-
我国面临农田土壤重金属污染类型增多,污染面积扩大,程度加深等问题[1-2]。我国各行政区农田呈现南方重金属污染远高于北方的特点,而城市化区域重金属污染则呈现北方高于南方的特点,虽然检测方法不一样,但均表明Cd污染最严重,Cu、Pb、Zn、Cr、Ni也均有不同程度的污染[3],我国省会城市Cd、Pb、Ni、Cu、Zn和Hg的含量均显示超标[4],土壤重金属污染控制与治理亟待开展。
铜渣是铜冶炼和精炼过程中获得的副产品,我国产量呈逐年上升趋势,几乎每7年就翻一番,2017年产量更是占世界产量的30%[5]。如此大量的铜渣积聚,不合理处置会导致一系列环境和空间问题,尤其是存在金属浸出风险[6-7]。铜渣的处置方法主要是金属回收,生产增值产品以及在炉渣堆或库存中处置[8],而金属回收存在工艺复杂、回收不彻底、成本较高等问题。基于磷酸镁水泥的形成,已有大量研究致力于铁基磷酸盐材料的合成[9-10],由于铁基磷酸盐凝胶的存在,铁基磷酸盐材料不仅具有良好的力学性能,而且拥有较好的吸附重金属能力。铜渣中铁含量较高,为实现铜渣的充分利用,已有人研究了铜渣系磷酸盐材料的合成,磷酸盐对铜渣具有一定的凝结性,通过与铜渣中的Fe形成FeH2P3O10·H2O、Fe(H2PO2)3、FeH2P3O10、FePO4、Fe(H2PO4)3等铁基磷酸盐凝胶体系,该体系对重金属有较好的固化作用[11]。而铜渣中的Fe主要以Fe2SiO4和Fe3O4为主,考虑添加一定的改性剂破坏铜渣中的Fe2SiO4结构,释放Fe离子,增强磷酸盐对铜渣的凝结性能,提高吸附重金属性能。Liu等[12]利用铜渣和含砷石膏泥混合发现可以有效的稳定固化重金属尤其是Pb和Cu。Zuo等[13]发现CaO的添加可以打破铜渣中的反应体系的平衡,促进2FeO·SiO2中Fe和Si分离,有利于铜渣的还原。Li等[14]发现,CaO比FeO更容易与SiO2结合生成硅酸钙,取代2FeO·SiO2中的FeO,增加了FeO的生成。大量的亚铁离子在硅酸盐四面体中形成硅酸酯单元共聚网络,在常温搅拌的情况下,CaO的添加同样能够破坏硅酸盐网络中的桥接氧[15]。但目前对CaO在铜渣系磷酸盐体系中的影响研究较少,对加入磷酸盐后,CaO能否破坏铜渣中的Fe2SiO4结构,以及促进铁基磷酸盐凝胶的生成尚不清楚。CaO对铜渣系磷酸盐内部结构的作用机理有待揭示。
基于磷酸铁材料的形成机制,结合铜渣中铁的含量高达50%,本研究将以磷酸盐作为激发剂,激发铜渣生成吸附材料,利用CaO改性铜渣系磷酸盐吸附材料,研究其对Pb、Cr、Cd、Cu、Ni 的5种重金属的吸附行为及机理,为解决重金属污染提供可行的方法。
-
本研究所用铜渣来自安徽省某有色金属冶炼厂冶炼尾渣,由XRF(Shimadzu-1800)测得其元素组成(表1)。实验所需试剂均为分析纯,重金属离子液由该离子硝酸盐配置,所需去离子水由纯水机(宏科DZG-303A)提供。
-
根据前人实验研究,确定铜渣系磷酸盐的制作方案如下:铜渣与磷酸二氢铵按4:1混合,加入2%的硼砂(占铜渣质量)作为缓凝剂[11],添加适量去离子水搅拌至粘稠状,记为M0。在铜渣系磷酸盐材料的基础上,添加3%、5%、10%的CaO替代铜渣作为改性剂,分别记为M3、M5、M10。表2为吸附剂具体添加比例制作比例,水胶比含量变化是由于CaO比铜渣更易与水发生反应,CaO作为胶体的一部分,硼砂含量表示占总胶体的比例。室温条件下养护3 d,后破碎,经球磨机(QM-3SP04)350 r·min−1下研磨30 min,过200目筛。
-
吸附剂喷金处理后,使用热场扫描电镜(德国蔡司Gemini 500) 二次电子,放大70000倍,1 μm、100 nm下对吸附材料进行形貌扫描。使用X射线衍射仪(DX-2700)扫描仪进行矿物结构分析,Cu靶,步长0.02,2θ范围10°—70°。
-
对比M0、M3、M5、M10在5种重金属(Pb、Cr、Cd、Cu和 Ni)中的吸附性能。单个重金属的浓度设置为100 mg·L−1,取50 mg吸附剂加入50 mL重金属溶液;混合重金属由5种500 mg·L−1的重金属各取10 mL组成,取50 mg吸附剂加入50 mL混合溶液中,吸附时间设置为120 min。
-
选择M3研究对5种重金属的吸附动力学行为,取50 mg吸附剂加入50 mL重金属离子液,每种重金属离子液的浓度设置200、400、600 mg·L−1的3个梯度,取样时间设置为5、10、20、40、60、90 min。所有吸附结果均由激光剥蚀等离子体质谱仪(Agilent 7500 四极杆质谱)测量。
-
使用50 mg的M3吸附50 mL的Pb、Cr、Cd、Cu和Ni离子液,每种重金属浓度均为200 mg·L−1,吸附时间120 min,过滤后取滤渣烘干由红外光谱仪IS50采用晶体KBr压片法测试FTIR图谱分析机理。
-
通过重金属去除率η和吸附量来判断其吸附性能,按公式(1)和(2)计算:
式中,C0是重金属离子液初始浓度, mg·L−1;Ce是平衡吸附浓度, mg·L−1;η是吸附率,%;V是离子液体积,L;m是吸附剂质量,g;q是吸附量,mg·g−1.
通过准一级动力学方程[式(3)]和准二级动力学方程(式(4))分析M3对重金属的吸附行为:
式中,Qt为t时刻吸附量,mg·g−1;Qe为平衡吸附量,mg·g−1;k1(min−1)、k2[g·(mg·min)−1]为吸附速率常数[16].
-
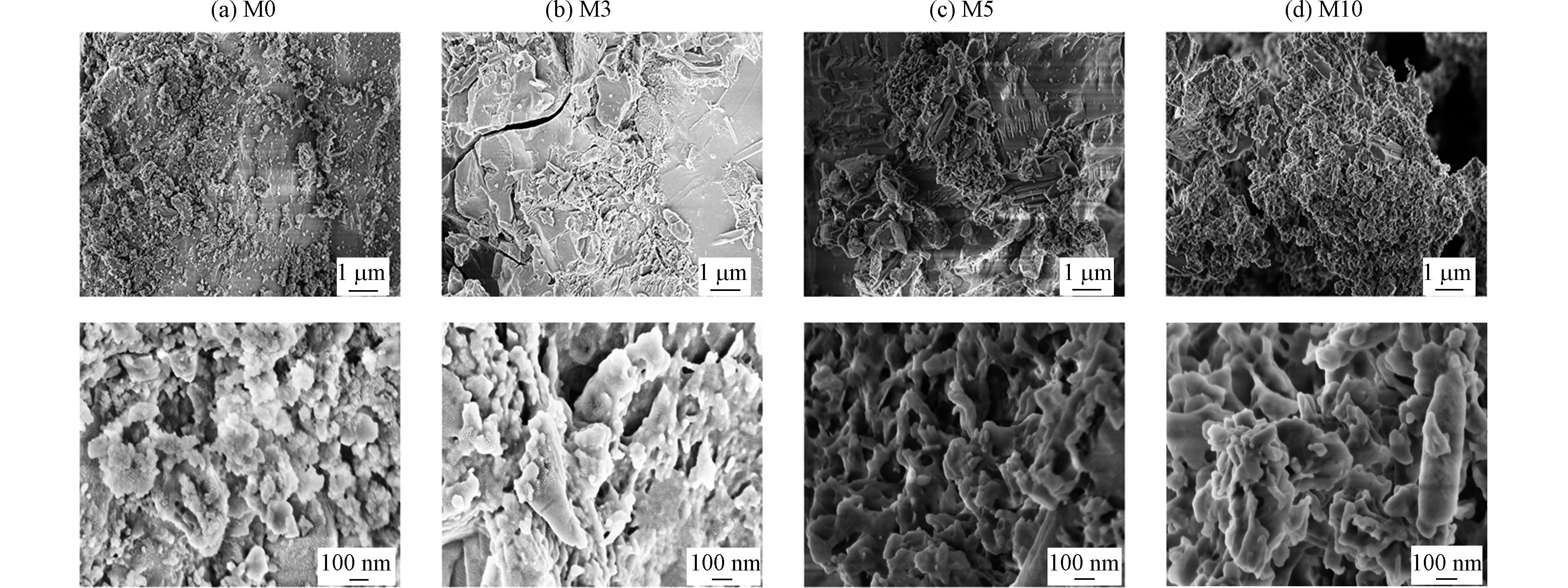
张理群等[17]研究表明,铜渣表面光滑,呈块状结构,而本实验中以铜渣制作的吸附剂表面均有大量絮状物附着在块状结构上,絮状物为胶凝相。图1(a)是未添加CaO时表面呈棉团状附着在块状上。图1(b)中棉团块状结构变大且部分脱离层块状结构,而随着CaO的添加,块状结构出现解体,棉团状逐渐展开,孔隙增加。尤其是M10,棉团状和层块状结构几乎完全消失,形成了以孔隙为主的絮状结构。
从图2中可以看出,铜渣(CS)的主要相是铁橄榄石,玻璃态物质和磁铁矿,铜渣中的主要物相为Fe2SiO4和Fe3O4,且其成峰明显,比较尖锐,说明结晶度较高[17]。对比M0和铜渣可见,铜渣中的Fe2SiO4、Fe3O4明显被削弱,尤其是Fe2SiO4,说明磷酸盐可以在一定程度上分解铜渣矿物结构,可能是磷酸盐比硅酸盐更易与铁形成新的结构体系[18]。
M0中铁基磷酸盐未形成明显峰相,表明结构较弱,而随着CaO的添加,M3、M5、M10在2θ为18°、22°、28°附近出现明显的铁基磷酸盐峰相,分别为Fe3(PO4)O3、FePO4·2H2O、Fe3(PO4)2(OH)2,表明CaO的添加促进了铜渣中的Fe的释放,增强了磷酸盐对铜渣的凝结性。M3中Ca主要以Ca2SiO4(31°、39°)、Ca3Si2O7(29°)、Ca(OH)2(34°)、(Ca, Fe)SiO3(61°)的形式存在,表明Ca离子取代了Fe离子在硅氧结构中的位置,证明在磷酸盐存在的条件下,CaO仍能促进铜渣中Fe2SiO4、Fe3O4相的分解[19]。M5中部分铁基磷酸盐结构加强,此外Ca离子开始与磷酸盐结合,34°处的Ca(OH)2被Ca5(PO4)3(OH)取代,52°—62°出现较多无定形的结构Ca2Fe2O5,表明Ca离子逐渐固定部分Fe离子。M10中34°处的Ca5(PO4)3(OH)峰变强,54°处的Ca2Fe2O5峰也变强,表明Ca离子不仅与Fe离子竞争磷酸盐结合加强,与磷酸根竞争Fe离子作用也越来越明显。
-
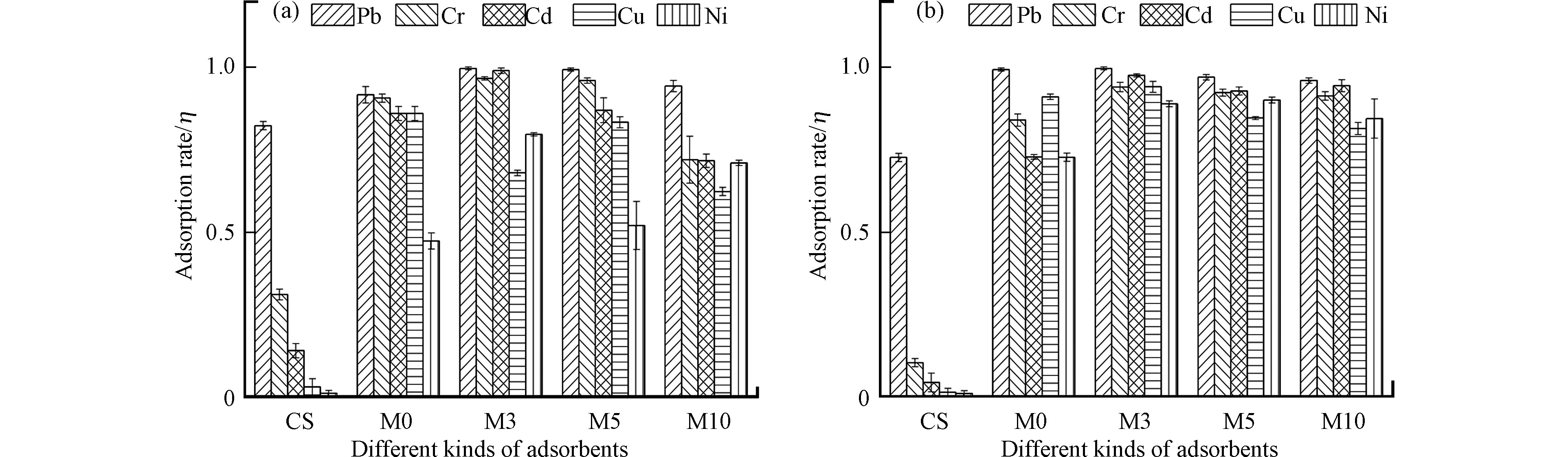
图3 (a)和图3 (b)分别表示4种吸附剂在单个重金属中和混合重金属中的吸附率,其中铜渣为对照,铜渣对除对Pb离子具有一定的吸附作用外,对其他离子几乎无作用。由图3 (a)可知,4种吸附剂对于单个重金属Pb离子的吸附效果最好,其次是Cr离子,而对于Ni和Cu的吸附效果较差,M0对于Ni的吸附效果最差,仅有47.33%,吸附量为47.33 mg·g−1。吸附单个重金属Pb、Cr和Cd时,M3和M5吸附率呈上升趋势,M10吸附率则出现降低,可能的原因是CaO的添加量过高出现了抑制吸附的现象,综合4种吸附剂在单个重金属中的表现,M3和M5拥有较好的吸附性能。由图3 (b)可知,4种吸附剂在混合离子中对Pb的吸附效果都极好,几乎达到100%,而对于Ni和Cu的吸附效果依旧较差,尤其是M0,对于添加了CaO的M3、M5、M10在混合重金属中吸附性能明显优于M0,可见CaO的添加改进了原有的吸附剂,提高了其在混合重金属中的吸附性能,M3在混合重金属中吸附性能最好,对5种重金属的吸附率分别为99.67%、94.03%、97.57%、94.10%、88.92%,吸附量分别为19.93、18.81、19.51、18.82、17.78 mg·g−1。但是随着CaO的增加,同样出现了吸附率降低现象。这与几种材料的结构表征相一致,M0中棉团状结构小且分散,仍为层块状的附着产物,而M3中的棉团状凝胶形貌则呈块状且聚合,M5中棉团状开始出现解体,M10中几乎完全为解体后的絮状形貌,表明棉团状结构为主要吸附结构。从材料结构变化可明显看出,Ca离子的过度添加会出现与Fe离子竞争磷酸根的现象,不利于铁基磷酸盐的形成,降低了磷酸盐对铜渣的凝结性,与吸附结果一致。相同重金属浓度条件下,CaO改进的吸附剂在混合重金属吸附性能比在单个重金属中吸附性能更好,推测吸附剂对于几种重金属的吸附机理不同,吸附剂中至少存在两种吸附作用,在单一重金属离子中仅有一种起主要作用,而在混合重金属中几种吸附机理协同作用,增加了吸附效率。而CaO添加量过高吸附率反而降低的现象表明Ca离子过量会与五种重金属离子存在竞争吸附关系。综上所述,添加3%氧化钙改性最适宜,此时吸附性能最好,尤其是在混合重金属中。
-
由图4和表3可知,M3在各浓度Pb离子下的R22均大于R12,更接近1,可见对于Pb的吸附更加满足准二级动力学模型,准二级理论值饱和吸附值略高于实际值,Pb离子为400 mg·L−1时吸附性能最佳,吸附量达到476.19 mg·g−1。通过比较R12和R22可知,在200 mg·L−1时,M3对Cr的吸附更加符合准一级动力学模型,此时饱和吸附量为82.09 mg·g−1,而在400、600 mg·L−1,均更加符合准二级动力学模型,吸附量分别为86.21、74.07 mg·g−1,综合比较可知,M3对Cr的最佳吸附在400 mg·L−1达到86.21 mg·g−1。M3对于Cd的吸附在200、400 mg·L−1时更加符合准二级动力学模型,而高浓度600 mg·L−1时更符合准一级动力学模型,最大饱和吸附量在400 mg·L−1时达到最大为112.36 mg·g−1。比较R12和R22可知,M3对Cu的吸附在600 mg·L−1更加符合准一级动力学模型,此时饱和吸附量为75.64 mg·g−1,而在200、400 mg·L−1,均更加符合准二级动力学模型,吸附量分别为58.14、81.97 mg·g−1,综合可知,M3对Cu的最佳吸附在400 mg·L−1达到81.97 mg·g−1。M3对于Ni的吸附与Pb相似,各浓度下均是更适合准二级动力学模型,但是远没有对Pb的吸附性能好,吸附最高浓度出现在200 mg·L−1,达到90.09 mg·g−1。M3对5种重金属的饱和吸附均是出现在200 mg·L−1或400 mg·L−1时,高浓度条件下则出现抑制现象。推测可能与铁凝胶吸附位点饱和有关[20]。
综合比较M3在5种重金属离子中的吸附性能可见,吸附剂对于Pb离子吸附性能是最好的,5种实际饱和吸附能力由大到小依次为Pb>Cd>Cr>Cu>Ni,理论饱和吸附值大小为Pb>Cd>Ni >Cr>Cu,均表明对Pb的吸附性能远大于其余4种,主要原因可能是铁凝胶作为吸附剂时,对Pb离子的亲和性明显优于其他重金属[21-23]。出现偏差的原因是Cr和Cu的理论和实际饱和值出现在不同浓度下,导致最大吸附值不一致。但是M3对Cr的吸附性能比Cu好,与Laura等[24]研究的铁亲和性一致,表明吸附剂中对Cr和Cu可能存在铁凝胶吸附。综上可知,M3的吸附能力主要来源于其中铁相,但是从铜渣直接吸附重金属表现来看,吸附性能与CaO-SiO2-FexOy -P2O5四元体系的关系更密切,而铁凝胶是吸附过程的主要承担者。推测作用机理可能是在四元体系中,Ca离子逐步取代铜渣中硅氧网络结构固定的Fe离子,硅氧网络结构对Fe离子的束缚作用减弱甚至消失[25],促进磷酸盐与Fe离子结合,有利于形成铁基磷酸盐胶凝材料吸附重金属共沉降。与M3的XRD结果中铁基磷酸盐峰相加强一致。
-
如图5所示,M3在吸附Pb重金属后3224 cm−1处峰偏移至3495 cm−1,是由于—OH的伸缩振动[26-28],形成了含水铁基磷酸盐凝胶[29],吸附Pb离子形成共沉降。此处比其他几种吸附重金属峰更强烈也说明了吸附Pb离子效果更好。1642 cm−1处是由于结晶H2O变角和无定形铁中—OH的振动[27,29],表明含水铁基磷酸盐胶凝的发生吸附。而1460 cm−1、1395 cm−1处的 Si—O—Me(Me指硅氧网络结构中非桥氧所连接的网外阳离子)伸缩振动峰消失形成1439 cm−1处单峰表明原来的非桥氧结构中的Fe离子被释放[30],重金属离子取代原来Fe离子位置,形成Si—O—Me非桥氧结构。1100 cm−1处峰偏移至1130 cm−1处发生了Si—O—Si的伸缩振动[31],表明Pb离子进入Si—O—Si四面体结构中,而随着1062 cm−1处峰相变强,Si—O—Si的反对称伸缩振动加强[30],表明形成了Si—O—Me的新结构,Pb离子被固定在硅氧网络结构中。1016—950 cm−1处短峰消失是由于磷酸二氢盐中P—OH伸缩振动[32],形成987 cm−1处新峰,此处部分游离Fe离子被P—OH固定,形成铁基磷酸盐凝胶。
吸附Cr后3224 cm−1处峰出现延伸,2868 cm−1处短峰消失,表明形成少量铁基磷酸盐凝胶,1460 cm−1处峰消失、1395cm−1处的 Si—O—N伸缩振动峰消失形成,1062 cm−1处峰变强变宽(1011、970 cm−1两处短峰消失)是由于Si—O—Si的反对称伸缩振动加强,536 cm−1峰变弱是由于Fe3O4中Fe—O的振动导致,部分Fe离子被释放[26],形成铁基磷酸盐凝胶,466 cm−1处峰变强,发生Si-O-Me振动,表明结合了重金属离子[33]。Cr的吸附与吸附Pb类似,但是无定形铁胶体吸附部分远不如Pb,大部分均是固定在Si—O网络结构中,形成Si—O—Me结构。
吸附Cd后3224 cm−1峰偏移至3492 cm−1处,峰相明显变弱,2868 cm−1处短峰消失,表明—OH出现伸缩振动,形成铁基磷酸盐凝胶量较小,1460 cm−1处峰消失、1395cm−1处的 Si—O—Me伸缩振动峰消失形成1439 cm−1处单峰表明原来的Si—O—Me结构中的Fe离子被释放,Cd取代形成新的Si—O—Me结构,1100 cm−1处峰偏移至1130 cm−1处发生了Si—O—Si的伸缩振动,表明Cr离子进入Si—O—Si四面体结构中,1062 cm−1处峰略强,表明Cd取代Si形成Si—O—Me结构。1016—950 cm−1处短峰消失,形成987 cm−1处新峰与Pb相似均是Fe离子被P—OH固定。
吸附Cu后1300—700 cm−1变化不明显,意味着Si—O—Si四面体结构无明显振动,表明Cu离子几乎没有被四面体结构固定。而主要变化集中在3224 cm−1处的峰偏移至3492 cm−1处(—OH振动),1460 cm−1处峰消失、1395 cm−1处形成较宽的峰(原来硅酸盐中的Si—O—Me结构中的Fe离子释放),536 cm−1峰变弱(Fe3O4:Fe—O vibration),表明Cu的吸附主要是与形成的铁基磷酸盐凝胶形成共沉降。吸收Ni后1062 cm−1处峰变强,Si—O—Si的反对称伸缩振动加强,表明Ni离子在四面体中形成了Si—O—Me的新结构,其余峰相与吸附Cu后相似,表明铁基磷酸盐凝胶也是吸附Ni的主要原因。
综上所述,M3在吸附Pb、Cd时铁基磷酸盐凝胶和Si—O—Si四面体结构同时参与,但吸附Pb离子时铁基磷酸盐凝胶表现更优异。而Cr的吸附主要依靠Si—O—Si四面体结构形成Si—O—Me非桥接氧结构固定,Cu的吸附主要依靠铁基磷酸盐凝胶的共沉降。对Ni离子的吸附主要靠铁基磷酸盐凝胶的共沉降,但也存在Si—O—Si四面体固定。从吸附机理推断吸附能力大小为Pb>>Cd≈Ni>Cr≈Cu,与吸附动力学的理论结果相一致。
-
(1)在CaO-SiO2-FexOy-P2O5四元体系中,CaO通过破坏Si—O—Fe之间的化学键,释放Fe离子,促进铁基磷酸胶凝材料的生成,增强了磷酸盐对铜渣的凝结性,从而提高吸附性能。随着CaO的增加,铜渣结构破坏更加明显,当添加量为10%时,铜渣层块状结构(Fe2SiO4相)几乎完全被孔隙絮状物替代。
(2)CaO添加量为3%时,吸附剂对重金属的吸附效果最好,尤其是在混合重金属中,对五种重金属的吸附率分别为99.67%、94.03%、97.57%、94.10%、88.92%,吸附量分别为19.93、18.81、19.51、18.82、17.78 mg·g−1。结合材料的形貌表征可知,M3中的棉团状凝胶呈现大块状且聚集,M0中棉团状小且分散,附着在层块状结构上,M5、M10中棉团状出现不同程度的解体,表明棉团状结构为主要吸附结构。结合材料结构变化可看出,Ca离子的过度添加会与磷酸根离子形成Ca5(PO4)3(OH),同时与Fe离子形成Ca2Fe2O5,导致磷酸盐对铜渣的凝结性下降,不利于铁基磷酸盐的形成,吸附性能减弱,与 CaO增加反而吸附率降低一致。混合重金属中吸附效果比单个重金属更好表明吸附剂对5种重金属存在不同的吸附机理。
(3)吸附动力学结果表明,添加3%的CaO改性的铜渣系磷酸盐材料吸附重金属能力依次为Pb>Cd>Ni>Cr>Cu,对Pb离子的吸附能力远大于其余4种,原因可能是铁基磷酸盐凝胶对于Pb离子的亲和性更好。FTIR结果表明在CaO-SiO2-FexOy-P2O5体系中对于重金属离子的吸附以Fe离子形成的铁基磷酸盐凝胶共沉降和Si—O—Si(Si—O—Me)四面体形成的网络结构固定为主,对于Pb离子的吸附性能远大于其余重金属,是由于铁基磷酸盐凝胶对Pb的亲和性更好,硅氧网络结构的固定也更明显。Cr的吸附主要由Si—O—Si四面体结构形成Si—O—Me非桥接氧结构固定,Cu的吸附主要由铁基磷酸盐凝胶的共沉降,吸附Cd、Ni时二者均参与,但吸收Ni时仅有少量Si—O—Si网络结构的固定。
基于CaO改性的铜渣系磷酸盐材料及其重金属吸附机制
Heavy metals adsorption mechanism based on copper slag phosphate material modified by CaO
-
摘要: 我国铜渣堆积导致的占地效应和污染效应问题日益严重。本研究以CaO为改性材料,制备一种高效去除重金属(Pb、Cr、Cd、Cu和 Ni)的铜渣系磷酸盐吸附材料。通过扫描电子显微镜(SEM)、X射线衍射仪(XRD)等测试技术表征了CaO的添加量对铜渣系磷酸盐材料结构的影响,利用吸附动力学和傅里叶红外分析仪(FTIR)探讨了改性材料在CaO-SiO2-FexOy-P2O5四元体系中对重金属的吸附机理。结果表明:(1)随着CaO的增加材料逐渐由层块状解体生成多孔结构。CaO的添加会使Fe离子从Fe2SiO4和Fe3O4结构中释放,促进铁基磷酸盐凝胶的生成,从而增强磷酸盐对铜渣的凝结性;(2)CaO添加量为铜渣的3%时,四元体系对重金属的吸附能力最强,除Cd和Cu在600 mg·L−1,Cr在200 mg·L−1符合准一级动力学外,其余浓度下均更加符合准二级动力学模型,理论吸附能力依次为Pb(476.19 mg·g−1)>Cd(112.36 mg·g−1)>Ni(90.09 mg·g−1)>Cr(86.21 mg·g−1)>Cu(81.97 mg·g−1);(3)在CaO-SiO2-FexOy-P2O5四元体系中,对Pb、Cd、Ni的吸附主要由铁基磷酸盐凝胶共沉降和Si—O—Si(Si—O—Me)四面体形成的网络结构固定协同作用,然而对Cr的吸附以Si—O—Si四面体形成Si—O—Me非桥接氧结构固定为主,对Cu的吸附则主要以铁基磷酸盐凝胶共沉降为主。本研究提供了以废治污的新思路,为重金属污染提供新的解决办法。
-
关键词:
- 铜渣系磷酸盐吸附材料 /
- CaO /
- 四元体系 /
- 铁基磷酸盐凝胶 /
- 共沉降 /
- Si—O—Si四面体
Abstract: The land occupation and environment pollution caused by copper slag accumulation are increasing greatly in China. The copper slag phosphate adsorption material modified by CaO was prepared to remove heavy metals (Pb, Cr, Cd, Cu and Ni). The effect of CaO on the structure of copper slag phosphate material was determined by both SEM and XRD. Meanwhile, the adsorption properties and mechanisms of heavy metals in CaO-SiO2-FexOy-P2O5 quaternary system were studied by both adsorption kinetics and FTIR. Results show that (1) the modified material disintegrates into porous structures gradually with the increasing of CaO addition. The free Fe ions were releasing from Fe2SiO4 and Fe3O4 crystal phases by adding of CaO, resulting in the promotion of the coagulability of phosphate to copper slag and formation of iron based phosphate gel. (2) all the adsorbents with 3% CaO are preferred to fix with the pseudo second-order kinetics model except for Cd and Cu at 600 mg·L−1 and Cr at 200 mg·L−1, the theoretical adsorption capacities were decreased in the order of Pb (476.19 mg·g−1) >Cd (112.36 mg·g−1) >Ni (90.09 mg·g−1) >Cr (86.21 mg·g−1) >Cu (81.97 mg·g−1). (3) Pb, Cd and Ni were adsorbed by the synergy of iron based phosphate gel co-precipitation and the network structure of Si-O-Si (Si-O-Me) tetrahedron formation in CaO-SiO2-FexOy-P2O5 system. Meanwhile, Cr was mainly adsorbed by Si—O—Si tetrahedron forming non bridged oxygen (Si—O—Me) structure, Cu was mostly existed in the co-precipitation of iron gel. This study provides useful information for utilization of solid waste and heavy metal treatment. -

-
表 1 铜渣的化学组成 (%)
Table 1. Chemical composition of copper slag (%)
成分Components FexOy SiO2 Al2O3 CaO ZnO K2O SO3 W 54.81 28.09 3.80 3.58 1.58 1.35 1.19 表 2 不同CaO添加量的吸附剂
Table 2. Adsorbents with different CaO additions
序号
Number名称
Name铜渣/g
Copper slag氧化钙/g
CaO铜渣/磷酸二氢铵
CS/ADP水胶比
W/B硼砂/%
Na2B4O7·
10H2O1 M0 10 0 4∶1 0.14 2 2 M3 9.7 0.3 4∶1 0.18 2 3 M5 9.5 0.5 4∶1 0.18 2 4 M10 9.0 1.0 4∶1 0.18 2 表 3 M3在不同重金属浓度下的吸附动力学参数
Table 3. The adsorption kinetic parameters of M3 at different concentrations of heavy metals
重金属
Heavy Metal浓度/(mg·L−1)
Concen-tration平衡吸附量/
(mg·g−1)Qmax准一级动力学方程
Pseudo-first-order kinetic equation准二级动力学方程
Pseudo-second-order kinetic equationQ1/(mg·g−1) k1 R12 Q2/(mg·g−1) k2 R22 Pb 200 237.21 219.31 5.3905 0.9831 256.41 0.0218 0.9987 400 430.23 401.10 5.9942 0.9887 476.19 0.0167 0.9973 600 400.73 350.41 5.8591 0.9864 416.67 0.0078 0.9991 Cr 200 90.02 82.09 4.4078 0.9880 113.64 0.2009 0.9680 400 81.76 57.05 4.0439 0.9969 86.21 0.1409 0.9985 600 71.22 40.34 3.6974 0.9869 74.07 0.1073 0.9995 Cd 200 95.12 85.84 4.4525 0.9881 104.17 0.0649 0.9903 400 106.13 105.18 4.6667 0.9962 112.36 0.0400 0.9988 600 78.12 67.19 4.2075 0.9997 82.64 0.0460 0.9986 Cu 200 55.43 38.19 3.6426 0.9822 58.14 0.0599 0.9982 400 75.27 70.89 4.2611 0.9951 81.97 0.0737 0.9964 600 81.22 75.64 4.3260 0.9981 86.96 0.0580 0.9952 Ni 200 80.23 78.26 4.3601 0.9974 90.09 0.1032 0.9983 400 66.85 55.65 4.0191 0.9702 73.53 0.1128 0.9878 600 50.12 40.56 3.7027 0.9927 54.64 0.1290 0.9988 -
[1] LI M, XI X H, XIAO G Y, et al. National multi-purpose regional geochemical survey in China [J]. Journal of Geochemical Exploration, 2014, 139: 21-30. doi: 10.1016/j.gexplo.2013.06.002 [2] TENG Y G, WU J, LU S J, et al. Soil and soil environmental quality monitoring in China: A review [J]. Environment International, 2014, 69: 177-199. doi: 10.1016/j.envint.2014.04.014 [3] 陈文轩, 李茜, 王珍, 等. 中国农田土壤重金属空间分布特征及污染评价 [J]. 环境科学, 2020, 41(6): 2822-2833. CHEN W X, LI Q, WANG Z, et al. Spatial distribution characteristics and pollution evaluation of heavy metals in arable land soil of China [J]. Environmental Science, 2020, 41(6): 2822-2833(in Chinese).
[4] 谷阳光, 高富代. 我国省会城市土壤重金属含量分布与健康风险评价 [J]. 环境化学, 2017, 36(1): 62-71. doi: 10.7524/j.issn.0254-6108.2017.01.2016051705 GU Y G, GAO F D. Spatial distribution and health risk assessment of heavy metals in provincial capital cities, China [J]. Environmental Chemistry, 2017, 36(1): 62-71(in Chinese). doi: 10.7524/j.issn.0254-6108.2017.01.2016051705
[5] 谭晓恒, 郭少毓, 喻相标, 等. 焙烧铜渣中磁铁矿的物性转变研究 [J]. 有色金属科学与工程, 2020, 11(5): 83-89. TAN X H, GUO S Y, YU X B, et al. Study on the physical property transformation of magnetite in roasted copper slag [J]. Nonferrous Metals Science and Engineering, 2020, 11(5): 83-89(in Chinese).
[6] GORAI B, JANA R K, PREMCHAND. Characteristics and utilisation of copper slag—a review [J]. Resources, Conservation and Recycling, 2003, 39(4): 299-313. doi: 10.1016/S0921-3449(02)00171-4 [7] LEMOUGNA P N, YLINIEMI J, ADESANYA E, et al. Reuse of copper slag in high-strength building ceramics containing spodumene tailings as fluxing agent [J]. Minerals Engineering, 2020, 155: 106448. doi: 10.1016/j.mineng.2020.106448 [8] KHANZADI M, BEHNOOD A. Mechanical properties of high-strength concrete incorporating copper slag as coarse aggregate [J]. Construction and Building Materials, 2009, 23(6): 2183-2188. doi: 10.1016/j.conbuildmat.2008.12.005 [9] WAGH A S, JEONG S Y. Chemically bonded phosphate ceramics: III, reduction mechanism and its application to iron phosphate ceramics [J]. Journal of the American Ceramic Society, 2003, 86(11): 1850-1855. doi: 10.1111/j.1151-2916.2003.tb03571.x [10] MASTALSKA-POPŁAWSKA J, PERNECHELE M, TROCZYNSKI T, et al. Chemically bonded phosphate ceramics based on silica residues enriched with iron(III) oxide and silicon carbide [J]. Journal of Molecular Structure, 2019, 1180: 215-219. doi: 10.1016/j.molstruc.2018.11.087 [11] 李娜秋. 铜渣基铁系磷酸盐化学键合材料的制备及其对重金属的固化研究[D]. 昆明: 昆明理工大学, 2019. LI N Q. Preparation of copper slag based iron phosphate chemical bonding material and its application in heavy metal solidification[D]. Kunming: Kunming University of Science and Technology, 2019(in Chinese).
[12] LIU D G, MIN X B, KE Y, et al. Co-treatment of flotation waste, neutralization sludge, and arsenic-containing gypsum sludge from copper smelting: Solidification/stabilization of arsenic and heavy metals with minimal cement clinker [J]. Environmental Science and Pollution Research, 2018, 25(8): 7600-7607. doi: 10.1007/s11356-017-1084-x [13] ZUO Z L, YU Q B, LIU J X, et al. Effects of CaO on reduction of copper slag by biomass based on ion and molecule coexistence theory and thermogravimetric experiments [J]. ISIJ International, 2017, 57(2): 220-227. doi: 10.2355/isijinternational.ISIJINT-2016-402 [14] LI Z, MA G J, LIU M K, et al. Calculation model for activity of FeO in quaternary slag system SiO2-CaO-Al2O3-FeO [J]. Metals, 2018, 8(9): 714. doi: 10.3390/met8090714 [15] FENG Y, CHEN Q S, ZHOU Y L, et al. Modification of glass structure via CaO addition in granulated copper slag to enhance its pozzolanic activity [J]. Construction and Building Materials, 2020, 240: 117970. doi: 10.1016/j.conbuildmat.2019.117970 [16] 冀泽华, 吴晓芙, 李芸, 等. 水溶液重金属离子在蛭石上的动态吸附行为与化学势变 [J]. 环境化学, 2015, 34(11): 2109-2117. doi: 10.7524/j.issn.0254-6108.2015.11.2015032309 JI Z H, WU X F, LI Y, et al. Kinetic adsorption and change in chemical potential of heavy metal ions in aqueous solutions [J]. Environmental Chemistry, 2015, 34(11): 2109-2117(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.11.2015032309
[17] 张理群,周慧慧,郑刘根,等. 尾渣中砷的精细化学结构、赋存形态 [J]. 环境化学, 2021, 40(5): 1611-1618. ZHANG L Q, ZHOU H H, ZHENG L G, et al. The fine chemical structure and occurrence patterns of arsenic in tailings [J]. Environmental Chemistry, 2021, 40(5): 1611-1618(in Chinese).
[18] ZHANG J D, SHEN Z M, MEI Z J, et al. Removal of phosphate by Fe-coordinated amino-functionalized 3D mesoporous silicates hybrid materials [J]. Journal of Environmental Sciences, 2011, 23(2): 199-205. doi: 10.1016/S1001-0742(10)60393-2 [19] KU J G, ZHANG L, FU W, et al. Mechanistic study on calcium ion diffusion into fayalite: A step toward sustainable management of copper slag [J]. Journal of Hazardous Materials, 2021, 410: 124630. doi: 10.1016/j.jhazmat.2020.124630 [20] WANG T, WANG S G, LUO Q Q, et al. Hydrogen adsorption structures and energetics on iron surfaces at high coverage [J]. The Journal of Physical Chemistry C, 2014, 118(8): 4181-4188. doi: 10.1021/jp410635z [21] KINNIBURGH D G, JACKSON M L, SYERS J K. Adsorption of alkaline earth, transition, and heavy metal cations by Hydrous oxide gels of iron and aluminum [J]. Soil Science Society of America Journal, 1976, 40(5): doi:10.2136/sssaj1976.03615995004000050047x. [22] NGUYEN T C, LOGANATHAN P, NGUYEN T V, et al. Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies [J]. Chemical Engineering Journal, 2015, 270: 393-404. doi: 10.1016/j.cej.2015.02.047 [23] LI Z T, WANG L, MENG J, et al. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil [J]. Journal of Hazardous Materials, 2018, 344: 1-11. doi: 10.1016/j.jhazmat.2017.09.036 [24] CASTRO L, BLÁZQUEZ M L, GONZÁLEZ F, et al. Heavy metal adsorption using biogenic iron compounds [J]. Hydrometallurgy, 2018, 179: 44-51. doi: 10.1016/j.hydromet.2018.05.029 [25] BOGAERTS M, SCHMIDT M W. Experiments on silicate melt immiscibility in the system Fe2SiO4-KAlSi3O8-SiO2-CaO-MgO-TiO2-P2O5 and implications for natural magmas [J]. Contributions to Mineralogy and Petrology, 2006, 152(3): 257-274. doi: 10.1007/s00410-006-0111-6 [26] LI Y K, ZHU X, QI X J, et al. Removal and immobilization of arsenic from copper smelting wastewater using copper slag by in situ encapsulation with silica gel [J]. Chemical Engineering Journal, 2020, 394: 124833. doi: 10.1016/j.cej.2020.124833 [27] SAIKIA B J, PARTHASARATHY G. Fourier transform infrared spectroscopic characterization of kaolinite from Assam and Meghalaya, northeastern India [J]. Journal of Modern Physics, 2010, 1(4): 206-210. doi: 10.4236/jmp.2010.14031 [28] PETIT T, PUSKAR L. FTIR spectroscopy of nanodiamonds: Methods and interpretation [J]. Diamond and Related Materials, 2018, 89: 52-66. doi: 10.1016/j.diamond.2018.08.005 [29] 姚时, 张鸣帅, 李林璇, 等. 茶渣负载纳米四氧化三铁复合材料制备及其对亚甲基蓝的吸附机理 [J]. 环境化学, 2018, 37(1): 96-107. doi: 10.7524/j.issn.0254-6108.2017050401 YAO S, ZHANG M S, LI L X, et al. Preparation of tea waste-nano Fe3O4 composite and its removal mechanism of methylene blue from aqueous solution [J]. Environmental Chemistry, 2018, 37(1): 96-107(in Chinese). doi: 10.7524/j.issn.0254-6108.2017050401
[30] ZHAO Z W, CHAI L Y, PENG B, et al. Arsenic vitrification by copper slag based glass: Mechanism and stability studies [J]. Journal of Non-Crystalline Solids, 2017, 466/467: 21-28. doi: 10.1016/j.jnoncrysol.2017.03.039 [31] YANG Z H, LIN Q, XIA J X, et al. Preparation and crystallization of glass-ceramics derived from iron-rich copper slag [J]. Journal of Alloys and Compounds, 2013, 574: 354-360. doi: 10.1016/j.jallcom.2013.05.091 [32] ARROYAVE J M, PUCCIA V, ZANINI G P, et al. Surface speciation of phosphate on goethite as seen by InfraRed Surface Titrations (IRST) [J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2018, 199: 57-64. doi: 10.1016/j.saa.2018.03.043 [33] YANG Z H, LIN Q, LU S C, et al. Effect of CaO/SiO2 ratio on the preparation and crystallization of glass-ceramics from copper slag [J]. Ceramics International, 2014, 40(5): 7297-7305. doi: 10.1016/j.ceramint.2013.12.071 -



 下载:
下载: