-
近年来,页岩气开发作为我国重大能源战略发展迅速。然而,页岩气开采需要将大量压裂液(由水、支撑剂、杀菌剂和防腐剂等物质配置而成)高压注入页岩层[1-2],开采后约10% —80%的压裂液返回地表成为返排水。返排水中大量有机污染物给人类生存和生态环境健康带来重大挑战,引起了社会的广泛关注。目前常规的返排水处理技术主要包括物理法,化学法以及生物法,其中生物法由于工艺简单,运行稳定,成本低和环境友好等特点具有良好的应用前景。然而由于地层结构和压裂液成分不同,导致返排水中有机物种类及浓度差别较大。同时,研究表明,返排水中含有大量难降解有毒有机物,例如多环芳烃(PAHs)和季铵盐类化合物(QACs)[3]等,使得返排水的B/C值仅为0.1—0.3,可生化性极差,导致返排水直接生物处理难度较大。因此,在生物处理前提高废水的可生化性是一个重要的解决思路。
高级氧化是一种常用的降解难有机物处理方法,与光催化和超临界氧化等物化处理技术相比,电催化技术具有效率高、通用性强以及环境友好等优点。电催化氧化技术主要是通过阳极与阴极之间的电场作用生成羟基自由基、单线态氧等具有强氧化性的活性物质,氧化有机物使其矿化或转化成易降解的小分子有机物。已有研究表明电催化处理能有效降低工业废水中的COD,同时还能够提高废水可生化降解性[4-8],尤其在处理含有芳香化合物、酚类化合物、染料和氰化物等物质的废水时效果显著[9-12]。近年来,研究者尝试采用电催化技术与其他工艺联合的手段来处理返排水,并取得一定效果[13-16]。而在电催化过程中,选取高效稳定的电极材料非常重要,钌铱钛电极由于其具有较强的催化活性,较高的电极稳定性备受青睐。同时,返排水具有高氯特性,钌铱钛电极对于高氯环境具有较强适应性,研究发现随着Cl−升高,钛涂氧化物电极的阳极寿命增强,析氯阻力变小,电化学体系的电流效率增加[17]。依托返排水的高氯特性,Cl−主要作为电催化过程中的电解质参与反应,参考传统电化学界面反应机理[18],有如下反应过程:
其中,“S”代表钛电极表面的钌铱涂层。从式(1)和式(4),界面反应的第一步为水分子和氯离子吸附结合在钌铱电极表面活性位点上,水分子和氯离子产生竞争吸附,氯浓度增大利于反应正向进行,促进·OH等活性氧成分产生进而促进有机污染物的氧化过程。同时,Cl2析出(反应4反应5)进一步溶于水在电场中通过电子传递产生ClOx−等氯的高价态酸根离子(反应6),可能与污染物发生部分氧化还原反应。因此,电催化氧化技术有潜力发展成为返排水治理的预处理手段。然而,目前对于电催化技术预处理实际页岩气开采返排水的效果、影响因素及其优化以及有机污染物转化过程与产物等仍然缺乏深入了解。
本论文建立了电催化氧化预处理技术处理实际返排水,建立了响应曲面模型并基于模拟对电极板间距、电压和反应时间等工艺参数的影响进行了识别与优化,得到了电催化氧化技术处理返排水的优化工艺条件;进一步通过实验对模拟优化结果进行了验证,分析电催化氧化过程对返排水中有机物组分的转化过程;为认识电催化预处理对实际返排水的作用效果及其机制提供支撑,为返排水电催化预处理技术开发应用提供技术指导。
-
页岩气返排水取自重庆市各页岩气平台,实验中常规水质指标的测试均采用相关国标或行标方法进行测定。色度的分析方法采用的是分光光度比色法,pH采用玻璃电极法测定,化学需氧量(COD)采用快速密闭消解法进行测定,总溶解性固体(TDS)和总悬浮性固体(TSS)采用称量法测定,TOC通过燃烧氧化-非色散红外吸收法测定。水样水质表征结果见表1。
-
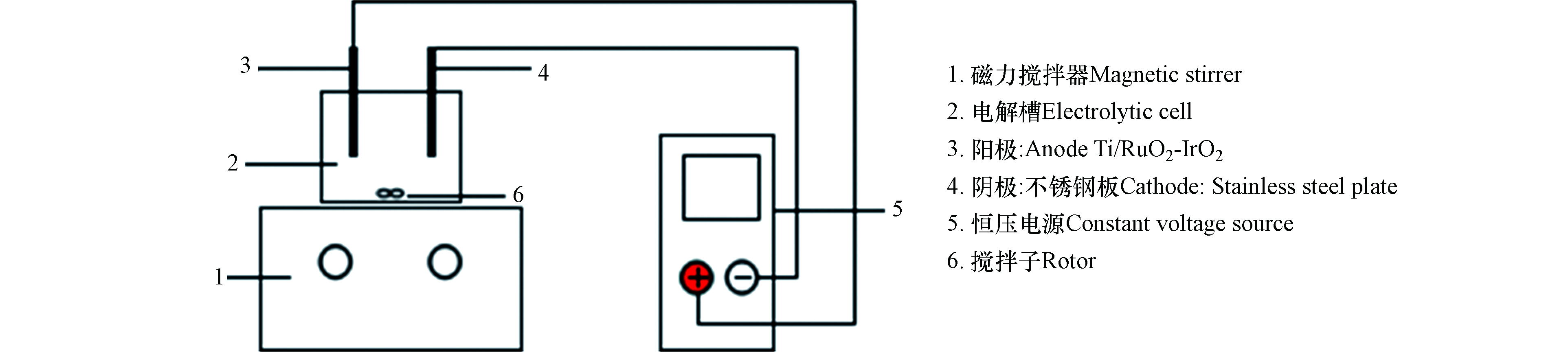
本研究采用的电催化氧化实验装置组成如图1所示,由阴阳电极板、磁力搅拌器、恒压电源和电解槽等构成。其中,阳极是三维形稳电极(Dimensionally Stable Anodes, DSA),以金属Ti为基底, RuO2和IrO2作涂层,阴极则由不锈钢板构成。电极尺寸为:6.5 cm×5.5 cm×2 mm(涂层厚度为:0.5 µm),电解槽尺寸为:95 mm×60 mm×70 mm。装置通过凹槽来固定电极板,电极板外接恒压电源,并通过磁力搅拌来维持电解槽中溶液均匀。
-
本研究通过响应曲面分析(RSM)来优化电催化氧化技术的工艺条件,根据前期单因素预实验确定变量的范围并设计具体响应曲面试验方案。通过Design-expert 11.0软件设计了响应曲面中心复合试验,设计规模为三因素五水平。响应曲面法研究选取的影响因素包括:电极板间距(X1)、电压(X2)和反应时间(X3),而相应的响应值分别是:B/C值(Y1)和COD去除率(Y2)。通过RSM设计对实际返排水进行电催化氧化处理实验,得到COD去除率和B/C的结果如表2所示。
基于RSM优化工艺参数,在最佳工况下开展电催化处理不同地区返排水的实验。通过对电催化前后返排水的COD以及BOD5变化情况,考察电催化氧化对返排水作用特性。同时,通过GC-MS及统计分析,识别电催化前后返排水中有机物组分变化,分析返排水中有机物转化过程特性。
通过多元二次回归方程来拟合不同变量与响应值之间的函数关系[19],如方程7所示。
其中,
$ {\beta }_{0} $ 为常数项,$ {X}_{i}\mathrm{和}{X}_{j} $ 为自量,$ {\beta }_{i}\mathrm{、}{\beta }_{ii}\mathrm{和}{\beta }_{ij} $ 表示各项的回归系数,k为影响因素的数量,e为误差(主要由实验误差和拟合不足误差组成,其中拟合不足误差包括高阶项和交互项)。 -
对返排水样品进行定性分析之前通过固相萃取系统(Dionex Auto Trace 280TM, USA)来提取有机物。具体来说,先用SPE填料来吸附目标化合物,然后用液体溶剂(二氯甲烷和乙酸乙酯)收集从样品中提取的有机物。有机物提取后通过GC-MS(TQ8040,Shimadzu Co., Japan)来检测其中的有机组分。
GC-MS分析采用的色谱柱是HP-5MS(30 m×0.25 mm×0.25 μm),恒温箱采用的升温程序是:初始温度60 ℃保持6 min,然后以2 ℃·min−1 速率升温到300 ℃并保持15 min;进样口温度设置为285 ℃(自动化注射器),接口温度为300 ℃。载气为高纯氦气,自动液体以1 mL·min−1恒定流速不分流进样,进样量为2 μL,质量扫描范围是50—500 amu。
-
对表2的实验结果进行线性回归分析可得到响应值(B/C值和COD去除率)与3个变量(板间距、电压和反应时间)之间的二次回归模型(方程8)以及两因素交互作用模型(方程9)。
针对得到的二次回归模型以及两因素交互作用模型,通过Design expert 11.0对模型进行各因素项显著性检验(表3)及回归模型方差分析(表4),从而对模型的显著性及各因素的显著性进行分析。其中,P值用来判定假设检验结果显著性,P值越小表示结果越显著(P值若大于0.05表明模型不显著)。分析结果显示,以B/C为响应值的模型中P值为0.0177,以COD去除率为响应值的Y2模型中P值小于0.0001,表明得到的回归模型具有显著性,在95%置信水平上回归模型具有统计学意义。
此外,F值表示相关系数的显著性,F值越大表示该变量对结果的影响越显著[20]。以B/C为响应值的模型中,板间距、电压和时间等变量的F值分别为:24.36、0.95和0.06,这表明相比于电压和反应时间,板间距对返排水的可生化降解性能影响最为显著。这是因为板间距大小决定电极间的有效电阻,板间距越小电流效率就越高,较高的电流密度导致返排水中难降解有机物得到有效的去除[21]。而以COD去除率为响应值的模型中,电压、反应时间和板间距3个变量对应的F值分别为:74.01、29.69和26.65,这表明电压对COD去除率的影响程度最大,这可能是因为电极板上的外加电压直接决定在阳极和阴极之间形成电场强度,电场强度又与活性物种的生成速率如·OH等活性氧成分密切相关,最终影响有机污染物的降解转化速率[22]。
-
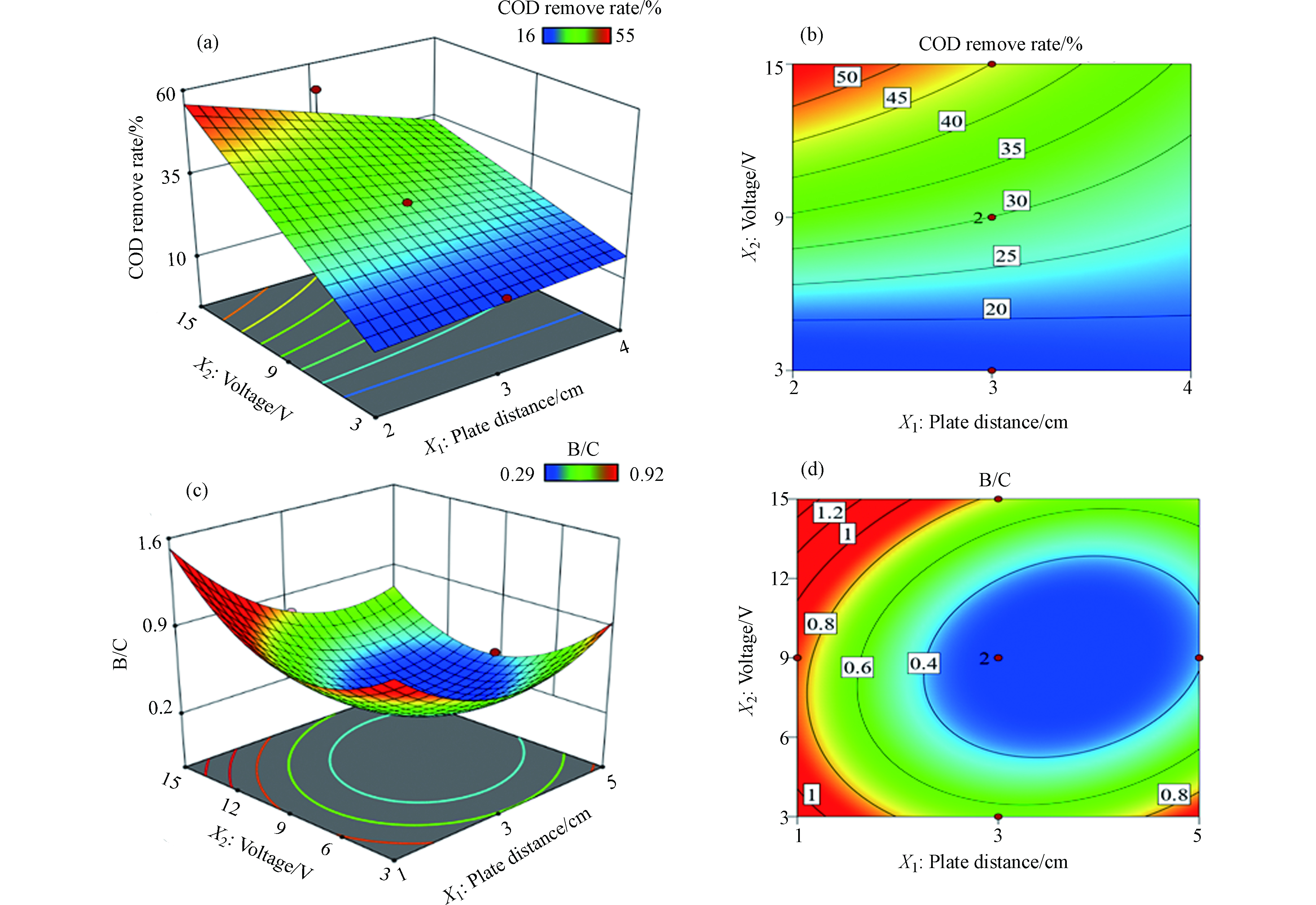
通过响应曲面法研究不同变量及其交互作用对COD去除率和B/C值的影响。电压和板间距之间交互作用对COD去除率影响如图2a和2b所示,结果显示电压增加会导致COD去除率显著提高,如固定板间距和反应时间,随着电压由3 V提高到15 V,COD去除率可由20%增加到50%,而板间距变化对COD去除率影响较小。
返排水中存在难生化降解有机物和易生化降解有机物,经过电催化氧化处理后部分难生化降解有机物被直接矿化,而另一部分转化成易生化降解的小分子有机物,宏观指标都会表现出B/C值增加且可生化性增强;而返排水中易生化降解有机物的矿化过程则会导致B/C值的降低。图2c和2d展示了电压和电极板间距交互作用对B/C值的影响,结果显示板间距增加会使得B/C值降低,而电压增加则使得B/C值呈现出了先降低后增加的趋势。其中,当板间距处于较低范围(1—2.5 cm)时,B/C值始终保持在0.3以上,说明板间距处于较低水平时返排水中难生化降解有机物的转化或矿化过程占据主导地位。此外,结果显示B/C值较高的区域主要出现在低电压低板间距(电压小于6 V,板间距为1—2.5 cm)、高电压低板间距(电压大于9 V,板间距为1—2.5 cm)和低电压高板间距(电压小于4 V,板间距为4.5—5 cm)等区域。其中,在低板间距高电压条件下由于大量·OH的生成,难降解组分直接完全矿化成为主导反应,导致BOD5增加并伴随着COD下降,进而导致返排水可生化降解性能增强。此外,在低板间距低电压以及高板间距低电压条件下,难降解有机污染物向可生化降解组分的转化作为主导反应(难以直接完全矿化),最终导致BOD5和B/C值的增加。而当电压和极板间距都处于较高水平时,主导反应是易生化降解有机污染物发生完全矿化,剩余大量难降解组分无法转化或矿化,最终导致COD去除率增加而可生化降解性能降低。
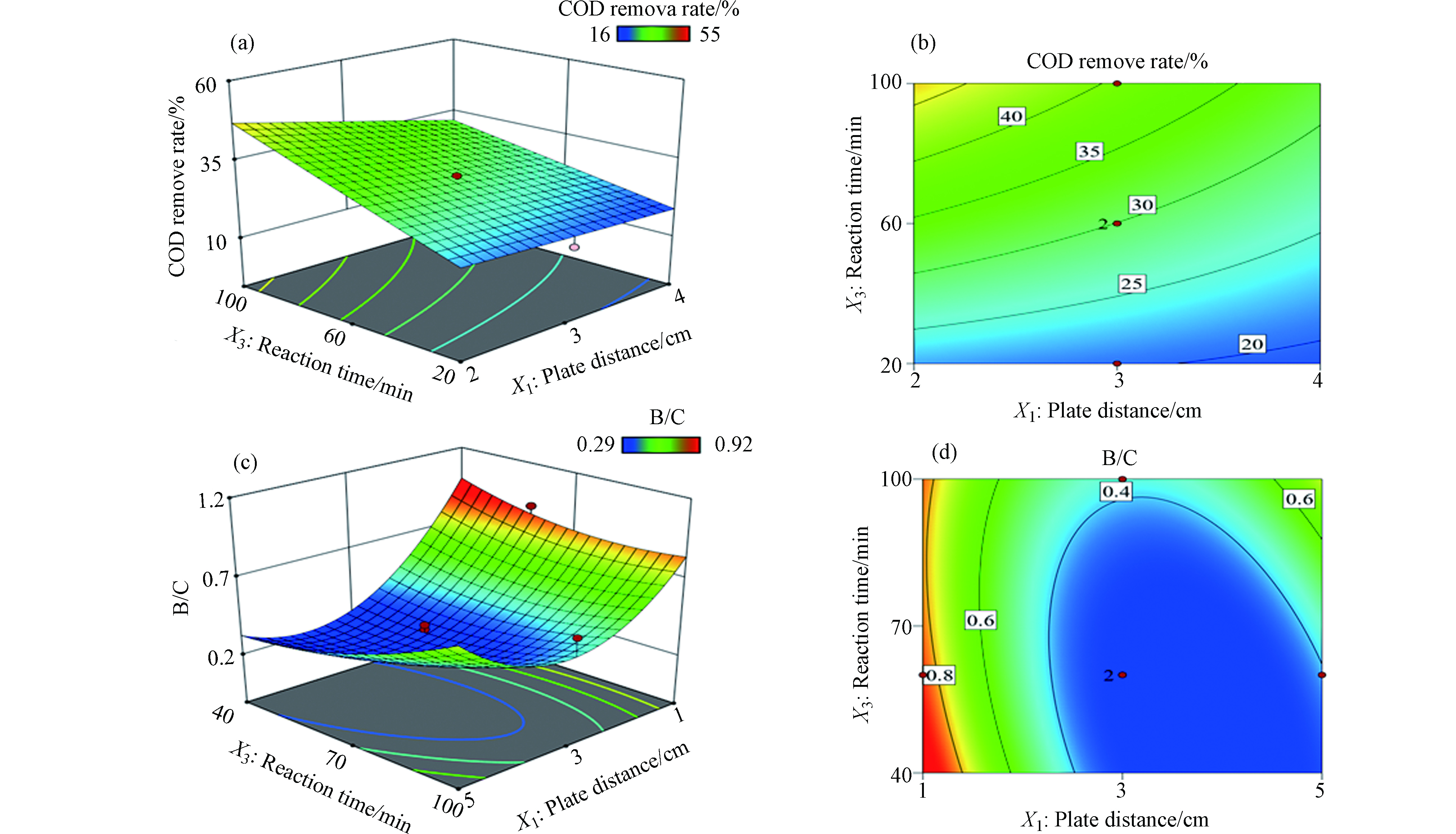
图3a和3b展示了反应时间和板间距对COD去除率的影响,结果显示该响应曲面平缓且等高线较为疏松,这表明反应时间和板间距对COD去除率影响较小。其中,COD去除率随着反应时间增加而呈现缓慢上升趋势,而板间距的增加则会导致COD去除率稍有下降。由响应曲面图可知COD去除率较高区域集中在低板间距且反应时间较长(板间距为1—2 cm,反应时间为70—90 min)区域。图3c和3d展示了反应时间和板间距对B/C值的影响,结果显示反应时间变化对B/C值影响较小,而板间距增加则会导致B/C值显著减小。
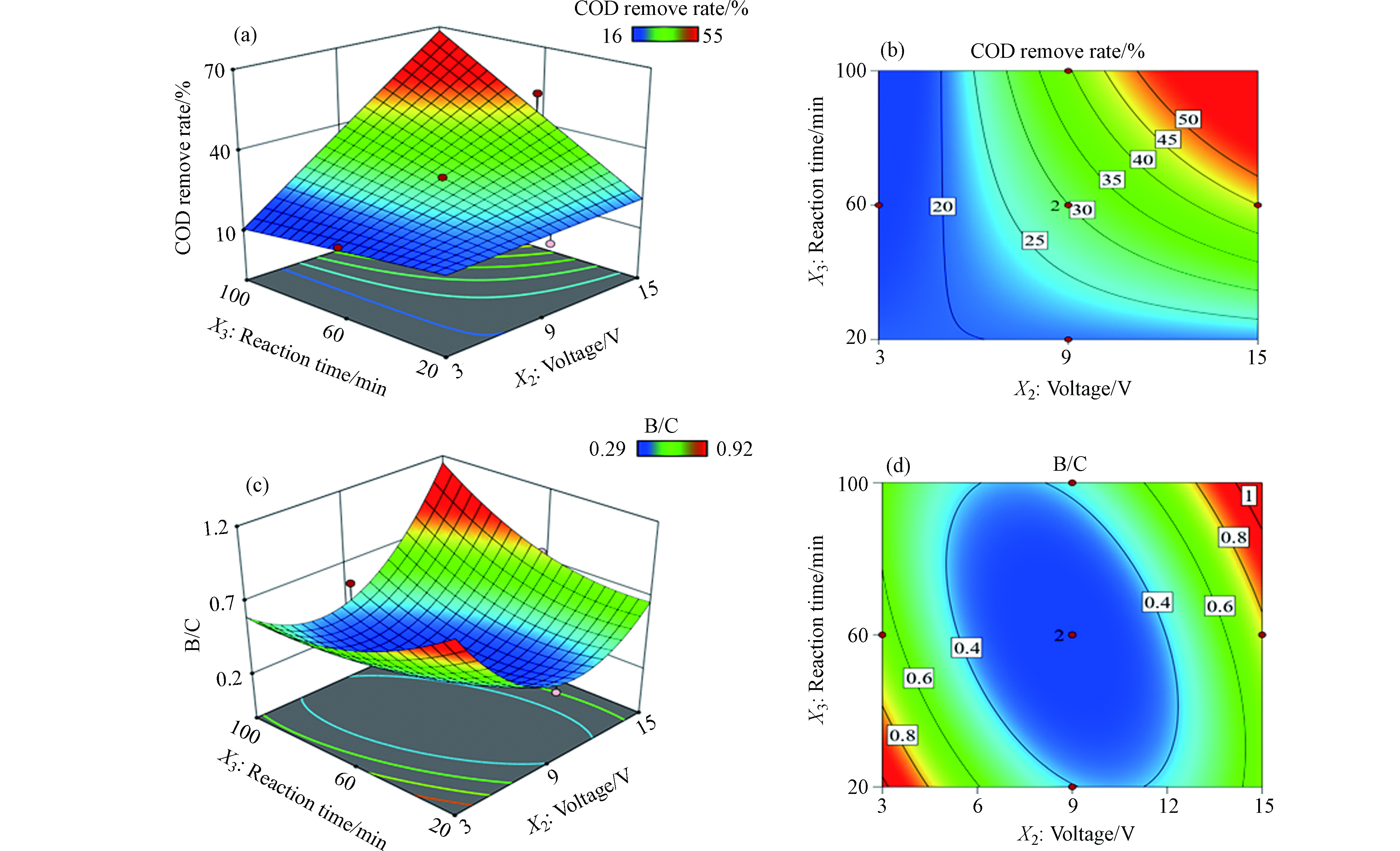
图4a和4b展示了电压和反应时间对COD去除率的影响,结果显示电压或反应时间增加都会提高返排水样品的COD去除率,这与图2和3中的结论一致。图4c和4d展示了电压和反应时间对B/C值的影响,结果显示电压和反应时间增加都会导致B/C值呈现先降低后增加的趋势,其中B/C值在低电压且反应时间较短(3—5 V,20—40 min)以及高电压且反应时间较长(13—15 V,80—100 min)条件下出现明显的峰值。这可能是因为在低电压且反应时间较短条件下有大量难生化组分转化为易生化组分,导致水样可生化降解性提高。而随着电压增加以及反应时间变长,体系中的易生化组分的矿化过程会逐渐会占据主导,导致B/C值逐渐下降。随着电压和反应时间继续提高并都达到较高水平,大量难生化降解有机组分被直接矿化,最终导致返排水可生化降解性能达到较高水平。
电压增加会使得体系电流变大,电流密度增加,而极板间距的减小则是通过减少体系阻抗,使得体系电流变大,两者都会促进电流密度增加。在一定范围内电压增加会使得电流密度增大,活性物种产率增加,导致更多污染物的氧化转化及矿化,宏观上表现为COD去除率的增加,与此同时能耗也会增加。而当电压增加到一定程度时,高电位条件下,阳极析氧副反应会不断加剧,反而会降低污染物的降解速率。极板间距在一定范围内减小,离子扩散距离减小,电流密度增大,在此情况下,一些难降解的有机物组分则能够在强氧化性物种作用下向小分子易降解组分转化甚至完全矿化,宏观上即BOD5增加,B/C值增加,可生化性增加。但是当极板间距太低时,容易发生电场击穿效应,导致电解失活,同时在实际电解工艺过程中,极板间距的减少意味着需要更多的电极板组数,导致一次投资大,安装和维护管理困难的问题。反应时间则是在时间尺度上影响电催化过程,反应时间越久,活性物种的作用时间越长,污染物的氧化作用越彻底,但是较长的反应时间也意味着更高的能耗。因此,在对于电催化工艺参数的考量时,要综合考虑以上问题设计工艺参数。通过Design-expert设置约束条件B/C值处于0.4—0.6,COD去除率为30%—55%,考虑最低的经济成本,得到的优化工艺参数为板间距:2.254 cm,电压:8.667 V,反应时间:80 min。
综上所述,电催化氧化处理返排水过程中有机物效应主要包括以下3个过程:
过程(1):难生化降解组分向易生化降解组分的转化过程,导致水样的B/C值增加,可生化性增强,COD去除率变化较小;
过程(2):易生化降解组分的直接矿化过程,导致水样COD去除率增加,B/C值降低,可生化性变差;
过程(3):难生化降解组分的直接矿化过程,导致水样COD去除率增加,B/C值增加,可生化性加强。
电压、板间距和反应时间变化均会对以上3个过程占据的比例产生影响,其中电压和电极板间距的影响更大。此外,从过程(1)到(2)再到(3),反应难度越来越大。总体来看,随着变量电压和反应时间的增加以及板间距的减小,返排水的电催化降解的主导反应逐渐由过程(1)向(2)再向(3)转变。
-
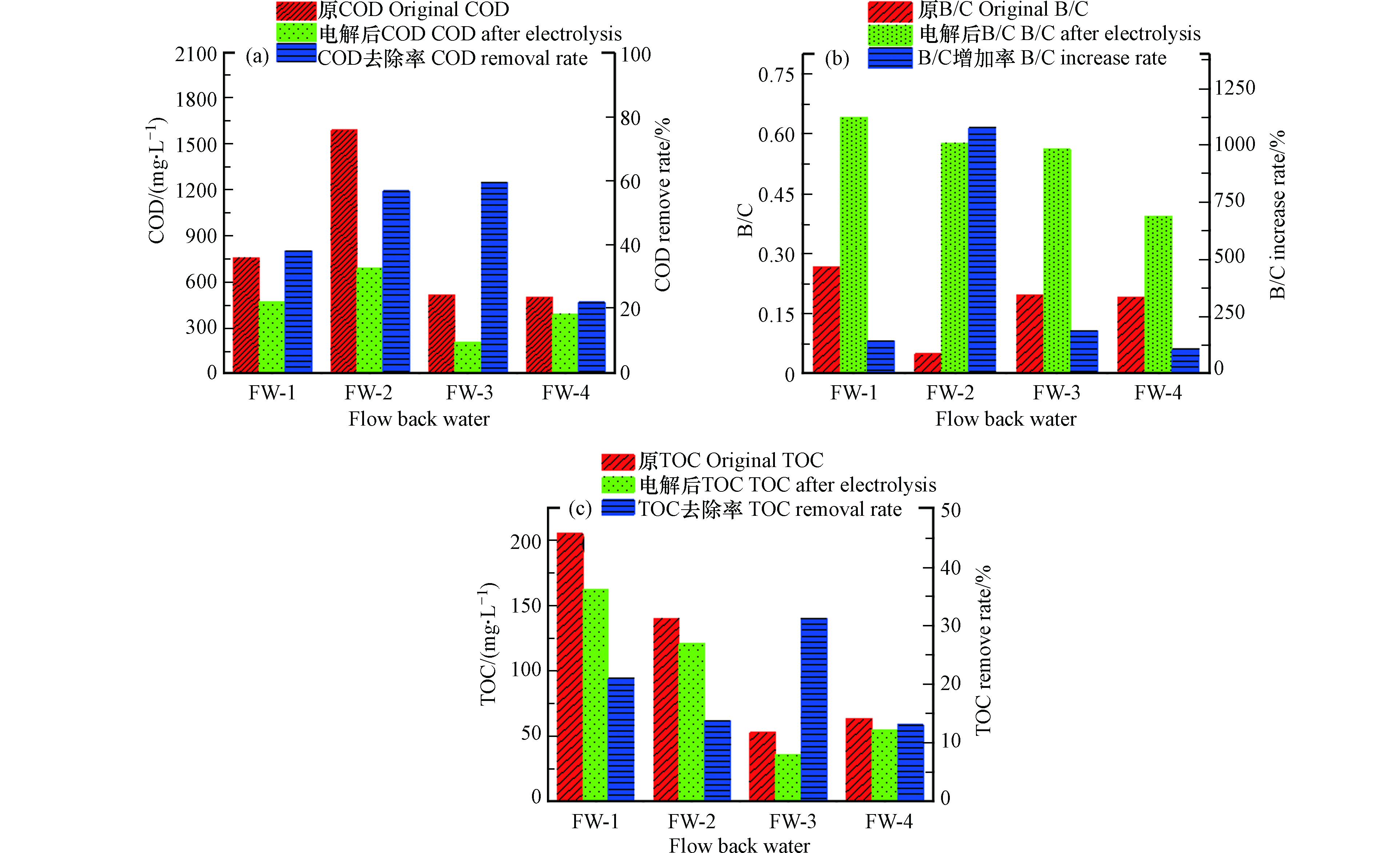
通过对不同地区采的返排水样品进行电催化氧化处理,分析了返排水样品的有机物的去除效果以及可生化性能。图5a—c分别展示了电催化氧化处理前后返排水样品中COD含量变化,B/C值变化以及TOC变化情况。结果表明电催化氧化处理对不同区域返排水样品的COD去除量约为100—900 mg·L−1,COD去除率约为22.2%—59.56%,水样的B/C值从0.04—0.26提高到0.39—0.64,不同返排液样品在电催化处理后B/C值都有所增加,但是增加率差异较大,这主要是由于返排水中物质组成存在很大差异,芳香族化合物、酚类物质、杀菌剂以及长链的大分子物质占比高的返排水样,在电催化处理后B/C增加率则较小。该实验结果与之前的研究结果一致。例如,杨德敏等发现采用电催化氧化处理压裂液返排废水时COD去除率可达49.58% [23]。另外,也有研究发现电催化氧化处理焦化废水时COD去除率可高达46.8%,处理过后B/C值由0.05提升至0.37[24]。不同返排水样品原水TOC存在差异,浓度为53—206 mg·L−1,在经过电催化处理后,总有机碳均有所下降,TOC去除率在13%—31%。以上结果表明电催化氧化处理可以有效去除返排水中部分有机物并提升其可生化降解性能。
为了进一步分析电催化氧化处理前后返排水中有机物组成成分的变化情况,GC-MS被用来分析电催化处理前后的返排水样品,电催化处理前后具体的有机物结构信息如表5和表6所示。结果显示原始返排水中有机物保留时间主要集中在15 min以后,而经电催化氧化处理后水样中有机物保留时间缩短,保留时间主要集中在15 min以前,这表明电催化处理导致水样中有机物的极性增加。通过图6展示了电催化处理前后不同种类有机物的相对含量。结果显示原始返排水中主要含有大量芳香族化合物(如萘菲等PAHs)和脂肪族化合物(烷烃类物质以及少量烯烃),二者含量分别达到25%和37.5%。之前的研究也发现了类似的现象,研究者认为这些芳香族化合物和脂肪族化合物可能源自于页岩层中有机物的溶解[9, 25-27]。此外,还有研究检测到原始返排水中存在酮类及酸类物质,部分酮类可能是聚合物或氧化剂的转化产物,这表明页岩气返排水中部分有机物已发生转化[28-29]。返排水在经过电催化氧化处理之后,水样主要包含脂肪族和酯类等易生化降解的有机物,而难降解的芳香族化合物相对含量也由25.5%下降至7.7%。值得注意的是,电催化处理后的水样中并未检测到酚类、酮类和萘菲等PAHs。另外,电催化氧化处理之后的水中出现2,6-二甲基-壬烷及3,7-二甲基癸烷等短链烷烃类中间产物,这说明电催化氧化处理对PAHs及芳香化合物等难降解有机物具有显著的转化或矿化作用。
综上,有机物种类的变化是返排水可生化降解性能提高的根本原因,返排水中部分难以被微生物利用或对微生物有毒性的多环芳烃和酚类等物质被转化成可以被微生物利用的中间产物,最终使得返排水的可生化降解性能得到提升。以上结果表明通过电催化氧化处理可有效提高页岩气返排水的可生化降解性能,该技术有潜力发展成为返排水生物降解的预处理技术。
-
(1)以电极板间距、电压和反应时间的3个因素作为变量,以B/C值和COD去除率作为响应值进行响应曲面优化。结果显示,电压增大可以显著提高返排水的COD去除率,而板间距减小可以显著提升返排水的可生化降解性能。
(2)电催化氧化处理返排水过程主要包括以下3个反应:1.难生化降解有机物转化成易生化有机物,2.易生化有机物直接矿化,3.难生化降解有机物直接矿化。不同电催化工艺参数下的主导反应过程不同,随着电压和反应时间的增加以及电极板间距减小,主导反应逐渐由过程1向2再向3转变。
(3)综合考虑返排水的COD去除率以及可生化降解性,并以降低经济成本为目标对电催化参数进行优化,得到的优化参数如下:电极板间距为2.254 cm,电压为8.667 V,反应时间为80 min。
(4)电催化氧化技术可有效去除返排水中22.2%—59.56% COD,同时显著提升返排水的可生化降解性,B/C值从0.04—0.26提高到0.39—0.64。有机物组分的变化是返排水经电催化氧化处理后可生化降解性能提升的主要原因,电催化氧化处理后返排水中毒性较高且难降解的有机物(如芳香族及酚类化合物)大幅减少,而易降解有机物(如脂肪族及酯类化合物)占比增加。
基于RSM模拟的返排水电催化工艺参数的影响特性及其优化实验
Effect of process parameters on electrocatalytic treatment of shale gas flowback water based on RSM simulation and its optimized experiment
-
摘要: 随着页岩气开发的大力推进,针对页岩气返排水处理技术成为当前的研究热点。为了去除页岩气返排水中复杂有机污染物,提升其可生化降解性,采用电催化氧化技术对页岩气返排水进行预处理。通过响应曲面分析研究了电压、电极板间距和反应时间3个参数对返排水中有机污染物转化过程的影响,并对3个电催化参数进行优化,得到最佳结果为板间距2.254 cm,电压8.667 V,反应时间80 min。基于优化结果开展的电催化实验结果表明,电催化氧化技术可有效去除不同区域返排水中22.2%—59.56% COD,同时显著提升返排水的可生化降解性,不同返排水样品BOD/COD值(B/C值)从0.04—0.26提高到0.39—0.64。此外,GC-MS分析结果表明,电催化氧化技术可以有效去除返排水中有毒且难生化降解的有机物(如芳香族化合物和酚类化合物),并生成易生化降解的脂肪族和酯类有机物。Abstract: With the vigorously promotion of shale gas industry, the shale gas flowback water treatment has become a research focus. To remove the complex organic pollutants and improve the biodegradability of flowback water, the electro-catalytic oxidation technology was used in this study for the pretreatment of the shale gas flowback water. Through response surface analysis, the influence of the three parameters (voltage, electrode plate spacing and reaction time) on the degradation process of organic pollutants in the flowback was studied and further optimized. The best result was the plate spacing: 2.254 cm, voltage: 8.667 V, response time: 80 min. Based on the optimization results, it showed that the electrocatalytic oxidation technology can effectively remove 22.2%—59.56% COD of different flowback water samples, and significantly improve the biodegradability. The BOD5/COD (B/C) value of different flowback water samples increased from 0.04—0.26 to 0.39—0.64. Additionally, GC-MS analysis indicated that electro-catalytic oxidation treatment could effectively remove plenty of non-biodegradable organic pollutants (such as aromatic compounds and phenolic compounds), with formation of abundant biodegradable organics such as aliphatic and esters.
-

-
图 3 板间距和反应时间对COD去除率影响的(a)响应曲面图和(b)等高线图,板间距和反应时间对B/C影响的(c)响应曲面图和(d)等高线图
Figure 3. (a) Response surface plot and (b) contour plot of the effect of plate distance and reactiontime on COD removal rate, (c) response surface plot and (d) contour plot of the effect of plate distance and reactiontime on B/C
表 1 返排水样品主要水质参数
Table 1. Water quality parameters of the flowback water sample
水质指标Water quality indexes FW-1 FW-2 FW-3 FW-4 pH 7.24 7.92 7.02 8.25 TSS/(mg·L−1) 14.257 193 146 257.5 TDS/(mg·L−1) 25648.3 20072 14206.67 19061.33 TOC/(mg·L−1) 205.74 430.88 63.747 140.76 COD/(mg·L−1) 758.3 1592 511.1 497 BOD5/(mg·L−1) 202 78 100 95 NH4+/(mg·L−1) 47.155 82.085 10.624 14 色度 25 20 20 — Cl−/(mg·L−1) 7047 9021 6272 11853 B/C 0.266 0.049 0.196 0.191 表 2 电催化氧化处理返排水响应曲面实验结果
Table 2. Response surface experimental results for the electro-catalytic treatment of flowback water
实验编号
No .X1 (电极板间距)/cm
Plate distanceX2 (电压)/V
VoltageX3 (反应时间)/min
Reaction timeY1(B/C) Y2(COD去除率)/% 1 2 6 40 0.65 24 2 4 6 40 0.39 21 3 2 12 40 0.68 33 4 4 12 40 0.30 22 5 2 6 80 0.35 27 6 4 6 80 0.32 23 7 2 12 80 0.65 55 8 4 12 80 0.42 33 9 1 9 60 0.92 38 10 5 9 60 0.41 22 11 3 3 60 0.74 16 12 3 15 60 0.76 52 13 3 9 20 0.36 17 14 3 9 100 0.55 36 15 3 9 60 0.29 30 16 3 9 60 0.32 30 表 3 各因素项显著性检验
Table 3. Significance test of various factors
响应值
Response value因素
Factor估计值
Predictive value标准差
Standard deviationF值
F-valueP值
P-valueB/C 常数项 0.268 0.064 A-板间距 −0.120 0.024 24.36 0.0026 B-电压 0.024 0.024 0.95 0.3664 C-反应时间 0.006 0.024 0.07 0.8057 AB −0.040 0.034 1.35 0.2889 AC 0.048 0.034 1.91 0.2164 BC 0.058 0.034 2.80 0.1455 A² 0.090 0.024 13.70 0.0101 B² 0.111 0.024 20.93 0.0038 C² 0.038 0.024 2.38 0.1739 COD 去除率 常数项 29.94 0.87 A-板间距 −4.50 0.87 26.65 0.0006 B-电压 7.50 0.87 74.01 0.0001 C-反应时间 4.75 0.87 29.69 0.0004 AB −3.25 1.23 6.95 0.0271 AC −1.50 1.23 1.48 0.2547 BC 3.50 1.23 8.06 0.0194 表 4 回归模型方差分析
Table 4. Analysis of variance of regression model
响应值
Response value差异源
Source statement自由度
df离差平方和
SS均方
MSF值
F-valueP值
P-valueB/C 回归 9 0.542 0.060 6.4 0.018 残差误差 6 0.057 0.009 失拟 5 0.056 0.011 25.1 0.151 纯误差 1 0.0005 0.0005 总计 15 0.598 R² /% 90 COD 去除率 回归 6 1785 297.6 24.5 0.0001 残差误差 9 109 12.2 失拟 8 109 13.7 纯误差 1 0 0 COD 去除率 总计 15 1894 R² /% 94 注:df表示自由度,SS表示离差平方和,MS 表示均方(MS=SS/df)。
Note: df denotes degrees of freedom, SS denotes sum of squares of deviations, and MS denotes mean square (MS=SS/df).表 5 原始返排水样品中检测到的有机物组分
Table 5. Organic component in the original flowback water sample
化学式
Chemical formula名称
Chemical name保留时间/min
Adjust retention timeC10H16 柠檬烯 4.71 C7H8O 对甲酚 5.36 C11H24 十二烷 5.67 C10H8 萘 6.97 C16H34 正十六烷 8.22 C14H30 4,6-二甲基十二烷 8.85 C9H9N 3-甲基吲哚 9.75 C14H30 正十四烷 9.82 S6 环六硫 11.23 C14H22O 2,4-二叔丁基苯酚 11.28 C20H42 正二十烷 11.58 C9H9NO 2,6-二甲基苯基异氰酸酯 11.89 C17H36 正十七烷 13.41 C14H10 菲 14.45 C21H44 正二十一烷 14.49 C17H24O3 7,9-二叔丁基-1-氧杂螺(4,5)癸-6,9-二烯-2,8-二酮 15.84 C16H32O2 棕榈酸 16.15 S8 环八硫 17.11 C54H110 五十四烷 17.81 C18H36O2 硬脂酸 18.03 C32H66 正三十二烷 19.22 C19H38O4 2-棕榈酰-外消旋-甘油 20.98 C21H42O4 单硬脂酸甘油酯 22.56 C30H50 全反-2,6,10,15,19,23-六甲基-2,6,10,14,18,22-廿四碳六烯 23.35 表 6 电催化处理后水样中检测到的有机物组分
Table 6. Organic components in the sample after electrocatalytic treatment
化学式
Chemical formula名称
Chemical name保留时间/min
Adjust retention timeC11H24 2,6-二甲基-壬烷 4.57 C13H28 2,7-二甲基十一烷 5.07 C12H26 3,7-二甲基癸烷 5.71 C9H10O 2,4-二甲基苯甲醛 7.41 C14H30 2,4-二甲基十二烷 8.00 C14H30 4,6-二甲基十二烷 8.22 C16H34 十六烷 8.85 C20H42 二十烷 11.04 C54H11O 五十四烷 15.78 C17H24O3 7,9-二叔丁基-1-氧杂螺(4,5)癸-6,9-二烯-2,8-二酮 15.84 C38H68O8 L-抗坏血酸基二棕榈酸酯 16.14 C19H38O4 2-棕榈酰-外消旋-甘油 20.98 C21H42O4 单硬脂酸甘油酯 22.55 -
[1] GREGORY K B, VIDIC R D, DZOMBAK D A. Water management challenges associated with the production of shale gas by hydraulic fracturing [J]. Elements, 2011, 7(3): 181-186. doi: 10.2113/gselements.7.3.181 [2] FERRAR K J, MICHANOWICZ D R, CHRISTEN C L, et al. Assessment of effluent contaminants from three facilities discharging Marcellus Shale wastewater to surface waters in Pennsylvania [J]. Environmental Science & Technology, 2013, 47(7): 3472-3481. [3] NELL M, HELBLING D E. Exploring matrix effects and quantifying organic additives in hydraulic fracturing associated fluids using liquid chromatography electrospray ionization mass spectrometry [J]. Environmental Science. Processes & Impacts, 2019, 21(2): 195-205. [4] SHAO L, HE P, XUE J, et al. Electrolytic degradation of biorefractory organics and ammonia in leachate from bioreactor landfill [J]. Water Science and Technology, 2006, 53(11): 143-150. doi: 10.2166/wst.2006.347 [5] WANG H, QUAN B X, AN X Q, et al. Advanced decomposition of coking wastewater in relation to total organic carbon using an electrochemical system [J]. Polish Journal of Environmental Studies, 2017, 26(2): 941-947. doi: 10.15244/pjoes/65358 [6] LI J, YANG Z H, XU H Y, et al. Electrochemical treatment of mature landfill leachate using Ti/RuO2–IrO2 and Al electrode: Optimization and mechanism [J]. RSC Advances, 2016, 6(53): 47509-47519. doi: 10.1039/C6RA05080H [7] LI T G, LI X F, CHEN J, et al. Treatment of landfill leachate by electrochemical oxidation and anaerobic process [J]. Water Environment Research, 2007, 79(5): 514-520. doi: 10.2175/106143006X115435 [8] 徐伟冬. 电芬顿氧化降解页岩气采出水中有机物过程研究[D]. 上海: 华东理工大学, 2020. XU W D. Study on the process of electro-Fenton oxidation and degradation of organic matter in shale gas produced water[D]. Shanghai: East China University of Science and Technology, 2020(in Chinese).
[9] PENG X H, PAN X H, WANG X, et al. Accelerated removal of high concentration p-chloronitrobenzene using bioelectrocatalysis process and its microbial communities analysis [J]. Bioresource Technology, 2018, 249: 844-850. doi: 10.1016/j.biortech.2017.10.068 [10] CAO D, WANG Y B, ZHAO X. Combination of photocatalytic and electrochemical degradation of organic pollutants from water [J]. Current Opinion in Green and Sustainable Chemistry, 2017, 6: 78-84. doi: 10.1016/j.cogsc.2017.05.007 [11] LIU S Q, WANG Y, ZHOU X Z, et al. Improved degradation of the aqueous flutriafol using a nanostructure macroporous PbO2 as reactive electrochemical membrane [J]. Electrochimica Acta, 2017, 253: 357-367. doi: 10.1016/j.electacta.2017.09.055 [12] JAYATHILAKA P B, HAPUHINNA K U K, BANDARA A, et al. Phenol contaminated water treatment on several modified dimensionally stable anodes [J]. Water Environment Research, 2017, 89(8): 687-693. doi: 10.2175/106143017X14839994522623 [13] 张永红, 金艳, 何化, 等. 页岩气采出水处理工艺试验研究 [J]. 天然气与石油, 2019, 37(3): 88-93. doi: 10.3969/j.issn.1006-5539.2019.03.017 ZHANG Y H, JIN Y, HE H, et al. Research on treatment process of shale gas wastewater [J]. Natural Gas and Oil, 2019, 37(3): 88-93(in Chinese). doi: 10.3969/j.issn.1006-5539.2019.03.017
[14] 冯岐, 何芳, 刘德蓉, 等. 基于RuO2-PPy复合电极的制备及其电催化性能研究 [J]. 表面技术, 2018, 47(12): 105-112. FENG Q, HE F, LIU D R, et al. Preparation and electrocatalysis performance of composite RuO2-PPy electrode [J]. Surface Technology, 2018, 47(12): 105-112(in Chinese).
[15] 肖波. 电催化氧化复合磁分离处理页岩气压裂返排液室内研究 [J]. 石油与天然气化工, 2017, 46(4): 109-114. XIAO B. Laboratory study on shale gas flowback water treatment applying electrochemical oxidation coupled with magnetic separation [J]. Chemical Engineering of Oil & Gas, 2017, 46(4): 109-114(in Chinese).
[16] 张太亮, 欧阳铖, 郭威, 等. 混凝—磁分离—电化学技术处理压裂返排液研究 [J]. 工业水处理, 2016, 36(4): 37-41. doi: 10.11894/1005-829x.2016.36(4).009 ZHANG T L, OUYANG C, GUO W, et al. Research on the treatment of fracturing flow-back fluid by coagulation-magnet separation-electrochemistry combined technology [J]. Industrial Water Treatment, 2016, 36(4): 37-41(in Chinese). doi: 10.11894/1005-829x.2016.36(4).009
[17] 张胜健, 辛永磊, 许立坤, 等. NaCl浓度对金属氧化物阳极电化学性能与失效影响研究 [J]. 热加工工艺, 2015, 44(6): 60-63, 66. doi: 10.14158/j.cnki.1001-3814.2015.06.017 ZHANG S J, XIN Y L, XU L K, et al. Study on electrochemical performance and deactivation behavior of metal oxide anode affected by different NaCl concentration [J]. Hot Working Technology, 2015, 44(6): 60-63, 66(in Chinese). doi: 10.14158/j.cnki.1001-3814.2015.06.017
[18] TOMCSÁNYI L, de BATTISTI A, HIRSCHBERG G, et al. The study of the electrooxidation of chloride at RuO2/TiO2 electrode using CV and radiotracer techniques and evaluating by electrochemical kinetic simulation methods [J]. Electrochimica Acta, 1999, 44(14): 2463-2472. doi: 10.1016/S0013-4686(98)00381-8 [19] TAN I A W, AHMAD A L, HAMEED B H. Optimization of preparation conditions for activated carbons from coconut husk using response surface methodology [J]. Chemical Engineering Journal, 2008, 137(3): 462-470. doi: 10.1016/j.cej.2007.04.031 [20] 张泽志, 韩春亮, 李成未. 响应面法在试验设计与优化中的应用 [J]. 河南教育学院学报(自然科学版), 2011, 20(4): 34-37. ZHANG Z Z, HAN C L, LI C W. Application of response surface method in experimental design and optimization [J]. Journal of Henan Institute of Education (Natural Science Edition), 2011, 20(4): 34-37(in Chinese).
[21] 万一会. 基于网格絮凝-电化学技术的水处理装置的数值模拟与实验研究[D]. 重庆: 重庆大学, 2018. WAN Y H. The study on simulation and experimental of water treatment equipment-based on flocculation-electrochemical technique[D]. Chongqing: Chongqing University, 2018(in Chinese).
[22] 陈建孟, 潘伟伟, 刘臣亮. 电化学体系中羟基自由基产生机理与检测的研究进展 [J]. 浙江工业大学学报, 2008, 36(4): 416-422. doi: 10.3969/j.issn.1006-4303.2008.04.015 CHEN J M, PAN W W, LIU C L. Research progress on generation mechanism and determination of hydroxyl radical in electrochemical system [J]. Journal of Zhejiang University of Technology, 2008, 36(4): 416-422(in Chinese). doi: 10.3969/j.issn.1006-4303.2008.04.015
[23] 杨德敏. 电化学催化氧化页岩气压裂返排废水的实验研究[J]. 环境工程, 2016, 34(增刊1): 159-161, 269. YANG D M. Research of shale gas fracturing wastewater treatment by electro-catalytic oxidation[J]. Environmental Engineering, 2016, 34(Sup 1): 159-161, 269(in Chinese).
[24] 宋迪慧, 安路阳, 张立涛, 等. 响应曲面法优化电化学耦合体系预处理焦化废水 [J]. 化工学报, 2018, 69(9): 4001-4011. SONG D H, AN L Y, ZHANG L T, et al. Optimization of electrochemical coupling system process for coking waste water pretreatment by response surface method [J]. CIESC Journal, 2018, 69(9): 4001-4011(in Chinese).
[25] LESTER Y, FERRER I, THURMAN E M, et al. Characterization of hydraulic fracturing flowback water in Colorado: Implications for water treatment [J]. Science of the Total Environment, 2015, 512/513: 637-644. doi: 10.1016/j.scitotenv.2015.01.043 [26] STRONG L C, GOULD T, KASINKAS L, et al. Biodegradation in waters from hydraulic fracturing: Chemistry, microbiology, and engineering[J]. Journal of Environmental Engineering, 2014, 140(5): 2015, 140: B4013001 [27] OREM W, TATU C L, VARONKA M, et al. Organic substances in produced and formation water from unconventional natural gas extraction in coal and shale [J]. International Journal of Coal Geology, 2014, 126: 20-31. doi: 10.1016/j.coal.2014.01.003 [28] RANI M, SHIM W J, HAN G M, et al. Qualitative analysis of additives in plastic marine debris and its new products [J]. Archives of Environmental Contamination and Toxicology, 2015, 69(3): 352-366. doi: 10.1007/s00244-015-0224-x [29] MOLDOVAN Z, MARINCAS O, POVAR I, et al. Environmental exposure of anthropogenic micropollutants in the Prut River at the Romanian-Moldavian border: A snapshot in the lower Danube river basin [J]. Environmental Science and Pollution Research, 2018, 25(31): 31040-31050. doi: 10.1007/s11356-018-3025-8 -



 下载:
下载: