化反应的关键步骤,反应速率与Cr(Ⅵ)在催化剂表面的吸附浓度呈相关性,两者的关系可以用Langmuir-Hinshelwood模型进行拟合[15]。同等反应条件下,Cr(Ⅵ)的初始浓度越高,Cr(Ⅵ)完全反应所需的反应时间就越长。图5a也正好验证了这一点。随着Cr(Ⅵ)初始浓度的增加,催化剂反应的初活性也逐渐增加,说明反应速率随着Cr(Ⅵ)初始浓度的增加而逐渐增加,图5b也表明,随着Cr(Ⅵ)初始浓度的增加,催化加氢还原反应的初活性也在逐渐增加。图5c是拟合后的Langmuir-Hinshelwood模型,由图可知,1/r0和1/C0的相关系数为0.977,证实了Cr(Ⅵ)在催化剂表面的催化加氢还原反应符合Langmuir-Hinshelwood模型,也就是反应过程受制于Cr(Ⅵ)在催化剂表面的吸附。
良好的稳定性是考察催化剂性能的重要指标,im-Pd/Al2O3催化剂和im-Pd/Al2O3@C-600催化剂的稳定性实验见图6。从图6a可以看出,im-Pd/Al2O3催化剂的活性在初次使用时很高,但是随着使用频次的增加,活性急剧下降。第3次使用在反应进行50 min时,Cr(Ⅵ)的浓度降低到0 mmol·L−1。第4次和第5次活性依然在下降,第5次循环使用时,Cr(Ⅵ)的浓度需要进行90 min才能够降到0 mmol·L−1。经历过5次循环,im-Pd/Al2O3@C-600催化剂的初活性基本保持在250(mmol·L−1)·g-1 Cat·h−1,而对于单金属的im-Pd/Al2O3催化剂来说,虽然说第1次循环时初活性高达414 mmol·L−1gCat−1h−1,但是第2次循环时初活性就降到了231(mmol·L−1)·g−1 Cat·h−1,降幅高达44%。第3次循环后初活性更是只有初始初活性的42%,第5次循环时活性则只剩下了初始活性的23%,基本丧失了催化活性。这从表1中im-Pd/Al2O3催化剂循环后的ICP测出的Pd含量也能够加以说明。这是由于反应过程中经过液相酸性条件的浸泡,剧烈的搅拌等因素造成了im-Pd/Al2O3催化剂有效活性组分的流失。而从图6b可以看出,im-Pd/Al2O3@C-600催化剂经过5次循环后,并没有看到明显的催化剂失活。im-Pd/Al2O3@C-600催化剂虽然开始时的活性没有im-Pd/Al2O3催化剂好,反应进行到第3次时,两者活性相当,之后第4次和第5次循环时im-Pd/Al2O3@C-600催化剂的活性依然保持最初的活性,并没有衰减的迹象。经过表面C修饰的im-Pd/Al2O3@C-600催化剂的稳定性远远高于未经表面修饰的im-Pd/Al2O3催化剂。经过在im-Pd/Al2O3催化剂的表面包裹C对催化剂的表面进行修饰,提高了Pd粒子与Al2O3载体之间的结合力,从而使im-Pd/Al2O3@C-600催化剂能够经受酸性条件的浸泡与剧烈的搅拌,表现出良好的催化稳定性。
2.1. 催化剂表征
2.2. Cr(Ⅵ)的液相催化加氢还原
-
本研究通过对im-Pd/Al2O3催化剂的表面进行包裹C和SiO2的方式进行修饰,并通过对Cr(Ⅵ)的催化加氢还原对催化剂的性能进行了考察。并通过一系列的表征手段对催化剂形貌组成进行了分析。催化剂的活性评价结果表明,im-Pd/Al2O3@C-600催化剂具有良好的催化活性和稳定性。通过在im-Pd/Al2O3催化剂的表面包裹C,有效的提高了催化剂表面的导电性,提高了催化剂中活性成分Pd粒子与载体Al2O3之间的结合力,将Pd粒子包埋在碳层下,有效的防止了催化反应过程中活性组份Pd粒子的流失。通过连续循环实验,也验证了经过表面修饰的im-Pd/Al2O3催化剂具有更好的稳定性。本研究结果表明,可以通过在im-Pd/Al2O3催化剂的表面包裹C的方式进行修饰,来提高催化剂的稳定性,实现了液相条件下Cr(Ⅵ)的高效稳定还原去除。



 下载:
下载: