-
抗生素被广泛用于治疗或预防人类和动物疾病,以多种途径进入环境,目前已在多种环境介质中被频繁检出[1-2]。随着其使用量的不断增加,环境中抗生素的浓度也随之提高,已然成为一个主要的全球公共卫生问题[3-4]。吸附法作为去除抗生素的有效方法之一,具有工艺简单、无二次污染、操作方便等特点[5]。然而,常用的吸附剂如金属有机纳米管[6] 、活性炭[7]、石墨烯[8]、碳纳米管[9]和生物碳材料[10]等,存在成本高且不能再生利用的缺点[5,11]。在众多的吸附剂中,黏土和黏土矿物因成本低和产量丰富而被广泛应用[12-13]。埃洛石(halloysite nanotubes, HNTs)是一种天然黏土矿物[14],它的晶体结构是由角共享硅氧四面体和边缘共享铝氧八面体组成的1:1型片层结构[15-16]。HNTs与其他铝硅酸盐矿物的主要区别在于其独特的纳米管状结构。HNTs具有相对较高的比表面积和总孔隙体积,这归因于HNTs结构中具有丰富的孔隙[17],可为吸附提供较多的反应位点。当HNTs用作吸附剂时,对客体分子仅表现出弱的亲和力(离子交换、氢键和范德华力),未修饰的HNTs由于含大量硅氧键,羟基较少,因此氢键作用力较小,且晶体结构中的孔道易因杂质而堵塞,降低了其对污染物的吸附[15-17]。为了提高HNTs的吸附性能,通常对HNTs进行改性。由于HNTs的外表面[18]、内腔表面[19]和层间表面[20]均可被改性,从而为HNTs的修饰提供了丰富的可能性。黏土表面具有天然的负电荷特性,可以通过吸附某些特定的阳离子来改性,迄今采用阳离子表面活性剂修饰黏土的研究较多,且修饰后对染料等有机污染物的吸附固定能力均有提高。而对于用阴阳离子表面活性剂复合改性及其应用的研究较少。因此,用离子表面活性剂对黏土矿物HNTs进行阴阳离子复合改性对抗生素吸附是否有作用,作用机理又是什么,这是一个值得探讨和验证的命题。
土霉素(OTC)作为渭河西安段检出频率最高的四环素类抗生素[21],在自然环境中的出现会影响微生物遗传变异的选择,随着其使用量的不断增加,不可避免地给人体健康和生态环境造成巨大压力[4]。基于此,为了探究阴-阳离子表面活性剂改性黏土矿物吸附水中抗生素的能力,本研究以HNTs为吸附剂,以土霉素(OTC)为吸附对象,采用十六烷基三甲基溴化铵(CTMAB)和十二烷基苯磺酸钠(SDBS)两种离子表面活性剂对HNTs进行改性,制备新型吸附材料阴阳离子改性埃洛石(C-S-HNTs)。考察了吸附剂投加量、吸附时间、初始浓度、温度和溶液pH对OTC在C-S-HNTs上吸附的影响,并对动力学模型、吸附等温模型及热力学模型进行拟合和讨论。结合XRD、FTIR、SEM、BET、zeta电位和XPS等多种表征手段,从吸附效果→表征→修饰机制的角度进行系统研究,目的在于建立起复配修饰黏土的复配修饰机制-表面特征-吸附效应之间的构效关系,为两性改性剂及其复配修饰黏土的理论及应用提供技术储备和数据支持。
-
天然HNTs购自天津福辰化学公司,纯矿物含量为99%,呈暗白色粉末状。通过氯化钡-硫酸法测定[22],其阳离子交换容量(CEC)为22.8 mmol·100g−1。阳离子表面活性剂CTAB和阴离子表面活性剂SDBS均购自凯美尔化学试剂有限公司(中国天津),纯度为90%。OTC购自阿达马斯试剂有限公司(上海)。本研究中使用的所有其他化学品,如HCl、NaOH均为分析级,无需进一步纯化。所有溶液均用蒸馏水配制。
-
使用前,将原生HNTs与适量去离子水混合,经过搅拌、超声使其在水中充分分散,清洗多次以去除HNTs表面可溶性盐类等杂质。然后于65 ℃的烘箱中干燥24 h,将其粉碎后筛过100目筛,装入透明密封袋并放置在干燥器备用。
表面活性剂改性HNTs样品的制备如下:用分析天平准确称取一定量的CTAB和SDBS,待其在装有适量蒸馏水的烧杯中溶解后,加入已提纯、干燥且过200目筛的原生HNTs,磁力搅拌器于25 ℃的条件下恒温搅拌24 h。待HNTs与表面活性剂交换反应后,以2500 r·min−1离心10 min固液分离,弃去上层溶液后用去蒸馏水清洗,重复7次以上操作以去除残留的有机改性剂。所得材料在65 ℃的烘箱烘干,然后于110 ℃的条件下活化1 h,研磨过200目筛,即制得C-S-HNTs。
-
为考察各种因素对吸附过程的影响,采取控制单一变量法进行批实验,所有试验均做3组平行,实验结果剔除异常值后取平均值绘制图表。通过高效液相色谱(戴安 U3000)测定吸附平衡前后的OTC浓度。按下式计算出吸附剂的吸附容量qe(mg·g−1)和吸附率Y(%)。
其中, C0 为吸附前OTC的初始浓度,mg·L−1; Ct为吸附后OTC的剩余浓度,mg·L−1;V 为OTC溶液的体积,L;m为吸附剂的投加量,g。
具体试验步骤如下:
(1)考察不同吸附时间的影响。OTC溶液初始浓度C0为50 mg·L−1,溶液初始pH=6.0,T=30 ℃,吸附剂投加量为1 g·L−1,吸附时间为0—48 h。
(2)考察污染物初始浓度的影响。OTC溶液初始pH=6.0,T=30 ℃,吸附剂投加量1 g·L−1,吸附时间24 h,OTC溶液初始浓度C0为10—200 mg·L−1。
(3)考察不同反应温度的影响。C0为50 mg·L−1,溶液初始pH=6.0,吸附剂投加量为1 g·L−1,吸附时间24 h,反应温度为30、40、50 ℃。
(4)考察不同初始pH的影响。C0为50 mg·L−1,T=30 ℃,吸附剂投加量为1 g·L−1,吸附时间24 h,溶液初始pH设定分别为3、4、5、6、7、8、9、10。
-
实验数据拟合所采用的吸附动力学方程公式如下:
(1)准一级动力学方程:
(2)准二级动力学方程:
(3)颗粒内扩散模型方程:
其中,qt 为在t时刻的吸附量,mg·g−1;qe 为平衡吸附量,mg·g−1;K1 为准一级动力学常数,min−1;K2 为准二级动力学常数,min−1;Kp 为颗粒内扩散速率常数,mg·g−1·min−0.5。
-
实验数据拟合所采用的等温吸附方程公式如下:
(1)Langmuir模型:
其中,qm 为最大吸附量,mg·g−1;qe 为平衡吸附量,mg·g−1;Ce 为 OTC吸附平衡浓度,mg·L−1;KL 为Langmuir模型参数。
(2)Freundlich模型:
其中,qe 为平衡吸附量,mg·g−1;Ce 为 OTC吸附平衡浓度,mg·L−1;KF为 Freundlich 模型参数。
(3)Dubinin-Radushkevich(D-R)模型:
其中,qe为平衡吸附量,mg·g−1;Qm为最大吸附量,mg·kg−1;Ce 为吸附平衡浓度,mol·L−1;R 为气体常数,8.314 J·mol−1·K−1;T 为反应温度,K;E 为吸附自由能,kJ·mol−1;ε 为波兰势能;β 为吸附参数。由E可判断吸附机制,当E小于8 kJ·mol−1时,物理吸附是体系吸附机制;当E在8 kJ·mol−1到16 kJ·mol−1之间时,主要的吸附机制为离子交换;当E大于16 kJ·mol−1时,吸附既包含物理吸附,又包含化学吸附。
-
实验数据所采用的吸附热力学方程公式如下:
其中,K为分配系数,mL·g−1;T 为吸附反应时的绝对温度,K;R 为理想气体状态常数,8.314 J·mol−1·K−1。
-
使用Bruker D8 Advance X射线粉末衍射仪对矿物的晶体结构进行测定,X射线源为CuKα,辐射波长为0.154 nm,扫描步长为0.02 °,速度为每步17.7 s,扫描电压/电流为40 kV/40 mA,样品采取压片处理,温度为室温条件下对材料在10°—80°范围内进行扫描分析;使用PerkinElmer 1725X 傅利叶变换红外光谱仪(FTIR)研究HNTs和C-S-HNTs所含有的官能团;样品采用KBr粉末压片法处理,分辨率为0.3 cm−1,扫描波长范围为400—4000 cm−1;使用Merlin型扫描电子显微镜对矿物表面形貌进行分析,电流为80 μA,加速电压为20 kV;使用Micromeritics ASAP 2020装置对矿物的比表面积进行测定,80 ℃下脱气12 h,孔径分析范围为3.5 Å到5000 Å,并依据Brunauer-Emmett-Teller(BET)理论进行分析;使用Nano ZS90型Zeta电位分析仪测定不同pH值下的zeta电位测量值,光源为He-Ne;使用配备Al-Ka单色仪X射线源的Thermo Scientific Escalab 250Xi光谱仪进行X射线光电选择光谱(XPS)分析,X射线束斑20—900 μm,能量扫描范围0—5000 eV。
-
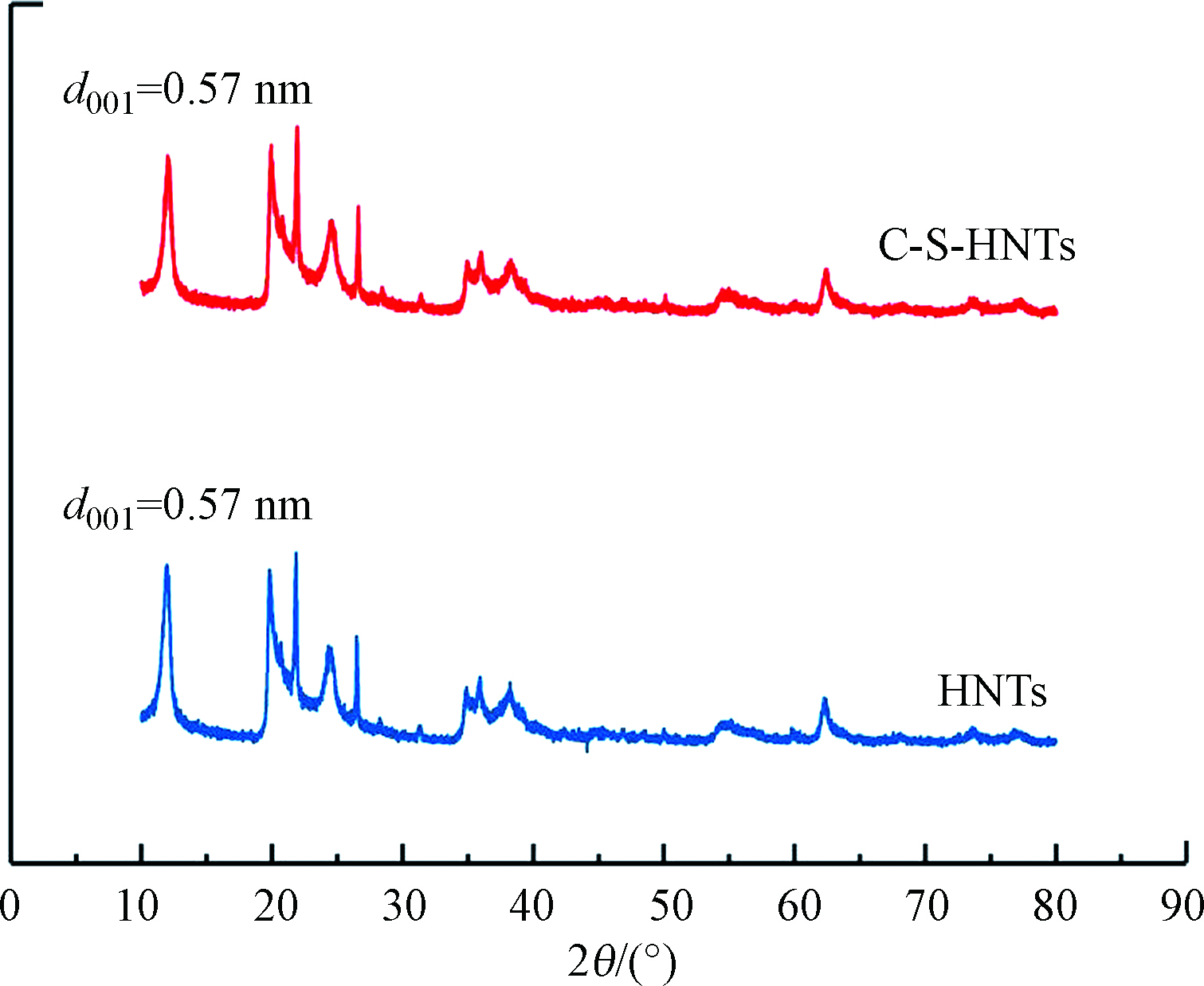
黏土矿物的不同结构和层间环境可能导致CTAB和SDBS在其层间的排列方式不同。图1所示,HNTs的2θ角为11.960°,对应层间距为0.57 nm,表明样品为0.7 nm HNTs。C-S-HNTs的2θ角为11.920°,对应层间距为0.57 nm。与HNTs相比,CTAB-SDBS修饰后的HNTs在XRD图上并未检测到新的衍射峰,峰的尖锐程度变化不大,同时也没有特征峰的偏移,C-S-HNTs层间距几乎保持不变,表明CTAB-SDBS分子未插入HNTs的层间,而是负载在HNTs的表面上,C-S-HNTs在修饰后依然保持了HNTs初始晶体结构。这主要是因为HNTs为 1∶1 型非膨胀型的黏土,层间可交换性阳离子较少[23],修饰剂难以进入HNTs的层间,使得CTAB和SDBS主要修饰在HNTs表面[24]。且HNTs的层间内表面Al-OH基团由于被层间强氢键所阻断,大多不可用于接枝。
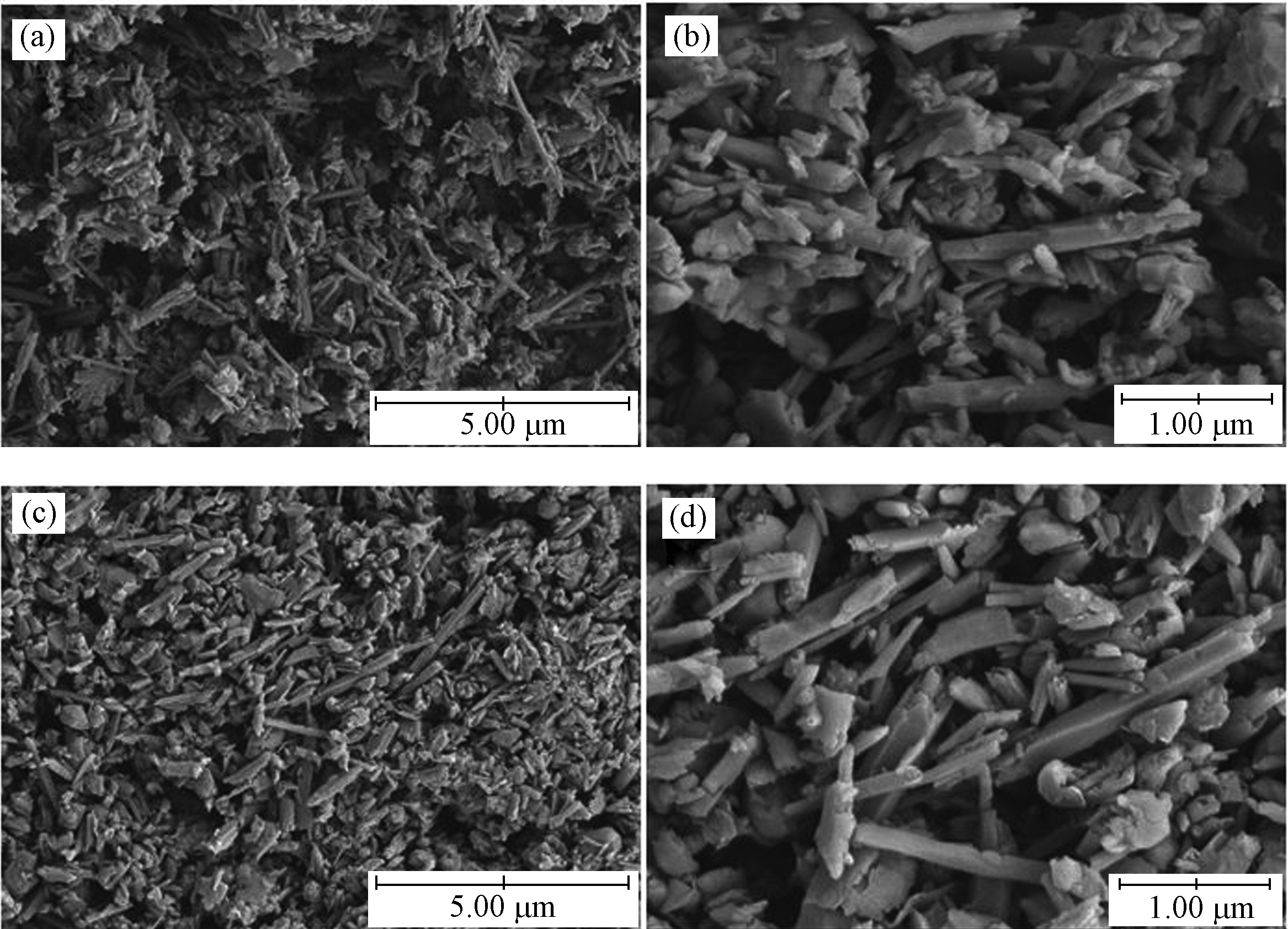
HNTs含有大量的纳米管和少量杂质的团聚体(图2)。HNTs的长度范围从0.05 μm到1.50 μm不等,内径为10—20 nm,壁厚为10—15 nm。与HNTs相比,有机改性后,C-S-HNTs仍能观察到中空管状结构,说明CTAB-SDBS改性对HNTs的管状形貌无明显影响。 结合XRD分析,可以得出CTAB-SDBS修饰对HNTs的晶体结构和形貌没有影响。
-
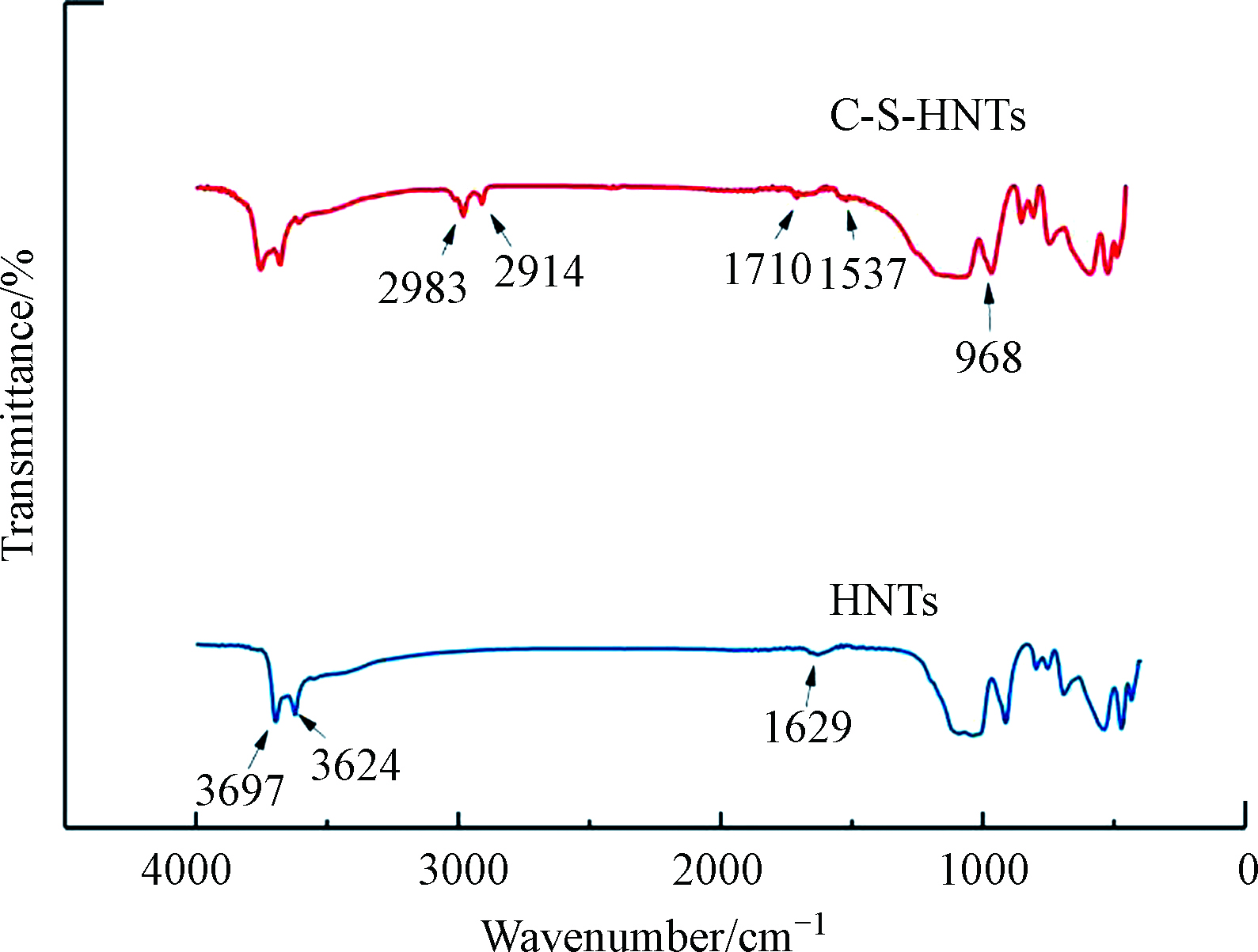
在3000—4000 cm−1范围内仅有两个羟基的吸收峰,分别在3624 cm−1和3697 cm−1处(图3)。这两处吸收峰分配给两个Al2OH拉伸带,每个羟基和两个Al原子相连[25]。这两个吸收峰归属于晶体中含有的两种类型的羟基基团:外羟基基团和内羟基基团。外羟基位于硅氧四面体和铝氧八面体构成的层状结构的外部非共享面上,而内羟基基团则位于硅氧四面体和铝氧八面体构成的层状结构的共享面上[26]。3697 cm−1附近吸收带表明羟基与层间H2O的氢键存在。在低波数400—2000 cm−1范围内,HNTs的吸收峰主要是一些Si—O和Al—O的吸收[27]。不同产地和纯度的HNTs的红外吸收峰略有不同。在中频区1618—1636 cm−1范围内出现吸附水中羟基弯曲振动带[28-29],在波数为1037 cm−1处出现Si—O的伸缩振动带以及在波数为914 cm−1处出现的震动带则是由内部羟基O—H变形引起[30]。在低频区682—754 cm−1范围内出现O—H的弯曲振动带以及540、470、432 cm−1等附近的Si—O弯曲振动带[30-31]。在796 cm−1和750 cm−1的波段可以被分配到HNTs O—H单元的O—H平移振动[30]。所有这些观察表明,在HNTs结构中存在不止一种类型的水。经过改性,与HNTs相比,C-S-HNTs中出现了两个新的吸收峰,分别位于2914 cm−1、2983 cm−1附近。这两个吸收峰分别对应—CH3、—CH2—的变形振动和伸缩振动[32],在1537cm−1处振动来自于CTAB的—CH2—失变振动。表明季铵盐阳离子成功接枝于HNTs表面,改性成功。与HNTs的光谱相比,C-S-HNTs的光谱显示内表面Al2OH基团的OH拉伸带(3656 cm−1)的强度并未降低,这表明改性并没有对内表面Al2OH进行消耗,即在这些基团之间没有发生接枝。其它吸收峰与处理前一致,FTIR结果进一步说明改性后基本结构并没有发生改变,能很好保持原有结构,这也与XRD结论一致。
-
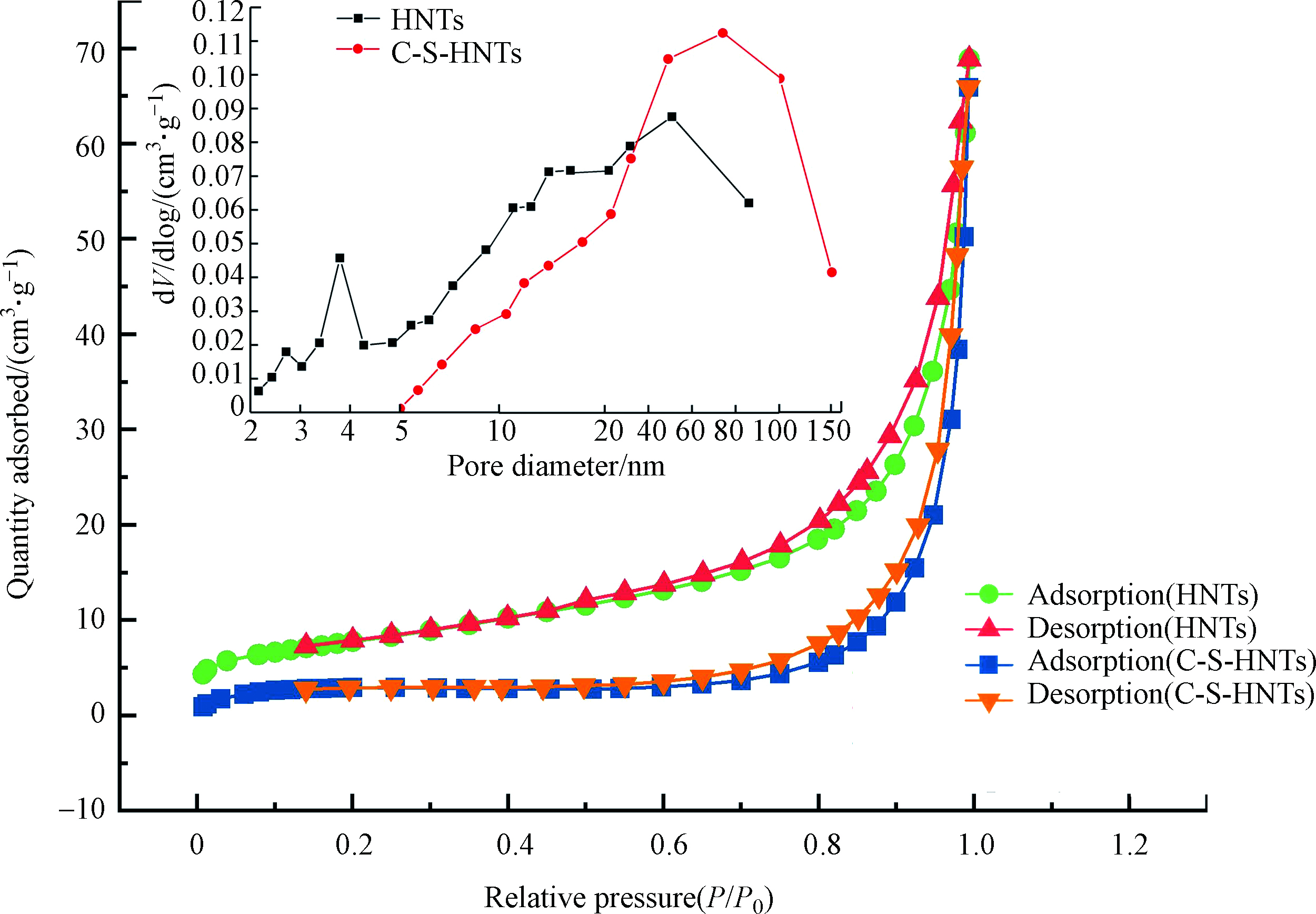
作为中空的纳米管状粒子,理论上HNTs应具有较高的比表面积,而实际测得HNTs比表面积为27.695 m2·g−1(表1)。这可能是由于HNTs表面缺陷较少从而导致其微孔较少。HNTs的氮气吸附脱附曲线呈现Ⅳ型吸附行为,且表现出H3滞后环,说明材料中存在介孔结构[33]。HNTs是表面多孔物质,可以看出HNTs的孔大小在2—100 nm范围(图4),表明HNTs既存在介孔也存在大孔。在3.7、16.8、50.9 nm处的峰分别对应于HNTs的表面结晶缺陷、管内径和HNTs管间搭接形成的孔,表明了HNTs的多孔性质。
值得注意的是,在CTAB-SDBS改性之后,HNTs的比表面积和孔容都表现出了明显的下降,而孔径却有所上升(表1)。比表面积的减小,可能是由于嫁接在HNTs表面的有机链阻断了一些结构通道,从而导致更少的N2能进入其间。而孔容的减少可能是存在其外表面的有机物引起的。比表面积减小后,吸附量却增加,这是由于OTC分子量有460.434 g·mol−1,巨大的分子空间构型使得OTC无法进入内部,HNTs内部的吸附位点无法利用,改性之后,虽然比表面积减小,但是由CTAB-SDBS的负载提供的吸附位点却增多,所以吸附量增大。另一方面,改性后的HNTs孔径增大的同时也增大了HNTs的吸附位点,也印证了C-S-HNTs对OTC的吸附性能优于 HNTs的实验结果。因此,可以推测,CTAB-SDBS成功负载在了HNTs表面但没有进入HNTs层间,XRD和FTIR表征同样证明了这一结论。
-
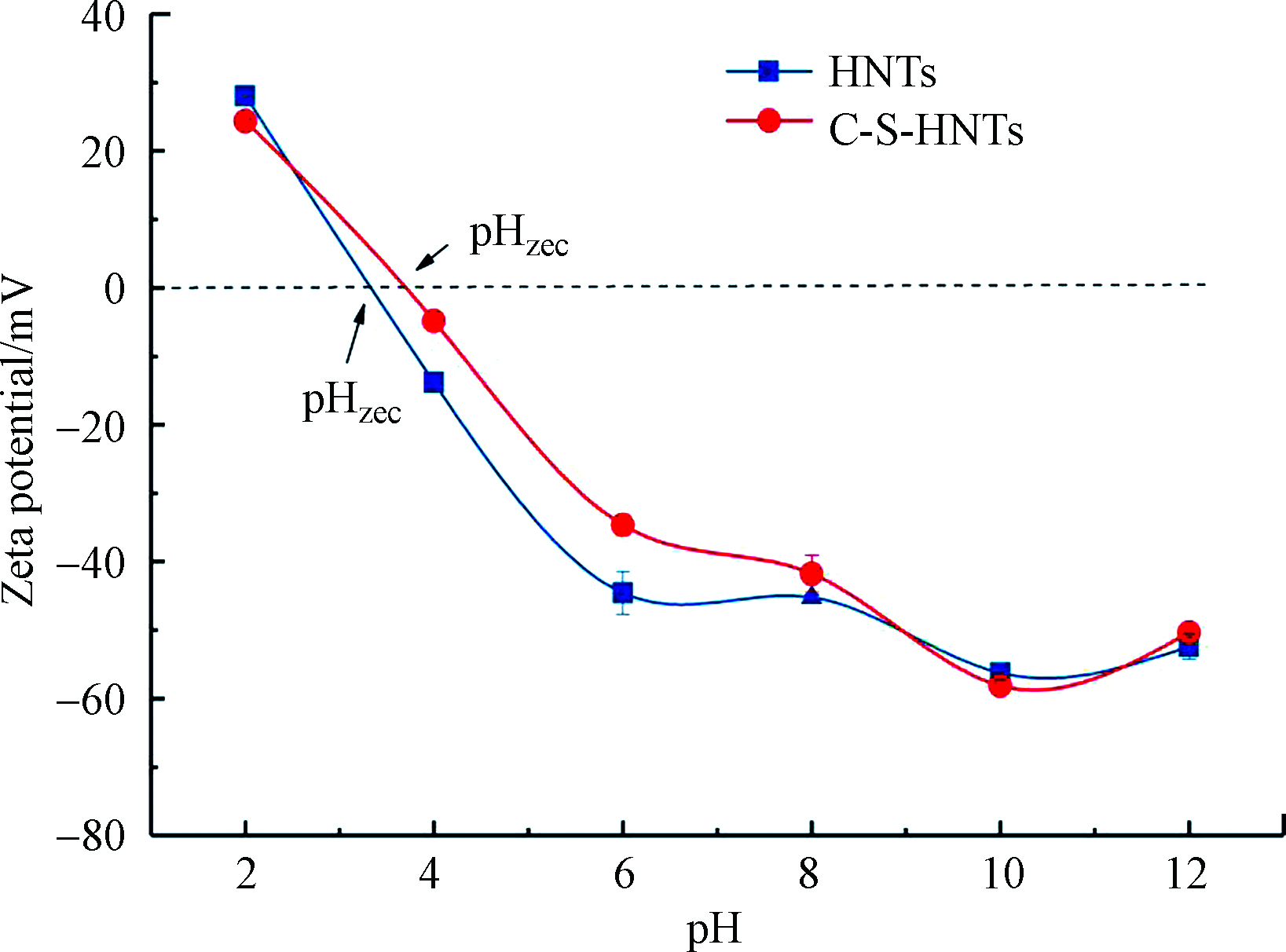
通过 Zeta 电位可以分析比较表面的电荷特性随溶液 pH 值变化的情况,这关系着吸附剂与污染物之间的静电吸附情况。经测试分析可知HNTs的等电点为3.32,当pH值大于等电点时HNTs的Zeta电位均为负值(图5),说明pH在3.32—12范围内HNTs表面均带负电荷,这是由于HNTs的表面主要是硅酸盐[34],因此在广泛的pH范围为负表面电势。经过改性后,C-S-HNTs在pH=2时的Zeta电位值为23.52 mV,此时材料表面带有正电荷;在pH值为3.0—4.0之间出现等电点(值为3.74),随后电位值全部为负值并逐渐下降,但其电位值均要小于同等条件下HNTs的Zeta电位值。正电荷的出现表明CTAB-SDBS上能在强酸性条件下(pH=2),使HNTs表面的负电性有所降低。阳离子间的静电相互作用也发挥了一定作用。此外,通过与HNTs的Zeta电位值相比,改性后的HNTs均带有较低的负电荷,这与CTAB-SDBS的结合减弱了HNTs表面的水解作用有关[24]。由于OTC分子的pKa为3.3、7.7和9.7[24],其在不同pH条件下以不同离子形式存在,故HNTs表面的电性及所带电荷量的不同会影响其对OTC的吸附能力。
-
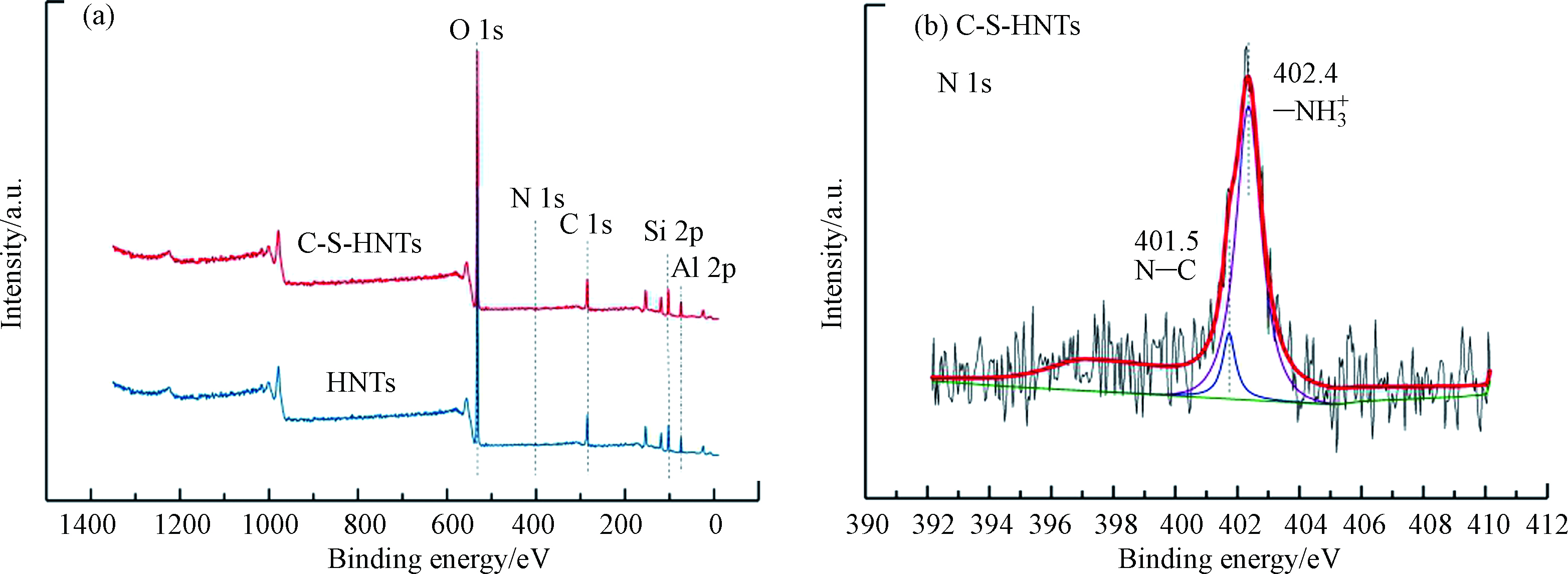
图6a给出了样品特征元素的吸收峰,可以清楚看出HNTs表面都有较强的O1s、C1s、Si2p和Al2p等特征峰。经过改性后,在C-S-HNTs中发现C元的信号增加,由20.31%提高至39.94%,且出现较弱N 1s特征峰,表明CTAB-SDBS成功附着在C-S-HNTs表面。C-S-HNTs中N 1s的高分辨率XPS光谱如图6b所示。在结合能401.5 eV和402.4 eV处有两个明显的峰,401.45 eV对应N—C[35],质子氨基(—NH3+)的标准结合能在400.5—402.5 eV区域[36],因此,402.4 eV的峰值属于—NH3+。—NH3+可能来自于SDBS通过氢键与HNTs的表面羟基相互作用[37]。
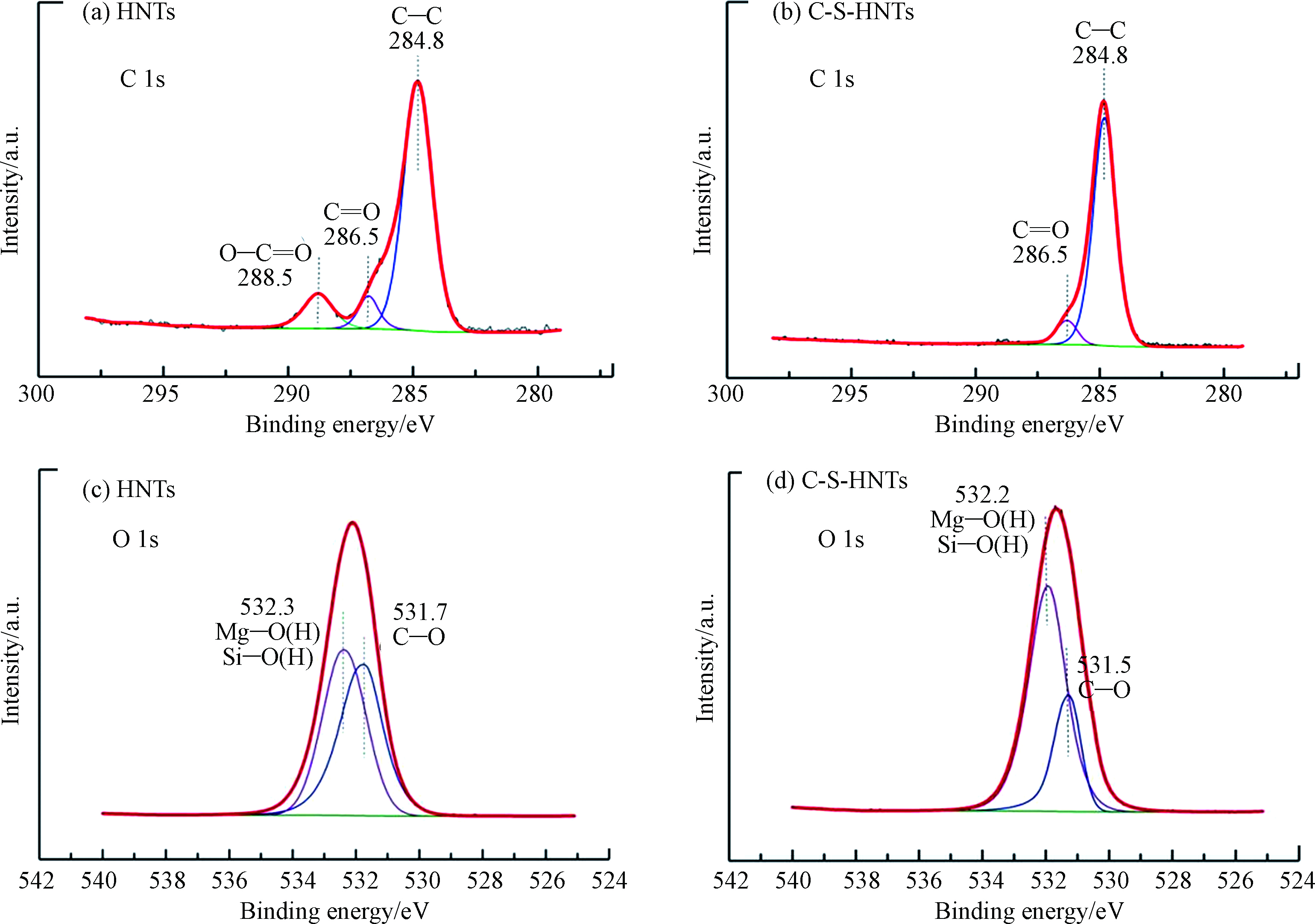
为进一步确定C-S-HNTs和HNTs表面元素化合态及其化学位移的变化情况,分别对C1s、O1s、N1s进行XPS分析。由图7a、b知,HNTs中C1s能谱可拟合为3个峰,结合能284.8、286.5、288.5 eV处分别对应为C—C、C=O[38]、O—C=O[39],C-S-HNTs中C1s能谱可拟合为2个峰,结合能284.8、286.5 eV处分别对应为C—C和C=O。在两个样品中,C—C和C=O键的结合能分别保持在284.8 eV和286.5 eV,分别对应于长烷基链中的C—C键和来自大气吸附的二氧化碳的C=O键[40]。与HNTs相比,C-S-HNTs中的C—C键对应的峰增强,这是因为表面活性剂会将长烷基链带到HNTs上,导致284.8 eV的信号增加。288.5 eV处对应的峰消失,可能与样品中的有机杂质有关,在改性过程中被冲洗掉。
图7c、d结合能532.3 eV和532.2 eV处分别对应硅氧四面体和铝氧八面体。图中未出现H2O特征峰,说明HNTs为0.7 nm HNTs,不含有层间结合水。与HNTs相比,C-S-HNTs [SiO4]和[AlO6]结合能降低了0.23 eV,表明[SiO4]和[AlO6]电子密度都增加。结合能的变化小于1 eV,证实了HNTs与表面活性剂之间的相互作用较弱。因此,表面活性剂很可能通过静电吸引在HNTs上负载。所有结果均表明在HNTs的表面存在有机表面活性剂。
-
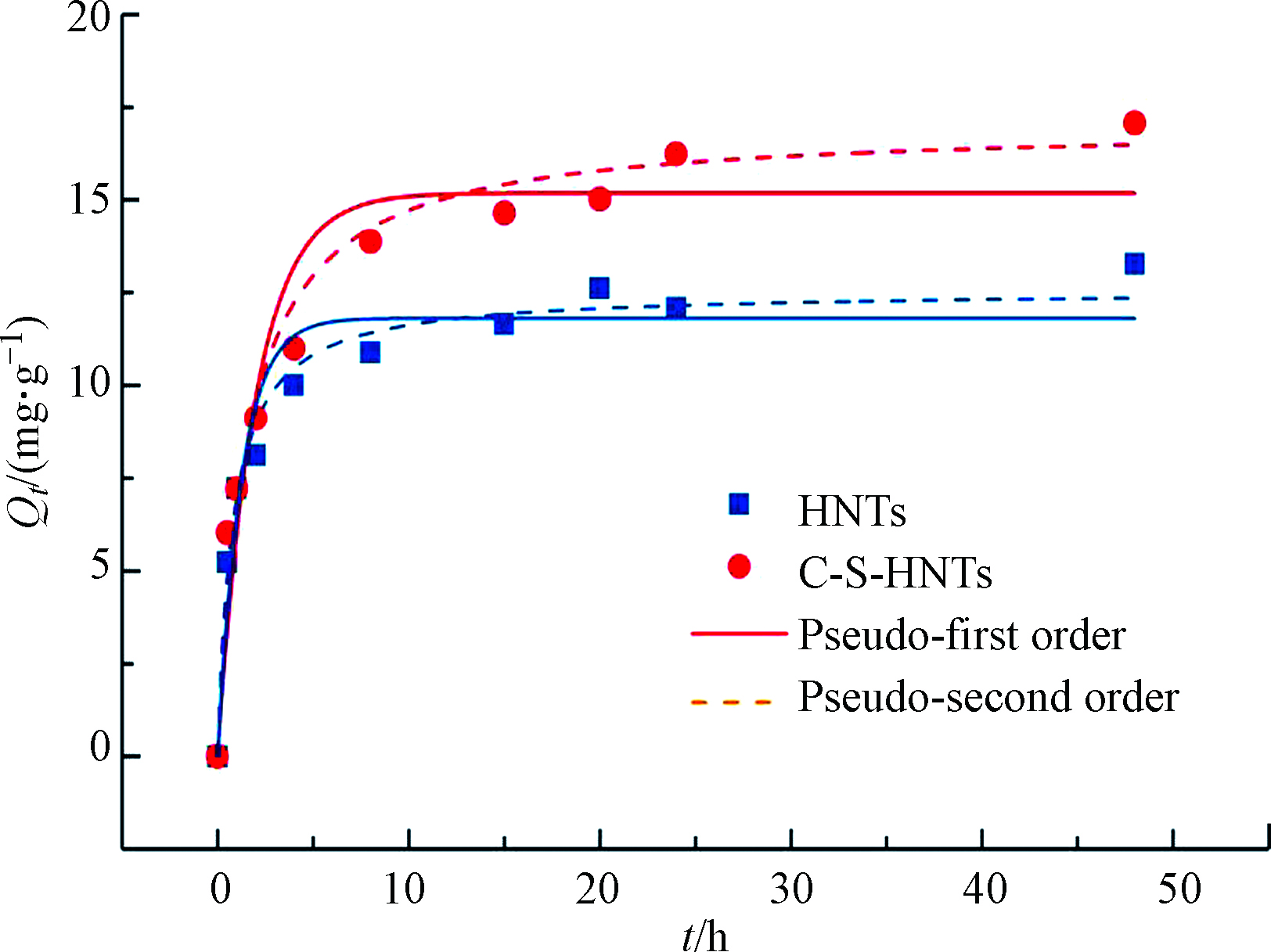
本实验采用阳离子表面活性剂CTAB和阴离子表面活性剂SDBS制备改性埃洛石。共制备单阳离子改性(C-HNTs)、单阴离子改性(S-HNTs)和阴阳离子复合改性(C-S-HNTs)三类埃洛石,对OTC的吸附率依次为C-S-HNTs > C-HNTs >HNTs>S-HNTs。根据预实验,复合改性HNTs的SDBS加入量为0.5倍的原生HNTs的CEC保持不变,此后,加入CTAB的量分别为0.25、0.5、1.0、1.5、2.0倍原生HNTs的CEC,分别记为0.25CTAB-0.5SDBS、0.5CTAB-0.5SDBS、1.0CTAB-0.5SDBS、1.5CTAB-0.5SDBS、2.0CTAB-0.5SDBS和2.5CTAB-0.5SDBS,共制备得到6种C-S-HNTs。结果表明0.5CTAB-0.5SDBS-HNTs对OTC吸附效果最好,故本实验选择原生HNTs及0.5CTAB-0.5SDBS-HNTs进行研究。如图8所示,HNTs和C-S-HNTs对OTC的吸附量随反应时间的增加而逐渐增大。初始的10 h为快速吸附阶段,两种黏土对OTC的吸附都较快,这可能是因为刚开始时HNTs和C-S-HNTs上存在大量的吸附位点,使得吸附反应得以快速进行。在10—24 h吸附反应以较慢的速率进行并最终达到饱和,在24 h时达到最大吸附量,此时OTC在HNTs上的吸附量与解吸量处于动态平衡状态。为保证吸附反应达到平衡,选定24 h作为后续实验的吸附反应时间。
HNTs和C-S-HNTs吸附动力学拟合结果如表2所示,准二级模型的相关系数R2分别为0.97和0.97,残差平方和<chi>分别为0.44和0.82,说明实验数据更符合准二级动力学模型,偏离程度很小,这与其他有机化合物如苯胺、硝基苯等在黏土矿物上的吸附结果一致[24],并且拟合出的理论吸附容量qe与实际黏土吸附量相近,因此,说明HNTs和C-S-HNTs吸附OTC过程更符合准二级动力学吸附模型,且C-S-HNTs对OTC的吸附过程存在化学吸附。李艳等[41]采用3-氨丙基三甲氧基硅烷改性的HNTs分别吸附亚甲基蓝和酸性橙Ⅱ,其吸附过程也都符合准二级动力学模型,与本研究的结果一致。
此外,HNTs对OTC的吸附速率(K2)要高于C-S-HNTs,可能是因为HNTs表面存在一定数量的吸附位点,随着时间的增加,吸附位点在短时间内就被完全占据,从而达到饱和,不再继续吸附溶液中多余的OTC。而经过改性后的C-S-HNTs表面的吸附位点增多,OTC与吸附位点结合直至达到饱和需要更长的时间,因此吸附速率与HNTs的相比较小。
考虑到准一级和准二级动力学吸附模型都不能分析HNTs和C-S-HNTs吸附OTC的扩散机制,利用颗粒内扩散模型对实验数据进行进一步的拟合分析。分析结果如表3所示。若颗粒内扩散模型拟合成经过原点的一条直线,则整个吸附过程只是由于内部粒子的扩散;如果拟合的直线偏离原点,那么主要是边界层控制吸附过程[42]。由表3数据可知,HNTs和C-S-HNTs线性拟合的相关系数分为0.853和0.86,说明直线无法很好地拟合,吸附过程不是单纯的一种扩散方式。结合图9分析,HNTs的Qt随时间的变化大致分为两段。第一阶段为表面或薄膜扩散过程,OTC分子从水相中逐渐转移到HNTs的外表面上;第二阶段为平衡吸附阶段,此时的吸附量保持相对稳定。C-S-HNTs对OTC的吸附过程与HNTs不同,大致可以分为3个阶段:第一个阶段为膜扩散过程;第二阶段为逐渐吸附阶段,OTC分子通过疏水作用逐渐扩散进入CTAB-SDBS在C-S-HNTs表面所形成的有机相中;第三阶段为平衡吸附阶段。
-
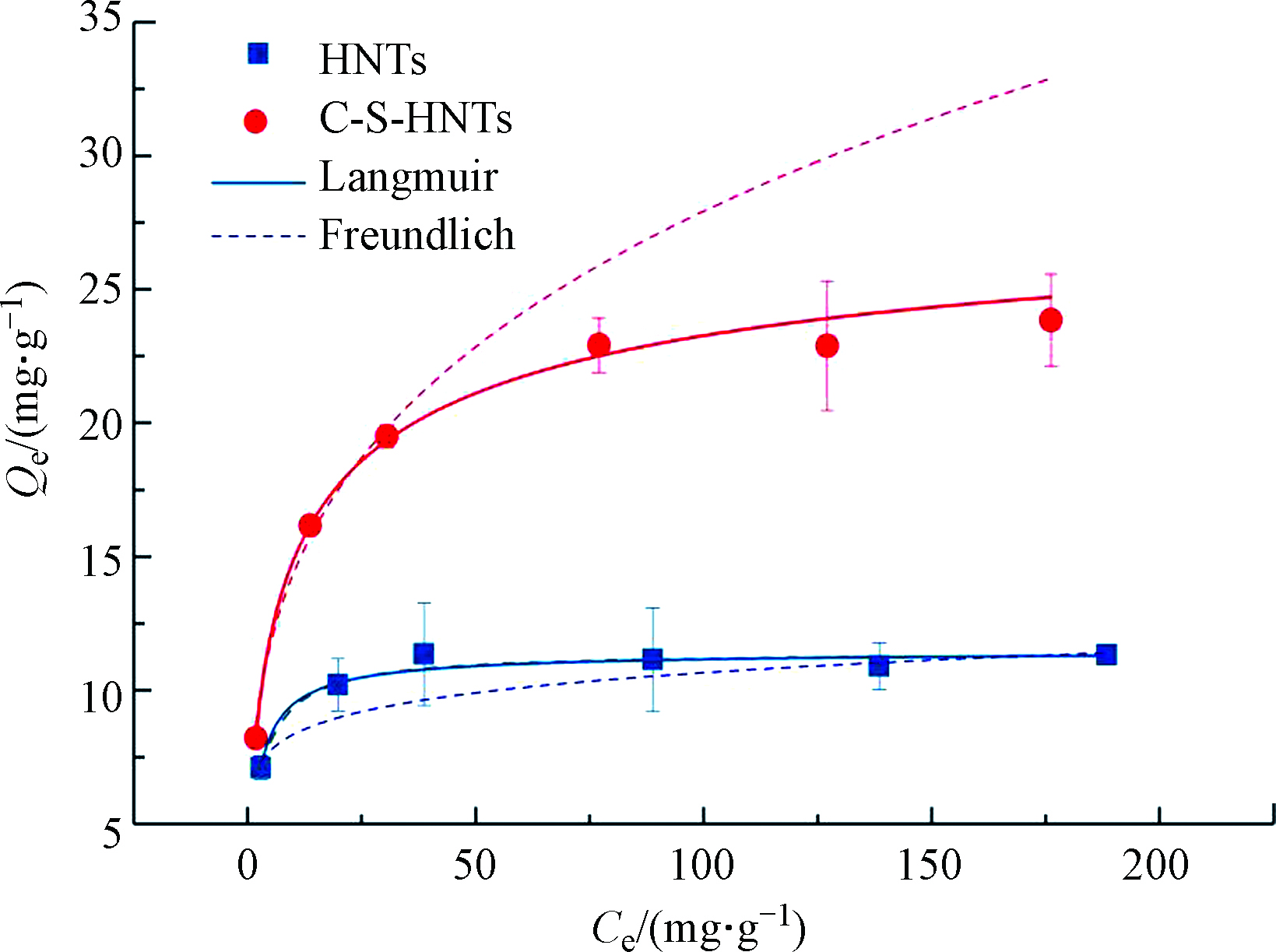
由图10可知,HNTs及C-S-HNTs的吸附量随OTC溶液初始浓度的增加而逐渐增加,直至达到饱和状态。是因为随着初始浓度的增加,吸附剂与OTC分子之间的碰撞概率也会增加,使得单位质量HNTs的吸附量增大。另一方面,OTC浓度增加使得HNTs的位点逐渐被完全占据,接近饱和位点后吸附量上升缓慢,几乎保持不变。对实验数据拟合结果如表4所示, HNTs和C-S-HNTs对OTC吸附的实验数据均更加符合Langmuir模型。表明HNTs和C-S-HNTs对OTC的吸附是单分子层均匀吸附[43],且吸附过程存在化学吸附,OTC被吸附到C-S-HNTs特定且均匀的活性位点上,吸附的过程为一个可逆的过程。吸附常数KL均小于1,因此可以初步判定HNTs和C-S-HNTs对OTC的吸附均属于优惠吸附;另外,表4中HNTs和C-S-HNTs吸附OTC的Freundlich吸附等温线拟合参数1/n分别为0.106和0.289,均在0—1的范围内,说明吸附反应容易进行。
为进一步了解HNTs和C-S-HNTs对OTC的吸附机制,用D-R模型对数据进行拟合。由该模型计算得出的参数E为吸附自由能,可由E来判断吸附机制。由表4可知,C-S-HNTs和HNTs吸附OTC的吸附自由能均小于8 kJ·mol−1,这也就意味着HNTs和C-S-HNTs对OTC的吸附过程存在物理吸附。
-
根据热力学公式,采用线性回归的方法,得到数据如表5所示,HNTs和C-S-HNTs吸附OTC的过程焓变ΔH分别为41.65 kJ·mol−1和27.16 kJ·mol−1,均大于0,证明吸附OTC的过程是吸热反应。吸附ΔS均大于0,这是因为单个OTC的体积要比单个H2O分子的体积要大,每吸附一个 OTC会伴随着更多H2O分子的解吸,导致熵增大于熵减,也说明HNTs和C-S-HNTs吸附OTC后,表面结构发生了改变,体系自由度变大,反应朝着混乱程度增大的方向进行。由表5可知,C-S-HNTs吸附OTC的过程中ΔG均小于0,说明此反应是自发进行的,不需要从外界获取能量。在一定程度上,吸附的类型可以从焓变量加以区分[44],物理吸附的ΔH小于84 kJ·mol−1,而化学吸附的ΔH在84—420 kJ·mol−1。但也有文献认为[45],通常物理吸附的ΔG在-20—0 kJ·mol−1, 而化学吸附的ΔG在-400—-80 kJ·mol−1。C-S-HNTs吸附OTC的ΔH均小于84 kJ·mol−1,ΔG均在-20—0 kJ·mol−1范围内,表明C-S-HNTs对OTC的吸附过程存在物理吸附。另一方面,准二级动力学方程说明存在化学吸附过程。因此,C-S-HNTs对OTC的吸附过程应该是物理吸附和化学吸附共同作用的结果。
-
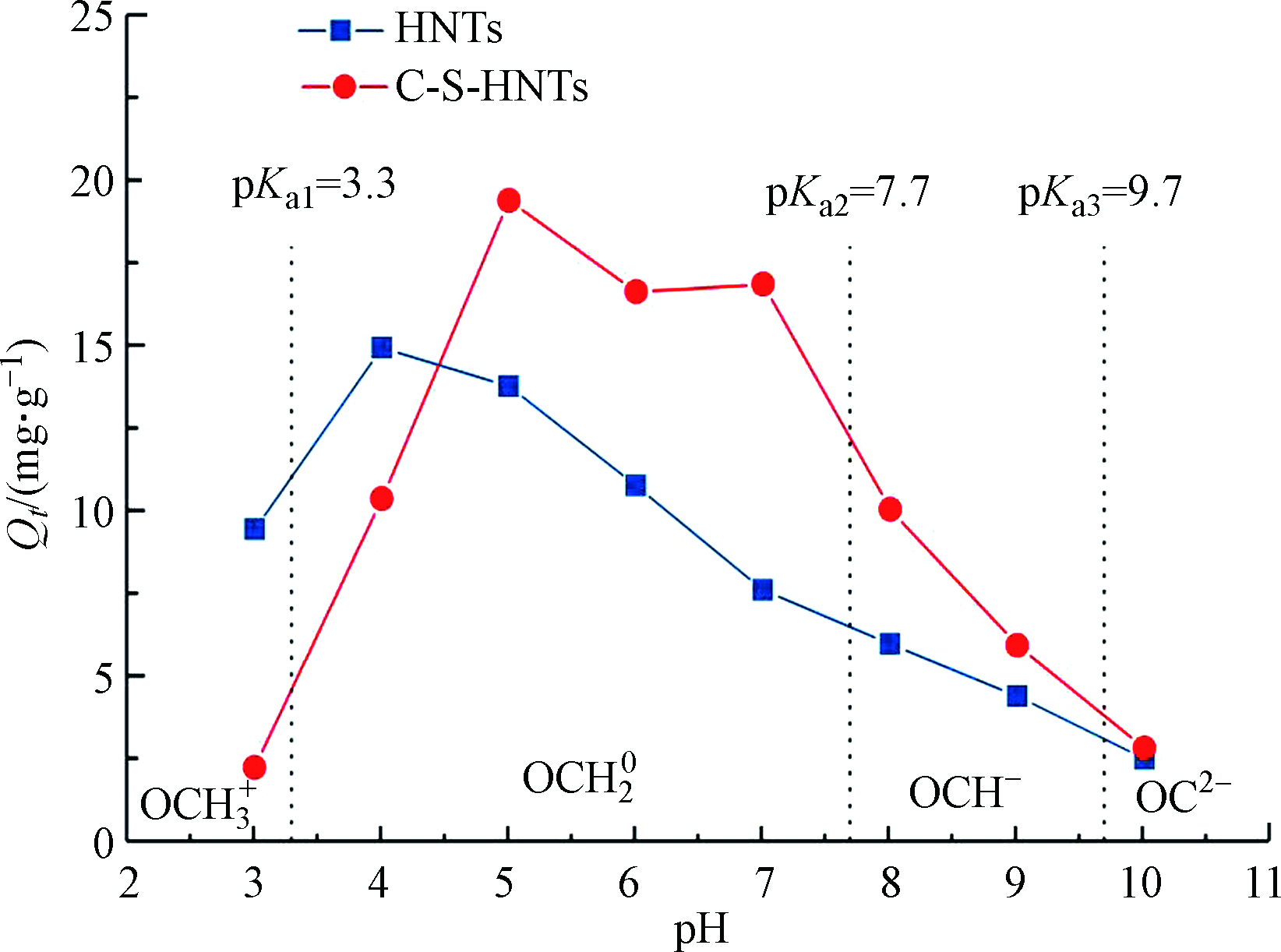
OTC在不同的pH下会呈现不同的形态,因此,HNTs和C-S-HNTs对OTC的吸附作用在不同的酸碱环境下是不同的。OTC是两性抗生素,分子结构中存在酸碱结构的酚羟基和氨基,共有3.3、7.7和9.7的3个解离常数(pKa)[24]。在不同的pH下,OTC分为4个组分:当pH小于3.3时,酚二酮基团失去1个质子,OTC结构中二甲氨基基团被质子化,主要以阳离子OCH3+的形式存在;当pH值为3.3—7.7时,酚二酮基团失去2个质子,OTC显现两性性质,形成两性离子形态,整体不带电荷,主要以OCH20的形式存在;pH大于7.7以后,含甲酰基氨基在内的三碳基团便失去H+,主要以OCH−和OC2-的形式存在[45-46](图11)。从图11可以看出,C-S-HNTs吸附OTC在pH 3时吸附容量比较低,吸附量随着溶液pH的增大而增加,当pH值在5—7时吸附容量最大,约为19 mg·g−1,此时pH增加吸附量迅速减小,在pH=10时其吸附量只有2 mg·g−1左右。这是因为在不同pH环境中,黏土矿物表面和抗生素的电荷属性会发生变化。当环境pH<pHZPC(黏土矿物的零点电荷pH)时,黏土表面带正电;当pH>pHZPC时,黏土表面带负电。通过Zeta电位测试得到HNTs的等电点pHZPC为3.34,C-S-HNTs的等电点pHZPC为3.67。在pH<3.3时,OTC主要以阳离子形式OCH3+ 存在,此时C-S-HNTs表面带正电,因此产生排斥作用,但由于此时体系中还存在部分OCH20分子,因此会仍有一定吸附量。在溶液pH值在5—7条件下,C-S-HNTs对OTC的吸附性能更强,这是因为OTC以OCH20分子形式存在,在该区域内OTC所带电荷几乎为零,此时黏土矿物与OTC之间的静电相互作用力几乎可以忽略,OCH20易通过分配作用与C-S-HNTs表面的有机相结合,吸附效果好且不易受pH的影响。有研究认为此时的高吸附量可能还与其他作用有关,比如络合作用[47]。由图11可知,在pH5时,C-S-HNTs吸附量达到最高,这是因为当pH=5.5时,OCH20达到最大比例。随着溶液pH的增加,溶液中的OTC主要以OCH−和OC2-负电荷的形式存在,且随着pH增大,体系中OTC以阴离子形式存在越来越多,此时HNTs和C-S-HNTs均带负电荷,因此产生排斥作用,从而吸附量随着pH的增大而减少。
-
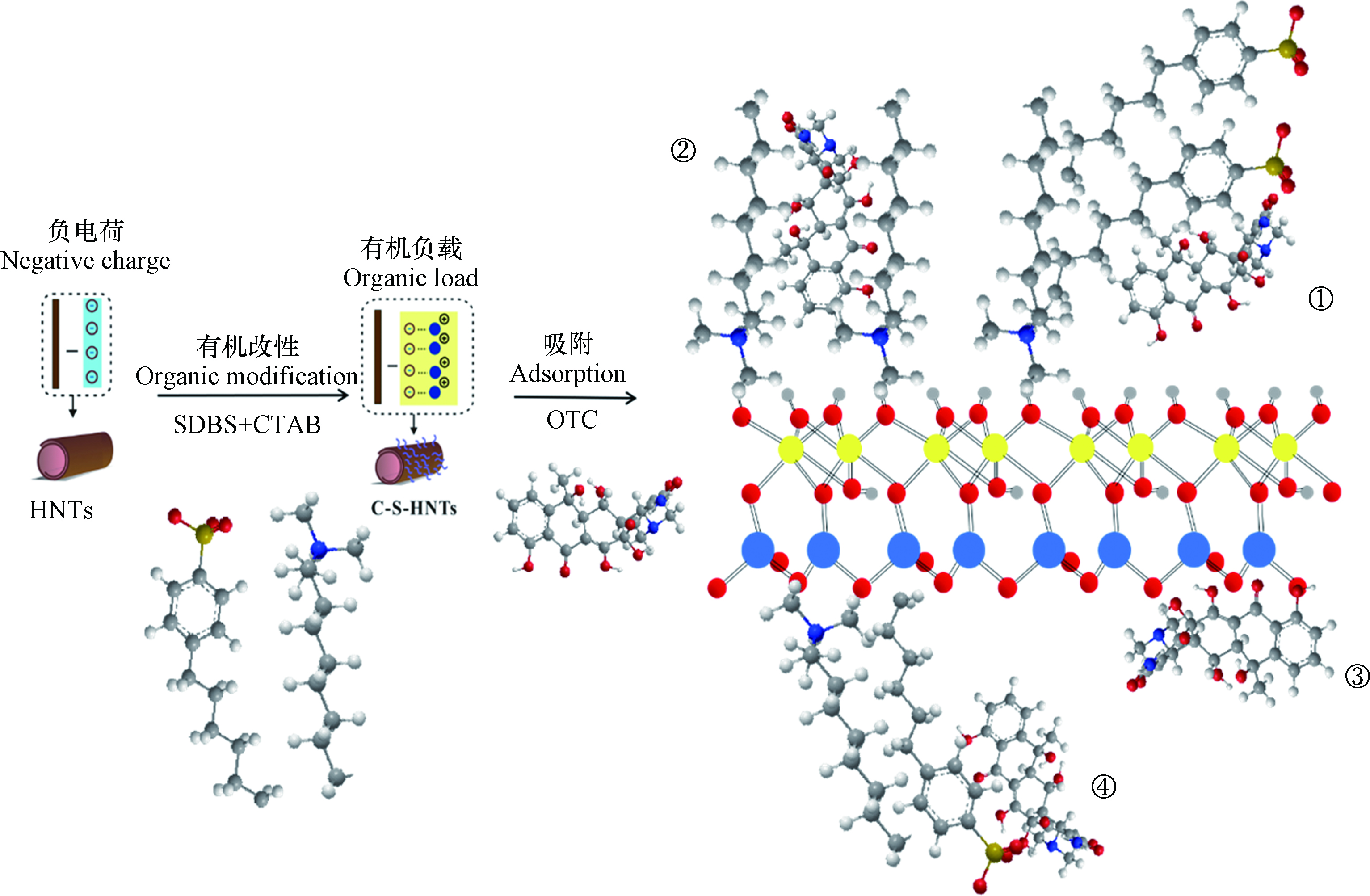
经过阴、阳离子表面活性剂复配改性得到的吸附材料C-S-HNTs,不仅保持了HNTs本身具有的吸附优势,还通过表面活性剂的修饰作用进一步提高了其对OTC的吸附能力。一方面,改性剂的加入使HNTs吸附OTC的分配作用有所增强;另一方面,阴、阳离子表面活性剂的负载,很大程度上丰富了HNTs表面的官能团,创造了更多的活性吸附位点,表面吸附作用的增强同样也提高了其吸附能力。
(1)分配作用:结合XRD和FTIR分析可得,CTAB和SDBS成功负载在HNTs外表面从而改变了HNTs的外表面性质。如图12,CTAB和SDBS通过疏水端结合形成混合胶束,CTAB带正电荷的季铵盐端与HNTs带负电的外表面结合,CTAB-SDBS一方面中和HNTs外表面部分负电荷,一方面增加HNTs表面有机相,为OTC吸附提供良好的有机分配介质,提高了OTC在HNTs表面的“溶解”能力,有利于OTC从水相中转移到C-S-HNTs的有机相上而发生吸附(图12①)。
(2)表面吸附作用:OTC属于两性抗生素,共有3.3、7.7和9.7的3个解离常数(pKa)。在不同的pH条件下,分别以阳离子OCH3+、两性离子OCH20和阴离子OCH−和OC2-等多种形式存在。HNTs表面带有较高的负电荷,CTAB季铵盐N端带正电,SDBS亲水端含有带负电的磺酸极性基(R-SO3−),以各种形式存在的OTC均能通过静电作用与C-S-HNTs结合(图12②③),从而增大吸附能力。有研究者发现用3-氨丙基三甲氧基硅烷改性的HNTs对酸性橙Ⅱ的吸附存在静电吸引作用[41],改性后吸附量也明显提高,这与本研究的结果 一致。此外,OTC上的羟基可以与SDBS上的磺酸基相互作用形成氢键作用(图12④)。Yuan 等[48]利用γ−氨基丙基三乙氧基硅烷对HNTs 进行改性用于染料吸附,也发现改性处理提高了 HNTs 表面的有机亲和性。
-
本研究以阳离子表面活性剂CTAB和阴离子表面活性剂SDBS改性HNTs,制备了C-S-HNTs以吸附水溶液中的OTC。两种表面活性剂成功地负载在HNTs表面,改性剂没有通过离子交换进入层间,使HNTs保持了原有的形貌和晶体结构。有机改性使得埃洛石对OTC的吸附率提高了50%。C-S-HNTs对OTC的吸附过程符合准二级动力学模型,其扩散机制为多过程吸附。Langmuir等温吸附模型更适合描述吸附数据,吸附为单层吸附。反应为吸热反应,自发进行且混乱程度增大。C-S-HNTs对OTC的吸附受pH影响较大。C-S-HNTs增加了HNTs表面的官能团,为OTC的吸附提供了一个良好的有机分配介质。C-S-HNTs对OTC的吸附机理主要包括分配作用和表面吸附作用。
埃洛石的复配改性及其对土霉素的吸附性能
Study on the compound modification of halloysite and its adsorption to oxytetracycline
-
摘要: 环境中的抗生素污染引起了全球的广泛关注。埃洛石(HNTs)由于价格低廉、储量丰富和独特的中空纳米管结构,是一种潜在的优良吸附剂。本研究以十六烷基三甲基溴化铵(CTMAB)和十二烷基苯磺酸钠(SDBS)对天然HNTs进行改性,获得阴阳离子改性埃洛石(C-S-HNTs),并应用于水中土霉素(OTC)的吸附。采用XRD、FTIR、SEM、BET、Zeta电位和XPS等表征手段研究了改性前后HNTs的晶体结构和理化性质的变化。采用静态吸附法研究了OTC在C-S-HNTs上的吸附性能和机理。结果表明,C-S-HNTs对OTC的吸附能力显着提高,吸附率提高了50%左右。通过FTIR分析,季铵盐阳离子成功接枝于HNTs表面,改性成功。通过SEM分析,仍然观察到C-S-HNTs的空心管状结构。CTMAB和SDBS成功负载到HNTs表面,但并没有进入HNTs 的层间结构域,保持了HNTs原有的晶体结构。C-S-HNTs对OTC的吸附过程符合准二级动力学模型和Langmuir等温吸附模型。通过Zeta电位分析,C-S-HNTs的吸附容量随pH的增大先增加后减少,在pH=5时,吸附容量达到最高。吸附反应为自发进行的混乱程度增大的吸热反应。OTC在C-S-HNTs上的吸附是分配作用和表面吸附相结合的结果。对天然HNTs进行改性,提高其吸附性能,扩大其应用范围,可为治理新污染物引起的环境问题提供数据支撑。Abstract: Antibiotics pollution in the environment has attracted extensive global attention. Halloysite (HNTs) is a potential excellent adsorbent due to its low price, abundant deposits and unique hollow nanotube structure. In the present study, natural HNTs was modified by cetyl trimethyl ammonium bromide (CTMAB) and sodium dodecyl benzene sulfonate (SDBS) to obtain cation and anion modified halloysite (C-S-HNTs), which was applied to the adsorption of oxytetracycline (OTC) in aqueous solution. XRD, FTIR, SEM, BET, Zeta potential and XPS were used to ascertain the changes in the crystal structure and physicochemical properties of HNTs before and after modification. The static batch adsorption experiments were used to explore the adsorption properties and adsorption mechanism of OTC on C-S-HNTs. The results showed that the adsorption capacity of C-S-HNTs towards OTC increased markedly, and the adsorption rate increased by around 50%. Through FTIR analysis, the quaternary ammonium salt cations were successfully grafted onto the surface of HNTs, and the modification was successful. According to SEM analysis, the hollow tubular structure of C-S-HNTs was still observed. CTMAB and SDBS were successfully loaded onto the surface of HNTs. However, they did not enter the interlayer domain of HNTs, maintaining the original crystal structure of HNTs. The adsorption process accorded with the pseudo-secondary kinetic model and Langmuir isothermal adsorption model. Through Zeta potential analysis, the adsorption capacity of C-S-HNTs first increased and then decreased with the increase of pH. When pH value was 5, the adsorption capacity reached the highest. The adsorption reaction was a spontaneous and endothermic reaction with increasing chaos. The adsorption of OTC on C-S-HNTs was the result of the combination of partitioning and surface adsorption. Modifying natural HNTs would improve their adsorption performance and expand their application range. These findings can provide data support for the treatment of environmental problems caused by emerging contaminants.
-
Key words:
- halloysite /
- modification /
- adsorption /
- oxtetracycline /
- antibiotics
-
抗生素被广泛用于治疗或预防人类和动物疾病,以多种途径进入环境,目前已在多种环境介质中被频繁检出[1-2]。随着其使用量的不断增加,环境中抗生素的浓度也随之提高,已然成为一个主要的全球公共卫生问题[3-4]。吸附法作为去除抗生素的有效方法之一,具有工艺简单、无二次污染、操作方便等特点[5]。然而,常用的吸附剂如金属有机纳米管[6] 、活性炭[7]、石墨烯[8]、碳纳米管[9]和生物碳材料[10]等,存在成本高且不能再生利用的缺点[5,11]。在众多的吸附剂中,黏土和黏土矿物因成本低和产量丰富而被广泛应用[12-13]。埃洛石(halloysite nanotubes, HNTs)是一种天然黏土矿物[14],它的晶体结构是由角共享硅氧四面体和边缘共享铝氧八面体组成的1:1型片层结构[15-16]。HNTs与其他铝硅酸盐矿物的主要区别在于其独特的纳米管状结构。HNTs具有相对较高的比表面积和总孔隙体积,这归因于HNTs结构中具有丰富的孔隙[17],可为吸附提供较多的反应位点。当HNTs用作吸附剂时,对客体分子仅表现出弱的亲和力(离子交换、氢键和范德华力),未修饰的HNTs由于含大量硅氧键,羟基较少,因此氢键作用力较小,且晶体结构中的孔道易因杂质而堵塞,降低了其对污染物的吸附[15-17]。为了提高HNTs的吸附性能,通常对HNTs进行改性。由于HNTs的外表面[18]、内腔表面[19]和层间表面[20]均可被改性,从而为HNTs的修饰提供了丰富的可能性。黏土表面具有天然的负电荷特性,可以通过吸附某些特定的阳离子来改性,迄今采用阳离子表面活性剂修饰黏土的研究较多,且修饰后对染料等有机污染物的吸附固定能力均有提高。而对于用阴阳离子表面活性剂复合改性及其应用的研究较少。因此,用离子表面活性剂对黏土矿物HNTs进行阴阳离子复合改性对抗生素吸附是否有作用,作用机理又是什么,这是一个值得探讨和验证的命题。
土霉素(OTC)作为渭河西安段检出频率最高的四环素类抗生素[21],在自然环境中的出现会影响微生物遗传变异的选择,随着其使用量的不断增加,不可避免地给人体健康和生态环境造成巨大压力[4]。基于此,为了探究阴-阳离子表面活性剂改性黏土矿物吸附水中抗生素的能力,本研究以HNTs为吸附剂,以土霉素(OTC)为吸附对象,采用十六烷基三甲基溴化铵(CTMAB)和十二烷基苯磺酸钠(SDBS)两种离子表面活性剂对HNTs进行改性,制备新型吸附材料阴阳离子改性埃洛石(C-S-HNTs)。考察了吸附剂投加量、吸附时间、初始浓度、温度和溶液pH对OTC在C-S-HNTs上吸附的影响,并对动力学模型、吸附等温模型及热力学模型进行拟合和讨论。结合XRD、FTIR、SEM、BET、zeta电位和XPS等多种表征手段,从吸附效果→表征→修饰机制的角度进行系统研究,目的在于建立起复配修饰黏土的复配修饰机制-表面特征-吸附效应之间的构效关系,为两性改性剂及其复配修饰黏土的理论及应用提供技术储备和数据支持。
1. 材料和方法(Materials and methods)
1.1 实验材料
天然HNTs购自天津福辰化学公司,纯矿物含量为99%,呈暗白色粉末状。通过氯化钡-硫酸法测定[22],其阳离子交换容量(CEC)为22.8 mmol·100g−1。阳离子表面活性剂CTAB和阴离子表面活性剂SDBS均购自凯美尔化学试剂有限公司(中国天津),纯度为90%。OTC购自阿达马斯试剂有限公司(上海)。本研究中使用的所有其他化学品,如HCl、NaOH均为分析级,无需进一步纯化。所有溶液均用蒸馏水配制。
1.2 埃洛石的提纯及改性
使用前,将原生HNTs与适量去离子水混合,经过搅拌、超声使其在水中充分分散,清洗多次以去除HNTs表面可溶性盐类等杂质。然后于65 ℃的烘箱中干燥24 h,将其粉碎后筛过100目筛,装入透明密封袋并放置在干燥器备用。
表面活性剂改性HNTs样品的制备如下:用分析天平准确称取一定量的CTAB和SDBS,待其在装有适量蒸馏水的烧杯中溶解后,加入已提纯、干燥且过200目筛的原生HNTs,磁力搅拌器于25 ℃的条件下恒温搅拌24 h。待HNTs与表面活性剂交换反应后,以2500 r·min−1离心10 min固液分离,弃去上层溶液后用去蒸馏水清洗,重复7次以上操作以去除残留的有机改性剂。所得材料在65 ℃的烘箱烘干,然后于110 ℃的条件下活化1 h,研磨过200目筛,即制得C-S-HNTs。
1.3 实验及数据分析
1.3.1 吸附实验
为考察各种因素对吸附过程的影响,采取控制单一变量法进行批实验,所有试验均做3组平行,实验结果剔除异常值后取平均值绘制图表。通过高效液相色谱(戴安 U3000)测定吸附平衡前后的OTC浓度。按下式计算出吸附剂的吸附容量qe(mg·g−1)和吸附率Y(%)。
qe=(C0−Ct)Vm (1) Y=C0−CtC0×100% (2) 其中, C0 为吸附前OTC的初始浓度,mg·L−1; Ct为吸附后OTC的剩余浓度,mg·L−1;V 为OTC溶液的体积,L;m为吸附剂的投加量,g。
具体试验步骤如下:
(1)考察不同吸附时间的影响。OTC溶液初始浓度C0为50 mg·L−1,溶液初始pH=6.0,T=30 ℃,吸附剂投加量为1 g·L−1,吸附时间为0—48 h。
(2)考察污染物初始浓度的影响。OTC溶液初始pH=6.0,T=30 ℃,吸附剂投加量1 g·L−1,吸附时间24 h,OTC溶液初始浓度C0为10—200 mg·L−1。
(3)考察不同反应温度的影响。C0为50 mg·L−1,溶液初始pH=6.0,吸附剂投加量为1 g·L−1,吸附时间24 h,反应温度为30、40、50 ℃。
(4)考察不同初始pH的影响。C0为50 mg·L−1,T=30 ℃,吸附剂投加量为1 g·L−1,吸附时间24 h,溶液初始pH设定分别为3、4、5、6、7、8、9、10。
1.3.2 动力学吸附模型
实验数据拟合所采用的吸附动力学方程公式如下:
(1)准一级动力学方程:
ln(qe−qt)=lnqe−k1t (3) (2)准二级动力学方程:
tqt=1k2qe2+tqe (4) (3)颗粒内扩散模型方程:
qt=kpt0.5+C (5) 其中,qt 为在t时刻的吸附量,mg·g−1;qe 为平衡吸附量,mg·g−1;K1 为准一级动力学常数,min−1;K2 为准二级动力学常数,min−1;Kp 为颗粒内扩散速率常数,mg·g−1·min−0.5。
1.3.3 等温吸附模型
实验数据拟合所采用的等温吸附方程公式如下:
(1)Langmuir模型:
Ceqe=1qmKL+Ceqm (6) 其中,qm 为最大吸附量,mg·g−1;qe 为平衡吸附量,mg·g−1;Ce 为 OTC吸附平衡浓度,mg·L−1;KL 为Langmuir模型参数。
(2)Freundlich模型:
lnqe=lnKF+1nlnCe (7) 其中,qe 为平衡吸附量,mg·g−1;Ce 为 OTC吸附平衡浓度,mg·L−1;KF为 Freundlich 模型参数。
(3)Dubinin-Radushkevich(D-R)模型:
lnqe=lnqm−β⋅ε2 (8) ε=RT×ln(1+1/Ce) (9) {E}\text={\text{(2}{β}\text{)}}^{{-1/2}} (10) 其中,qe为平衡吸附量,mg·g−1;Qm为最大吸附量,mg·kg−1;Ce 为吸附平衡浓度,mol·L−1;R 为气体常数,8.314 J·mol−1·K−1;T 为反应温度,K;E 为吸附自由能,kJ·mol−1;ε 为波兰势能;β 为吸附参数。由E可判断吸附机制,当E小于8 kJ·mol−1时,物理吸附是体系吸附机制;当E在8 kJ·mol−1到16 kJ·mol−1之间时,主要的吸附机制为离子交换;当E大于16 kJ·mol−1时,吸附既包含物理吸附,又包含化学吸附。
1.3.4 热力学吸附模型
实验数据所采用的吸附热力学方程公式如下:
\text{Δ}{G}\text{=Δ}{H}-{T}\text{Δ}{S} (11) \text{Δ}{G}=-{RT}\text{ln}{K} (12) {K}\text=\frac{{{q}}_{\text{e}}}{{{C}}_{\text{e}}} (13) 其中,K为分配系数,mL·g−1;T 为吸附反应时的绝对温度,K;R 为理想气体状态常数,8.314 J·mol−1·K−1。
1.4 表征方法
使用Bruker D8 Advance X射线粉末衍射仪对矿物的晶体结构进行测定,X射线源为CuKα,辐射波长为0.154 nm,扫描步长为0.02 °,速度为每步17.7 s,扫描电压/电流为40 kV/40 mA,样品采取压片处理,温度为室温条件下对材料在10°—80°范围内进行扫描分析;使用PerkinElmer 1725X 傅利叶变换红外光谱仪(FTIR)研究HNTs和C-S-HNTs所含有的官能团;样品采用KBr粉末压片法处理,分辨率为0.3 cm−1,扫描波长范围为400—4000 cm−1;使用Merlin型扫描电子显微镜对矿物表面形貌进行分析,电流为80 μA,加速电压为20 kV;使用Micromeritics ASAP 2020装置对矿物的比表面积进行测定,80 ℃下脱气12 h,孔径分析范围为3.5 Å到5000 Å,并依据Brunauer-Emmett-Teller(BET)理论进行分析;使用Nano ZS90型Zeta电位分析仪测定不同pH值下的zeta电位测量值,光源为He-Ne;使用配备Al-Ka单色仪X射线源的Thermo Scientific Escalab 250Xi光谱仪进行X射线光电选择光谱(XPS)分析,X射线束斑20—900 μm,能量扫描范围0—5000 eV。
2. 结果与讨论(Results and discussion)
2.1 表征分析
2.1.1 X射线衍射(XRD)和扫描电子显微镜(SEM)分析
黏土矿物的不同结构和层间环境可能导致CTAB和SDBS在其层间的排列方式不同。图1所示,HNTs的2θ角为11.960°,对应层间距为0.57 nm,表明样品为0.7 nm HNTs。C-S-HNTs的2θ角为11.920°,对应层间距为0.57 nm。与HNTs相比,CTAB-SDBS修饰后的HNTs在XRD图上并未检测到新的衍射峰,峰的尖锐程度变化不大,同时也没有特征峰的偏移,C-S-HNTs层间距几乎保持不变,表明CTAB-SDBS分子未插入HNTs的层间,而是负载在HNTs的表面上,C-S-HNTs在修饰后依然保持了HNTs初始晶体结构。这主要是因为HNTs为 1∶1 型非膨胀型的黏土,层间可交换性阳离子较少[23],修饰剂难以进入HNTs的层间,使得CTAB和SDBS主要修饰在HNTs表面[24]。且HNTs的层间内表面Al-OH基团由于被层间强氢键所阻断,大多不可用于接枝。
HNTs含有大量的纳米管和少量杂质的团聚体(图2)。HNTs的长度范围从0.05 μm到1.50 μm不等,内径为10—20 nm,壁厚为10—15 nm。与HNTs相比,有机改性后,C-S-HNTs仍能观察到中空管状结构,说明CTAB-SDBS改性对HNTs的管状形貌无明显影响。 结合XRD分析,可以得出CTAB-SDBS修饰对HNTs的晶体结构和形貌没有影响。
2.1.2 傅立叶红外光谱(FTIR)分析
在3000—4000 cm−1范围内仅有两个羟基的吸收峰,分别在3624 cm−1和3697 cm−1处(图3)。这两处吸收峰分配给两个Al2OH拉伸带,每个羟基和两个Al原子相连[25]。这两个吸收峰归属于晶体中含有的两种类型的羟基基团:外羟基基团和内羟基基团。外羟基位于硅氧四面体和铝氧八面体构成的层状结构的外部非共享面上,而内羟基基团则位于硅氧四面体和铝氧八面体构成的层状结构的共享面上[26]。3697 cm−1附近吸收带表明羟基与层间H2O的氢键存在。在低波数400—2000 cm−1范围内,HNTs的吸收峰主要是一些Si—O和Al—O的吸收[27]。不同产地和纯度的HNTs的红外吸收峰略有不同。在中频区1618—1636 cm−1范围内出现吸附水中羟基弯曲振动带[28-29],在波数为1037 cm−1处出现Si—O的伸缩振动带以及在波数为914 cm−1处出现的震动带则是由内部羟基O—H变形引起[30]。在低频区682—754 cm−1范围内出现O—H的弯曲振动带以及540、470、432 cm−1等附近的Si—O弯曲振动带[30-31]。在796 cm−1和750 cm−1的波段可以被分配到HNTs O—H单元的O—H平移振动[30]。所有这些观察表明,在HNTs结构中存在不止一种类型的水。经过改性,与HNTs相比,C-S-HNTs中出现了两个新的吸收峰,分别位于2914 cm−1、2983 cm−1附近。这两个吸收峰分别对应—CH3、—CH2—的变形振动和伸缩振动[32],在1537cm−1处振动来自于CTAB的—CH2—失变振动。表明季铵盐阳离子成功接枝于HNTs表面,改性成功。与HNTs的光谱相比,C-S-HNTs的光谱显示内表面Al2OH基团的OH拉伸带(3656 cm−1)的强度并未降低,这表明改性并没有对内表面Al2OH进行消耗,即在这些基团之间没有发生接枝。其它吸收峰与处理前一致,FTIR结果进一步说明改性后基本结构并没有发生改变,能很好保持原有结构,这也与XRD结论一致。
2.1.3 比表面积和孔结构分析(BET)
作为中空的纳米管状粒子,理论上HNTs应具有较高的比表面积,而实际测得HNTs比表面积为27.695 m2·g−1(表1)。这可能是由于HNTs表面缺陷较少从而导致其微孔较少。HNTs的氮气吸附脱附曲线呈现Ⅳ型吸附行为,且表现出H3滞后环,说明材料中存在介孔结构[33]。HNTs是表面多孔物质,可以看出HNTs的孔大小在2—100 nm范围(图4),表明HNTs既存在介孔也存在大孔。在3.7、16.8、50.9 nm处的峰分别对应于HNTs的表面结晶缺陷、管内径和HNTs管间搭接形成的孔,表明了HNTs的多孔性质。
表 1 HNTs、C-S-HNTs的比表面积和孔结构参数Table 1. Surface area and porous structure parameters of the HNTs and C-S-HNTs样品Sample 比表面积/(m2·g−1)Specific surface 平均孔径/nmAverage porediameter 孔容/(cm3·g−1)Total pore volume HNTs 27.695 14.563 0.104 C-S-HNTs 9.429 42.065 0.00197 值得注意的是,在CTAB-SDBS改性之后,HNTs的比表面积和孔容都表现出了明显的下降,而孔径却有所上升(表1)。比表面积的减小,可能是由于嫁接在HNTs表面的有机链阻断了一些结构通道,从而导致更少的N2能进入其间。而孔容的减少可能是存在其外表面的有机物引起的。比表面积减小后,吸附量却增加,这是由于OTC分子量有460.434 g·mol−1,巨大的分子空间构型使得OTC无法进入内部,HNTs内部的吸附位点无法利用,改性之后,虽然比表面积减小,但是由CTAB-SDBS的负载提供的吸附位点却增多,所以吸附量增大。另一方面,改性后的HNTs孔径增大的同时也增大了HNTs的吸附位点,也印证了C-S-HNTs对OTC的吸附性能优于 HNTs的实验结果。因此,可以推测,CTAB-SDBS成功负载在了HNTs表面但没有进入HNTs层间,XRD和FTIR表征同样证明了这一结论。
2.1.4 Zeta电位分析
通过 Zeta 电位可以分析比较表面的电荷特性随溶液 pH 值变化的情况,这关系着吸附剂与污染物之间的静电吸附情况。经测试分析可知HNTs的等电点为3.32,当pH值大于等电点时HNTs的Zeta电位均为负值(图5),说明pH在3.32—12范围内HNTs表面均带负电荷,这是由于HNTs的表面主要是硅酸盐[34],因此在广泛的pH范围为负表面电势。经过改性后,C-S-HNTs在pH=2时的Zeta电位值为23.52 mV,此时材料表面带有正电荷;在pH值为3.0—4.0之间出现等电点(值为3.74),随后电位值全部为负值并逐渐下降,但其电位值均要小于同等条件下HNTs的Zeta电位值。正电荷的出现表明CTAB-SDBS上能在强酸性条件下(pH=2),使HNTs表面的负电性有所降低。阳离子间的静电相互作用也发挥了一定作用。此外,通过与HNTs的Zeta电位值相比,改性后的HNTs均带有较低的负电荷,这与CTAB-SDBS的结合减弱了HNTs表面的水解作用有关[24]。由于OTC分子的pKa为3.3、7.7和9.7[24],其在不同pH条件下以不同离子形式存在,故HNTs表面的电性及所带电荷量的不同会影响其对OTC的吸附能力。
2.1.5 X-射线光电子能(XPS)分析
图6a给出了样品特征元素的吸收峰,可以清楚看出HNTs表面都有较强的O1s、C1s、Si2p和Al2p等特征峰。经过改性后,在C-S-HNTs中发现C元的信号增加,由20.31%提高至39.94%,且出现较弱N 1s特征峰,表明CTAB-SDBS成功附着在C-S-HNTs表面。C-S-HNTs中N 1s的高分辨率XPS光谱如图6b所示。在结合能401.5 eV和402.4 eV处有两个明显的峰,401.45 eV对应N—C[35],质子氨基(—NH3+)的标准结合能在400.5—402.5 eV区域[36],因此,402.4 eV的峰值属于—NH3+。—NH3+可能来自于SDBS通过氢键与HNTs的表面羟基相互作用[37]。
为进一步确定C-S-HNTs和HNTs表面元素化合态及其化学位移的变化情况,分别对C1s、O1s、N1s进行XPS分析。由图7a、b知,HNTs中C1s能谱可拟合为3个峰,结合能284.8、286.5、288.5 eV处分别对应为C—C、C=O[38]、O—C=O[39],C-S-HNTs中C1s能谱可拟合为2个峰,结合能284.8、286.5 eV处分别对应为C—C和C=O。在两个样品中,C—C和C=O键的结合能分别保持在284.8 eV和286.5 eV,分别对应于长烷基链中的C—C键和来自大气吸附的二氧化碳的C=O键[40]。与HNTs相比,C-S-HNTs中的C—C键对应的峰增强,这是因为表面活性剂会将长烷基链带到HNTs上,导致284.8 eV的信号增加。288.5 eV处对应的峰消失,可能与样品中的有机杂质有关,在改性过程中被冲洗掉。
图7c、d结合能532.3 eV和532.2 eV处分别对应硅氧四面体和铝氧八面体。图中未出现H2O特征峰,说明HNTs为0.7 nm HNTs,不含有层间结合水。与HNTs相比,C-S-HNTs [SiO4]和[AlO6]结合能降低了0.23 eV,表明[SiO4]和[AlO6]电子密度都增加。结合能的变化小于1 eV,证实了HNTs与表面活性剂之间的相互作用较弱。因此,表面活性剂很可能通过静电吸引在HNTs上负载。所有结果均表明在HNTs的表面存在有机表面活性剂。
2.2 吸附研究
2.2.1 吸附动力学
本实验采用阳离子表面活性剂CTAB和阴离子表面活性剂SDBS制备改性埃洛石。共制备单阳离子改性(C-HNTs)、单阴离子改性(S-HNTs)和阴阳离子复合改性(C-S-HNTs)三类埃洛石,对OTC的吸附率依次为C-S-HNTs > C-HNTs >HNTs>S-HNTs。根据预实验,复合改性HNTs的SDBS加入量为0.5倍的原生HNTs的CEC保持不变,此后,加入CTAB的量分别为0.25、0.5、1.0、1.5、2.0倍原生HNTs的CEC,分别记为0.25CTAB-0.5SDBS、0.5CTAB-0.5SDBS、1.0CTAB-0.5SDBS、1.5CTAB-0.5SDBS、2.0CTAB-0.5SDBS和2.5CTAB-0.5SDBS,共制备得到6种C-S-HNTs。结果表明0.5CTAB-0.5SDBS-HNTs对OTC吸附效果最好,故本实验选择原生HNTs及0.5CTAB-0.5SDBS-HNTs进行研究。如图8所示,HNTs和C-S-HNTs对OTC的吸附量随反应时间的增加而逐渐增大。初始的10 h为快速吸附阶段,两种黏土对OTC的吸附都较快,这可能是因为刚开始时HNTs和C-S-HNTs上存在大量的吸附位点,使得吸附反应得以快速进行。在10—24 h吸附反应以较慢的速率进行并最终达到饱和,在24 h时达到最大吸附量,此时OTC在HNTs上的吸附量与解吸量处于动态平衡状态。为保证吸附反应达到平衡,选定24 h作为后续实验的吸附反应时间。
HNTs和C-S-HNTs吸附动力学拟合结果如表2所示,准二级模型的相关系数R2分别为0.97和0.97,残差平方和<chi>分别为0.44和0.82,说明实验数据更符合准二级动力学模型,偏离程度很小,这与其他有机化合物如苯胺、硝基苯等在黏土矿物上的吸附结果一致[24],并且拟合出的理论吸附容量qe与实际黏土吸附量相近,因此,说明HNTs和C-S-HNTs吸附OTC过程更符合准二级动力学吸附模型,且C-S-HNTs对OTC的吸附过程存在化学吸附。李艳等[41]采用3-氨丙基三甲氧基硅烷改性的HNTs分别吸附亚甲基蓝和酸性橙Ⅱ,其吸附过程也都符合准二级动力学模型,与本研究的结果一致。
表 2 吸附动力学拟合参数Table 2. Kinetic parameters of adsorption样品Sample 准一级动力学模型Pseudo-first-order 准二级动力学模型Pseudo-second-order K1/min−1 qe/(mg·g−1) R2 <chi> K2/(g·mg−1min−1) qe/(mg·g−1) R2 <chi> HNTs 0.87 11.76 0.91 1.42 0.10 12.57 0.97 0.44 C-S-HNTs 0.51 15.19 0.92 2.44 0.036 17.028 0.97 0.82 此外,HNTs对OTC的吸附速率(K2)要高于C-S-HNTs,可能是因为HNTs表面存在一定数量的吸附位点,随着时间的增加,吸附位点在短时间内就被完全占据,从而达到饱和,不再继续吸附溶液中多余的OTC。而经过改性后的C-S-HNTs表面的吸附位点增多,OTC与吸附位点结合直至达到饱和需要更长的时间,因此吸附速率与HNTs的相比较小。
考虑到准一级和准二级动力学吸附模型都不能分析HNTs和C-S-HNTs吸附OTC的扩散机制,利用颗粒内扩散模型对实验数据进行进一步的拟合分析。分析结果如表3所示。若颗粒内扩散模型拟合成经过原点的一条直线,则整个吸附过程只是由于内部粒子的扩散;如果拟合的直线偏离原点,那么主要是边界层控制吸附过程[42]。由表3数据可知,HNTs和C-S-HNTs线性拟合的相关系数分为0.853和0.86,说明直线无法很好地拟合,吸附过程不是单纯的一种扩散方式。结合图9分析,HNTs的Qt随时间的变化大致分为两段。第一阶段为表面或薄膜扩散过程,OTC分子从水相中逐渐转移到HNTs的外表面上;第二阶段为平衡吸附阶段,此时的吸附量保持相对稳定。C-S-HNTs对OTC的吸附过程与HNTs不同,大致可以分为3个阶段:第一个阶段为膜扩散过程;第二阶段为逐渐吸附阶段,OTC分子通过疏水作用逐渐扩散进入CTAB-SDBS在C-S-HNTs表面所形成的有机相中;第三阶段为平衡吸附阶段。
表 3 颗粒内扩散模型拟合参数Table 3. The fitting parameters of intra-particle diffusion models样品Sample 拟合公式Fitting formula Kp/(mg·g-1·min-0.5) R2 HNTs y = 0.1486x + 6.5964 0.1486 0.853 C-S-HNTs y = 0.2348x + 6.5711 0.2348 0.860 2.2.2 吸附等温线
由图10可知,HNTs及C-S-HNTs的吸附量随OTC溶液初始浓度的增加而逐渐增加,直至达到饱和状态。是因为随着初始浓度的增加,吸附剂与OTC分子之间的碰撞概率也会增加,使得单位质量HNTs的吸附量增大。另一方面,OTC浓度增加使得HNTs的位点逐渐被完全占据,接近饱和位点后吸附量上升缓慢,几乎保持不变。对实验数据拟合结果如表4所示, HNTs和C-S-HNTs对OTC吸附的实验数据均更加符合Langmuir模型。表明HNTs和C-S-HNTs对OTC的吸附是单分子层均匀吸附[43],且吸附过程存在化学吸附,OTC被吸附到C-S-HNTs特定且均匀的活性位点上,吸附的过程为一个可逆的过程。吸附常数KL均小于1,因此可以初步判定HNTs和C-S-HNTs对OTC的吸附均属于优惠吸附;另外,表4中HNTs和C-S-HNTs吸附OTC的Freundlich吸附等温线拟合参数1/n分别为0.106和0.289,均在0—1的范围内,说明吸附反应容易进行。
表 4 吸附等温线拟合参数Table 4. Isotherm parameters for adsorption样品Samples Langmuir Freundlich D-R qmax/(mg·g−1) KL R2 <chi> KF 1/n R2 <chi> β R2 E/(kJ·mol−1) HNTs 11.483 0.654 0.993 0.0822 6.547 0.106 0.943 0.702 0.782 0.962 1.228 C-S-HNTs 29.602 0.277 0.999 0.251 7.381 0.289 0.961 18.037 0.754 0.886 1.251 为进一步了解HNTs和C-S-HNTs对OTC的吸附机制,用D-R模型对数据进行拟合。由该模型计算得出的参数E为吸附自由能,可由E来判断吸附机制。由表4可知,C-S-HNTs和HNTs吸附OTC的吸附自由能均小于8 kJ·mol−1,这也就意味着HNTs和C-S-HNTs对OTC的吸附过程存在物理吸附。
2.2.3 吸附热力学
根据热力学公式,采用线性回归的方法,得到数据如表5所示,HNTs和C-S-HNTs吸附OTC的过程焓变ΔH分别为41.65 kJ·mol−1和27.16 kJ·mol−1,均大于0,证明吸附OTC的过程是吸热反应。吸附ΔS均大于0,这是因为单个OTC的体积要比单个H2O分子的体积要大,每吸附一个 OTC会伴随着更多H2O分子的解吸,导致熵增大于熵减,也说明HNTs和C-S-HNTs吸附OTC后,表面结构发生了改变,体系自由度变大,反应朝着混乱程度增大的方向进行。由表5可知,C-S-HNTs吸附OTC的过程中ΔG均小于0,说明此反应是自发进行的,不需要从外界获取能量。在一定程度上,吸附的类型可以从焓变量加以区分[44],物理吸附的ΔH小于84 kJ·mol−1,而化学吸附的ΔH在84—420 kJ·mol−1。但也有文献认为[45],通常物理吸附的ΔG在-20—0 kJ·mol−1, 而化学吸附的ΔG在-400—-80 kJ·mol−1。C-S-HNTs吸附OTC的ΔH均小于84 kJ·mol−1,ΔG均在-20—0 kJ·mol−1范围内,表明C-S-HNTs对OTC的吸附过程存在物理吸附。另一方面,准二级动力学方程说明存在化学吸附过程。因此,C-S-HNTs对OTC的吸附过程应该是物理吸附和化学吸附共同作用的结果。
表 5 吸附热力学拟合参数Table 5. Thermodynamic parameters for adsorption样品Samples 热力学常数Thermodynamic Constant 温度/K lnK ΔG/(kJ·mol−1) ΔH/(kJ·mol−1) ΔS/(J·mol−1·K−1) HNTs 303 −1.28 1.30 41.65 133.16 313 −0.95 −0.029 323 −0.60 −1.36 C-S- HNTs 303 −0.0027 −0.0069 27.16 89.65 313 0.35 −0.90 323 0.67 −1.80 2.2.4 溶液pH的影响
OTC在不同的pH下会呈现不同的形态,因此,HNTs和C-S-HNTs对OTC的吸附作用在不同的酸碱环境下是不同的。OTC是两性抗生素,分子结构中存在酸碱结构的酚羟基和氨基,共有3.3、7.7和9.7的3个解离常数(pKa)[24]。在不同的pH下,OTC分为4个组分:当pH小于3.3时,酚二酮基团失去1个质子,OTC结构中二甲氨基基团被质子化,主要以阳离子OCH3+的形式存在;当pH值为3.3—7.7时,酚二酮基团失去2个质子,OTC显现两性性质,形成两性离子形态,整体不带电荷,主要以OCH20的形式存在;pH大于7.7以后,含甲酰基氨基在内的三碳基团便失去H+,主要以OCH−和OC2-的形式存在[45-46](图11)。从图11可以看出,C-S-HNTs吸附OTC在pH 3时吸附容量比较低,吸附量随着溶液pH的增大而增加,当pH值在5—7时吸附容量最大,约为19 mg·g−1,此时pH增加吸附量迅速减小,在pH=10时其吸附量只有2 mg·g−1左右。这是因为在不同pH环境中,黏土矿物表面和抗生素的电荷属性会发生变化。当环境pH<pHZPC(黏土矿物的零点电荷pH)时,黏土表面带正电;当pH>pHZPC时,黏土表面带负电。通过Zeta电位测试得到HNTs的等电点pHZPC为3.34,C-S-HNTs的等电点pHZPC为3.67。在pH<3.3时,OTC主要以阳离子形式OCH3+ 存在,此时C-S-HNTs表面带正电,因此产生排斥作用,但由于此时体系中还存在部分OCH20分子,因此会仍有一定吸附量。在溶液pH值在5—7条件下,C-S-HNTs对OTC的吸附性能更强,这是因为OTC以OCH20分子形式存在,在该区域内OTC所带电荷几乎为零,此时黏土矿物与OTC之间的静电相互作用力几乎可以忽略,OCH20易通过分配作用与C-S-HNTs表面的有机相结合,吸附效果好且不易受pH的影响。有研究认为此时的高吸附量可能还与其他作用有关,比如络合作用[47]。由图11可知,在pH5时,C-S-HNTs吸附量达到最高,这是因为当pH=5.5时,OCH20达到最大比例。随着溶液pH的增加,溶液中的OTC主要以OCH−和OC2-负电荷的形式存在,且随着pH增大,体系中OTC以阴离子形式存在越来越多,此时HNTs和C-S-HNTs均带负电荷,因此产生排斥作用,从而吸附量随着pH的增大而减少。
2.2.5 吸附机理
经过阴、阳离子表面活性剂复配改性得到的吸附材料C-S-HNTs,不仅保持了HNTs本身具有的吸附优势,还通过表面活性剂的修饰作用进一步提高了其对OTC的吸附能力。一方面,改性剂的加入使HNTs吸附OTC的分配作用有所增强;另一方面,阴、阳离子表面活性剂的负载,很大程度上丰富了HNTs表面的官能团,创造了更多的活性吸附位点,表面吸附作用的增强同样也提高了其吸附能力。
(1)分配作用:结合XRD和FTIR分析可得,CTAB和SDBS成功负载在HNTs外表面从而改变了HNTs的外表面性质。如图12,CTAB和SDBS通过疏水端结合形成混合胶束,CTAB带正电荷的季铵盐端与HNTs带负电的外表面结合,CTAB-SDBS一方面中和HNTs外表面部分负电荷,一方面增加HNTs表面有机相,为OTC吸附提供良好的有机分配介质,提高了OTC在HNTs表面的“溶解”能力,有利于OTC从水相中转移到C-S-HNTs的有机相上而发生吸附(图12①)。
(2)表面吸附作用:OTC属于两性抗生素,共有3.3、7.7和9.7的3个解离常数(pKa)。在不同的pH条件下,分别以阳离子OCH3+、两性离子OCH20和阴离子OCH−和OC2-等多种形式存在。HNTs表面带有较高的负电荷,CTAB季铵盐N端带正电,SDBS亲水端含有带负电的磺酸极性基(R-SO3−),以各种形式存在的OTC均能通过静电作用与C-S-HNTs结合(图12②③),从而增大吸附能力。有研究者发现用3-氨丙基三甲氧基硅烷改性的HNTs对酸性橙Ⅱ的吸附存在静电吸引作用[41],改性后吸附量也明显提高,这与本研究的结果 一致。此外,OTC上的羟基可以与SDBS上的磺酸基相互作用形成氢键作用(图12④)。Yuan 等[48]利用γ−氨基丙基三乙氧基硅烷对HNTs 进行改性用于染料吸附,也发现改性处理提高了 HNTs 表面的有机亲和性。
3. 结论(Conclusion)
本研究以阳离子表面活性剂CTAB和阴离子表面活性剂SDBS改性HNTs,制备了C-S-HNTs以吸附水溶液中的OTC。两种表面活性剂成功地负载在HNTs表面,改性剂没有通过离子交换进入层间,使HNTs保持了原有的形貌和晶体结构。有机改性使得埃洛石对OTC的吸附率提高了50%。C-S-HNTs对OTC的吸附过程符合准二级动力学模型,其扩散机制为多过程吸附。Langmuir等温吸附模型更适合描述吸附数据,吸附为单层吸附。反应为吸热反应,自发进行且混乱程度增大。C-S-HNTs对OTC的吸附受pH影响较大。C-S-HNTs增加了HNTs表面的官能团,为OTC的吸附提供了一个良好的有机分配介质。C-S-HNTs对OTC的吸附机理主要包括分配作用和表面吸附作用。
-
表 1 HNTs、C-S-HNTs的比表面积和孔结构参数
Table 1. Surface area and porous structure parameters of the HNTs and C-S-HNTs
样品Sample 比表面积/(m2·g−1)Specific surface 平均孔径/nmAverage porediameter 孔容/(cm3·g−1)Total pore volume HNTs 27.695 14.563 0.104 C-S-HNTs 9.429 42.065 0.00197 表 2 吸附动力学拟合参数
Table 2. Kinetic parameters of adsorption
样品Sample 准一级动力学模型Pseudo-first-order 准二级动力学模型Pseudo-second-order K1/min−1 qe/(mg·g−1) R2 <chi> K2/(g·mg−1min−1) qe/(mg·g−1) R2 <chi> HNTs 0.87 11.76 0.91 1.42 0.10 12.57 0.97 0.44 C-S-HNTs 0.51 15.19 0.92 2.44 0.036 17.028 0.97 0.82 表 3 颗粒内扩散模型拟合参数
Table 3. The fitting parameters of intra-particle diffusion models
样品Sample 拟合公式Fitting formula Kp/(mg·g-1·min-0.5) R2 HNTs y = 0.1486x + 6.5964 0.1486 0.853 C-S-HNTs y = 0.2348x + 6.5711 0.2348 0.860 表 4 吸附等温线拟合参数
Table 4. Isotherm parameters for adsorption
样品Samples Langmuir Freundlich D-R qmax/(mg·g−1) KL R2 <chi> KF 1/n R2 <chi> β R2 E/(kJ·mol−1) HNTs 11.483 0.654 0.993 0.0822 6.547 0.106 0.943 0.702 0.782 0.962 1.228 C-S-HNTs 29.602 0.277 0.999 0.251 7.381 0.289 0.961 18.037 0.754 0.886 1.251 表 5 吸附热力学拟合参数
Table 5. Thermodynamic parameters for adsorption
样品Samples 热力学常数Thermodynamic Constant 温度/K lnK ΔG/(kJ·mol−1) ΔH/(kJ·mol−1) ΔS/(J·mol−1·K−1) HNTs 303 −1.28 1.30 41.65 133.16 313 −0.95 −0.029 323 −0.60 −1.36 C-S- HNTs 303 −0.0027 −0.0069 27.16 89.65 313 0.35 −0.90 323 0.67 −1.80 -
[1] HE L Y, LIU Y S, SU H C, et al. Dissemination of antibiotic resistance genes in representative broiler feedlots environments: Identification of indicator ARGs and correlations with environmental variables [J]. Environmental Science & Technology, 2014, 48(22): 13120-13129. [2] QIAO M, YING G G, SINGER A C, et al. Review of antibiotic resistance in China and its environment [J]. Environment International, 2018, 110: 160-172. doi: 10.1016/j.envint.2017.10.016 [3] SUN Y B, XU Y, XU Y M, et al. Reliability and stability of immobilization remediation of Cd polluted soils using sepiolite under pot and field trials [J]. Environmental Pollution, 2016, 208: 739-746. doi: 10.1016/j.envpol.2015.10.054 [4] YU H, ZHU Y F, HUI A P, et al. Removal of antibiotics from aqueous solution by using porous adsorbent templated from eco-friendly Pickering aqueous foams [J]. Journal of Environmental Sciences, 2021, 102: 352-362. doi: 10.1016/j.jes.2020.09.010 [5] 王盈盈, 余静, 曾红杰, 等. 磁性吸附剂CeO2/MZFS去除水中盐酸四环素 [J]. 环境科学学报, 2020, 40(9): 3250-3258. WANG Y Y, YU J, ZENG H J, et al. Adsorption of tetracycline hydrochloride by magnetic adsorbent CeO2/MZFS [J]. Acta Scientiae Circumstantiae, 2020, 40(9): 3250-3258(in Chinese).
[6] JOSEPH L, JUN B M, JANG M, et al. Removal of contaminants of emerging concern by metal-organic framework nanoadsorbents: A review [J]. Chemical Engineering Journal, 2019, 369: 928-946. doi: 10.1016/j.cej.2019.03.173 [7] SHEN Y, CHEN B L. Sulfonated graphene nanosheets as a superb adsorbent for various environmental pollutants in water [J]. Environmental Science & Technology, 2015, 49(12): 7364-7372. [8] XIONG W P, ZENG G M, YANG Z H, et al. Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53(Fe) as new adsorbent [J]. Science of the Total Environment, 2018, 627: 235-244. doi: 10.1016/j.scitotenv.2018.01.249 [9] 侯嫔, 杨晓瑜, 霍燕龙, 等. 超声氧化多壁碳纳米管对水中Ni(Ⅱ)的吸附效能 [J]. 环境工程学报, 2021, 15(7): 2256-2264. doi: 10.12030/j.cjee.202102127 HOU P, YANG X Y, HUO Y L, et al. Adsorption efficiency of Ni(Ⅱ) in water by ultrasonically oxidized multi-walled carbon nanotubes [J]. Chinese Journal of Environmental Engineering, 2021, 15(7): 2256-2264(in Chinese). doi: 10.12030/j.cjee.202102127
[10] ZHANG B P, HAN X L, GU P J, et al. Response surface methodology approach for optimization of ciprofloxacin adsorption using activated carbon derived from the residue of desilicated rice husk [J]. Journal of Molecular Liquids, 2017, 238: 316-325. doi: 10.1016/j.molliq.2017.04.022 [11] SOPHIA A C, LIMA E C. Removal of emerging contaminants from the environment by adsorption [J]. Ecotoxicology and Environmental Safety, 2018, 150: 1-17. doi: 10.1016/j.ecoenv.2017.12.026 [12] 杜明阳, 邹京, 豆俊峰, 等. 钾改性蒙脱石磁性微球对铯的吸附性能 [J]. 环境化学, 2021, 40(3): 779-789. doi: 10.7524/j.issn.0254-6108.2019110202 DU M Y, ZOU J, DOU J F, et al. Adsorption properties of potassium modified montmorillonite magnetic microspheres for cesium [J]. Environmental Chemistry, 2021, 40(3): 779-789(in Chinese). doi: 10.7524/j.issn.0254-6108.2019110202
[13] GULEN B, DEMIRCIVI P. Adsorption properties of flouroquinolone type antibiotic ciprofloxacin into 2: 1 dioctahedral clay structure: Box-Behnken experimental design [J]. Journal of Molecular Structure, 2020, 1206: 127659. doi: 10.1016/j.molstruc.2019.127659 [14] ZHANG B F, YUAN P, GUO H Z, et al. Effect of curing conditions on the microstructure and mechanical performance of geopolymers derived from nanosized tubular halloysite [J]. Construction and Building Materials, 2021, 268: 121186. doi: 10.1016/j.conbuildmat.2020.121186 [15] BEN M'BAREK JEMAÏ M, SDIRI A, ERRAIS E, et al. Characterization of the Ain Khemouda halloysite (western Tunisia) for ceramic industry [J]. Journal of African Earth Sciences, 2015, 111: 194-201. doi: 10.1016/j.jafrearsci.2015.07.014 [16] JAUKOVIĆ V, KRAJIŠNIK D, DAKOVIĆ A, et al. Influence of selective acid-etching on functionality of halloysite-chitosan nanocontainers for sustained drug release [J]. Materials Science and Engineering:C, 2021, 123: 112029. doi: 10.1016/j.msec.2021.112029 [17] YUAN P, TAN D Y, ANNABI-BERGAYA F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects [J]. Applied Clay Science, 2015, 112/113: 75-93. doi: 10.1016/j.clay.2015.05.001 [18] CHANG P R, XIE Y F, WU D L, et al. Amylose wrapped halloysite nanotubes [J]. Carbohydrate Polymers, 2011, 84(4): 1426-1429. doi: 10.1016/j.carbpol.2011.01.038 [19] JOO Y, JEON Y, LEE S U, et al. Aggregation and stabilization of carboxylic acid functionalized halloysite nanotubes (HNT-COOH) [J]. The Journal of Physical Chemistry C, 2012, 116(34): 18230-18235. doi: 10.1021/jp3038945 [20] MATUSIK J, WŚCISŁO A. Enhanced heavy metal adsorption on functionalized nanotubular halloysite interlayer grafted with aminoalcohols [J]. Applied Clay Science, 2014, 100: 50-59. doi: 10.1016/j.clay.2014.06.034 [21] 王嘉玮. 渭河西安段表层水体中抗生素的分布特征及生态风险评价[D]. 西安: 西安理工大学, 2018. WANG J W. Distribution characteristics and ecological risk assessment of antibiotics in surface water of xi’an section of Weihe river[D]. Xi’an: Xi’an University of Technology, 2018(in Chinese).
[22] COSTA R F, FIRMANO R F, COLZATO M, et al. Sulfur speciation in a tropical soil amended with lime and phosphogypsum under long-term no-tillage system [J]. Geoderma, 2022, 406: 115461. doi: 10.1016/j.geoderma.2021.115461 [23] ZHANG W, WANG L, SU Y G, et al. Indium oxide/Halloysite composite as highly efficient adsorbent for tetracycline Removal: Key roles of hydroxyl groups and interfacial interaction [J]. Applied Surface Science, 2021, 566: 150708. doi: 10.1016/j.apsusc.2021.150708 [24] WU J Y, WANG Y H, WU Z X, et al. Adsorption properties and mechanism of sepiolite modified by anionic and cationic surfactants on oxytetracycline from aqueous solutions [J]. Science of the Total Environment, 2020, 708: 134409. doi: 10.1016/j.scitotenv.2019.134409 [25] ZHANG Y G, BAI L B, CHENG C, et al. A novel surface modification method upon halloysite nanotubes: A desirable cross-linking agent to construct hydrogels [J]. Applied Clay Science, 2019, 182: 105259. doi: 10.1016/j.clay.2019.105259 [26] FAKHRUDDIN K, HASSAN R, KHAN M U A, et al. Halloysite nanotubes and halloysite-based composites for biomedical applications [J]. Arabian Journal of Chemistry, 2021, 14(9): 103294. doi: 10.1016/j.arabjc.2021.103294 [27] CAN M, DEMIRCI S, YILDRIM Y, et al. Modification of halloysite clay nanotubes with various alkyl halides, and their characterization, blood compatibility, biocompatibility, and genotoxicity [J]. Materials Chemistry and Physics, 2021, 259: 124013. doi: 10.1016/j.matchemphys.2020.124013 [28] SZCZEPANIK B, SŁOMKIEWICZ P, GARNUSZEK M, et al. The effect of chemical modification on the physico-chemical characteristics of halloysite: FTIR, XRF, and XRD studies [J]. Journal of Molecular Structure, 2015, 1084: 16-22. doi: 10.1016/j.molstruc.2014.12.008 [29] SHANG S, MA X, YUAN B H, et al. Modification of halloysite nanotubes with supramolecular self-assembly aggregates for reducing smoke release and fire hazard of polypropylene [J]. Composites Part B:Engineering, 2019, 177: 107371. doi: 10.1016/j.compositesb.2019.107371 [30] CHENG H F, FROST R L, YANG J, et al. Infrared and infrared emission spectroscopic study of typical Chinese kaolinite and halloysite [J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2010, 77(5): 1014-1020. doi: 10.1016/j.saa.2010.08.039 [31] TAHERIAN S, RAHMANI S, SHARIF A, et al. In-situ polymerization of aliphatic-aromatic polyamide nanocomposites in the presence of Halloysite nanotubes [J]. Polymers for Advanced Technologies, 2019, 30(3): 538-544. doi: 10.1002/pat.4489 [32] ZHENG Y, WANG L F, ZHONG F L, et al. Site-oriented design of high-performance halloysite-supported palladium catalysts for methane combustion [J]. Industrial & Engineering Chemistry Research, 2020, 59(13): 5636-5647. [33] LIU S, WU P X, CHEN M Q, et al. Amphoteric modified vermiculites as adsorbents for enhancing removal of organic pollutants: Bisphenol A and Tetrabromobisphenol A [J]. Environmental Pollution, 2017, 228: 277-286. doi: 10.1016/j.envpol.2017.03.082 [34] 刘晨, 陈元涛, 张炜, 等. KH-550改性埃洛石对水中铀酰离子吸附性能的研究 [J]. 环境科学学报, 2017, 37(1): 243-248. LIU C, CHEN Y T, ZHANG W, et al. The adsorption of uranyl ion in aqueous solution by halloysite modified KH-550 [J]. Acta Scientiae Circumstantiae, 2017, 37(1): 243-248(in Chinese).
[35] LI J, YU G W, PAN L J, et al. Study of ciprofloxacin removal by biochar obtained from used tea leaves [J]. Journal of Environmental Sciences, 2018, 73: 20-30. doi: 10.1016/j.jes.2017.12.024 [36] YAN Z L, FU L J, ZUO X C, et al. Green assembly of stable and uniform silver nanoparticles on 2D silica nanosheets for catalytic reduction of 4-nitrophenol [J]. Applied Catalysis B:Environmental, 2018, 226: 23-30. doi: 10.1016/j.apcatb.2017.12.040 [37] SUN Y B, SHAO D D, CHEN C L, et al. Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline [J]. Environmental Science & Technology, 2013, 47(17): 9904-9910. [38] LI F B, LI X Y, CUI P. RETRACTED: Adsorption of U(VI) on magnetic iron oxide/Paecilomyces catenlannulatus composites [J]. Journal of Molecular Liquids, 2018, 252: 52-57. doi: 10.1016/j.molliq.2017.12.136 [39] JIN Z X, WANG X X, SUN Y B, et al. Adsorption of 4-n-nonylphenol and bisphenol-A on magnetic reduced graphene oxides: A combined experimental and theoretical studies [J]. Environmental Science & Technology, 2015, 49(15): 9168-9175. [40] WU Y, LUO H J, WANG H. Efficient removal of Congo red from aqueous solutions by surfactant-modified hydroxo aluminum/graphene composites [J]. Separation Science and Technology, 2014, 49(17): 2700-2710. doi: 10.1080/01496395.2014.942741 [41] 李艳, 张证, 孙瑜, 等. 埃洛石对阳离子型和阴离子型染料的吸附研究 [J]. 矿物岩石地球化学通报, 2020, 39(2): 187-192,169. LI Y, ZHANG Z, SUN Y, et al. A study on the adsorption of cationic and anionic dyes by halloysite [J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2020, 39(2): 187-192,169(in Chinese).
[42] ABUNOWARA M, SUFIAN S, BUSTAM M A, et al. Experimental and theoretical investigations on kinetic mechanisms of low-pressure CO2 adsorption onto Malaysian coals [J]. Journal of Natural Gas Science and Engineering, 2021, 88: 103828. doi: 10.1016/j.jngse.2021.103828 [43] 周莉, 童裳伦. 碘氧化铋/钨酸铜复合材料的制备及对氟喹诺酮类抗生素的吸附性能 [J]. 环境科学学报, 2021, 41(10): 3993-4002. ZHOU L, TONG C L. The preparation of BiOI/CuWO4 composite and its adsorption performance for fluoroquinolone antibiotics [J]. Acta Scientiae Circumstantiae, 2021, 41(10): 3993-4002(in Chinese).
[44] MUNAGAPATI V S, KIM D S. Adsorption of anionic azo dye Congo Red from aqueous solution by Cationic Modified Orange Peel Powder [J]. Journal of Molecular Liquids, 2016, 220: 540-548. doi: 10.1016/j.molliq.2016.04.119 [45] SONG Y L, SACKEY E A, WANG H, et al. Adsorption of oxytetracycline on kaolinite [J]. PLoS One, 2019, 14(11): e0225335. doi: 10.1371/journal.pone.0225335 [46] SUN Y Y, YUE Q Y, GAO B Y, et al. Preparation of activated carbon derived from cotton linter fibers by fused NaOH activation and its application for oxytetracycline (OTC) adsorption [J]. Journal of Colloid and Interface Science, 2012, 368(1): 521-527. doi: 10.1016/j.jcis.2011.10.067 [47] CHANG P H, LI Z H, JIANG W T, et al. Adsorption and intercalation of tetracycline by swelling clay minerals [J]. Applied Clay Science, 2009, 46(1): 27-36. doi: 10.1016/j.clay.2009.07.002 [48] YUAN P, SOUTHON P D, LIU Z W, et al. Organosilane functionalization of halloysite nanotubes for enhanced loading and controlled release [J]. Nanotechnology, 2012, 23(37): 375705. doi: 10.1088/0957-4484/23/37/375705 -






 下载:
下载: