-
能源消耗的与日剧增和化石燃料的日渐匮乏及其引发的环境问题,促使新型清洁能源发展迅猛[1]。核能具有清洁稳定、经济高效等特点,在新型能源中发挥着越来越重要的作用[2]。铀资源是核工业持续发展的基础原料,然而在铀矿的开采、核燃料的加工使用等过程中会产生大量放射性的含铀废水,如果得不到妥善处理将会对环境造成污染[2]。此外,突发的核泄漏也会产生大量高辐射的含铀废水,对环境和生态造成难以修复的灾难[3]。因此,亟需建立快速高效的铀富集及检测方法来对铀污染进行预防和治理。
铀在自然界中主要以U(Ⅳ)和U(Ⅵ) 2种价态存在[4]。U(Ⅳ)主要存在于还原条件下,当溶液为氧化条件时,正四价的铀会被氧化成正六价。此外,U(Ⅳ)容易与溶液中溶解的无机碳发生络合反应,形成沉淀[5-6]。通常情况下,水中铀的主要价态为正六价,常见的含铀废水处理方法包括化学沉淀、离子交换、膜分离和吸附等[7]。吸附法因为操作方法简单,成本可控,是目前公认的最好的铀富集手段之一[8]。吸附材料可以借助多孔结构和较大的比表面积增大吸附效果[9],通过对其表面官能团进行修饰,对铀酰离子进行选择性富集。ZHAO等[10]设计了一种具有纳米孔道的互联开孔结构的聚偕胺肟纤维,该吸附剂在天然水体中对铀的吸附量高达17.6 mg·g−1,可至少重复使用30次,大大降低了生产成本,极具实际应用价值。WANG等[11]受海洋海绵独特的生理结构特性的启发,通过简单的浸渍-干燥-交联的过程,将聚酰亚胺二肟/藻酸盐互穿网络水凝胶层均匀包裹在三聚氰胺海绵基材的骨架上,聚酰亚胺二肟/藻酸盐复合海绵5 t海水中吸附56 d后铀吸附量达到5.8 mg·g−1。LUO等[12]报道了一种可从海水中高效、低成本的提取铀的微孔膜。这种微孔膜处理来自我国东海的 10 L天然海水后可获取27.81 μg铀,而它的生产成本大约为275 $·kg−1。
水体中的铀质量浓度的检测也是保障核安全的重要环节[13],根据《铀矿冶辐射防护和环境保护规定》[14],可排放的含铀废水中铀的质量浓度不能超过50 µg·L−1。世界卫生组织要求饮用水中铀的最高质量浓度不得高于14.4 µg·L−1[15]。目前,较为主流的检测方法有电感耦合等离子体(ICP)联用技术,包括电感耦合等离子体发射光谱法(ICP-OES)[16]、电感耦合等离子体质谱法(ICP-MS)[17]、火焰原子吸收光谱法(FAAS)、拉曼光谱法和分光光度法[18],此外还包括根据颜色差值进行检测的比色法。以上方法虽然在检测时可以达到较高的精确度,但是需要额外添加显色剂,对操作精确度的要求高,且均需要在实验室条件下进行分析,这使得铀的检测过程繁琐,增加了人力及时间成本。为了高效、快速地进行铀的检测,必须寻找到一种快速、高效的现场原位检测技术。
铀是一种天然放射性元素,可以放射出α射线,因而可以将铀富集后利用α表面污染探测仪,快速便捷地检测出其表面α粒子数,实现对铀质量浓度的检测。但是α射线射程极短、穿透力很弱,在几何形状不规则的污染表面不易被探测器检测,影响检测效果。因此,使用α表面污染探测仪需要重点考虑如何将水体中的铀富集到形状规则的表面[19]。在众多吸附材料中,平板膜型吸附剂因其表面平整,比粉末状、纤维状、凝胶状等吸附材料更容易满足α表面探测仪的探测条件。此外,平板膜型吸附剂不仅具有吸附能力,也结合了膜过滤技术具有的高效、操作性强、节省时间和空间等优势,在重金属过滤去除领域有很好的应用前景[7,20]。
本研究利用高性能的琥珀酰-β-环糊精膜对水体中的铀进行富集,使用可调节转速的齿轮泵和膜组件对污染水体进行连续、自动化地过滤吸附,通过调节操作压力和时间来优化铀的富集条件,使用α辐射探测仪对富集到膜表面的α粒子进行计数,计算得到不同质量浓度含铀废水的检测结果并绘制水体铀质量浓度与膜表面α粒子的关系曲线,确定水体中U(Ⅵ)的质量浓度且验证检测方法的精确度,旨在为α粒子检测仪检测水体中铀质量浓度的实际应用提供参考。
-
聚丙烯腈膜(PAN膜,孔径100 nm)购于广州海涛水处理有限公司。三羟甲基氨基甲烷(Tris,99%)、盐酸多巴胺(PDA-HCl,98%)、聚醚酰亚胺(PEI,相对分子质量25 000)、吗啉乙磺酸(MES,99.5%)、N-羟基琥珀酰亚胺(NHS,98%)、1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(EDC,98.5%)均购于麦克林生化科技有限公司;琥珀酰-β-环糊精(SβCD,99%)购于苏州卡博森斯化学科技有限公司;六水合硝酸铀酰(99%),偶氮胂Ⅲ(>95%)均购自与国药化学试剂有限公司。实验用水均为去离子水。
-
本实验使用的实验仪器包括场发射电子扫描显微镜(SEM,S-4800,日立公司)、原子力显微镜(AFM,Dimension Icon,美国维Bruker公司)、傅里叶变换红外光谱(FT-IR,pectrum BX00,Perkin-Elmer)、X射线光电子能谱(XPS,K-alpha,美国Thermo公司)、X射线能谱(EDS,NORAN 7,美国Thermo公司),α粒子检测仪(自研)。
-
本实验参考同课题组已发表成果中的方法[6]制备琥珀酰-β-环糊精膜,分别使用乙醇和去离子水浸泡PAN膜24 h,以去除表面保护膜并进行适当的扩孔。在Tris-HCl缓冲溶液(pH 为8.5 ± 0.2)中,加入PDA,搅拌均匀,并加入适量PEI,将PAN膜在室温下浸泡2 h,在膜表面形成具有黏附作用的PDA和PEI混合层。将琥珀酰-β-环糊精上的羧基通过在NHS和EDC 混合溶液中进行活化。将PDA/PEI黏附的PAN膜完全浸泡在琥珀酰-β-环糊精溶液中,在温度为25 ℃,转速为150 r·min−1条件下振荡24 h,制备了琥珀酰-β-环糊精膜,命名为CDM[6]。
-
用 SEM对膜的表面和断面形貌进行分析;借助AFM来观察样品表面的粗糙度;使用FT-IR和XPS用于检测膜表面化学组成;使用EDS对吸附铀之后的复合膜进行分析,并探究膜表面铀元素的分布情况。
-
将CDM放置在定制膜组件中,进水口通过硅胶软管连接齿轮泵的出水口。在膜组件的上方安装1个压力表,实时检测膜表面的压力。齿轮泵的进水口连接铀溶液,通过调节齿轮泵的转速,改变水流大小来调节膜表面的压力。所有软管用橡胶保温海绵进行保温。
-
α粒子探测器采用平面硅(PIPS)半导体探测器探测α粒子,α粒子在探测器的灵敏体积内产生电子-空穴对,电子-空穴对在外电场的作用下漂移而输出电流信号,电流信号的大小与α粒子能量相关,电流信号通过电荷灵敏前置放大镜进行电荷放大,形成脉冲信号,脉冲信号通过多道分析器进行采样、峰值检测等形成多道数字信号,此信号发送到上位机软件进行能谱分析,在整个过程中,使用真空泵控制压力并进行气压检测。
在进行测量前,要先对仪器进行能量刻度。能谱数据平滑的作用是去除能谱中的噪声计数,提高信噪比。初步选用多项式最小二乘拟合的方法对数据进行平滑。之后寻找U能量区间的计数,通过式(1)计算水中U(Ⅵ)质量浓度水平。
式中:C为铀质量浓度,μg·L−1;N为铀计数;
$ \epsilon $ 为仪器探测效率;V为水样总富集量;L;$ \eta $ 为富集效率;T为测量时间,s。在实验过程中,制备出不同铀质量浓度(80~8 000 µg·L−1)的溶液,并将其作为初始溶液。将吸附U(Ⅵ)的CDM(CDM-U)从膜过滤装置中取出,在55 ℃的烘箱中烘干,然后放置在α粒子检测仪中,对膜表面的α粒子数进行检测。使用恒温水浴槽控制进料液温度(所有管道均用保温棉进行保温),调节齿轮泵的转速来改变水体的流速,从而控制膜表面的压力,从过滤装置上方的压力表可以观察膜表面的压力。最后,将吸附膜放置到烘箱中进行干燥,使用α粒子检测仪计算膜表面的α粒子数目。
-
图1(a)和图1(b)分别显示了PAN和CDM的表面形态。可以看出,PAN表面较为光滑,而CDM的膜表面出现岛状颗粒,这与报道中的结果[6]一致。此外,借助图1(c)和图1(d)的AFM图像也可以确定浸泡过SβCD溶液的膜表面出现了形态上的改变。PAN的平均粗糙度为11.3 nm,CDM的平均粗糙度为9.17 nm,表明PAN膜表面负载了其他物质。
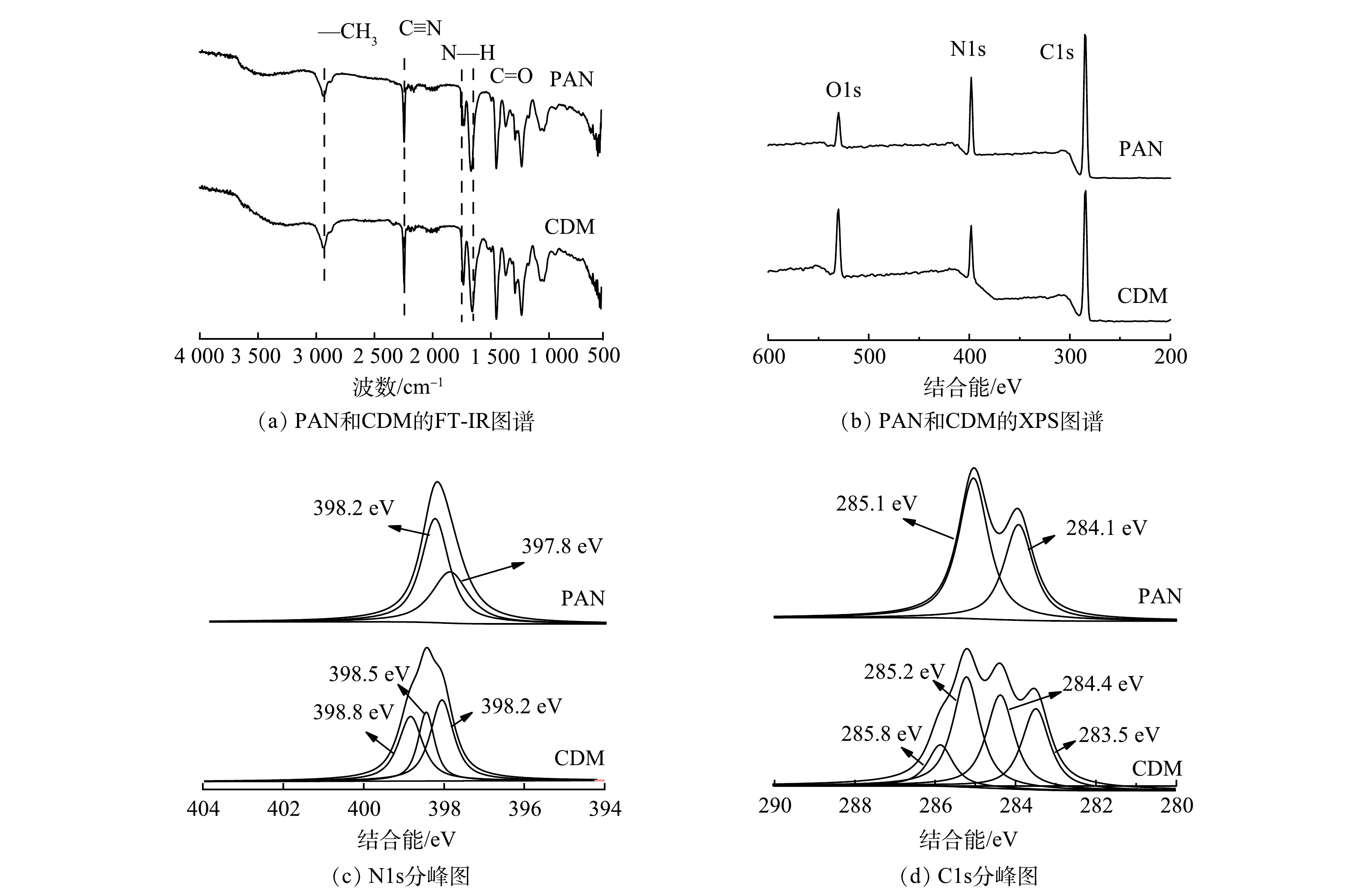
为进一步确定表面的颗粒是否为SβCD,利用FT-IR和XPS检测表面组成,结果如图2所示。通过FT-IR光谱对膜表面上官能团的化学性质进行研究,结果如图2(a)所示。可以看出,PAN在3 418 cm−1(—OH),2 950 cm−1(—CH3),2 253 cm−1(C≡N)和1 680 cm−1 (C=O)处的存在特征峰[3]。在CDM的FT-IR光谱中,C=O(1 679 cm−1)的峰值相较于PAN的峰值变高,而其他的峰值无明显变化。这可能是SβCD上的羧基导致的,证明SβCD被成功接枝到PAN膜表面。此外,还通过XPS评估了制备的膜的化学组成。由图2(b)可以看出,与PAN相比,CDM中N元素相对含量更低,O元素相对含量更高,也证明了SβCD被成功接枝到PAN膜表面[21]。PAN的N1s光谱可分为2个主要峰,位于397.8 eV和398.2 eV,分别对应于吡啶N和吡咯N[22];而在CDM的N1s光谱中,除了吡啶N(398.2 eV) 和吡咯N(398.5 eV)之外,出现了NH2(398.8 eV),该基团来自于膜表面添加的多巴胺[6,20] 。PAN膜的C1s光谱在284.1 eV和285.1 eV处的2个峰分别对应于C—C和C≡N。在CDM的C1s光谱中,由于引入了PDA和PEI,C—C峰出现了0.6 eV的负移,C≡N 峰产生了更高结合能,为285.2 eV。此外,还出现C=O和C—N的2个新峰,分别位于284.4 eV和285.8 eV,证明SβCD成功结合到膜表面[10,23]。
-
在静态条件下测试吸附动力学数据,并使用拟一级和拟二级速率模型进行拟合,以研究物理化学过程和吸附的动力学机理[24],得到的拟合参数见表1。在25 ℃的U(Ⅵ)溶液(80 mg·L−1)中进行吸附动力学实验,接触时间分别为5、20、30、60、100、150、200和300 min。根据拟合结果,伪二级吸附模型的拟合度(R2)高于伪一级吸附模型的拟合度,这表明吸附剂与被吸附物之间的吸附是化学吸附,铀酰离子是通过金属络合的方式被环糊精腔内的羟基和琥珀酰部分的羧酸根捕获。
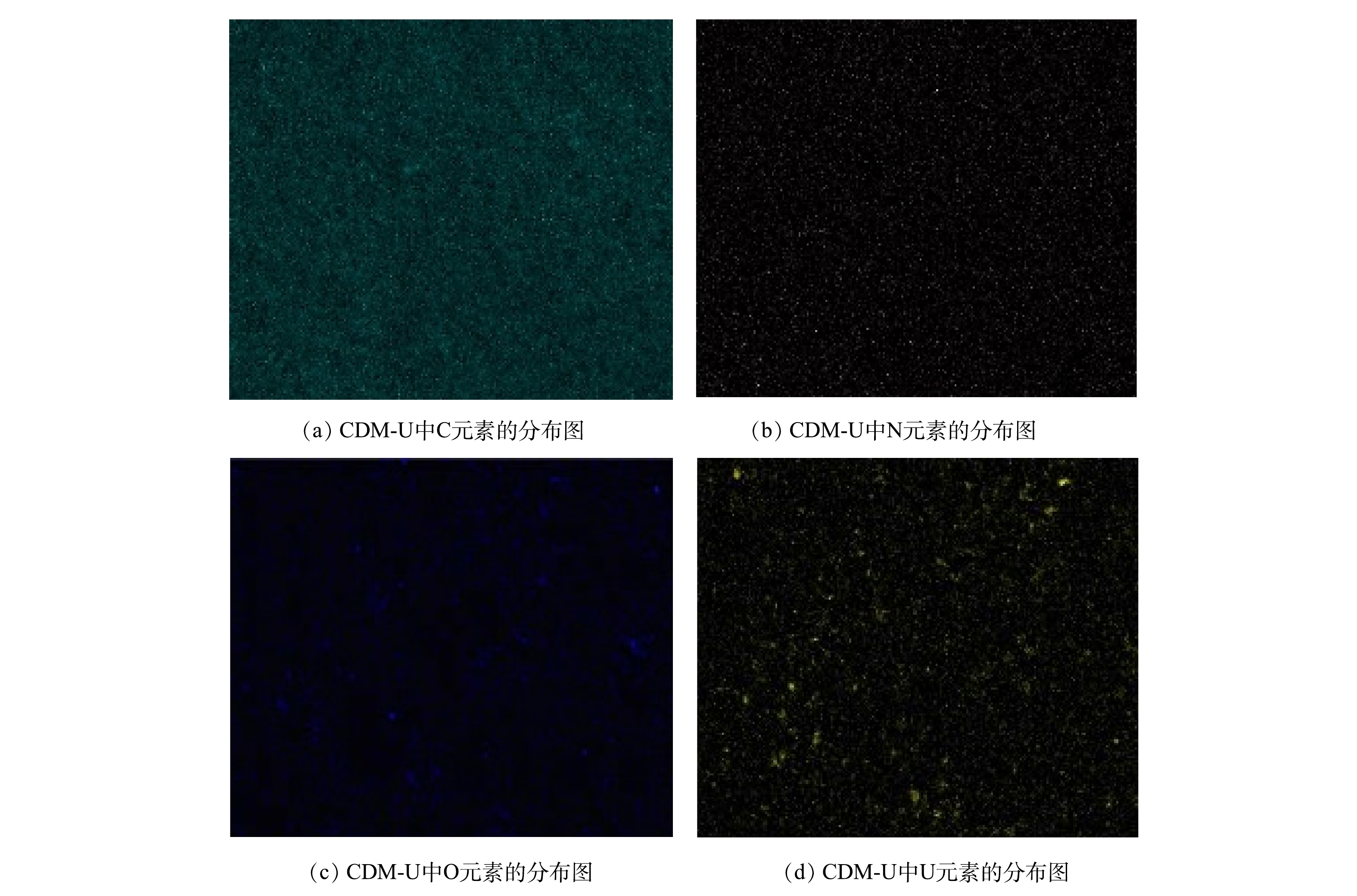
通过EDS能谱观察CDM-U表面的吸附情况。为增大EDS的检测效果,本实验对80 mg·L−1的含铀溶液进行过滤,在吸附2 h后,膜表面的EDS能谱结果见表2,膜表面U的质量分数只有1.68%。通过图3观察膜表面铀的分布情况,可以看出,在颗粒较大的位置,铀的吸附比较集中,但整体来看,铀元素基本均匀地分布在膜表面,为后续α粒子的检测提供了很好的条件。
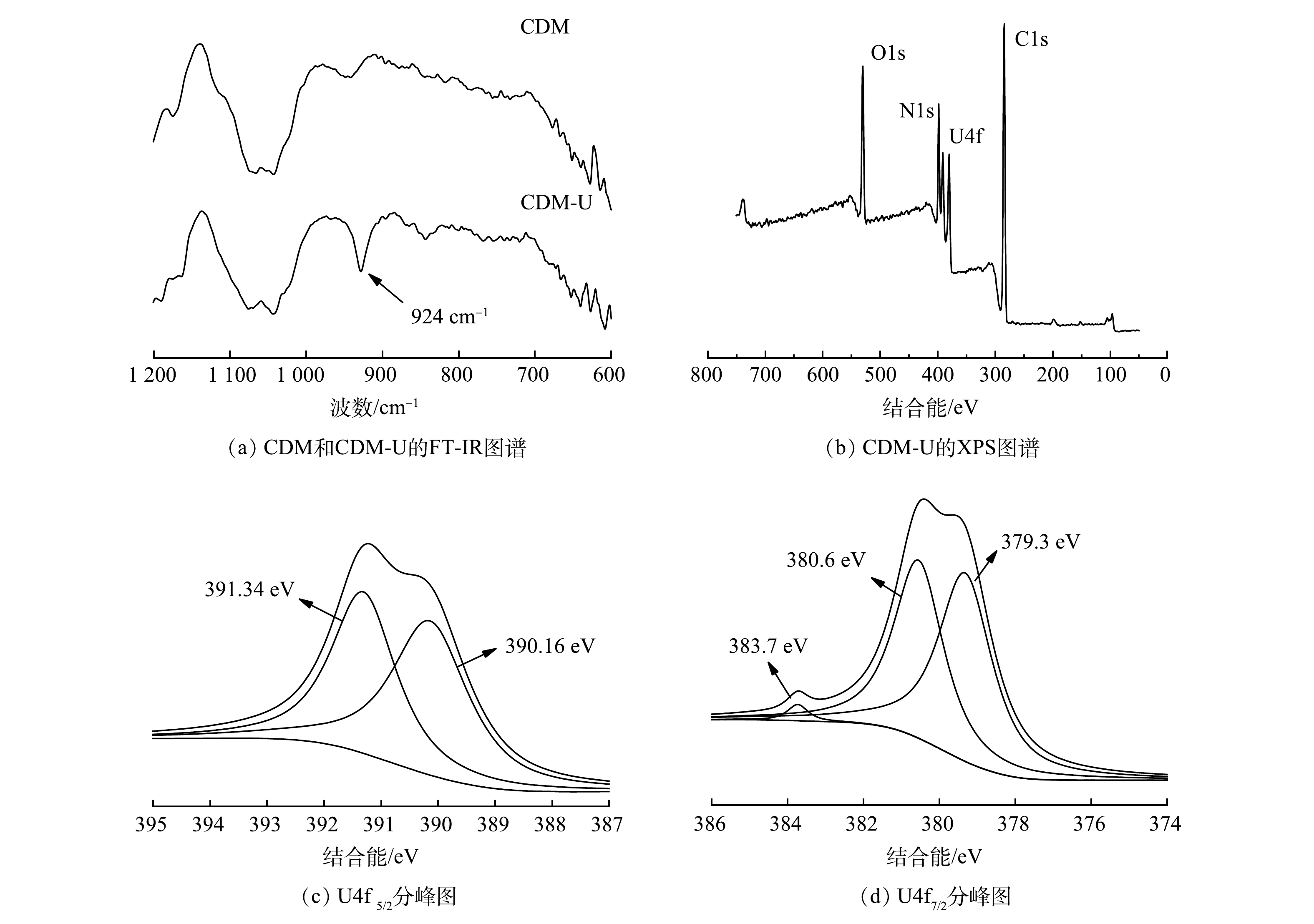
使用FT-IR和XPS对CDM-U的组成及吸附机理进行深入研究,结果如图4所示。在吸附U(Ⅵ)的CDM(CDM-U)FT-IR光谱中,在924 cm−1处出现了新峰[25],这是由于SβCD与U(Ⅵ)之间的络合作用形成的。图4(b)的XPS光谱中也得到了类似的结果,在400.7 eV处出现的新峰表明U(Ⅵ)和SβCD的羧基之间存在相互作用[26]。U4f7/2(391.5 eV)和U4f7/2(381.6 eV)的分峰结果如图4(c)和图4(d)所示,可以看出铀的价态在吸附过程中没有发生改变。
-
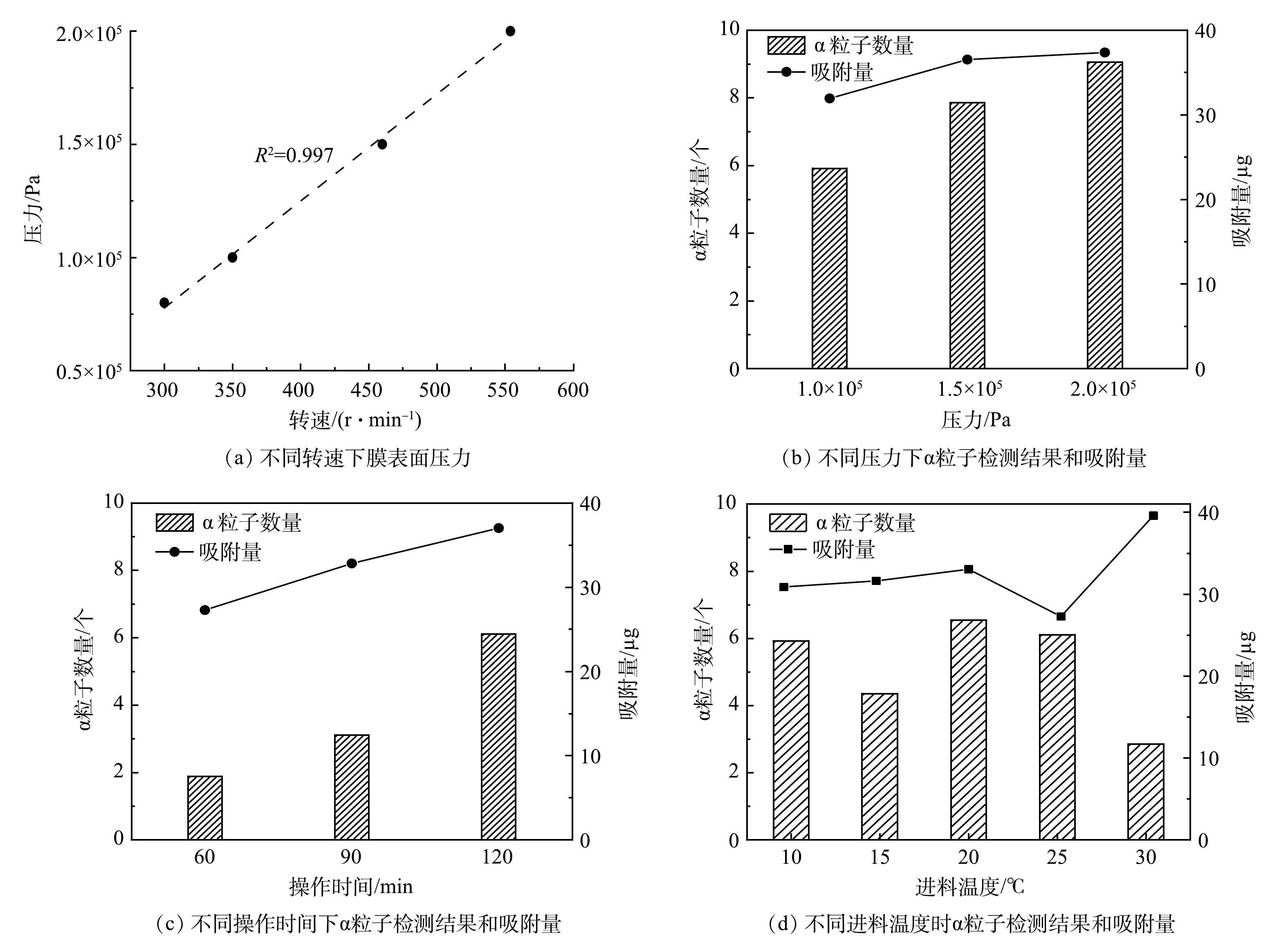
实验研究了CDM在不同压力和操作时间下对U(Ⅵ)的吸附情况。选择质量浓度为200 µg·L−1的U(Ⅵ)溶液进行过滤吸附,检测膜表面U(Ⅵ)的吸附量,结果如图5所示。由图5(a)可以看出,在转速为300~560 r·min−1时,压力和水通量与转速呈现很好的线性关系,拟合度为0.997。这说明在此转速范围内,可以通过改变转速调整实验所需的压力。由图5(b)可以看出,控制操作时间为120 min,调整压力1.0×105 ~ 2.0×105 Pa,检测的α粒子数随着压力的增大逐渐增多,这可能是水通量的增加导致更多的铀吸附到膜表面造成的。同样,由图5(c)可以看出,操作时间的增加也有助于让更多的U(Ⅵ)富集到膜表面,从而提高膜表面的α粒子数。
在实际应用中,不同流域的水温对CDM的过滤吸附有较大影响,因此,本实验选取了5个不同的温度对膜表面α粒子数进行计算,结果如图5(d)所示。可以看出,U(Ⅵ)的吸附整体呈波动状态。这可能是受到膜结构改变和吸附反应影响的双重作用导致的。进料温度改变膜材料的结构,受热胀冷缩影响,膜表面孔径变大,水流速度加快,增加了与铀酰离子的接触,从而增加了膜对U(Ⅵ)的吸附效果。但由于CDM对U(Ⅵ)的吸附是放热反应[6],温度的变化会影响吸附基团与铀的相互作用。随着温度的增加,膜材料对铀酰离子的吸附降低,从而影响了CDM的过滤效果,因此,膜的吸附效率与温度变化并非成正比例变化。
为确定该方法对铀溶液中U(Ⅵ)的检测情况,实验制备了不同质量浓度的U(Ⅵ)溶液。为防止制备过程中U(Ⅵ)溶液的质量浓度出现较大误差,采用ICPMS精确测量溶液中的U(Ⅵ)质量浓度, ICPMS仪器的重复性实验相对标准偏差(RSD)≤1%。
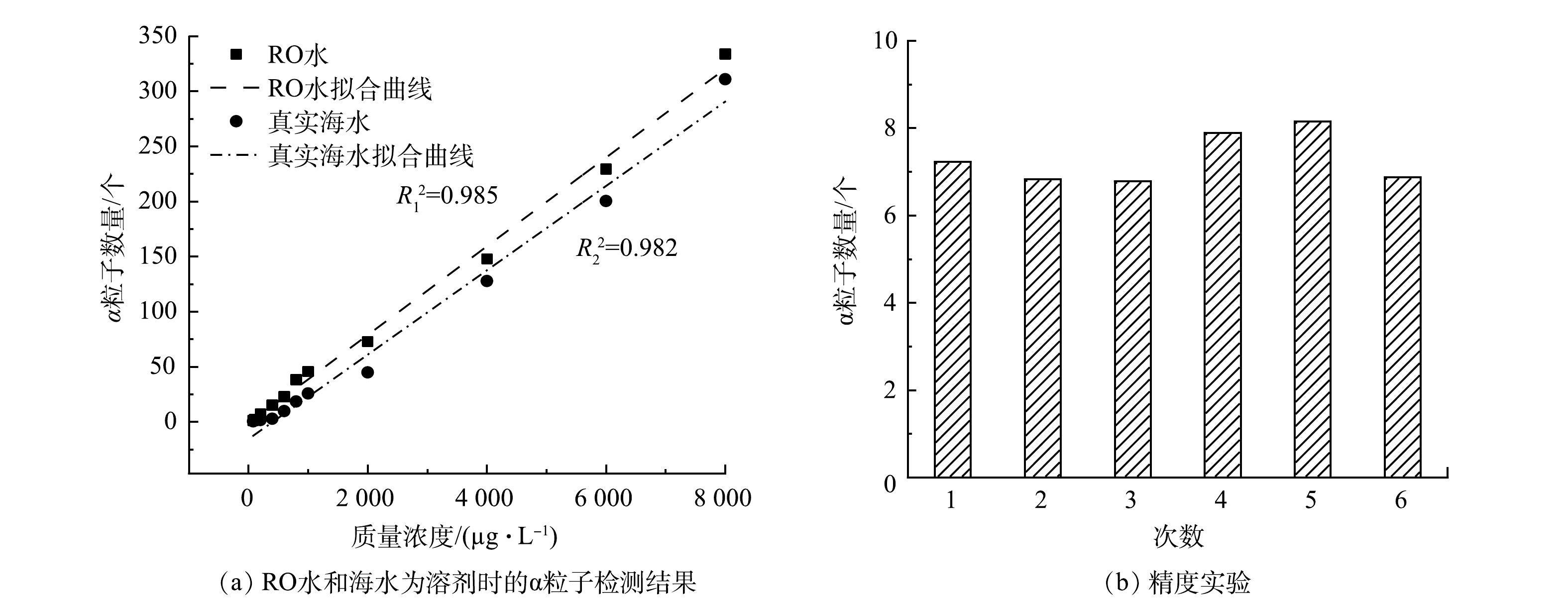
在25 ℃下,以8×104 Pa 的过滤压力过滤120 min不同浓度的U(Ⅵ)溶液,结果如图6(a)所示。可以看出,随着质量浓度的增加,α粒子的数量逐渐增大。这是因为质量浓度的增加提高了溶液中溶质分子的密度,增加了铀与吸附位点的接触。将数据进行线性拟合,得到拟合度R12=0.985,证明U(Ⅵ)的质量浓度与膜表面α粒子数量具有很好的线性关系。同样,将实验条件模拟到海水中(使用微滤膜对海水进行过滤净化),检测结果表明,受海水中其他离子的影响,CDM对U(Ⅵ)的吸附量降低,膜表面检测到的α数量降低,将数据进行线性拟合后,得到拟合度R22=0.982。
为确定检测方法的精确度,实验使用200 µg·L−1的U(Ⅵ)溶液为初始溶液(使用ICPMS确定溶液的质量浓度,为(200±2) µg·L−1),控制齿轮泵转速为300 r·min−1,在室温下过滤吸附,结果如图6(b)所示。重复操作6次,得到实验的精密度为1.11%。
-
1)利用多巴胺的黏合作用,将SβCD接枝到聚丙烯腈膜表面,制备了琥珀酰-β-环糊精膜。SEM、AFM、FT-IR、XPS检测结果证明材料被成功合成。
2)联合膜过滤与吸附技术将水体中的U(Ⅵ)富集到琥珀酰-β-环糊精膜表面,借助FT-IR、EDS、XPS等手段,均证明U(Ⅵ)可以被均匀地富集到琥珀酰-β-环糊精膜表面。较低的操作压力和较长的操作时间有利于水体中的U(Ⅵ)被均匀地富集到琥珀酰-β-环糊精膜表面;进料温度会影响过滤和吸附2个过程,富集效果呈现波动状态。
3)分别以离子水和海水为溶剂,制备不同质量浓度的U(Ⅵ)溶液进行实验,对琥珀酰-β-环糊精膜表面的α粒子进行计数,与溶液中U(Ⅵ)质量浓度进行拟合,分别得到拟合度R12=0.985和R22=0.982。这证明使用α粒子探测仪检测水体中的铀酰离子质量浓度具有很好的线性关系,此方法可以用于确定水溶液中的铀的质量浓度。同时该方法实验设备简单,可实现原位检测水中U(Ⅵ)质量浓度的目的。
基于琥珀酰-β-环糊精膜的铀酰离子富集与α粒子检测效果分析
Effect analysis of α particle detection and uranyl ion enrichment based on succinyl-β-cyclodextrin membrane
-
摘要: 为实现原位、高效检测水体中铀质量浓度的目的,采用层层负载的方法,制备了具有高效吸附铀的琥珀酰-β-环糊精膜;通过齿轮泵将进料液运输到膜表面,结合吸附和膜过滤技术将水体中的铀富集到膜表面,借助铀具有放射α粒子的特性,使用α粒子检测仪对膜表面的α粒子进行计算,从而推定水体中铀的质量浓度;通过改变操作压力、操作时间、进料温度,探究α粒子在膜表面的富集趋势,确定最佳操作条件。结果表明:当转速控制在300~560 r·min−1时,操作压力与转速呈线性关系,拟合度为0.997,吸附量随着操作压力和时间的增加而增加;进料液温度对膜结构和吸附反应有双重影响,膜表面的α粒子数量随着温度的变化呈波动状态,当温度为25 ℃时,膜表面α粒子数量为6.41个;在25 ℃下,调节压力为8×104 Pa,对不同质量浓度的铀溶液进行过滤,溶质分别选择去离子水和海水,利用α检测技术对膜表面的铀进行计算,拟合溶液中铀质量浓度和膜表面α粒子数目,得到拟合度为0.985和0.982的线性曲线;通过膜表面α粒子数目确定未知溶液中铀的质量浓度,并确定检测方法的精确度,得到相对标准偏差为1.11%。与传统检测技术相比,该技术简化了操作流程,提高了检测效率,实现了原位检测水体中铀的目的。Abstract: In order to achieve the purpose of in-situ and efficient detection of uranium content in water, a type of succinyl-β-cyclodextrin membrane was prepared by a layered loading method. The feed liquid was transported to the membrane surface by a gear pump, the adsorption and membrane filtration technologies was combined to enrich uranium in water to the membrane surface. The α particles released by uranium on the surface of membrane could be captured by α particle detector, so as to estimate the content of uranium in water. The enrichment trend of α particles on the membrane surface was investigated by changing the operating pressure, operating time and feed temperature, then the best operating conditions were determined. The results show that the operating pressure had a linear relationship with the rotating speed from 300 to 560 r·min−1 , and the fitting degree was 0.997, the adsorption amount increased with the increase of operating pressure and time. The feed liquid temperature had a dual effect on the membrane structure and adsorption reaction, the number of α particles on the membrane surface fluctuated with the change of temperature, which was 6.41 at 25 ℃. At 25 °C and the pressure of 8×104 Pa, uranium solutions with different mass concentrations were filtered with RO water and seawater as solutes, the uranium on the membrane surface was calculated by α detector, the uranium mass concentration in the solution and the number of particles on the membrane surface were fitted, and the linear curves with a fitting degree of 0.985 and 0.982 were obtained, respectively. The mass concentration of uranium in the unknown solution could be determined by the number of α particles on the membrane surface. The accuracy of the method was determined with RSD of 1.11%. Compared with the traditional detection technology, it simplifies the operation process, improves the detection efficiency, and realizes the purpose of in-situ detection of uranium in water.
-
Key words:
- uranyl ion /
- succinyl-β-cyclodextrin /
- adsorption /
- filtrate /
- detection of α particle
-

-
表 1 伪一级模型和伪二级模型的吸附动力学参数
Table 1. Adsorption kinetic parameters of pseudo first-order model and pseudo second-order model
方程 qe,cal /(mg·g−1) R2 K/min−1 伪一级动力学方程 100.29 0.972 9.39×10-3 伪二级动力学方程 126.58 0.982 6.84×10-5 表 2 CDM-U的 EDS能谱结果
Table 2. Results on EDS of CDM-U
元素 质量分数/% 原子占比/% C 67.79 72.71 N 22.88 21.04 O 7.65 6.16 U 1.68 0.09 -
[1] AO J, ZHANG H, XU X, et al. A novel ion-imprinted amidoxime-functionalized UHMWPE fiber based on radiation-induced crosslinking for selective adsorption of uranium[J]. RSC Advances, 2019, 49(9): 28588-28597. [2] AHMED W, MEHMOOD S, NUNES-DELGADO A, et al. Utilization of citrullus lanatus L. seeds to synthesize a novel MnFe2O4-biochar adsorbent for the removal of U(VI) from wastewater: Insights and comparison between modified and raw biochar[J]. Science of the Total Environment, 2021, 771: 144955. doi: 10.1016/j.scitotenv.2021.144955 [3] ZHANG B, GUO X, XIE S, et al. Synergistic nanofibrous adsorbent for uranium extraction from seawater[J]. RSC Advances, 2016, 85(6): 81995-82005. [4] 昝金晶, 董一慧, 张卫民. 铀在地下水系统中的赋存与迁移[J]. 有色金属(矿山部分), 2019, 71(6): 69-73. [5] 汪向伟, 梁漫春, 李钢, 等. 水中铀的分离富集方法综述[J]. 清华大学学报(自然科学版), 2021, 61(1): 64-69. [6] LI N, YANG L, JI X, et al. Bioinspired succinyl-β-cyclodextrin membranes for enhanced uranium extraction and reclamation[J]. Environmental Science:Nano, 2020, 7(10): 3124-3135. doi: 10.1039/D0EN00709A [7] LI W, LIU Q, LIU J, et al. Removal U(VI) from artificial seawater using facilely and covalently grafted polyacrylonitrile fibers with lysine[J]. Applied Surface Science, 2017, 403: 378-388. doi: 10.1016/j.apsusc.2017.01.104 [8] HU R, SHAO D, WANG X. Graphene oxide/polypyrrole composites for highly selective enrichment of U(VI) from aqueous solutions[J]. Polymer Chemistry, 2014, 21(5): 6207-6215. [9] ZHU J, LIU Q, LIU J, et al. Ni-Mn LDH-decorated 3D Fe-inserted and N-doped carbon framework composites for efficient uranium(VI) removal[J]. Environmental Science:Nano, 2018, 5(2): 467-475. doi: 10.1039/C7EN01018D [10] ZHAO H, LIU X, YU M, et al. A study on the degree of amidoximation of polyacrylonitrile fibers and its effect on their capacity to adsorb uranyl ions[J]. Industrial & Engineering Chemistry Research, 2015, 54(12): 3101-3106. [11] WANG D, SONG J, LIN S, et al. A marine‐inspired hybrid sponge for highly efficient uranium extraction from seawater[J]. Advanced Functional Materials, 2019, 29(32): 1901009. doi: 10.1002/adfm.201901009 [12] LUO W, XIAO G, TIAN F, et al. Engineering robust metal-phenolic network membranes for uranium extraction from seawater[J]. Energy & Environmental Science, 2019, 12(2): 607-614. [13] 余志强, 杨仁伟, 汪帅马. 饮用水铀污染处理方法简述[J]. 江西化工, 2016(3): 8-14. doi: 10.3969/j.issn.1008-3103.2016.03.003 [14] 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 铀矿冶辐射防护和辐射环境保护规定: GB 23727-2020[S]. 北京: 中国标准出版社, 2020. [15] MA F, GUI Y, LIU P, et al. Functional fibrous materials-based adsorbents for uranium adsorption and environmental remediation[J]. Chemical Engineering Journal, 2020, 390: 124597. doi: 10.1016/j.cej.2020.124597 [16] NOVAES C G, BEZERRA M A, DA SILVERE G P, et al. A review of multivariate designs applied to the optimization of methods based on inductively coupled plasma optical emission spectrometry (ICP- OES)[J]. Microchemical Journal, 2016, 128: 331-346. doi: 10.1016/j.microc.2016.05.015 [17] POINTURIER F, HUBERT A, BAGLAN N, et al. Evaluation of a new generation quadrupole-based ICP-MS for uranium isotopic measurements in environmental samples[J]. Journal of Radioanalytical and Nuclear Chemistry, 2008, 276(2): 505-511. doi: 10.1007/s10967-008-0534-9 [18] 杜浪, 李玉香, 马雪, 等. 偶氮胂Ⅲ分光光度法测定微量铀[J]. 冶金分析, 2015, 35(1): 68-71. doi: 10.13228/j.boyuan.issn1000-7571.009388 [19] 付军. 长距离α污染测量仪的研制[D]. 成都: 成都理工大学, 2008. [20] PEI H J, HUI G, JIAN W B, et al. Construction of gel-like swollen-layer on polyacrylonitrile surface and its swelling behavior and uranium adsorption properties[J]. Journal of Colloid and Interface Science, 2020, 576: 109-118. doi: 10.1016/j.jcis.2020.04.080 [21] QIAN Y, QUAN Y, WANG H, et al. Highly efficient uranium adsorption by salicylaldoxime/polydopamine graphene oxide nanocomposites[J]. Journal of Materials Chemistry A, 2018, 48(6): 24676-24685. [22] ZHAO Y, LI J, ZHANG S, et al. Amidoxime-functionalized magnetic mesoporous silica for selective sorption of U(VI)[J]. RSC Advances, 2014, 62(4): 32710. [23] YUAN Y, ZHAO S, WEN J, et al. Rational design of porous nanofiber adsorbent by blow spinning with ultrahigh uranium recovery capacity from seawater[J]. Advanced Functional Materials, 2019, 29(2): 1805380. doi: 10.1002/adfm.201805380 [24] WANG D, SONG J, WEN J, et al. Significantly enhanced uranium extraction from seawater with mass produced fully amidoximated nanofiber adsorbent[J]. Advanced Energy Materials, 2018, 8: 1802607. doi: 10.1002/aenm.201802607 [25] QIAN Y, YUAN Y, WANG H. Highly efficient uranium adsorption by salicyl aldoxime/polydopamine graphene oxide nanocomposites[J]. Journal of Materials Chemistry A, 2018, 6: 24676-24685. doi: 10.1039/C8TA09486A [26] ZHU M X, LIU L J, FENG J. Efficient uranium adsorption by amidoximized porouspolyacrylonitrile with hierarchical pore structure prepared by freeze-extraction[J] Molecular Liquids, 2021, 328: 115304. -




 下载:
下载: