-
氨基甲酸酯类农药(carbamate pesticides, CPs)是氨基甲酸的N-甲基取代酯类化合物,具有选择性强、高效、广谱、易分解等特点,且其原料易得、合成简单,可作为新型杀虫、杀螨、除草剂等农药,被广泛应用在农业、林业、牧业等领域[1-3]。然而,大多数氨基甲酸酯类农药在水中有较高溶解度,其生产和使用过程中易被排入水体环境中。据报道[4-11],我国生活污水、工业废水、畜禽养殖场废水等实际废水以及海洋、河流、湖泊等天然水环境中已经被检测出的氨基甲酸酯类农药多达30多种。已有研究[11]表明,在湖北省中部66份地表水样品有机农药残留量检测的结果中,有机农药残留检出率为9.09%,超标率为3.03%,检出甲萘威质量分数为1 110 μg·kg−1,远远超过国家安全标准。在水环境中已检出的氨基甲酸酯类农药中,部分农药的生态毒性较高,引发的水生态安全问题不容忽视[5, 9-10]。生物毒性实验数据显示,对水中鱼类、水蚤类和藻类来说,甲萘威等氨基甲酸酯类农药属于强毒或中等毒性化学物,如果未被有效处理就排入水体环境,将对水生生物构成极大威胁[9-10]。因此,针对水环境中甲萘威等氨基甲酸酯类农药的去除研究具有重要意义。
有机农药污染控制一直是水处理研究领域关注的热点和难点问题[12-14]。电化学氧化技术因为具有高效率、低污染和占地小等优点,通常被用于水中有机农药的去除[14-16]。在电化学去除和矿化水中有机农药方面,Ti/IrO2-RuO2、Ti/SnO2、Ti/Sb2O5、硼掺杂金刚石(BDD)、Gd-PO2等高活性阳极已经取得了较好的效果[17-22]。陈灿等[17]分别用Ti/IrO2-RuO2、Ti/SnO2、Ti/Sb2O5、石墨阳极处理氨基甲酸酯类农药废水,发现Ti/IrO2-RuO2阳极效果最好,在电流密度为20 mA·cm−2、pH为7~8、反应时间为4 h条件下,其化学需氧量(COD)去除率可达85.0%;邹徐[18]用BDD阳极降解水中浓度为8.5 μmol·L−1的敌草隆,在10 mA·cm−2的电流密度下,反应40 min后,敌草隆降解率高达97%;ÇELEBI等[19]用BDD阳极降解水中浓度为0.1 mmol·L−1的甲萘威,在300 mA电流下,反应14 min后,甲萘威几乎全部去除,反应2 h后,溶液总有机碳(TOC)去除率可达90.2%;SANTOS等[20]用Ti/RuO2阳极降解水中浓度为50 μmol·L−1的甲萘威,在30 mA·cm−2的电流密度下,反应2 h后,甲萘威降解率达到了96.4%。然而,Ti/IrO2-RuO2、Ti/SnO2、Ti/RuO2、Ti/Sb2O5等阳极使用寿命有限,石墨阳极容易被氧化成CO2而失活,BDD阳极制备成本较高[21-22]。因此,仍需要开发对水中有机农药去除率和矿化率较高、制备成本较低、稳定性较好的电化学阳极。
近年来,亚氧化钛(Ti4O7)电极材料具有较高的析氧电位(OEP),优良的导电性能、电化学活性和稳定性,可用于制备高活性电化学阳极[23-30]。WANG等[23]通过等离子喷涂技术制备了钛基亚氧化钛(Ti/Ti4O7)阳极,其OEP值(2.6 V,vs. SCE)与BDD阳极接近(2.3~2.7 V,vs. SCE),制备成本(42 568 元·m−2)比BDD阳极(105 000~153 000 元·m−2)低60%~70%。韩金名[26]用亚氧化钛修饰泡沫(TF/Ti4O7)阳极降解磺胺甲基嘧啶,在电流密度为10 mA·cm−2、初始pH为2.0的最佳条件下,反应8 h,磺胺甲基嘧啶和溶液TOC去除率分别为99.48%和48.04%。YOU等[27]利用Ti4O7阳极处理工业废水,废水中的COD和TOC的去除率分别为66%和57%。目前,使用Ti4O7阳极去除水中有机农药和氨基甲酸酯类农药的相关研究鲜见报道,且Ti4O7阳极电活性还有继续优化的空间。
本研究拟通过电化学氧化-自掺杂还原法制备亚氧化钛纳米管(Ti/Ti4O7-NTA)阳极,通过微观结构、电化学性能和稳定性分析,研究其结构和性能特点;以甲萘威为目标污染物,分析和比较Ti/Ti4O7-NTA、Ti/Ti4O7、Ti/Ti4O7-PbO2-Ce等阳极降解甲萘威时的甲萘威去除率和溶液TOC去除率,评估Ti/Ti4O7-NTA阳极电化学降解甲萘威活性;开展Ti/Ti4O7-NTA阳极电化学降解水中甲萘威实验,分析电流密度、甲萘威初始质量浓度、电解质种类、溶液初始pH等参数对甲萘威降解率的影响;分析最优参数条件下Ti/Ti4O7-NTA阳极电化学降解水中甲萘威时的电流效率和反应能耗,以期为Ti4O7阳极开发和应用以及电化学氧化技术处理有机农药废水相关研究提供参考。
-
钛板面积为5 cm×5 cm,厚为1 mm,附有1 cm×3 cm接线柱,购自宝鸡智铭特种金属有限公司。Ti4O7粉末(99.9%,粒径1~3 μm)购自湖南科莱新材料有限公司。甲萘威(C10H7OCONHCH3,分析纯,99%)、甲醇 (色谱纯) 、其他试剂(分析纯),均通过湖南省政府采购电子卖场购买,生产厂家为国药集团化学试剂有限公司。
-
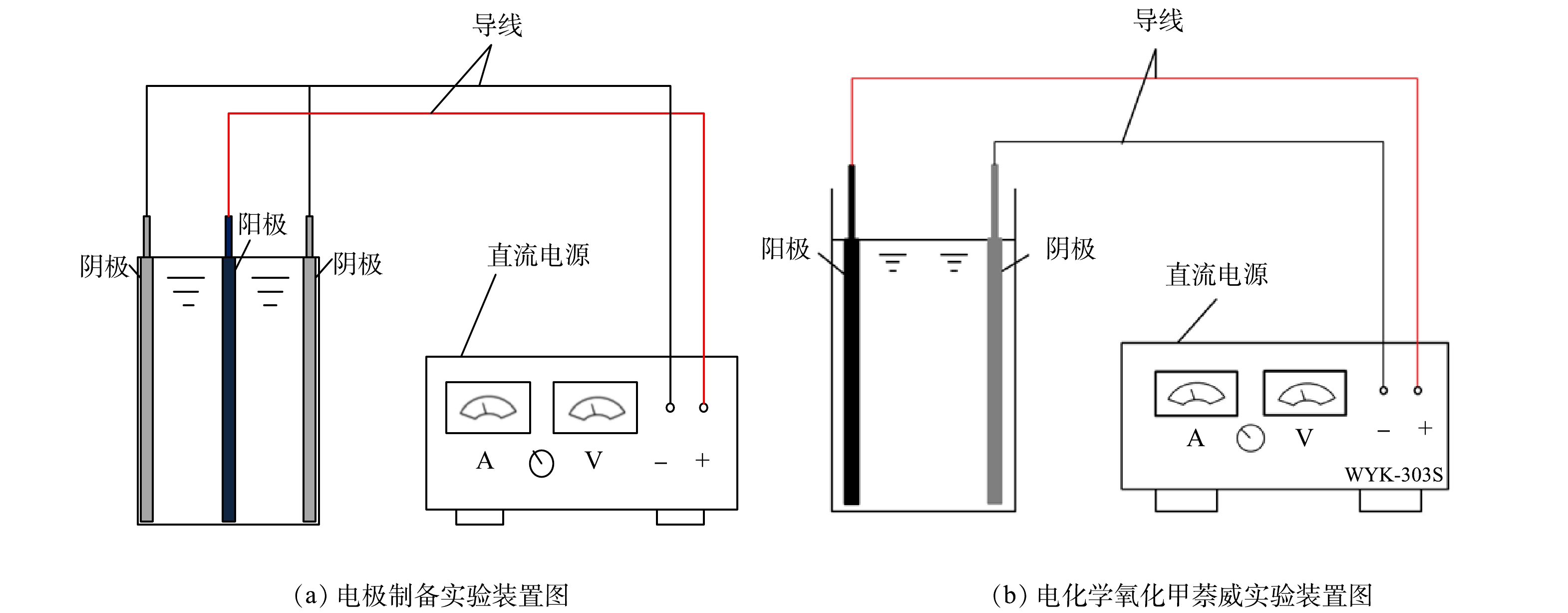
Ti/Ti4O7-NTA阳极通过电化学氧化-自掺杂还原法进行制备,即先通过电化学阳极氧化法和高温煅烧程序将Ti氧化为Ti/TiO2-NTA电极[31-35],再将Ti/TiO2-NTA电极电化学还原为Ti/Ti4O7-NTA阳极[35]。电化学氧化和电化学还原具体条件参考已有研究[31-35]。用1块钛板(使用前进行预处理[23])作阳极和2块钛板作阴极,将阳极板和阴极板放置在乙二醇(体积分数为95%)、超纯水(体积分数为5%)和氟化钠(质量分数为0.5%)混合溶液中。如图1(a)所示,2块钛板阴极放置于钛板阳极两侧,3块极板两两相距25 mm。阳极和阴极分别与直流电源正负极相连,接通直流电源,使混合溶液在40 V恒压条件下连续反应4.5 h。然后,将反应过后的阳极板取下浸泡在无水乙醇中30 min,以去除极板表面残留的F−,并清洗烘干。将烘干后的阳极板放入450 ℃马弗炉中煅烧2 h,程序升温和降温速率均设置为4.5 ℃·min−1,以此得到较稳定的二氧化钛纳米管阵列(Ti/TiO2-NTA)电极。之后,使用超纯水作溶剂,配制含1 mol·L−1 (NH4)2SO4和0.5 mmol·L−1 H2PtCl6·6H2O的电解液。与阳极氧化法反应装置相同(图1(b)),以TiO2-NTA电极作为阴极,以等离子喷涂法制备的Ti/Ti4O7电极[23]作为阳极,阳极和阴极间距为25 mm,电流密度为4 mA·cm−2,持续还原10 min。按此方式制备Ti/Ti4O7-NTA阳极。
-
电化学氧化降解水中甲萘威的反应装置如图1(b)所示。以制备的Ti/Ti4O7-NTA电极为阳极,以钛板为阴极,以长5 cm、宽5 cm、高6.5 cm的有机玻璃反应槽为反应器,阳极和阴极放置在反应器两端,经导线与直流电源正负极相连。在进行电化学氧化降解甲萘威实验时,反应器中加入5 cm高的甲萘威溶液和30 mmol·L−1的电解质溶液,分析0~40 min内电流密度(4~20 mA·cm−2)、甲萘威初始质量浓度(5~20 mg·L−1)、电解质种类(Na2SO4、NaCl、NaNO3)、溶液初始pH(3.0~11.0)对溶液中甲萘威电化学降解率、TOC去除率的影响。
-
电极形貌结构和表面元素组成采用QUANTA F250扫描电子显微镜(SEM)进行分析,扫描电压为30 kV,分辨率为128.8 eV。电极表面晶型和强度采用D8 Advance X射线衍射仪(XRD)进行分析,扫描模式设置为全扫模式,加速电压设置为40 kV。电极表面元素组成及其化学态等化学性质采用Thermo Scientific K-AlphaX射线光电子能谱(XPS)进行分析。电极线性扫描曲线(LSV)采用电化学工作站(Chenhua, CHI660D, Shanghai)测试,辅助电极采用的是Pt电极,参比电极采用的是饱和甘汞电极。
甲萘威质量浓度通过高效液相色谱仪(Agilent, 1100, USA)测试。色谱柱选用WondaSil C18色谱柱,柱温设置为35 ℃,以甲醇和水体积比为65: 35的混合溶液作为流动相,流速设置为1.0 mL·min−1,波长设置为280 nm,进样量取20 μL。使用重铬酸钾氧化法分析溶液COD变化。使用总有机碳测定仪(Shimadzu, TOC-L CPH, Japan)分析溶液TOC变化。使用pH计(FE20K, Mettler-Toledo, Switzerland)测量溶液pH,溶液酸碱度通过10% H2SO4和1 mg·L−1 NaOH水溶液调节。
平板电极电化学氧化降解甲萘威的能耗计算方法[36]见式(1)。
式中:E为能耗(以去除1 kg TOC所消耗的电能计算),kWh;U为电压,V;I为电流,A;t为反应时间,h;C为时间t内溶液TOC去除量,mg·L−1;V为溶液体积,L。
电极电流效率计算方法[24]见式(2)。
式中:η为电极电流效率;D为时间t内溶液COD去除量,mg·L−1;t为反应时间,h;V为溶液体积,L;I为电流,A;F为法拉第常数,96 487 C·mol−1。
-
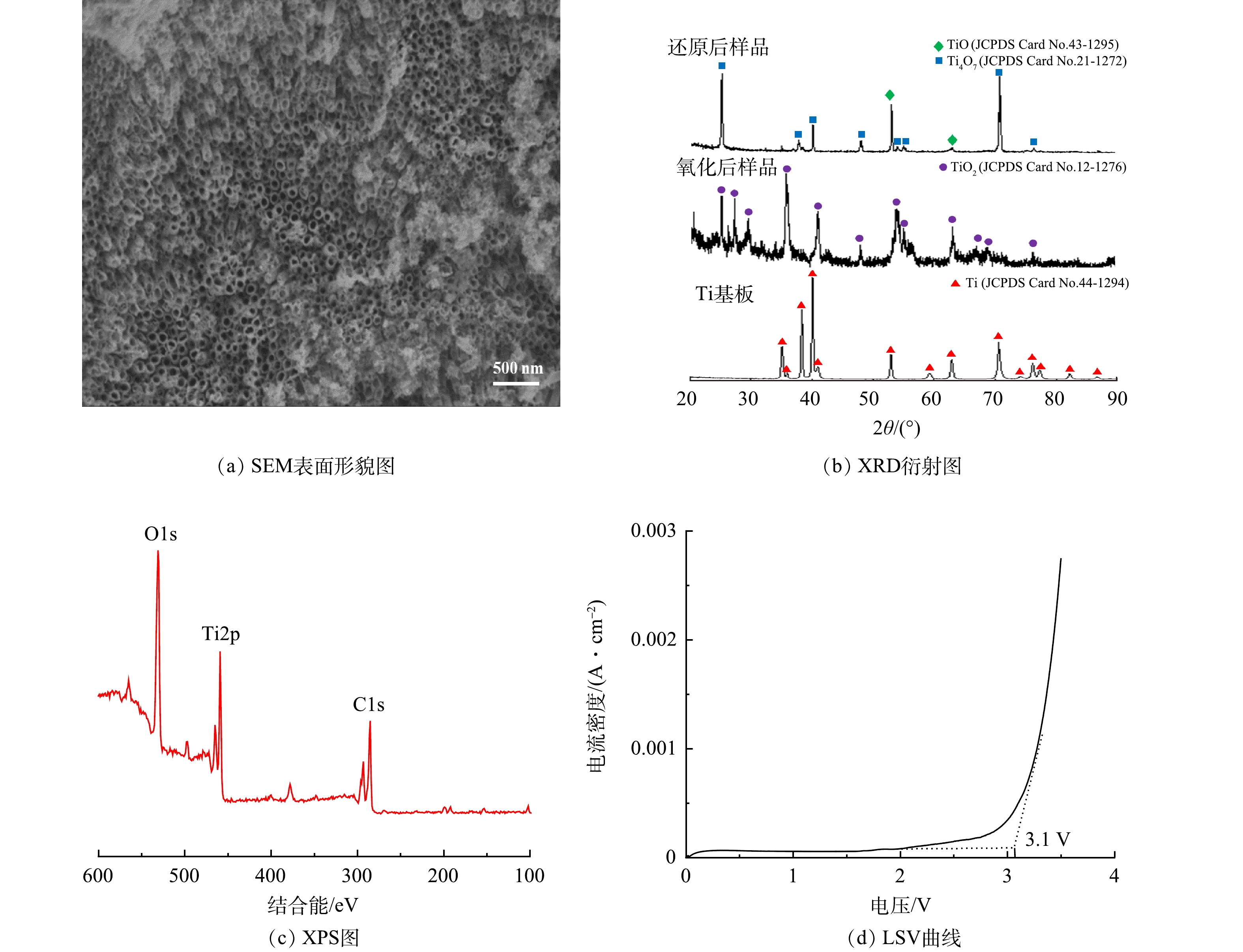
1)Ti/Ti4O7-NTA阳极表面形态表征研究。Ti/Ti4O7-NTA阳极的表面形态表征结果如图2所示。在进行电化学氧化-自掺杂还原法制备Ti/Ti4O7-NTA阳极之前,钛基板表面呈粗糙、均匀的表面形态[23],因其导电性能较好且耐腐蚀被广泛用作电化学氧化材料研究领域电极涂覆材料载体[37]。由SEM表征数据(图2(a))可以看出,制备的Ti/Ti4O7-NTA阳极表面呈致密、连续的管状结构分布,这种纳米管阵列结构有助于增加阳极电化学反应面积,进而提高阳极电化学氧化活性[33]。由能谱定量数据可以看出,Ti/Ti4O7-NTA阳极表面主要组成元素是Ti元素和O元素,其原子数量占比分别为46.75%和53.25%。由XRD扫描数据(图2(b))可以看出,所用钛基板表面的衍射峰位置为2θ ≈ 35.1°、35.9°、38.3°、40.1°、40.9°、52.9°、59.1°、62.9°、70.6°、74.1°、76.1°、77.3°、82.2°和86.7°,与Ti的标准衍射峰位置吻合(JCPDS Card No. 44-1294);经电化学氧化和高温煅烧后,电极样品表面的衍射峰位置为2θ ≈ 25.1°、27.5°、29.5°、36.1°、41.2°、47.7°、54.3°、56.6°、63.7°、67.1°、68.5°和76.5°,与TiO2标准衍射峰位置吻合JCPDS (Card No. 21-1276);经电化学还原后,电极样品表面存在Ti4O7的衍射峰(JCPDS Card No. 21-1272)以及少许的TiO的衍射峰(JCPDS Card No. 43-1295),这可能是由于TiO2在还原过程中反应不够充分,生成了少量TiO。由XPS分析数据(图2(c))可以看出,Ti/Ti4O7-NTA阳极表面含有Ti、O、C元素,O1s在530.1 eV和532.4 eV位置处存在2个明显轨道的峰,分别符合钛氧化物中的晶格氧(Ti—O)和影响电极催化活性的氢氧基团(H—O)的结合能。Ti2p在457.95 eV和458.58 eV位置处存在2个明显对称峰,分别对应+3价Ti和+4价Ti,Ti3+的存在证明了电化学还原过程中四价钛向低价态转化的情况;同时,Ti3+的占比大于Ti4+的占比,可以证明大量二氧化钛均实现了向低价钛的转化。依据Ti/Ti4O7-NTA阳极表面形态表征结果,该阳极在钛板表面蚀刻出了以Ti4O7为主要组分的纳米管阵列。
2)Ti/Ti4O7-NTA阳极电化学性能表征。LSV曲线能反映极板OEP值的变化规律,OEP值越高,电化学析氧副反应越难发生,因而电化学氧化有机物的电流效率越高[23, 38]。在30 mmol·L−1 Na2SO4溶液中,对Ti/Ti4O7-NTA阳极进行LSV测试,扫描电压为0~3.5 V,扫面速率为0.1 V·s−1,测试结果如图2(d)所示。以饱和甘汞电极为参比电极,Ti/Ti4O7-NTA阳极在30 mmol·L−1 Na2SO4溶液中的OEP值约为3.1 V,比在相同介质条件下Ti/Ti4O7阳极(2.6 V)、PbO2电极(1.9 V)、SnO2电极(1.9 V)、IrO2电极(1.6 V)和铂电极(1.3~1.6 V)高[23]。可见,Ti/Ti4O7-NTA阳极析氧电位较高,其电化学氧化有机物的电流效率会更高。
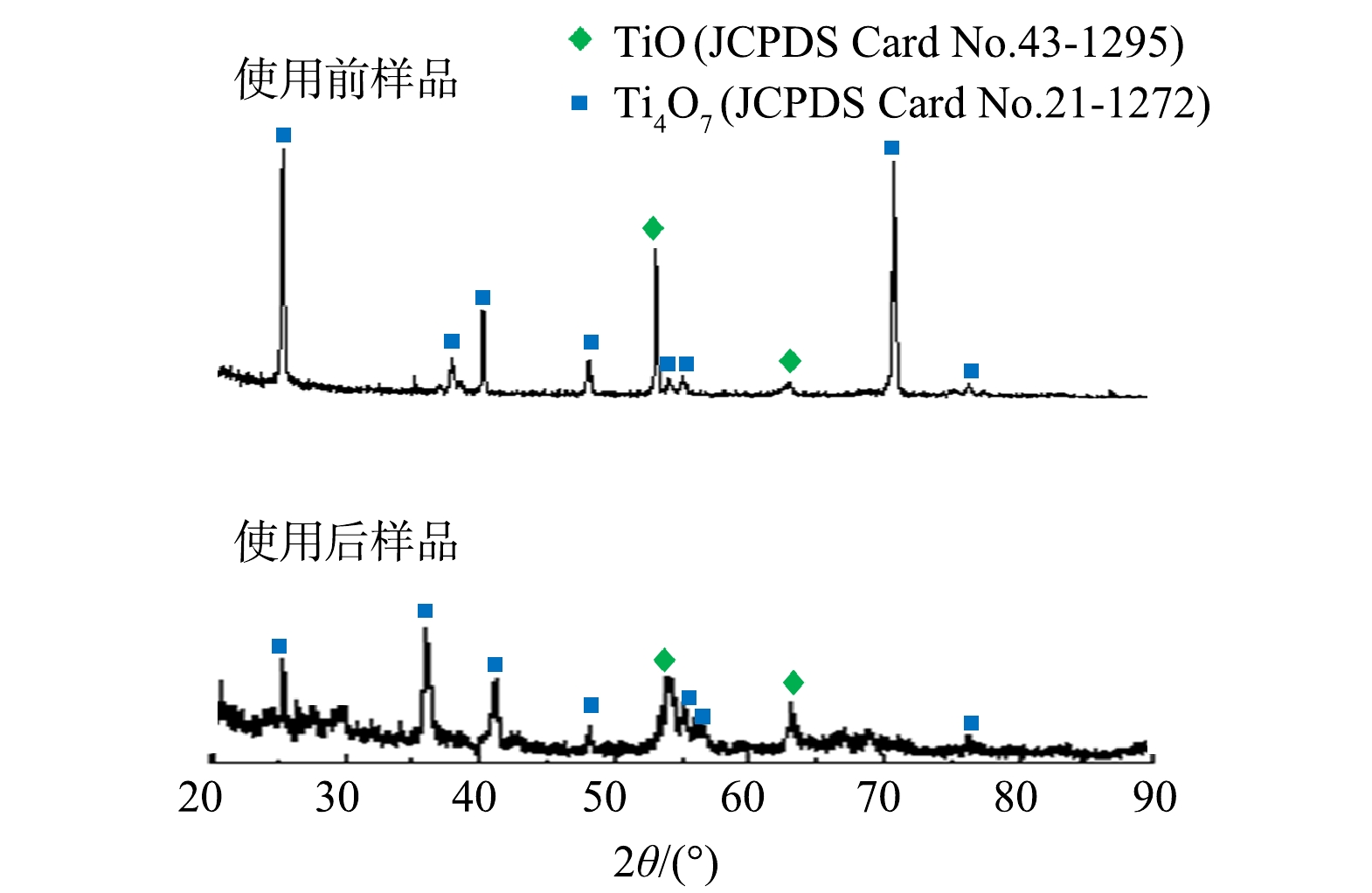
3) Ti/Ti4O7-NTA阳极反复使用性能。在电流密度为12 mA·cm−2、甲萘威初始质量浓度为10 mg·L−1、Na2SO4电解质浓度为30 mmol·L−1、pH为7.0的最优反应参数条件下,将Ti/Ti4O7-NTA阳极用于电化学降解甲萘威实验并重复使用30次(每次40 min),其电化学降解甲萘威去除率基本没有变化。Ti/Ti4O7-NTA阳极电极使用后,SEM表征数据显示其表面仍呈致密、连续的管状结构分布;能谱定量数据显示其表面主要组成元素Ti和O的原子数量占比分别为47.16%和52.84%,与使用前差别不大;XRD扫描数据显示其表面特征峰位置与使用前一致,主要存在Ti4O7衍射峰(图3)。由此可见,Ti/Ti4O7-NTA阳极稳定性较好。与WANG等[23]通过等离子喷涂技术制备的Ti/Ti4O7平板阳极相比,该Ti/Ti4O7-NTA阳极不仅表面涂层纯度高且覆盖致密、电极稳定性较好;其表面蚀刻出的Ti4O7纳米管阵列有助于增加阳极电化学反应面积,其较高的OEP值有助于增强电化学氧化有机物电流效率。
-
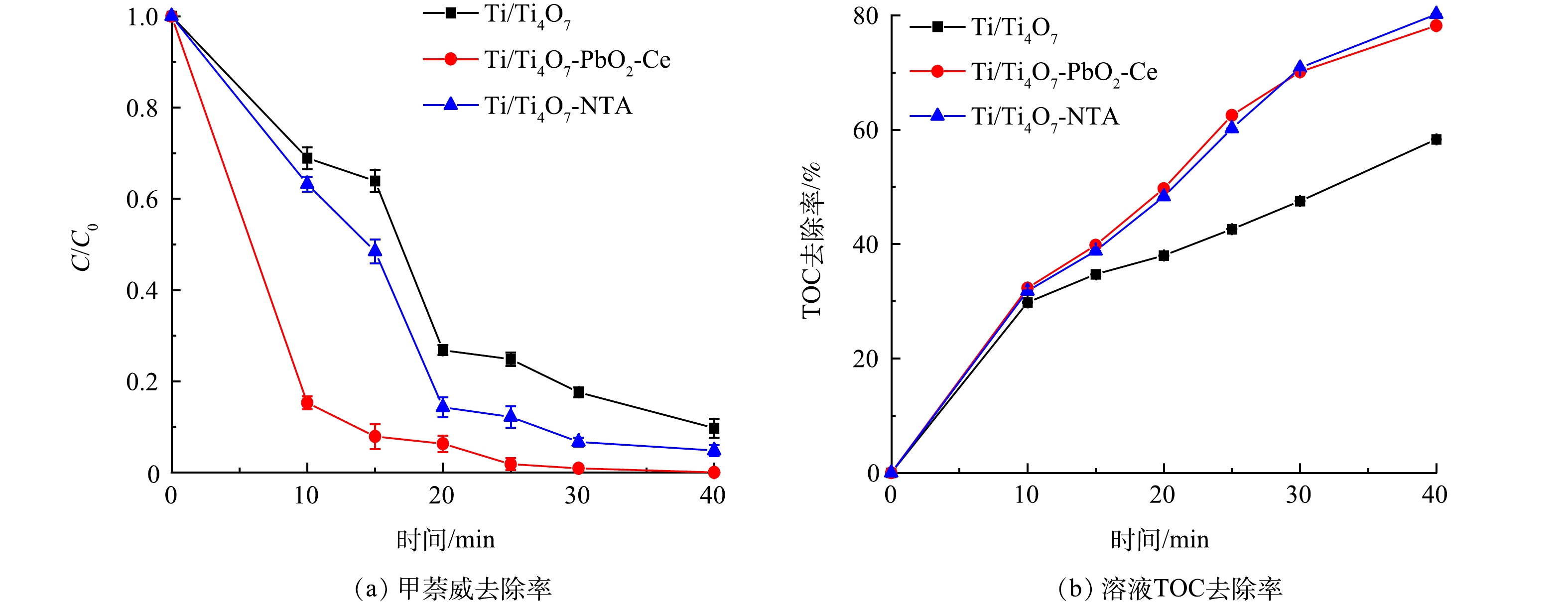
为了评估Ti/Ti4O7-NTA阳极电化学降解和矿化水中有机农药效能,本研究将Ti/Ti4O7-NTA阳极电化学降解甲萘威实验的甲萘威去除率和溶液TOC去除率与Ti/Ti4O7阳极(等离子喷涂法[23]制备)和Ti/Ti4O7-PbO2-Ce阳极(电化学沉积法[39]制备)进行了比较,结果如图4所示。随着反应时间的延长,3种Ti4O7平板阳极对水中甲萘威降解率和溶液TOC去除率均呈增加的趋势。当电化学反应到40 min时,Ti/Ti4O7-NTA、Ti/Ti4O7和Ti/Ti4O7-PbO2-Ce阳极降解甲萘威效率分别为96.1%、90.5%、100%(图4(a))。可见,3种Ti4O7阳极电化学降解甲萘威效率均比较高,Ti/Ti4O7-NTA和Ti/Ti4O7-PbO2-Ce阳极略高于Ti/Ti4O7阳极。当电化学反应到40 min时,Ti/Ti4O7-NTA、Ti/Ti4O7和Ti/Ti4O7-PbO2-Ce阳极降解甲萘威时溶液TOC去除率分别为80.2%、58.3%、78.2%(图4(b))。可见,3种Ti4O7阳极电化学降解甲萘威时,Ti/Ti4O7-NTA和Ti/Ti4O7-PbO2-Ce阳极对溶液TOC去除率明显高于Ti/Ti4O7阳极。由此可见,与Ti/Ti4O7阳极相比,Ti/Ti4O7-NTA和Ti/Ti4O7-PbO2-Ce阳极对水中甲萘威降解和矿化效果相对较好。LIN等[40]和AI等[41]研究发现,Magneli相钛氧化物(TinO2n−1, 4≤n≤10)是PbO2涂层的良好支撑材料,且通过掺杂Ce元素,可以形成纳米尺寸的PbO2晶粒,这可以提高比表面积,从而提高电催化活性。考虑到二氧化铅类电极可能会因为铅溶出使溶液毒性增加[42],且Ti/Ti4O7-PbO2-Ce阳极制备工艺相对较复杂[39],Ti/Ti4O7-NTA阳极制备工艺较简单、安全性较好,更适合用于有机农药的降解和矿化。
-
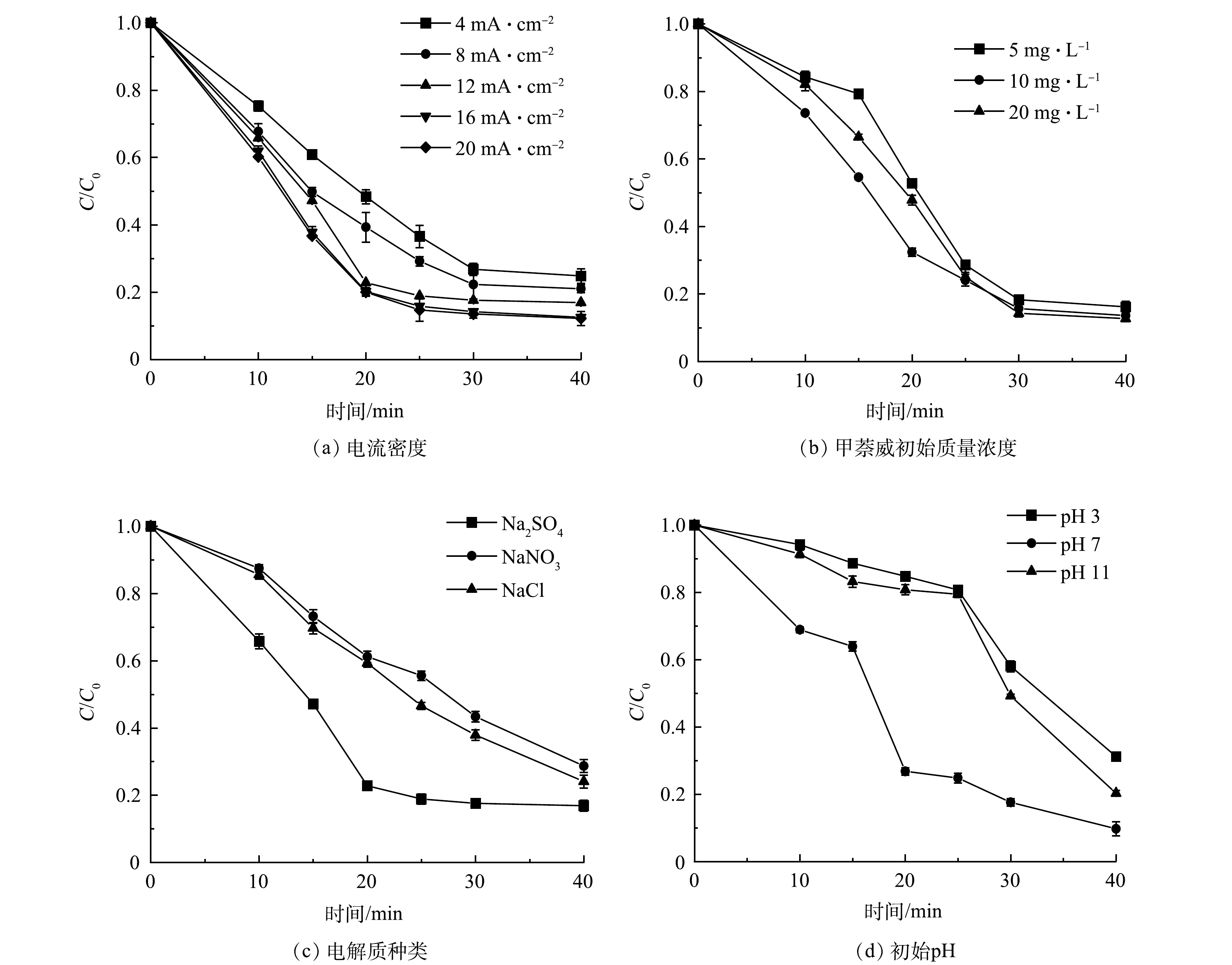
在Ti/Ti4O7-NTA阳极电化学降解水中甲萘威实验中,通过测定不同电流密度、甲萘威初始质量浓度、电解质种类、溶液初始pH等参数来验证反应过程中甲萘威质量浓度的变化,分析甲萘威去除率变化规律,结果如表1和图5所示。通过对实验结果进行半对数线性回归拟合分析,发现甲萘威电化学降解的反应均遵循一级反应动力学规律,反应速率常数R2均大于0.91。
电流密度对甲萘威降解率和速率的影响如图5(a)和表1所示。整体来看,甲萘威电化学降解率随电流密度的增大而增大。随着电流密度从4 mA·cm−2增至20 mA·cm−2,甲萘威降解率由75.2%增至95.8%,反应速率常数k由0.035 min−1增至0.079 min−1(表1),半衰期t1/2由18 min降至11 min(表1)。可见,随着电流密度的增大,甲萘威电化学降解速率加快。当电流密度由4 mA·cm−2增至12 mA·cm−2时,甲萘威电化学降解率(75.2%~92.1%)和降解速率(3.5×10−2~6.3×10−2 min−1)增大趋势明显。当电流密度由12 mA·cm−2增至20 mA·cm−2时,甲萘威电化学降解率(92.1%~95.8%)和降解速率(6.3×10−2~7.9×10−2 min−1)增大趋势变得不明显。WANG等[23]在用Ti/Ti4O7平板电极降解四环素时也发现了类似的规律。这可能是由于当电流密度在4~12 mA·cm−2时,Ti/Ti4O7-NTA阳极电化学降解甲萘威反应处于半传质控制,甲萘威电化学降解率和速率与电流密度有关,随电流密度的增大而增大;甲萘威电化学降解反应随着电流密度的增加逐渐处于传质控制,当电流密度在12~20 mA·cm−2时,Ti/Ti4O7-NTA阳极电化学降解甲萘威反应处于传质控制,反应符合一级反应动力学规律,甲萘威电化学降解率和速率不随电流密度明显增大。
甲萘威初始质量浓度对降解率和速率的影响如图5(b)和表1所示。可以看出,甲萘威电化学降解率随其初始质量浓度增大有降低趋势。甲萘威初始质量浓度由5 mg·L−1增至20 mg·L−1,其电化学降解率从95.8%降低到87.3%,k值从0.079降低到0.052(表1),半衰期t1/2由9 min增至16 min(表1)。可见,随着甲萘威初始质量浓度的增大,其电化学速率变慢。ZHI等[43]在用Ti/SnO2-Sb阳极降解四环素时也发现了类似的规律。这可能是由于在一定的电流密度条件下,电化学反应体系在单位时间内产生的活性自由基数量一定[23, 43]。而甲萘威初始质量浓度越高,扩散到阳极板表面的甲萘威分子数量越多。当扩散到阳极表面的甲萘威分子数量大于氧化这些甲萘威分子所需的活性自由基数量时,甲萘威的降解率和速率降低。此外,在甲萘威初始质量浓度为5~20 mg·L−1时,甲萘威电化学降解速率可达到87.3%~95.8%,其降解速率可达到5.2×10−2~7.9×10−2 min−1,可见Ti/Ti4O7-NTA阳极对甲萘威电化学降解活性较高。
电解质种类对甲萘威降解率和速率的影响如图5(c)和表1所示。以Na2SO4为电解质时,甲萘威电化学降解率和速率分别为92.1%和6.3 min−1,高于以NaCl和NaNO3为电解质时的甲萘威电化学降解率和速率 (表1) 。已有研究[24, 44]表明,水溶液中电解质的种类会影响电化学阳极氧化效率和有机物去除反应机理。电化学过程中常用的电解质有硫酸盐、硝酸盐、高氯酸盐和氯化物。硫酸盐和氯化物被认为是排水和自然水中的活性电解质[44]。ZHI等[24]用Ti/Ti4O7阳极电化学处理焦化废水和煤气化废水时,发现以NaCl为电解质时的COD去除率(85.3%)高于以Na2SO4为电解质时的COD去除率(78.7%)。这可能是因为,Cl−更容易被氧化成活性氯,从而提高有机物的阳极氧化效率。FRONTISTIS等[45]研究发现,以NaCl为电解质电化学降解有机物时,NaCl可能会与有机物反应生成有机氯化合物,进而导致溶液毒性增加。因此,综合考虑废水的安全性和电化学阳极氧化效率,硫酸盐是一种合适且有效的辅助电解质。
溶液初始pH对甲萘威降解率和速率的影响如图5(d)和表1所示。在溶液初始pH为3.0~11.0时,甲萘威电化学降解率为88.8%~92.1%(表1)。可见,Ti/Ti4O7-NTA阳极可在较大的溶液初始pH范围内实现甲萘威的高效降解。关于溶液初始pH对有机物电化学氧化降解率的影响,学者们得到了一些矛盾的结论[29]。例如,KLAMKLANG等[46]发现碱性条件下废水的电氧化效率更高,而SCIALDONE等[47]发现酸性条件更有利于水中草酸电氧化过程的效率。溶液初始pH对有机物电化学氧化效率的影响与有机物种类和电极材料性质有关。电极材料性质对有机物在不同溶液初始pH下的电化学阳极氧化有明显的影响[29]。可见,溶液初始pH对有机物电化学降解率的影响还需进一步深入研究。
-
为了评估Ti/Ti4O7-NTA阳极用于水中有机农药去除的经济性,本研究对最优反应参数条件下Ti/Ti4O7-NTA阳极电化学降解甲萘威反应能耗和电流效率进行分析。电化学反应到40 min时,Ti/Ti4O7-NTA阳极电化学降解甲萘威能耗为215.3 kWh。YANG等[37]研究发现,平板电极电化学降解水中有机能耗大约在50~1 000 kWh。可见,Ti/Ti4O7-NTA阳极电化学降解甲萘威反应能耗相对较低。电化学反应到40 min时,Ti/Ti4O7-NTA阳极电化学降解甲萘威电流效率为66.1%。ZHI等[24]用Ti/Ti4O7阳极处理焦化废水和煤气化废水时,电流效率为65%~66%。可见,Ti/Ti4O7-NTA阳极电化学降解甲萘威电流效率较好。目前,平板电极电化学降解甲萘威等氨基甲酸酯类农药反应能耗和电流效率研究鲜有报道。可见,Ti/Ti4O7-NTA阳极用于电化学降解甲萘威等氨基甲酸酯类农药具有实用性。
-
1)电化学氧化-自掺杂还原法制备的Ti/Ti4O7-NTA阳极表面呈致密、连续的管状结构分布,电化学反应面积较大;其在30 mmol·L−1 Na2SO4电解质溶液中的析氧电位为3.1 V,电化学活性较高;重复使用多次,其电化学降解甲萘威效率基本不变,稳定性较好。
2)与Ti/Ti4O7和Ti/Ti4O7-PbO2-Ce阳极相比,Ti/Ti4O7-NTA阳极对水中甲萘威和TOC去除效果相对较好,电化学降解甲萘威效率和溶液TOC去除率可达96.1%和80.2%,且制备工艺较简单、安全性较好,更适合用于有机农药的降解和矿化。
3)Ti/Ti4O7-NTA阳极电化学降解甲萘威反应符合一级反应动力学规律(R2>0.91)。甲萘威在pH为3~11时均可被高效降解,降解率随电流密度的增大而增大,随甲萘威初始质量浓度的增大而减小。综合考虑废水的安全性和电化学阳极氧化效率,Na2SO4是一种合适且有效的辅助电解质。
4)在电流密度为12 mA·cm−2、甲萘威初始质量浓度为10 mg·L−1、Na2SO4电解质浓度为30 mmol·L−1、pH为7.0的最适宜电化学条件下,Ti/Ti4O7-NTA阳极电化学降解甲萘威反应能耗和电流效率分别为215.3 kWh和66.1%,具有较好的实用性。
亚氧化钛纳米管阳极对水中甲萘威的电化学降解
Electrochemical degradation of carbaryl in water over the Ti/Ti4O7-NTA anode
-
摘要: 为探究电化学阳极氧化技术降解水中甲萘威的效率和能耗,通过电化学氧化-自掺杂还原法制备了高活性亚氧化钛纳米管(Ti/Ti4O7-NTA)阳极,比较了其与Ti/Ti4O7、Ti/Ti4O7-PbO2-Ce阳极电化学降解甲萘威活性;考察了电流密度、甲萘威初始质量浓度、电解质种类、溶液初始pH等参数对甲萘威降解率的影响;分析了甲萘威电化学反应能耗和电流效率。结果表明:Ti/Ti4O7-NTA阳极电化学活性较高,电化学降解甲萘威效率和溶液总有机碳去除率可达96.1%和80.2%;在pH为3~11时,甲萘威降解率为88.8%~92.1%,降解率随电流密度增大而增大,随初始质量浓度增大而减小;甲萘威电化学反应能耗和电流效率分别为215.3 kWh和66.1%。由此可知,Ti/Ti4O7-NTA阳极应用于电化学氧化处理有机农药废水具有良好潜力。本研究成果可为电化学氧化技术处理有机农药废水提供参考。
-
关键词:
- Ti/Ti4O7-NTA阳极 /
- 甲萘威电化学氧化 /
- 降解率 /
- 反应能耗
Abstract: To explore the performance and energy consumption of electro-oxidation technology for carbaryl degradation in water, a highly active Ti/Ti4O7-NTA anode was synthesized via the electro-oxidation within self-doping reduction technology, and its reactivity for carbaryl electro-oxidation was analyzed and compared with the Ti/Ti4O7 and Ti/Ti4O7-PbO2-Ce anodes. The effects of influencing parameters including current density, initial carbaryl concentration, electrolyte type, and initial pH on the electro-degradation kinetics of carbaryl were investigated. The energy consumption and current efficiency of electro-oxidizing carbaryl over the Ti/Ti4O7-NTA anode were also highlighted. The results showed that Ti/Ti4O7-NTA anode had a high electrochemical activity for carbaryl electro-oxidation, and the carbaryl electro-degradation and TOC removal efficiencies reached 96.1% and 80.2%, respectively. At pH 3~11, the degradation efficiencies of carbaryl were 88.8%~92.1%, and they increased with current density increasing, and decreased with the increase of initial carbaryl concentration. The energy consumption and current efficiency of carbaryl electro-oxidation were 215.3 kWh and 66.1% in 40 minutes reaction, respectively. It can be concluded that the Ti/Ti4O7-NTA anode may have a satisfactory application potential for the treatment of organic pesticide wastewater. This study can provide a reference for the treatment of organic pesticide wastewater by electrochemical oxidation technology. -

-
表 1 Ti/Ti4O7-NTA阳极降解甲萘威的效率以及反应动力学常数
Table 1. Efficiency and kinetics of carbaryl electrochemical oxidation by the Ti/Ti4O7-NTA anode
因素 数值或物质 降解率/% 反应速率常数k/min−1 半衰期t1/2/min R2 电流密度 4 mA·cm−2 75.2±0.2 0.035±0.000 2 18 0.934 8 mA·cm−2 79.0±0.7 0.039±0.000 1 14 0.928 12 mA·cm−2 92.1±0.4 0.063±0.000 7 12 0.918 16 mA·cm−2 92.5±0.5 0.065±0.000 6 12 0.925 20 mA·cm−2 95.8±0.7 0.079±0.000 2 11 0.931 甲萘威初始质量浓度 5 mg·L−1 95.8±0.4 0.079±0.000 3 9 0.936 10 mg·L−1 92.1±0.4 0.063±0.000 7 12 0.918 20 mg·L−1 87.3±0.6 0.052±0.000 2 16 0.929 电解质 Na2SO4 92.1±0.4 0.063±0.000 7 12 0.918 NaNO3 81.3±0.3 0.042±0.000 2 22 0.989 NaCl 85.9±0.6 0.049±0.000 7 19 0.985 pH 3.0 88.8±0.9 0.055±0.000 8 19 0.96 7.0 92.1±0.4 0.063±0.000 7 12 0.918 11.0 91.7±0.5 0.062±0.000 4 17 0.955 -
[1] 郭姣艳, 孙艳波, 马向东, 等. 濮阳市蔬菜中有机磷和氨基甲酸酯类农药残留调查分析[J]. 中国卫生检验杂志, 2022, 32(4): 487-490. [2] 董恒涛, 姚劲挺, 郝红元, 等. 超高效液相色谱三重四极杆质谱联用法测定地表水中的氨基甲酸酯类农药残留[J]. 环境化学, 2018, 37(2): 367-370. [3] WEI J C, WEI B, YANG W, et al. Trace determination of carbamate pesticides in medicinal plants by a fluorescent technique[J]. Food and Chemical Toxicology, 2018, 119: 430-437. doi: 10.1016/j.fct.2017.12.019 [4] BORDBAR M, NGUYEN T, ARDUINI F, et al. A paper-based colorimetric sensor array for discrimination and simultaneous determination of organophosphate and carbamate pesticides in tap water, apple juice, and rice[J]. Microchimica Acta, 2020, 187(11): 621. doi: 10.1007/s00604-020-04596-x [5] DE SIQUEIRA A, RODRIGUES K, GON ALVES-JUNIOR V, et al. Exhumation of Wistar rats experimentally exposed to the carbamate pesticides aldicarb and carbofuran: A pathological and toxicological study[J]. Experimental & Toxicologic Pathology, 2016, 68(6): 307-314. [6] 郭斌, 陈灿, 李士永, 等. Qu EChERS-柱后衍生高效液相色谱法测定蔬菜中6种氨基甲酸酯类农药及其代谢物[J]. 中国卫生检验杂志, 2022, 32(13): 1556-1559. [7] 孙晓静. 海水中氨基甲酸酯类农药的光降解研究[D]. 青岛: 中国海洋大学, 2015. [8] 太欧. 地下水中六种氨基甲酸酯类农药UPLC-MS/MS方法的建立与应用[D]. 北京: 中国地质大学(北京), 2019. [9] CHANDRAKAR C. Occurrence of carbaryl, DDT and deltamethrin residues in bovine milk in Chhattisgarh, India and risk assessment to human health[J]. Journal of Animal Research, 2020, 10(2): 178. [10] 燕娅娅, 刘峰, 徐飞. 一起克百威致急性中毒事件的调查分析[J]. 中国卫生检验杂志, 2022, 32(13): 1623-1625. [11] 李国强. 应城市农产品及其生长环境农药残留现状评估[J]. 化学分析计量, 2011, 20(2): 5. doi: 10.3969/j.issn.1008-6145.2011.02.022 [12] 姚梦东, 姚梦东, 徐雪婧, 等. 球磨硫化零价铁活化过硫酸盐降解水体中有机氯农药[J]. 环境工程学报, 2021, 15(8): 2563-2575. doi: 10.12030/j.cjee.202103052 [13] WASEEM H. 活化过硫酸盐氧化降解水中农药的研究[D]. 广州: 华南理工大学, 2020. [14] 赵泽华, 张后虎, 许彬, 等. 三维电催化氧化技术处理草铵膦农药废水[J]. 环境工程学报, 2017, 11(1): 93-97. doi: 10.12030/j.cjee.201508162 [15] 张丽曼. 二氧化铅电极电催化降解农药废水的研究[D]. 天津: 河北工业大学, 2020. [16] 智丹, 王建兵, 周云惠, 等. 钛基锡锑阳极电化学氧化去除水中的四环素[J]. 环境工程学报, 2018, 12(1): 57-64. doi: 10.12030/j.cjee.201705098 [17] 陈灿, 秦岳军, 张燕. 电催化氧化技术处理氨基甲酸酯类农药废水[J]. 广州化工, 2014, 42(6): 58-60. doi: 10.3969/j.issn.1001-9677.2014.06.021 [18] 邹徐. BDD电极电化学氧化去除水中典型农药特性及机理研究[D]. 长沙: 湖南大学, 2018. [19] ÇELEBI M S, OTURAN N, ZAZOU H, et al. Electrochemical oxidation of carbaryl on platinum and boron-doped diamond anodes using electro-Fenton technology[J]. Separation and Purification Technology, 2015, 156: 996-1002. doi: 10.1016/j.seppur.2015.07.025 [20] SANTOS T É S, SOLVA R S, CARLESI J C, et al. The influence of the synthesis method of Ti/RuO2 electrodes on their stability and catalytic activity for electrochemical oxidation of the pesticide carbaryl[J]. Materials Chemistry and Physics, 2014, 148(1): 39-47. [21] YANG Y, XIA Y, WEI F, et al. Electrochemical oxidation of the pesticide nitenpyram using a Gd-PbO2 anode: Operation parameter optimization and degradation mechanism[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(8): 2120-2128. [22] HAI H, XING X, LI S, et al. Electrochemical oxidation of sulfamethoxazole in BDD anode system: Degradation kinetics, mechanisms and toxicity evaluation[J]. Science of the Total Environment, 2020, 738: 139909. doi: 10.1016/j.scitotenv.2020.139909 [23] WANG J, ZHI D, ZHOU H, et al. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode[J]. Water Research, 2018, 137: 324-334. doi: 10.1016/j.watres.2018.03.030 [24] ZHI D, ZHANG J, WANG J, et al. Electrochemical treatments of coking wastewater and coal gasifcation wastewater with Ti/Ti4O7 and Ti/RuO2-IrO2 anodes[J]. Journal of Environmental Management, 2020, 265: 110571. doi: 10.1016/j.jenvman.2020.110571 [25] 智丹, 王建兵, 王维一, 等. Ti/Ti4O7阳极电化学氧化降解水中的美托洛尔[J]. 环境科学学报, 2017, 38(5): 1858-1867. [26] 韩金名. NF/CN-TF/Ti4O7电化学体系对磺胺甲基嘧啶降解机制研究[D]. 哈尔滨: 哈尔滨工业大学, 2019. [27] YOU S, LIU B, GAO Y, et al. Monolithic porous magnéli-phase Ti4O7 for electro-oxidation treatment of industrial wastewater[J]. Electrochimica Acta, 2016, 214: 326-335. doi: 10.1016/j.electacta.2016.08.037 [28] LIN H, NIU J, LIANG S, et al. Development of macroporous magnéli phase Ti4O7 ceramic materials: As an efficient anode for mineralization of poly- and perfluoroalkyl substances[J]. Chemical Engineering Journal, 2018, 354: 1058-1067. doi: 10.1016/j.cej.2018.07.210 [29] ZHANG J, ZHOU Y, YAO B, et al. Current progress in electrochemical anodic-oxidation of pharmaceuticals: Mechanisms, influencing factors, and new technique[J]. Journal of Hazardous Materials, 2021, 418: 126313. doi: 10.1016/j.jhazmat.2021.126313 [30] LIANG S, LIN H, YAN X, et al. Electro-oxidation of tetracycline by a Magnéli phase Ti4O7 porous anode: Kinetics, products, and toxicity[J]. Chemical Engineering Journal, 2018, 332: 628-636. doi: 10.1016/j.cej.2017.09.109 [31] 谭志谋. 阳极氧化二氧化钛纳米管和纳米孔阵列的形成机理[D]. 杭州: 浙江大学, 2013. [32] 李洪义, 陈言慧, 郑雄领, 等. 二氧化钛纳米管的制备和应用[J]. 金属功能材料, 2022, 29(5): 487-490. doi: 10.13228/j.boyuan.issn1005-8192.20220116 [33] GENG P, SU J, CHRISTOS C, et al. Highly-ordered magneli Ti4O7 nanotube arrays as effective anodic material for electro-oxidation[J]. Electrochimica Acta, 2015, 153: 316-324. doi: 10.1016/j.electacta.2014.11.178 [34] WANG C, NIU J, YIN L, et al. Electrochemical degradation of fluoxetine on nanotube array intercalated anode with enhanced electronic transport and hydroxyl radical production[J]. Chemical Engineering Journal, 2018, 346: 662-671. doi: 10.1016/j.cej.2018.03.159 [35] XU C, NIU J, XIE H, et al. Effective degradation of aqueous carbamazepine on a novel blue-colored TiO2 nanotube arrays membrane filter anode[J]. Journal of Hazardous Materials, 2021, 402: 123530. doi: 10.1016/j.jhazmat.2020.123530 [36] ZHI D, WANG J, ZHOU Y, et al. Development of ozonation and reactive electrochemical membrane coupled process: Enhanced tetracycline mineralization and toxicity reduction[J]. Chemical Engineering Journal, 2020, 383: 123149. doi: 10.1016/j.cej.2019.123149 [37] YANG Y, HOFFMANN M. Synthesis and stabilization of blue-black TiO2 nanotube arrays for electrochemical oxidant generation and wastewater treatment[J]. Environmental Science & Technology, 2016, 50(21): 11888-11894. [38] LI D, TANG J, ZHOU X, et al. Electrochemical degradation of pyridine by Ti/SnO2-Sb tubular porous electrode[J]. Chemosphere, 2016, 149: 49-56. doi: 10.1016/j.chemosphere.2016.01.078 [39] 张峰源. 高活性电极的制备及电化学氧化处理煤化工废水的研究[D]. 北京: 中国矿业大学(北京), 2019. [40] LIN H, NIU J, XU Y, et al. Electrochemical mineralization of sulfamethoxazole by Ti/SnO2-Sb/Ce-PbO2 anode: kinetics, reaction pathways, and energy cost evolution[J]. Electrochimica Acta, 2013, 97: 167-174. doi: 10.1016/j.electacta.2013.03.019 [41] AI S, GAO M, ZHANG W, et al. Preparation of Ce-PbO2 modified electrode and its application in detection of anilines[J]. Talanta, 2004, 62: 445-450. doi: 10.1016/j.talanta.2003.08.019 [42] ZHOU C, WANG Y, CHEN J, et al. Electrochemical degradation of sunscreen agent benzophenone-3 and its metabolite by Ti/SnO2-Sb/Ce-PbO2 anode: Kinetics, mechanism, toxicity and energy consumption[J]. Science of the Total Environment, 2019, 688: 75-82. doi: 10.1016/j.scitotenv.2019.06.197 [43] ZHI D, QIN J, ZHOU H, et al. Removal of tetracycline by electrochemical oxidation using a Ti/SnO2-Sb anode: Characterization, kinetics, and degradation pathway[J]. Journal of Applied Electrochemistry, 2017, 47(12): 1313-1322. doi: 10.1007/s10800-017-1125-7 [44] RADJENOVIC J, SEDLAK D L. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water[J]. Environmental Science & Technology, 2015, 49(19): 11292-11302. [45] FRONTISTIS Z, ANTONOPOULOU M, YAZIRDAGI M, et al. Boron-doped diamond electrooxidation of ethyl paraben: The effect of electrolyte on by-products distribution and mechanisms[J]. Journl of Environment. Management, 2017, 195: 148-156. [46] KLAMKLANG S, VERGNES H, SENOCQ F, et al. Deposition of tin oxide, iridium and iridium oxide films by metal-organic chemical vapor deposition for electrochemical wastewater treatment[J]. Journal of Applied Electrochemistry, 2010, 40(5): 997-1004. doi: 10.1007/s10800-009-9968-1 [47] SCIALDONE O, GALIA A, GUARISCO C, et al. Electrochemical incineration of oxalic acid at boron doped diamond anodes: Role of operative parameters[J]. Electrochimica Acta, 2018, 53(5): 2095-2108. -



 下载:
下载: