-
近年来,我国海水循环水养殖因高效节能、环境友好等优势发展迅速[1],但养殖循环水盐度高、养殖系统水力停留时间短、传统的生物处理法易出现氨氮及亚硝氮积累的问题,当氨氮含量超过0.2 mg·L−1会对水生生物造成毒害作用[2],因此,传统的水处理技术难以达到有效处理养殖循环水的目的。为了更好的处理养殖循环水,发展出了一系列新型处理技术,如生物滤器优化[3]、臭氧氧化[4]、电化学氧化等[5]。电化学氧化去除海水养殖循环水中的氨氮具有显著优势,不仅由于海水的高电导率降低了能耗,高氯离子浓度也保证了较高的间接氧化效率,使电化学氧化法在循环水养殖系统中的应用成为可能[6-7]。
电化学氧化过程与电极材料的催化活性及对目标污染物的选择性有直接关系,选择电活性高的催化剂可以增强电氧化效率。在各种电极材料中,Pt由于具有较高催化脱氢能力而成为目前最为理想的氨氮电催化材料[8]。反应中间产物Nads能够化学强吸附在电极表面,从而使催化活性下降;此外,Nads极大地消除了活性位点的数量,阻止氨在失活电极上氧化。由于Pt价格昂贵且资源匮乏,一般可将Pt负载在载体上以达到降低成本的目的,而且合适的载体可以削弱Nads在Pt上的吸附[9]。碳作为最常见的纳米颗粒载体具有比表面积大、电导率高和成本低等特点。但惰性材料不会增强电催化活性,而氮原子在外层轨道上比碳原子多一个电子,可增强复合碳材料的给电子能力,从而增强其催化活性[10]。因此,研究人员将碳与氮掺杂作为纳米颗粒载体[11]。Ribeiro等研究表明,Pt/CN 5上2 h的电流密度比Pt/C高约400%,碳掺杂氮载体对Pt纳米粒子的电氧化性能有改善作用[12]。
为了提高电催化氧化法处理海水养殖循环水中氨氮的效率,本文采用碳掺杂氮(CN)作为Pt纳米颗粒的载体,制备并优化复合电氧化催化剂CN-Pt,通过电催化氧化法处理模拟海水养殖循环水,研究电压、转速、催化剂负载试剂浓度、pH参数对氨氮去除率的影响并优化实验条件;同时考察电解过程中可能产生的羟基自由基、硫酸根自由基、活性氯等活性氧化物质对氨氮去除的影响,为电化学氧化技术在海水养殖循环水处理中的应用提供技术参考。
-
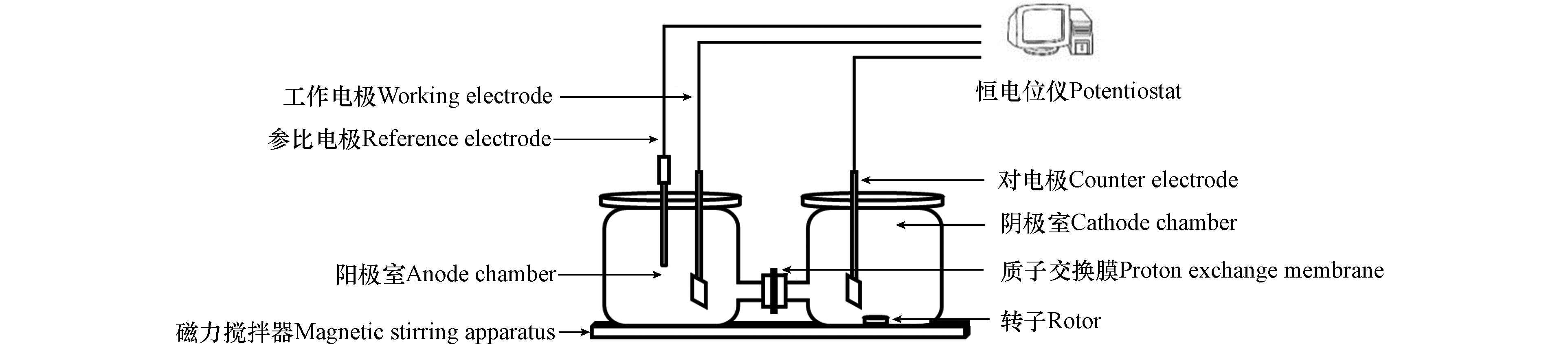
实验装置(图1)主要由 H 型双室结构电化学反应器(主体为玻璃,密封盖为聚四氟乙烯,单个反应池体积 200 mL,天津艾达恒晟科技发展有限公司)、恒电位仪、磁力搅拌器组成。系统采用三电极体系,工作电极为一定量催化剂负载PVDF 复合碳纤维电极(4 cm2),对电极为铂片电极(1 cm2),参比电极为饱和甘汞电极(SCE),工作电极与对电极间距 9.5 cm,两室间采用质子交换膜(Nafion 117,Dupont,美国)分隔,使用恒电位仪提供恒压直流电。
主要仪器为恒电位仪(DJS-292,上海雷磁创益仪器仪表有限公司)、总有机碳分析仪(TOC-L CPN CN200,岛津)、YSI多参数水质分析仪(Professional Plus)、可见分光光度计(SP-722E)、电化学工作站(PARSTAT 3000A-DX,Princeton Applied Research,USA)、场发射扫描电镜(Nova Nano SEM 4570,FEI,USA)、铂片电极(天津艾达恒晟科技发展有限公司)、甘汞电极(上海雷磁创益仪器仪表有限公司)等。
-
将尿素(质量分数分别为1%、2.5%、5%)和碳的混合物在氮气气氛下于管式炉中800 ℃加热1 h。混合物取出离心、清洗后,在50 ℃ 真空干燥24 h。不同C、N比例载体记为CNx。
通过醇还原法制备CN-Pt:将 H2PtCl6·6H2O(质量分数分别为10%、15%、20%)、45 mL乙二醇和15 mL水均匀混合,向混合液中添加CN,超声20 min,在开放气氛环境下150 ℃回流3 h。混合物取出离心、清洗后,在50 ℃真空干燥24 h,即得所需CN-Pt(CNx-y%Pt)催化剂。
-
为考察不同电压、转速、催化剂负载试剂浓度、pH对氨氮去除效果的影响,本实验采取正交实验对操作参数进行优化,如表1所示。实验用水为模拟海水养殖循环水:氨氮质量浓度4 mg·L−1,SO42-质量浓度2.71 g·L−1,NaCl质量浓度 33 g·L−1,根据不同情况调节溶液pH,溶液 pH 用质量分数为5% H2SO4和25% NaOH调节。反应时间为60 min。
-
为考察电氧化过程中可能产生的羟基自由基、硫酸根自由基对氨氮去除的影响,使用最优实验条件对模拟养殖循环水进行处理,反应时间20 min。模拟海水养殖循环水组成:氨氮质量浓度4 mg·L−1,NaCl质量浓度 33 g·L−1,向反应体系中分别加入硫酸钠(SO42-质量浓度2.71 g·L−1)及自由基淬灭剂(质量浓度10 mmol·L−1)。
-
实际养殖水体取自欧洲舌齿鲈(Dicentrarchus labrax)循环水养殖系统,反应时间为40 min,在处理过程中并未改变其水质参数。水质参数:pH 8、溶解氧浓度5.64 mg·L−1、氨氮浓度1.35 mg·L−1、亚硝氮浓度0.33 mg·L−1、硝氮浓度5.31 mg·L−1。
-
实验过程中各水质指标测定方法均参照《海洋监测规范》(GB 17378.4—2007)。氨氮浓度采用次溴酸盐氧化法,亚硝酸盐浓度采用萘乙二胺分光光度法,硝酸盐浓度采用锌镉还原法,pH使用 YSI 多参数分析仪现场测定。
对催化剂采用循环伏安法(CV)进行电化学性能评价,电解质溶液分别为KOH(1 mol·L−1)、KOH(1 mol·L−1)+ NH3·H2O(0.5 mol·L−1)、KOH(1 mol·L−1)+ NH4Cl(0.5 mol·L−1)、KOH(1 mol·L−1)+(NH4)2SO4(0.25 mol·L−1)。循环伏安测试在电化学工作站上进行,采用三电极体系,其中工作电极为玻碳(GC)电极,工作面积为 0.03 cm2,电极上涂覆一层催化剂和nafion溶液的混合液;对电极为铂片电极;参比电极为氧化汞(Hg/HgO)。使用扫描电子显微镜(SEM)对催化剂进行观察和分析;采用X射线光电子能谱(XPS)、X射线衍射(XRD)对催化剂物理化学性能进行分析。
-
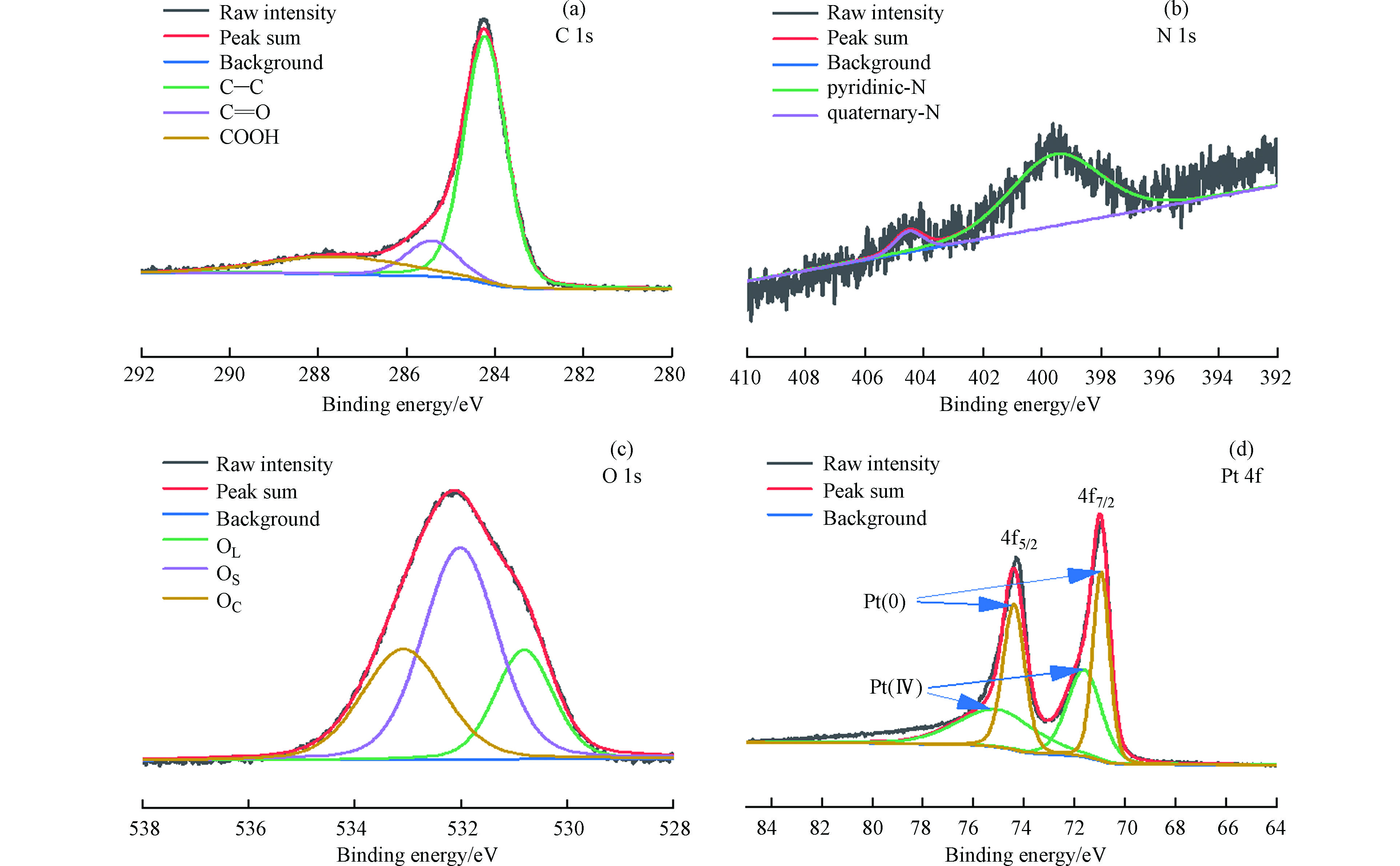
CN1+15%Pt样品的扫描电子显微镜(SEM)图像如图2a、2b所示,Pt纳米粒子沉积在载体表面;元素映射(图2c)进一步证明Pt纳米粒子负载在载体表面且分散性较强。在催化剂CN1+15%Pt的XRD图谱中(图2d),峰值为39°、46°、67°、81° 2θ归属于Pt的面心立方(fcc)结构,分别对应于(111)、(200)、(220)和(311)晶面[13-14];通过Scherrer方程和(220)峰[11, 15]估算 CN1+15%Pt 晶体尺寸为1.56 nm,与SEM观察结果吻合。
为了获得催化剂结合状态的信息,记录了XPS C 1s、N 1s、O 1s和Pt 4f核水平谱。C 1s谱(图3a)被分解成3个峰,284.22 eV左右的主导峰归属于石墨碳相,而另两个峰对应于碳氧键结构[16-17]。在399.54 eV、404.5 eV出现两个峰,分别对应于吡啶型氮及季铵盐(图3b)[18],表面存在的N非常少,然而,很明显,N存在于掺尿素的碳中。
在530.81 eV、532.02 eV和533.09 eV处观察到与表面氧有关的3个峰(图3c),分别对应于晶格氧(OL),表面氧种与金属原子结合(OS),氧原子与碳以双键结合(OC)[16]。反褶积后的Pt 4f谱由两个自旋轨道双峰组成(图3d),分别为金属Pt和PtO2相,分别具有约70.09 eV和71.62 eV的Pt 4f 7/2组分的束缚能[13, 19]。
-
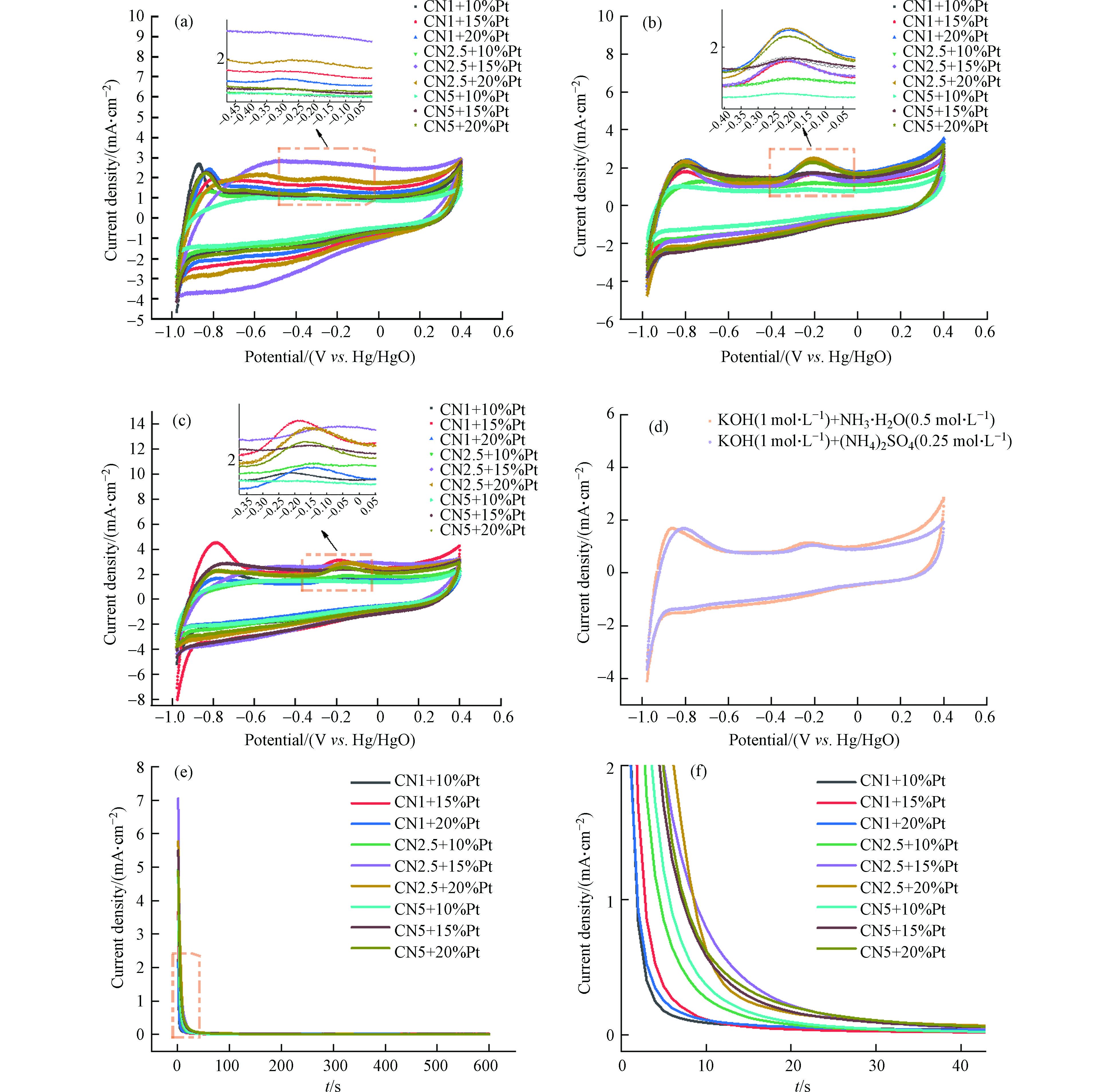
在图4a中,多数样品表现出典型的铂基催化剂电化学行为,所有催化剂的氢吸附/解吸区域位于−1.0 V到−0.4 V(vs. Hg/HgO)的电位范围内[13, 20]。在正向扫描过程中,-0.45 V到 +0.1 V电位范围内的峰值是由于Pt表面的氧化[13, 21]。
${\rm{NH}}_4^{+} $ 存在时(图4b),在约−0.2 V出现明显氧化峰,系氨的电化学氧化峰。${\rm{NH}}_4^{+} $ 、Cl− 同时存在时(图4c),在−0.18 V左右出现明显的氨氧化峰,且CN1+15%Pt的起始电位最低。有研究表明较低的起始电位,催化剂表面Nads的密度就越低,因此低起始电位有利于氨氧化活性的增强[22],故本实验中 CN1+15%Pt的氨氧化能力最强。在${\rm{SO}}_4^{2-} $ 存在的情况下,氨氧化峰无显著变化,说明硫酸根或可能产生的硫酸根自由基对氨氧化均无影响(图4d)。由于催化剂载体元素组成相同,只有比例变化,故所有催化剂的计时安培曲线形状整体相似(图4e、4f)。但可以看出,电流在初始阶段随时间显著降低,该过程与催化剂失活有关。失活主要是由于非活性中间产物(如Nads)化学强吸附在电极表面,随后电流密度的逐渐衰减是由于电极表面上电活性物质的消耗[13, 15, 23]。值得注意的是,所有催化剂的稳态电流只有细微变化,但CN2.5+15%Pt 催化剂的劣化速率最慢。
使用CN1+15%Pt、CN2.5+15%Pt、CN5+20%Pt 对氨氮去除进行分析。通过去除氨氮的量确定催化剂最优配比。实验结果表明(图5),CN1+15%Pt对氨氮去除量最大。
-
采用正交实验优化反应条件,通过极差分析法分析实验结果(表2),各因子对氨氮去除效果的主次顺序为:催化剂负载试剂浓度C0、电压、磁力搅拌转速、pH。最优水平组合为C0 100 mg·mL−1,电压1.7 V vs. SCE,转速300 r·min−1,pH 10。当转速为0时,氨氮去除效果不明显;催化剂负载试剂浓度越高,处理的效果越好;电压从1.5 V提升到1.7 V,氨氮去除效率明显升高。但催化剂负载试剂浓度过高,会导致成本升高;电压过大会造成电流效率低下、能耗高等问题。在最优实验参数下处理模拟养殖废水,20 min氨氮去除率为79.89%。
-
前期研究已表明,电化学氧化处理养殖海水可通过电氧化及其产生的活性氯,实现氨氮的直接/间接氧化[24],然而在高浓度Cl−存在情况下,直接氧化对于氨氮去除的贡献可以忽略不计[25]。另外在电化学氧化过程中也可产生其他具有强氧化性的活性物质,如羟基自由基、硫酸根自由基及活性氧等[26-27]。因此,为探明本实验中是否存在除活性氯外的其他氧化性物质参与氨氮去除过程(包括羟基自由基和硫酸根自由基),通过考察电解液中添加·OH淬灭剂叔丁醇(10 mmol·L−1)[28-29]或硫酸根离子对氨氮去除率的影响,研究是否存在羟基自由基或硫酸根自由基去除氨氮的作用,进而阐明氨氮去除的主要作用因子。
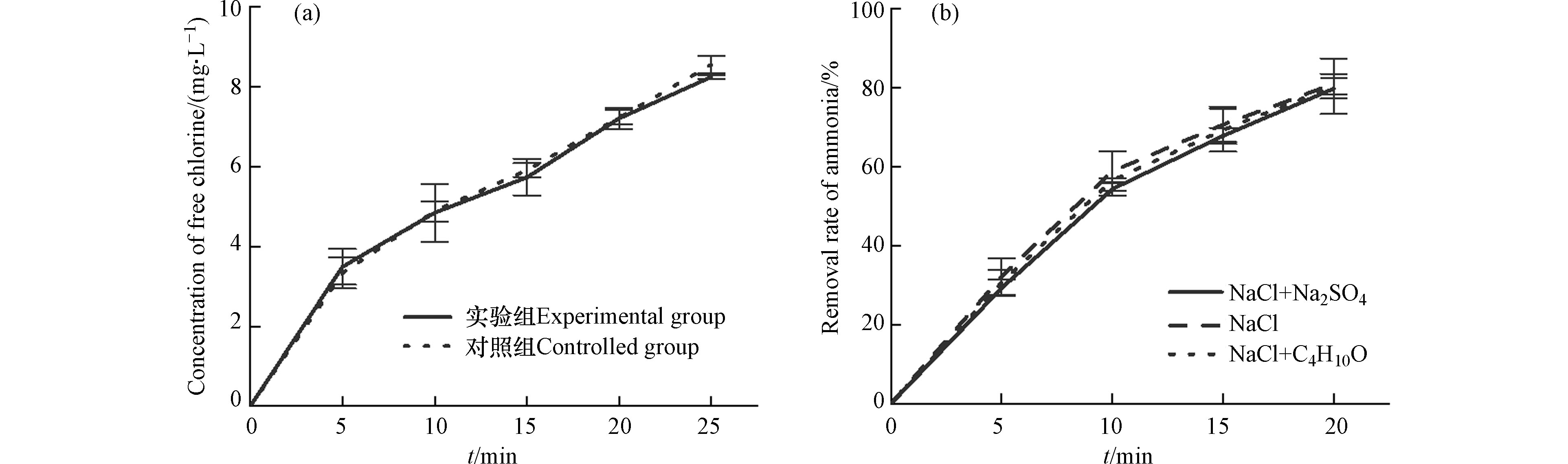
首先,在NaCl配置的模拟海水中,添加叔丁醇对活性氯浓度无影响,可排除叔丁醇对活性氯的淬灭作用(图6a)。其次,在NaCl和氨氮配置的模拟养殖海水中,叔丁醇对电解20 min的氨氮去除率无显著影响,说明系统中基本不存在羟基自由基参与氨氮去除过程。最后,模拟养殖海水中添加硫酸钠对氨氮去除效率无显著影响,说明硫酸根或可能产生的硫酸根自由基对氨氮去除均无影响。故本实验中氨氮去除的主要作用因子为活性氯。
实际养殖循环海水的处理实验中,除pH值外其他实验条件均采用正交实验的最优结果,主要原因是:(1)为了更符合实际水处理条件;(2)pH值对氨氮去除影响最小。因此,在实验条件(C0 100 mg·mL−1、电压1.7 V vs. SCE、转速300 r·min−1、pH 8)下对实际养殖循环水进行处理,电解40 min时,氨氮的去除率达97.04%,在10 min内亚硝氮完全去除,硝氮含量由5.31 mg·L−1增加至7.43 mg·L−1(图7)。在10 min后,硝氮含量保持不变,但氨氮浓度持续降低,40 min时,氨氮浓度为0.04 mg·L−1。
-
通过CV、计时安培及氨氮去除效果优化催化剂配比,最优催化剂配比为CN1+15%Pt,其氧化起始电位为−0.34 V vs. Hg/HgO,峰值电流密度为0.69 mA·cm−2,氨氮去除量最高,电氧化氨氮能力最强。SEM、XRD、XPS结果表明,Pt纳米粒子成功负载在CN载体上,且分散性较好,晶体直径约1.56 nm。
通过正交实验得出影响氨氮去除的主次顺序依次为:催化剂负载试剂浓度C0、电压、磁力搅拌速度、pH;最优实验参数为C0 100 mg·mL−1、电压1.7 V vs. SCE、转速300 r·min−1、pH 10。该实验条件下处理模拟养殖循环水,20 min氨氮去除率可达79.89%。
氨氮去除主要作用因子是活性氯,无羟基自由基或硫酸根自由基的作用。未改变水质pH且在实验条件C0 100 mg·mL−1 、电压1.7 V vs. SCE、转速300 r·min−1下对实际养殖循环水进行处理,电解40 min氨氮去除率为97.04%。
CN负载Pt电催化氧化去除海水养殖循环水中氨氮
Ammonia removal in aquaculture recirculating seawater by electrocatalytic oxidation of CN supported Pt modified anode
-
摘要: 本研究制备CN负载Pt催化剂(CN-Pt)电催化氧化高效去除海水养殖循环水中的氨氮。结果表明,优化催化剂组分为CN1+15%Pt时,氨氮的电催化活性最高,氧化起始电位为−0.34 V vs. Hg/HgO;通过正交试验得出,最优实验参数为催化剂负载试剂浓度C0 100 mg·mL−1、阳极电势1.7 V vs. SCE、转速300 r·min−1、pH 10;该实验条件下处理模拟海水养殖循环水,20 min氨氮去除率为79.89%。氨氮去除的主要作用因子是活性氯,无羟基自由基或硫酸根自由基的作用。在不改变pH条件下对实际养殖循环水进行处理,40 min氨氮去除率达97.04%。CN-Pt改性阳极电催化氧化去除海水养殖循环水中的氨氮效率高、易于操控,在循环水养殖系统的水处理中具有较大的应用潜力。Abstract: In this study, platinum nanoparticles supported on nitrogen-doped activated charcoal powder (CN-Pt) was prepared for efficient ammonia removal in mariculture recirculating seawater by electrocatalytic oxidation. The catalyst CN1+15%Pt showed the highest electrocatalytic activity of ammonia oxidation. The oxidation onset potential was −0.34 V vs. Hg/HgO. The orthogonal experiment indicated the optimal parameters were C0 100 mg·mL−1, anodic potential 1.7 V vs. SCE, rotational speed 300 r·min−1, and pH 10. Under these conditions, the ammonia removal rate was 79.89% in 20 min for the simulated aquaculture recirculating water treatment. The main functional factor is active chlorine, not hydroxyl radical nor sulfate radical. Treatment of actual aquaculture recirculating water without changing the pH, the ammonia removal rate reached 97.04% in 40 min. The CN-Pt modified anode electrocatalytic oxidation is used to remove ammonia in the recirculating seawater, this method is efficient and easy to operate, and of great application potential in the water treatment of recirculating aquaculture system (RAS).
-
Key words:
- ammonia /
- active oxygen species /
- electrochemical oxidation /
- Pt /
- recirculating aquaculture system
-

-
图 4 循环伏安图(CV) (a)不同催化剂在KOH (1 mol·L− 1)中; (b)不同催化剂在KOH (1 mol·L− 1)+ NH3·H2O (0.5 mol·L− 1)中; (c)不同催化剂在KOH (1 mol·L− 1)+ NH4Cl (0.5 mol·L− 1)中; (d)CN1+15%Pt在不同电解液中; (e) (f)不同催化剂在−0.2 V (vs. Hg/HgO)、KOH (1 mol·L− 1)+ NH4Cl (0.5 mol·L− 1)电解质中的计时安培结果及局部放大图
Figure 4. Cyclic voltammogram (CV) (a)of different catalysts in KOH (1 mol·L− 1); (b)of different catalysts in KOH (1 mol·L− 1)+ NH3·H2O (0.5 mol·L− 1); (c)of different catalysts in KOH (1 mol·L− 1)+ NH4Cl (0.5 mol·L− 1); (d)of CN1+15%Pt in different electrolytes; (e)Chronoamperometric results at −0.2 V (vs. Hg/HgO)for different catalysts and (f)partial enlarged detail
表 1 正交实验表
Table 1. Orthogonal experimental Table
水平
LevelA 电压/V
PotentialB 转速/(r·min−1)
SpeedC 催化剂负载试剂浓度/(mg·mL−1)
Catalyst loading reagent concentrationD pH 1 1.3 0 10 6 2 1.5 300 20 8 3 1.7 600 100 10 表 2 正交实验结果及分析表
Table 2. Orthogonal experimental results and analysis table
实验号
Experiment1 2 3 4 出水氨氮浓度/(mg·L− 1)
Ammonia concentration
in effluent电压/V
Potential转速/(r·min− 1)
Speed催化剂负载试剂浓度/(mg·mL− 1)
Catalyst loading reagent concentrationpH 1 1.3 0 10 6 2.74 2 1.3 300 20 8 1.99 3 1.3 600 100 10 0.02 4 1.5 0 20 10 2.55 5 1.5 300 100 6 0.15 6 1.5 600 10 8 2.56 7 1.7 0 100 8 0.98 8 1.7 300 10 10 0.08 9 1.7 600 20 6 0.06 kgp kg1 4.75 6.27 5.38 2.96 kg2 5.26 2.22 4.60 5.53 kg3 1.12 2.63 1.15 2.64 Kgp Kg1 1.58 2.09 1.79 0.99 Kg2 1.75 0.74 1.53 1.84 Kg3 0.37 0.88 0.38 0.88 Rg 1.38 1.35 1.41 0.96 -
[1] 刘鹰. 海水工业化循环水养殖技术研究进展 [J]. 中国农业科技导报, 2011, 13(5): 50-53. doi: 10.3969/j.issn.1008-0864.2011.05.08 LIU Y. Research progress on marine industrial recirculating aquaculture technology [J]. Journal of Agricultural Science and Technology, 2011, 13(5): 50-53(in Chinese). doi: 10.3969/j.issn.1008-0864.2011.05.08
[2] PRZYBYLKO A R M, THOMAS C L P, ANSTICE P J, et al. The determination of aqueous ammonia by ion mobility spectrometry [J]. Analytica Chimica Acta, 1995, 311(1): 77-83. doi: 10.1016/0003-2670(95)00177-2 [3] 张海耿, 张宇雷, 张业韡, 等. 循环水养殖系统中流化床生物滤器净水效果影响因素 [J]. 环境工程学报, 2013, 7(10): 3849-3855. ZHANG H G, ZHANG Y L, ZHANG Y W, et al. Influencing factors of purifying effluent of recirculating aquaculture system by fluidized-sand biofilter [J]. Chinese Journal of Environmental Engineering, 2013, 7(10): 3849-3855(in Chinese).
[4] 姜妍君, 强志民, 董慧峪, 等. 海水循环养殖系统水处理工艺综述 [J]. 环境化学, 2013, 32(3): 410-418. JIANG Y J, QIANG Z M, DONG H Y, et al. Water treatment processes in marine recirculating aquaculture systems: A review [J]. Environmental Chemistry, 2013, 32(3): 410-418(in Chinese).
[5] 宋协法, 边敏, 黄志涛, 等. 电化学氧化法在循环水养殖系统中去除氨氮和亚硝酸盐效果研究 [J]. 中国海洋大学学报(自然科学版), 2016, 46(11): 127-135. SONG X F, BIAN M, HUANG Z T, et al. Studies of the ammonia and nitrite removal by electrochemical oxidation in recirculating aquaculture system [J]. Periodical of Ocean University of China, 2016, 46(11): 127-135(in Chinese).
[6] LAHAV O, BEN ASHER R, GENDEL Y. Potential applications of indirect electrochemical ammonia oxidation within the operation of freshwater and saline-water recirculating aquaculture systems [J]. Aquacultural Engineering, 2015, 65: 55-64. doi: 10.1016/j.aquaeng.2014.10.009 [7] 叶章颖, 裴洛伟, 林孝昶, 等. 微电流电解去除养殖海水中氨氮效果 [J]. 农业工程学报, 2016, 32(1): 212-217. doi: 10.11975/j.issn.1002-6819.2016.01.030 YE Z Y, PEI L W, LIN X C, et al. Ammonia removal effect by using micro-current electrolysis in aquaculture saline water [J]. Transactions of the Chinese Society of Agricultural Engineering, 2016, 32(1): 212-217(in Chinese). doi: 10.11975/j.issn.1002-6819.2016.01.030
[8] de VOOYS A C A, KOPER M T M, van SANTEN R A, et al. The role of adsorbates in the electrochemical oxidation of ammonia on noble and transition metal electrodes [J]. Journal of Electroanalytical Chemistry, 2001, 506(2): 127-137. doi: 10.1016/S0022-0728(01)00491-0 [9] WANG Y H, YANG Z H, YANG J, et al. Towards continuous ammonia electro-oxidation reaction on Pt catalysts with weakened adsorption of atomic nitrogen [J]. International Journal of Hydrogen Energy, 2020, 45(41): 21816-21824. doi: 10.1016/j.ijhydene.2020.05.180 [10] DAEMS N, SHENG X, VANKELECOM I J, et al. Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction[J]. Journal of Materials Chemistry A[J]. Journal of Materials Chemistry A, 2014, 2(12): 4085-4110. [11] LIU S H, WU M T, LAI Y H, et al. Fabrication and electrocatalytic performance of highly stable and active platinum nanoparticles supported on nitrogen-doped ordered mesoporous carbons for oxygen reduction reaction [J]. Journal of Materials Chemistry, 2011, 21(33): 12489. doi: 10.1039/c1jm11488c [12] RIBEIRO V A, de FREITAS I C, NETO A O, et al. Platinum nanoparticles supported on nitrogen-doped carbon for ammonia electro-oxidation [J]. Materials Chemistry and Physics, 2017, 200: 354-360. doi: 10.1016/j.matchemphys.2017.07.088 [13] ASSUMPÇÃO M H M T, PIASENTIN R M, HAMMER P, et al. Oxidation of ammonia using PtRh/C electrocatalysts: Fuel cell and electrochemical evaluation [J]. Applied Catalysis B: Environmental, 2015, 174/175: 136-144. doi: 10.1016/j.apcatb.2015.02.021 [14] SILVA J C M, da SILVA S G, de SOUZA R F B, et al. PtAu/C electrocatalysts as anodes for direct ammonia fuel cell [J]. Applied Catalysis A: General, 2015, 490: 133-138. doi: 10.1016/j.apcata.2014.11.015 [15] LOMOCSO T L, BARANOVA E A. Electrochemical oxidation of ammonia on carbon-supported bi-metallic PtM (M = Ir, Pd, SnOx) nanoparticles [J]. Electrochimica Acta, 2011, 56(24): 8551-8558. doi: 10.1016/j.electacta.2011.07.041 [16] ZHANG L, LI F. Helical nanocoiled and microcoiled carbon fibers as effective catalyst supports for electrooxidation of methanol [J]. Electrochimica Acta, 2010, 55(22): 6695-6702. doi: 10.1016/j.electacta.2010.06.002 [17] dos REIS F V E, ANTONIN V S, HAMMER P, et al. Carbon-supported TiO2-Au hybrids as catalysts for the electrogeneration of hydrogen peroxide: Investigating the effect of TiO2 shape [J]. Journal of Catalysis, 2015, 326: 100-106. doi: 10.1016/j.jcat.2015.04.007 [18] SONG J, LIU L F, ZHANG G Q, et al. Oxygen reduction at carbon nanotubes (CNTs)/cobaltous phthalocyanine (CoPc) and MFC electricity generation affected by air-cathode catalyst layer structure [J]. Journal of the Electrochemical Society, 2016, 163(10): F1209-F1216. doi: 10.1149/2.0761610jes [19] ALI M, WITKOWSKA A, ABBAS M, et al. Evolution of the nanostructure of Pt and Pt-Co polymer electrolyte membrane fuel cell electrocatalysts at successive degradation stages probed by X-ray photoemission [J]. Journal of Power Sources, 2014, 271: 548-555. doi: 10.1016/j.jpowsour.2014.08.028 [20] DU X W, LUO S P, DU H Y, et al. Monodisperse and self-assembled Pt-Cu nanoparticles as an efficient electrocatalyst for the methanol oxidation reaction [J]. Journal of Materials Chemistry A, 2016, 4(5): 1579-1585. doi: 10.1039/C5TA09261B [21] JIANG L, HSU A, CHU D, et al. Ethanol electro-oxidation on Pt/C and PtSn/C catalysts in alkaline and acid solutions [J]. International Journal of Hydrogen Energy, 2010, 35(1): 365-372. doi: 10.1016/j.ijhydene.2009.10.058 [22] GOOTZEN J F E, WONDERS A H, VISSCHER W, et al. A DEMS and cyclic voltammetry study of NH3 oxidation on platinized platinum [J]. Electrochimica Acta, 1998, 43(12/13): 1851-1861. [23] ASSUMPÇÃO M H M T, da SILVA S G, de SOUZA R F B, et al. Investigation of PdIr/C electrocatalysts as anode on the performance of direct ammonia fuel cell [J]. Journal of Power Sources, 2014, 268: 129-136. doi: 10.1016/j.jpowsour.2014.06.025 [24] SONG J, YIN Y M, LI Y H, et al. In-situ membrane fouling control by electrooxidation and microbial community in membrane electro-bioreactor treating aquaculture seawater [J]. Bioresource Technology, 2020, 314: 123701. doi: 10.1016/j.biortech.2020.123701 [25] LI L, LIU Y. Ammonia removal in electrochemical oxidation: Mechanism and pseudo-kinetics [J]. Journal of Hazardous Materials, 2009, 161(2/3): 1010-1016. [26] F ARAÚJO K C, P BARRETO J P, CARDOZO J C, et al. Sulfate pollution: Evidence for electrochemical production of persulfate by oxidizing sulfate released by the surfactant sodium dodecyl sulfate [J]. Environmental Chemistry Letters, 2018, 16(2): 647-652. doi: 10.1007/s10311-017-0703-6 [27] 刘敏. 氨氮电氧化技术及其在养猪废水中的应用研究[D]. 上海: 上海大学, 2014. LIU M. Electro-oxidation technology for ammonia removal and its application in swine wastewater[D]. Shanghai: Shanghai University, 2014(in Chinese).
[28] 戴慧旺, 陈建新, 苗笑增, 等. 醇类对UV-Fenton体系羟基自由基淬灭效率的影响 [J]. 中国环境科学, 2018, 38(1): 202-209. doi: 10.3969/j.issn.1000-6923.2018.01.024 DAI H W, CHEN J X, MIAO X Z, et al. Effect of alcohols on scavenging efficiencies to hydroxyl radical in UV-Fenton system [J]. China Environmental Science, 2018, 38(1): 202-209(in Chinese). doi: 10.3969/j.issn.1000-6923.2018.01.024
[29] 林晓璇, 孔青青, 曾泳钦, 等. 酮洛芬在臭氧作用下的降解机制、产物及毒性 [J]. 环境化学, 2018, 37(5): 1063-1070. LIN X X, KONG Q Q, ZENG Y Q, et al. Study on mechanism, intermediates and toxicity of ketoprofen degradation by ozone [J]. Environmental Chemistry, 2018, 37(5): 1063-1070(in Chinese).
-



 下载:
下载: