-
纳米银(silver nanoparticles, AgNPs)是三维空间中至少有一维处于1—100 nm的单质银颗粒[1],其拥有高效、广谱的杀菌性能,因此广泛应用于医药、食品、化妆品、纺织品等领域[2]. 随着近年来AgNPs技术的不断发展[3],越来越多的AgNPs产品在生产、使用和废弃过程中释放进入水环境[4-5],并对水生生物产生毒害[6],因此有必要深入了解其环境归宿及潜在危害.
AgNPs化学性质活泼,进入水环境后很容易在氧气(O2)和质子(H+)的作用下发生氧化溶解,释放出银离子(silver ions, Ag+)[7]. 由于银的氧化还原电位适中(Ψ(Ag+/Ag0=0.80 V)),自然界中Ag+也可被环境中普遍存在的天然有机质以及一些动物、植物和微生物等还原成零价的AgNPs[8-10]. 因此,水环境中AgNPs与Ag+会相互转化,呈高度动态性. 而由于形态不同,AgNPs和Ag+的毒性效应存在较大差异[11-12]. 例如,虽然AgNPs和Ag+都会对蚯蚓产生细胞毒性,但Ag+主要积聚在含胞质溶胶的部分,而AgNPs主要破坏细胞膜隔室[13]. 此外,AgNPs和Ag+的生物利用度及在生物体的富集过程也存在差异[14-15]. 因此,研究水环境中的AgNPs与Ag+的转化过程对评估AgNPs的生态风险具有重要意义.
溶解性有机物(dissolved organic matter, DOM) 是一类广泛存在于自然水体,由各种活性有机物(如腐殖酸(humic acid, HA)和富里酸(fluvic acid, FA)、蛋白质、多糖和胞外聚合物(extracellular polymeric substances, EPS))组成的非均质复合物[16]. DOM具有多种活性官能团,如硫醇(—SH)、醇/酚羟基(—OH)、醛、羰基、酮、醚基、羧基(—COOH)、胺和甲氧基等,因此其具有较强的氧化还原性,能够介导水体中重金属的迁移转化、毒性和生物利用度的改变[17-18].
现有研究表明,DOM是影响AgNPs和Ag+相互转化的重要因素之一[19-21]. 然而DOM对AgNPs/Ag+的氧化还原存在双面性[22-24],既可氧化AgNPs释放Ag+,又可还原Ag+生成AgNPs,因此,在含有DOM的水环境中AgNPs/Ag+如何转化,环境风险会有多大,目前仍难以预测.
本文首先介绍了DOM促进/抑制AgNPs氧化溶解的机理,然后阐述了DOM还原Ag+形成AgNPs的机理,在此基础上总结了环境因素对DOM介导AgNPs与Ag+相互转化的影响. 最后提出了目前研究存在的不足,并为未来研究方向提供一定的建议.
-
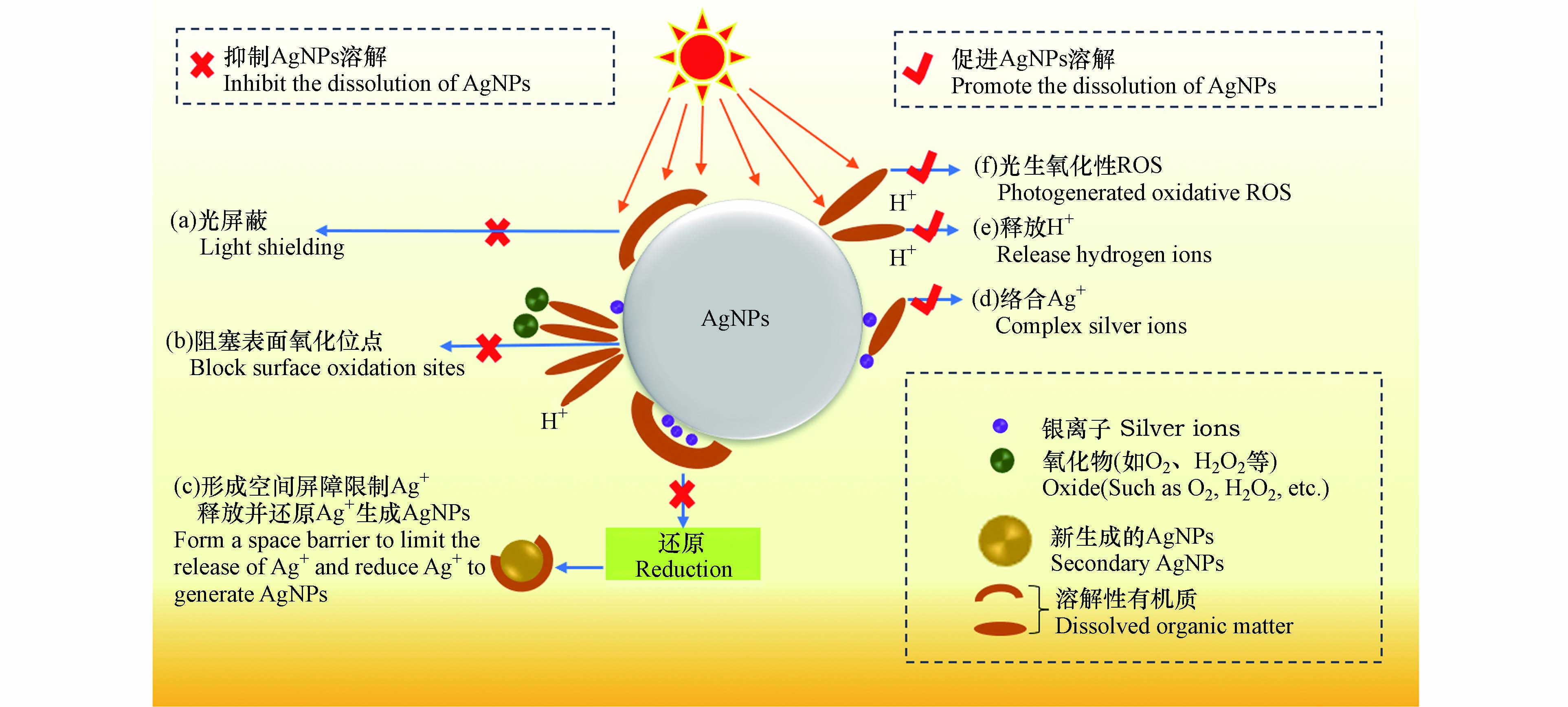
DOM可通过氢键、静电引力、疏水性作用、配体交换和离子架桥等方式吸附在无涂层AgNPs、聚乙烯吡咯烷酮包埋的AgNPs(PVP-AgNPs)或柠檬酸盐包埋的AgNPs(Cit-AgNPs)表面,改变其界面特性,进而影响AgNPs的溶解速率及溶解平衡[25-26]). 多数研究表明,DOM在AgNPs表面的吸附抑制了AgNPs的氧化溶解和Ag+的释放,其机理可总结为以下3点:
(a)吸附在AgNPs表面的DOM会屏蔽AgNPs对光子的吸收,进而抑制AgNPs的氧化蚀刻及Ag+释放[27-28]. Zhang等[29]研究发现,由于光屏蔽效应,AgNPs在含有聚苯乙烯微塑料溶液中光氧化释放的Ag+浓度显著低于纯水环境.
(b)阻塞AgNPs表面活性位点并降低其与水体氧化剂(如O2、H2O2和·OH)及H+的反应性[30],这是DOM抑制AgNPs氧化溶解的主要机理. Li等[31]发现,AgNPs的氧化与DOM在其表面的覆盖率呈反比,当全氟羧酸在Cit-AgNPs表面覆盖率为0、20%和50%时,Ag+释放量分别为35.5、31.4、18.8 µg·L−1.
(c)形成物理屏障限制AgNPs表面的Ag+扩散到溶液中,并将氧化释放的Ag+还原为新的AgNPs[32-33]. Fernando等[34]研究发现,HA介导下AgNPs在短时间内释放大量的Ag+,然而在较长时间后,溶液中Ag+会被还原成AgNPs,导致溶液中Ag+浓度降低.
-
DOM也可促进AgNPs氧化释放Ag+. 如Ostermeyer、Zhang和Yang [35-37]等研究发现,添加600 mg·L−1的牛血清白蛋白、10 mg·L−1的HA和总有机碳含量(Total Organic Carbon, TOC)为10 mg·L−1 C的EPS后,溶液中Ag+含量分别是未加DOM时的2倍、2.5倍和3倍. DOM促进AgNPs氧化溶解的机理可总结为以下3点:
(a)DOM可通过官能团(如—COOH、—OH和—SH等)与AgNPs、Ag2O相互作用形成复合物,削弱Ag—Ag键和Ag—O键,从而促进Ag+的释放[38]. 而且,DOM还可以通过与吸附在AgNPs表面的Ag+络合,使反应(1)平衡右移,促进AgNPs氧化溶解[39].
Gondikas等[40]发现,半胱氨酸(cysteine, Cys)能通过—SH与AgNPs释放的Ag+配位结合,促进溶液中AgNPs的氧化溶解.
(b)DOM中含量较多的酸性官能团(如羧基和酚羟基等)在水环境中会电离释放H+,较高的H+浓度会促进AgNPs表面氧化层的溶解,释放Ag+[41]. Zhang等[36]研究发现,HA在溶液中的酸释放促进了AgNPs的氧化溶解. 虽然吸附在AgNPs表面的DOM一定程度上阻碍了AgNPs与O2和H+的相互作用,但吸附层是可渗透的,AgNPs依然可与O2和H+反应[31].
(c)DOM具有很强的光化学活性,其在光照下可生成H2O2、1O2和·OH等强氧化性的活性氧物质(reactive oxygen species, ROS),氧化AgNPs[42]. Tong等[43]证实了光照下聚苯乙烯微塑料产生的1O2和·OH可诱导AgNPs氧化溶解.
DOM介导下促进/抑制AgNPs氧化释放Ag+的机理可总结为图1.
-
DOM/Ag浓度(物质的量)比会影响DOM对AgNPs表面活性位点的占用以及DOM-Ag配体的形成,进而影响AgNPs的氧化溶解. 一般来说,当DOM/Ag较高时,DOM会占据AgNPs更多反应活性位点,抑制AgNPs与O2和H+作用,Ag+释放显著减少[44]. 当系统中萨旺尼河腐殖酸(Suwannee River humic acid, SRHA)的TOC浓度由0增加至6.6 mg·L−1C时,2.78 mg·L−1的AgNPs释放Ag+浓度由1383 µg·L−1降低至339 µg·L−1[45]. 同样,Ag+的释放也随其他组分DOM(如多糖、蛋白质和胞外聚合物等)浓度的增加而显著降低[46-48].
而当DOM/Ag浓度比较低时,DOM占据AgNPs表面活性位点较少,其还可以通过鳌合Ag+而促进AgNPs氧化溶解[35]. Cáceres-Vélez等[49]研究发现,20 mg·L−1的HA促进了10 mg·L−1 AgNPs的溶解(DOM/Ag=2),而抑制了0.5、1、3 mg·L−1 AgNPs (DOM/Ag > 6)的溶解. Boehmler等[50]同样发现,当牛血清白蛋白浓度由0增加至2 nmol·L−1时,其可通过—SH鳌合Ag+使得粒径为10 nm的 Cit-AgNPs的溶解速率增加1.5倍.
-
DOM是非均质的混合物,其种类复杂,性质多变,如元素含量、官能团和芳香性会存在差异. 因此不同DOM种类作用下,AgNPs的氧化溶解差异明显. Gunsolus等[51]研究发现,小马湖富里酸(Pony Lake fluvic acid, PLFA)对Cit-AgNPs氧化溶解的抑制作用强于同样浓度的SRHA和萨旺尼河富里酸(Suwannee River fluvic acid, SRFA),进一步探究发现DOM介导下Ag+释放量与DOM的S、N含量呈负相关. 高S、N元素的DOM对AgNPs/Ag+有很强的亲和性,因此会占据更多AgNPs表面活性位点进而抑制AgNPs氧化溶解[52]. 而对Ag+有强亲和力的官能团也可促进AgNPs的氧化溶解. Liu[39]和Gondikas[40]均研究发现,DOM可通过—SH络合Ag+从而促进AgNPs的氧化溶解. 芳香性较强的DOM不会占据太多AgNPs的活性位点,进而增强AgNPs的反应性. Pokhrel等[53]研究发现,溶液中较高芳香性的风化褐煤腐殖酸(Leonardite humic acid, LHA)作用下Ag+释放量是无LHA条件下的4—5倍.
-
离子浓度会影响DOM的分子结构,改变DOM在AgNPs表面的吸附[54],进而影响DOM介导的AgNPs氧化释放Ag+. 较低离子浓度下,DOM分子结构较膨胀,DOM在AgNPs表面的吸附会占据更多氧化位点,且许多环境阴离子(
${\rm{CO}}_3^{2-} $ 、${\rm{SO}}_4^{2-} $ 、Cl−、${\rm{PO}}_4^{3-} $ )会与Ag+反应产生微溶或难溶性产物,覆盖在AgNPs表面,占据氧化位点,进一步抑制AgNPs的氧化溶解[55]. Zhao等[56]研究发现,在较低的离子浓度条件下(<0.01 mol·L−1 Cl−),随着DOM浓度的增加,溶液中Ag+释放量显著降低.在较高离子浓度环境中,DOM分子结构紧凑,吸附在AgNPs表面的DOM占据表面氧化位点较少,并且与释放的Ag+络合促进AgNPs氧化溶解[49, 57]. 高浓度的Cl−对AgNPs氧化溶解的影响最为显著[58]. 当Cl−/Ag <26750时,反应主要生成AgCl(s)沉淀;当Cl−/Ag ≥26750时,主要生成溶解性的配合物
${\rm{AgCl}}_x^{1-x}$ (如AgCl2−、${\rm{AgCl}}_3^{2-} $ 和${\rm{AgCl}}_4^{3-} $ )[59]. DOM会与${\rm{AgCl}}_x^{1-} $ 络合,促进AgNPs的氧化溶解平衡右移[56]. Li等[57]研究发现Cit-AgNPs暴露的天然微咸水盐度越高,则水体DOM介导的Ag+释放量越大.阳离子(如Na+、Ca2+、Mg2+)会促使AgNPs聚集,减小比表面积,从而抑制AgNPs氧化释放Ag+[60]. 虽然有研究证明DOM抑制了AgNPs的聚集,但在二价阳离子(如Ca2+、Mg2+)作用下,吸附在AgNPs表面的DOM会通过络合Ca2+、Mg2+而桥连,发生更强烈的聚集,使Ag+释放量显著降低[61]. Huang等[62]研究发现, AgNPs在HA与Ca2+共同作用下氧化释放Ag+的浓度依次低于其在HA作用下、Ca2+作用下和纯水中的Ag+释放量.
-
pH会影响AgNPs向Ag+的转化过程,且会改变DOM在水体中的分子结构,影响DOM的光化学反应,因此pH显然会影响DOM介导下AgNPs的氧化溶解. 在低pH条件下溶液中H+含量较高,会促进AgNPs的氧化溶解平衡(反应式1)右移[63];并且低pH时DOM的分子结构较紧凑,占据AgNPs氧化位点较少,削弱了其对Ag+释放的抑制作用[64];此外,相比碱性条件下,酸性溶液中DOM光生氧化性自由基显著增加[65]. 因此,低pH条件下促进了AgNPs的氧化溶解;反之,高pH条件下会抑制Ag+的释放.
-
AgNPs具有很强的光吸收能力,短时间光照会促进AgNPs的光裂解及氧化蚀刻,迅速释放Ag+. Shi等[28]研究发现,光照下三磷酸腺苷包埋的AgNPs会在短时间内(≤1 h)迅速氧化,其Ag+释放量显著高于黑暗条件. 但长时间光照会破坏AgNPs表面涂层,使AgNPs失稳聚集,表面活性位点减少,进而氧化速率降低. Yu等[32]研究发现在短时间光照(≤ 12 h)下,Cit-AgNPs的平均溶解速率常数是长时间光照(70 h)下的4.5倍,且AgNPs发生明显聚集.
光照会促进DOM介导的AgNPs氧化或降低DOM对AgNPs氧化溶解的抑制作用. Rong等[66]发现,当SRFA的浓度为0、5、10 mg·L−1时,10 min光照可使Cit-AgNPs氧化率分别比黑暗条件下提高了3.4%、4.6%和6.0%. Yu等[67]研究发现,黑暗条件下,TOC浓度为5 mg·L−1 C的SRHA使1.02 mg·L−1的AgNPs在20 h后的Ag+释放量由830 µg·L−1降至100 µg·L−1;而20 h的光照后,Ag+释放量由738 µg·L−1降至225 µg·L−1.
-
在黑暗/光照条件下,DOM均可介导Ag+还原成AgNPs,其还原机理可概括为以下3点,见图2
(1)黑暗条件下的自催化. 溶液中游离的Ag+通过沉积在Ag2O/AgNPs簇表面进而提高其氧化还原电位(游离状态:Ψ(Ag+/Ag0 = −1.8 V);吸附在固态表面的Ag+:Ψ(Ag+/Ag0 = 0.7996 V),进而使DOM还原Ag+的反应在热力学上是可行的,如式(2,3)所示[68].
自催化过程可以被描述为以下几个步骤[69]:
DOM首先发生脱质子化,然后通过静电作用/络合作用与Ag+结合[70-71],DOM-Ag复合物通过还原性官能团(如—COOH、—OH、—SH、醛基和酮基等)将e−转移给Ag+,生成AgNPs[39, 72].
(2)光照生成还原性自由基. 在光照条件下,DOM充当光吸收体,产生强还原性自由基(如
${\mathrm{e}}^{-}_{\mathrm{a}\mathrm{q}}$ 和${\mathrm{O}}_{2}^{•-}$ )使Ag+被迅速还原[73]. Yin等[74]研究发现,HA在Xe灯照射下产生的${\mathrm{O}}_{2}^{•-}$ 介导了Ag+还原成AgNPs;而在溶液中添加超氧化物歧化酶(superoxidase dismutase, SOD)去除${\mathrm{O}}_{2}^{•-}$ 后,溶液中没有生成AgNPs,证实了${\mathrm{O}}_{2}^{•-}$ 在Ag+还原过程中的重要作用.(3)光照条件下配体-金属电荷转移(ligand-to-metal charge transfer, LMCT). 当Ag+吸附到DOM表面后,光照促进DOM配体将e−转移至Ag+,进而生成AgNPs[75]. Hou等[76]研究发现,HA光还原Ag+生成AgNPs的速率随着溶液中Na+浓度的增加而显著降低,证实了Na+通过竞争HA表面金属离子结合位点进而抑制了HA通过LMCT还原Ag+.
-
DOM介导的AgNPs形成速率可由以下式子表示[74]:
其中,r为AgNPs形成速率,单位h−1;[Ag+]为Ag+浓度,单位mg·L−1;[DOM]为DOM浓度,单位mg·L−1;A为AgNPs表面等离子体共振(Surface Plasmon Resonance, SPR)峰吸光度.
由于DOM介导的AgNPs/Ag+氧化还原是同时进行的,当DOM浓度一定时,需要足够的Ag+浓度才能实现AgNPs团簇的快速生长,这个浓度称为临界诱导浓度. 低于临界诱导浓度时,Ag+还原生成AgNPs不稳定,会马上被氧化,无法实现AgNPs团簇的生长[77];而高于该浓度时,AgNPs生成速率与DOM浓度呈正相关. Xiong等[78]研究发现,初始浓度为30 µg·L−1的Ag+无法被EPS被还原成AgNPs;而Yin等[79]研究发现,初始浓度为0.2 mmol·L−1的Ag+会随DOM浓度的升高而加速转化为AgNPs.
-
不同组分DOM的性质差异(比如对Ag+的吸附能力、芳香性、分子量和官能团)会影响DOM介导的Ag+还原成AgNPs. 高Ag+吸附性的DOM能更好地通过LMCT还原Ag+. Liu等[75]比较了吸附性很强的溶解性黑炭和吸附性较弱的SRHA对Ag+的光还原能力,发现溶解性黑炭介导的AgNPs生成速率显著高于SRHA. Nie等[80]研究发现,DOM的芳香成分会限制Ag+与DOM的还原性官能团结合. 低芳香性的泥炭HA 和泥炭FA可在24 h内介导Ag+还原成AgNPs;而同等浓度的高芳香性商用HA需要120 h才能介导Ag+还原成AgNPs. 由于低分子量的DOM光屏蔽能力较弱,Guo等[81]研究发现,分子量<3 kDa的DOM作用下Ag+光还原效率远高于>3 kDa DOM. 此外,DOM官能团的还原性会影响Ag+的还原. Nie等[80]研究发现,酚基比羰基具有更强的还原性,其利用NaBH4将羰基转化为酚基后,发现DOM介导的AgNPs生成速率和浓度均显著增加.
-
环境共存离子会影响Ag+还原为AgNPs. 其中环境中常见的阴离子如
${\rm{CO}}_3^{2-} $ 、${\rm{SO}}_4^{2-} $ 、${\rm{PO}}_4^{3-} $ 、S2−和Cl−均可与Ag+反应生成微溶或不溶性银盐(如Ag2SO4 (Ksp=1.4×10−5)、Ag2CO3 (Ksp=8.5×10−12)、Ag3PO4 (Ksp=8.9×10−17)、Ag2S (Ksp=6×10−51)和AgCl (Ksp=1.8×10−10)等). 微溶性的银盐可为AgNPs的生成提供成核位点,促进Ag+还原成AgNPs. 而难溶性的银盐(如Ag3PO4、Ag2S、AgCl)在黑暗条件下会迅速聚集沉淀,不利于AgNPs的生成. Dong等[82]研究发现相比于没有离子添加的条件下,${\rm{SO}}_4^{2-} $ 和${\rm{CO}}_3^{2-} $ 促进了HA还原Ag+,而${\rm{PO}}_4^{3-} $ 、S2−和Cl−与HA无法在黑暗条件下将Ag+还原成AgNPs.此外,一些具有光敏性的银盐(如AgCl)被认为是AgNPs的前体物质,在光照下其会激发出从价带跃迁到导带的电子e−,e−通过界面电子转移至AgCl表面的Ag+,从而生成AgNPs[83]. Cl−介导下Ag+还原可以由以下式子表示[84]:
离子浓度对Ag+转化成AgNPs影响很大. 当离子浓度较低时(如Cl−/Ag+ ≤5000),Cl−与Ag+结合主要生成AgCl,在光照下可被还原成AgNPs;而在高离子浓度下(Cl−/Ag+ > 1.8×105),则AgCl会转化为光活性较弱的
${\rm{AgCl}}_x^{1-x} $ ,难以生成AgNPs[84]. DOM可作为AgCl纳米晶体还原时的电子源,加速光照下AgNPs的生成并使AgNPs稳定. Xiong等[78]发现,光照下TOC浓度为12 mg·L−1 C的EPS在无Cl−溶液中生成的AgNPs吸光度仅为0.15,当Cl−浓度增加至10 mg·L−1,生成的AgNPs吸光度为0.3,而当Cl−浓度高到150 mg·L−1时,AgNPs吸光度又降低至0.15.环境中常见的阳离子(如Ca2+、Mg2+、Na+、Fe2+和Fe3+)也会影响环境中Ag+转化为AgNPs. 如在光照下DOM会与Fe2+/Fe3+形成氧化还原循环,催化DOM对Ag+的还原,如化学式(12—14)所示[85]. Yin等[86]研究表明,添加10 µmol·L−1的Fe2+/Fe3+可使DOM-Ag+溶液中还原生成的AgNPs浓度显著增加.
Ca2+、Mg2+和Na+等阳离子的存在会竞争DOM表面的吸附位点,抑制DOM对Ag+的吸附及还原,并且这些阳离子会压缩AgNPs表面的双电层,使其失稳聚集,导致溶液中AgNPs浓度降低[87]. Yin等[79]研究发现,溶液中 Ca2+浓度越高则DOM还原Ag+生成的AgNPs浓度越低,且AgNPs的粒径显著增大. 而其他贵重金属离子如(Au3+)对应的纳米粒子具有较高的内聚能,成核速率较快,因此这类金属会优先与DOM作用并生成相应的纳米粒子,吸附Ag+在其表面并提供成核位点,促进了Ag+转化为AgNPs[88].
-
DOM还原Ag+受溶液pH影响显著. 随着pH的升高,DOM的氧化还原电位逐渐降低,这促进了Ag+转化为AgNPs[74];而且高pH条件下DOM的酸性官能团去质子化,其表面电性更负,带正电的Ag+与带负电的DOM之间的强静电引力增强了Ag+与DOM络合,促进了光照条件DOM通过LMCT途径还原Ag+或黑暗条件下DOM通过给电子官能团还原Ag+[76].
-
黑暗条件下DOM还原Ag+需要很高的活化能,而光照可以加快DOM介导的Ag+还原生成AgNPs速率[89]. 光照可作为AgNPs生成的催化剂,既会诱导DOM产生
${\mathrm{O}}_{2}^{•-}$ 和${\mathrm{e}}^{-}_{\mathrm{a}\mathrm{q}}$ ,又可激发AgNPs产生表面热电子,进而使Ag+快速转化为AgNPs[90]. Tan等[91]研究发现,模拟阳光照射下,90%以上的Ag+可在DOM介导下被还原成AgNPs,而在黑暗环境中Ag+还原率不到10%. Liu等[92]模拟了自然界的光暗交替环境,研究发现光照下HA可还原Ag+生成AgNPs,而黑暗中AgNPs则被氧化释放出Ag+. 但Dong等[82]的研究则表明,黑暗条件下DOM可还原Ag+生成AgNPs. 由此可见,环境中AgNPs与Ag+的相互转化是同时进行的,实验设置的差异导致Ag+还原速率与AgNPs氧化速率不一样,因此目前的研究结果常存在矛盾.鉴于研究DOM介导AgNPs/Ag+转化会得到DOM促进/抑制AgNPs氧化释放Ag+以及还原Ag+生成AgNPs 3种不同的结论,可能源于这些研究中设计的特定实验条件影响了DOM对
$ \mathrm{A}{\mathrm{g}}^{0}\rightleftarrows \mathrm{A}{\mathrm{g}}^{+} $ 的转化,如光照、pH、DOM种类、DOM/Ag物质的量比和电解质等. 现将这些研究中的反应条件展示于表1,以更好地看出先前研究中DOM介导AgNPs/Ag+不同转化的反应条件异同. -
目前研究表明DOM可通过占据氧化位点、还原Ag+、光衰弱来抑制AgNPs氧化,或释放H+、络合Ag+和光生氧化性ROS来促进Ag+释放,还可以通过自催化、光生还原性ROS和LMCT等途径将Ag+还原成AgNPs. 而环境因素(如DOM组分及浓度、AgNPs/Ag+浓度、离子、光照和pH等)会影响AgNPs/Ag+的转化过程,因此判断真实环境中DOM介导下AgNPs/Ag+的转化方向是比较困难的. 鉴于目前银基抗菌产品的市场在世界范围内进一步扩大,这将导致AgNPs与Ag+释放到生态环境中,并对生态系统和人体健康造成潜在危害,因此迫切需要更科学的理论基础去预测和评估其环境及健康风险.
目前DOM存在下AgNPs氧化/Ag+还原是AgNPs研究领域的热点之一,研究仍存在一些问题值得今后进一步研究:
(1)从表1可以看出,反应条件会显著影响DOM介导的AgNPs氧化及Ag+还原过程,区分和量化各种反应条件对这些过程的影响[26],将有利于判定AgNPs与Ag+转化的进行.
(2)光照对DOM介导的纳米银/银离子转化过程具有重要的影响,光照既可促进纳米银转化为银离子,也可促进Ag+还原生成纳米银,因此难以预测在现实环境中光暗交替下纳米银/银离子转化,未来可加强这方面的研究.
(3)目前关于DOM与AgNPs相互作用的研究存在使用的DOM模型简单(常将HA/FA作为DOM模型)的问题[93]. 未来研究中应考虑研究其他DOM组分(如溶解性黑炭[94]和人工合成类DOM)与AgNPs/Ag+相互作用的效应及作用机理.
(4)AgNPs粒径、形貌和表面包被等对AgNPs氧化溶解的影响已研究得较为透彻,但目前尚不清楚在DOM存在的条件下AgNPs粒径、形貌和表面包被会怎样影响DOM与AgNPs的相互作用及AgNPs的氧化溶解. 今后可研究上述因素与DOM耦合作用下的AgNPs氧化溶解,并深入探究其机理.
溶解性有机质对纳米银/银离子转化的影响
Effect of dissolved organic matter on the migration transformation and toxicity of silver nanoparticles
-
摘要: 纳米银(silver nanoparticles,AgNPs)的广泛应用导致其大量释放到水环境中,并在各种环境要素的作用下溶解产生银离子(silver ions,Ag+),对生态环境和人类健康造成了潜在威胁. 由于Ag+是AgNPs毒性的主要来源,探究水环境中AgNPs/Ag+相互转化有助于评估其环境风险. 溶解性有机物(dissolved organic matter,DOM)是地球上化学活性最强的物种之一,其在水环境中无处不在,既可介导AgNPs氧化释放Ag+,也能还原Ag+形成AgNPs. 由于反应条件的差异,先前研究DOM介导的AgNPs/Ag+相互转化常常得到相反的结论,因此难以预测具体环境中DOM介导的AgNPs/Ag+转化. 因此本文总结了DOM介导AgNPs氧化及Ag+还原的机理,并重点剖析不同环境因子对DOM介导AgNPs/Ag+氧化还原的影响. 本综述旨在为探究DOM介导AgNPs/Ag+氧化还原循环提供新的视野,并为AgNPs进入水环境后的归趋和风险预测提供科学依据.Abstract: The numerous applications of silver nanoparticles (AgNPs) leads to their spread in aquatic systems and the generation of silver ions (Ag+), which brings potential risks to environments and human health. Because Ag+ is the main toxicant source of AgNPs, the exploration of mutual transformations between AgNPs and Ag+ could help to evaluate the environmental risks in the process. Dissolved organic matters (DOMs) are ubiquitous on the earth and have high chemical activities. DOM could oxidize AgNPs to Ag+, and reduce Ag+ to AgNPs as well. Due to the different reaction conditions, previous studies always generate opposite conclusions in the DOM mediated transformations between AgNPs and Ag+, which causing the difficulty to predict the transformation in specific reactions. Here we summarized mechanisms of AgNPs oxidation and Ag+ reduction regulated by DOMs, and analyzed the environmental effects on DOM regulating the reactions between AgNPs and Ag+. The objective of review is to raise new perspectives to above mentioned processes, and provide references for the risk assessments while AgNPs entering into aquatic environments.
-
Key words:
- dissolved organic matter /
- silver nanoparticles /
- silver ions /
- oxidation /
- reduction.
-

-
表 1 DOM介导下的AgNPs氧化或Ag+还原条件汇总
Table 1. Summary of AgNPs oxidation or Ag+ reduction conditions mediated by DOM
离子
IonAg初始浓度
Initial concentration of Ag speciesDOM种类及初始浓度
DOM species and initial concentration光照
Illumination statepH 时间
Time结果
Result参考文献
Reference0.08—16.67 mg·L−1 盐度天然水 5 mg·L−1 AgNPs 3.8—7.2 mg·L−1 C 天然水体DOM 黑暗 — 7 d 高盐度↑
低盐度↓Li et al., 2020[57] 0、0.01、0.1、0.3 mol·L−1 NaCl 1 mg·L−1 AgNPs 1.5—2 mg·L−1 C 天然水体DOM — 5.5 168 h ↑/↓ Zhao et al., 2021[56] — 20 mg·L−1 Ag+ 5—20 mg·L−1 C HA 光暗交替 7 96 h 光: ←;暗:↑ Liu et al., 2020[92] 7 mmol·L−1 NaHCO3
10 mmol·L−1 NaNO38 µmol·L−1 AgNPs 400 µmol·L−1 Cys — 7.5— 8.1 50 h ↑ Gondikas et al., 2012[40] 280 mg·L−1 CaCO3 10 mg·L−1 AgNPs 2—20 mg·L−1 LHA — 7 4 h ↑ Pokhrel et al., 2014[53] — 1.08 mg·L−1 AgNPs 0—10 mg·L−1 C EPS 132 W·m−2 Xe灯 7.1 72 h ↑ Yang et al., 2021[37] 柠檬酸盐缓冲液 1 mg·L−1 AgNPs 0—2 nmol·L−1 BSA — 6.5 4 h ↑ Boehmler et al., 2020[50] 硼酸盐、硝酸根 20 mg·L−1 AgNPs 20 mg·L−1 PS MPs 550 W·m−2 Xe灯/黑暗 5.5 72 h ↑ Tong et al.,2022[43] 硼酸盐、硝酸根 20 mg·L−1 AgNPs 20 mg·L−1 PS MPs 550 W·m−2 Xe灯/黑暗 8.5 24 h ↓ Zhang et al.,2021[29] 0.1 mmol·L−1 KH2PO4 500 µg·L−1 AgNPs 0.1—10 µmol·L−1 Cys — 7 72 h ↓ Afshinnia et al., 2018[52] 人工介质ASW38 1 µg·L−1 AgNPs 0—50 µmol·L−1 BSA 自然光 8 15 h ↓ Levak et al., 2017[47] — 1.02 mg·L−1 AgNPs 5 mg·L−1 C SRHA 550 W·m−2 Xe灯/黑暗 5—8.3 48 h ↓ Yu et al., 2014[67] 0.1 mol·L−1 KH2PO4 5 mg·L−1 AgNPs 10 mg·L−1 PLFA、SRHA、 SRFA — 7 5 h ↓ Gunsolus et al., 2015[51] 硼酸盐缓冲液 1—1000 µg·L−1 Ag+ 25 mg·L−1 SRHA 黑暗 6—9 2 d ← Dong et al., 2019[82] 0—150 mg·L−1 NaCl 5 mg·L−1 Ag+ 20、40 mg·L−1 EPS 荧光灯/黑暗 8 36 h ← Xiong et al., 2021[78] 0—10 µmol·L−1 Fe2+/Fe3+ 1 mmol·L−1 Ag+ 30 mg·L−1 DOM 550 W·m−2Xe灯 6.3 8 h ← Yin et al., 2017[86] 12.7 mg·L−1 NaCl 10 mg·L−1 Ag+ 50 mg·L−1 DBC/SRHA 50 W Xe灯 7.3 2 h ← Liu et al., 2021[75] 磷酸盐–硼酸盐缓冲液 1 mmol·L−1 Ag+ 15—100 mg·L−1 HA 黑暗 8 5 d ← Nie et al., 2020[80] — 0.2 mmol·L−1 Ag+ 20 mg·L−1 C EPS Xe灯/黑暗 7.6 16 h ← Zhang et al., 2016[90] 注:↑,↓分别表示促进和抑制Ag+释放;←表示促进Ag+还原生成AgNPs;-为文献未提及该因素;PS MPs代表聚苯乙烯微塑料;DBC代表溶解性黑炭;BSA代表牛血清白蛋白. Note:↑ and ↓ respectively promote and inhibit the release of Ag+;← means promoting Ag+ reduction to generate AgNPs; - is not mentioned in the literature; PS MPs stands for polystyrene microplastics; DBC stands for dissolved black carbon; BSA stands for bovine serum albumin. -
[1] NEL A, XIA T, MÄDLER L, et al. Toxic potential of materials at the nanolevel [J]. Science, 2006, 311(5761): 622-627. doi: 10.1126/science.1114397 [2] SUN T Y, GOTTSCHALK F, HUNGERBÜHLER K, et al. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials [J]. Environmental Pollution, 2014, 185: 69-76. doi: 10.1016/j.envpol.2013.10.004 [3] HICKS A L, TEMIZEL-SEKERYAN S. Understanding the potential environmental benefits of nanosilver enabled consumer products [J]. NanoImpact, 2019, 16: 100183. doi: 10.1016/j.impact.2019.100183 [4] WU J T, LI C, ZHANG J, et al. Release of silver from nanoparticle-based filter paper and the impacts to mouse gut microbiota [J]. Environmental Science. Nano, 2020, 7(5): 1554-1565. doi: 10.1039/C9EN01387C [5] YANG T X, PAULOSE T, REDAN B W, et al. Food and beverage ingredients induce the formation of silver nanoparticles in products stored within nanotechnology-enabled packaging [J]. ACS Applied Materials & Interfaces, 2021, 13(1): 1398-1412. [6] XIANG Q Q, WANG D, ZHANG J L, et al. Effect of silver nanoparticles on gill membranes of common carp: Modification of fatty acid profile, lipid peroxidation and membrane fluidity [J]. Environmental Pollution, 2020, 256: 113504. doi: 10.1016/j.envpol.2019.113504 [7] ADAMCZYK Z, OĆWIEJA M, MROWIEC H, et al. Oxidative dissolution of silver nanoparticles: A new theoretical approach [J]. Journal of Colloid and Interface Science, 2016, 469: 355-364. doi: 10.1016/j.jcis.2015.12.051 [8] YIN Y G, YANG X Y, HU L G, et al. Superoxide-mediated extracellular biosynthesis of silver nanoparticles by the fungus Fusarium oxysporum [J]. Environmental Science & Technology Letters, 2016, 3(4): 160-165. [9] KANG F X, ALVAREZ P J, ZHU D Q. Microbial extracellular polymeric substances reduce Ag+ to silver nanoparticles and antagonize bactericidal activity [J]. Environmental Science & Technology, 2014, 48(1): 316-322. [10] MAKAROV V V, LOVE A J, SINITSYNA O V, et al. Green nanotechnologies: Synthesis of metal nanoparticles using plants [J]. Acta Naturae, 2014, 6(1): 35-44. doi: 10.32607/20758251-2014-6-1-35-44 [11] QUEVEDO A C, LYNCH I, VALSAMI-JONES E. Cellular repair mechanisms triggered by exposure to silver nanoparticles and ionic silver in embryonic zebrafish cells [J]. Environmental Science:Nano, 2021, 8(9): 2507-2522. doi: 10.1039/D1EN00422K [12] MALYSHEVA A, IVASK A, DOOLETTE C L, et al. Cellular binding, uptake and biotransformation of silver nanoparticles in human T lymphocytes [J]. Nature Nanotechnology, 2021, 16(8): 926-932. doi: 10.1038/s41565-021-00914-3 [13] LI L Z, WU H F, PEIJNENBURG W J G M, et al. Both released silver ions and particulate Ag contribute to the toxicity of AgNPs to earthworm Eisenia fetida [J]. Nanotoxicology, 2015, 9(6): 792-801. doi: 10.3109/17435390.2014.976851 [14] DANG F, JIANG Y Y, LI M, et al. Oral bioaccessibility of silver nanoparticles and ions in natural soils: Importance of soil properties [J]. Environmental Pollution, 2018, 243: 364-373. doi: 10.1016/j.envpol.2018.08.092 [15] DIEZ-ORTIZ M, LAHIVE E, KILLE P, et al. Uptake routes and toxicokinetics of silver nanoparticles and silver ions in the earthwormLumbricus rubellus [J]. Environmental Toxicology and Chemistry, 2015, 34(10): 2263-2270. doi: 10.1002/etc.3036 [16] NEBBIOSO A, PICCOLO A. Molecular characterization of dissolved organic matter (DOM): A critical review [J]. Analytical and Bioanalytical Chemistry, 2013, 405(1): 109-124. doi: 10.1007/s00216-012-6363-2 [17] GAO J, POWERS K, WANG Y, et al. Influence of Suwannee River humic acid on particle properties and toxicity of silver nanoparticles [J]. Chemosphere, 2012, 89(1): 96-101. doi: 10.1016/j.chemosphere.2012.04.024 [18] ZHANG H F, ZHENG Y C, WANG X C, et al. Characterization and biogeochemical implications of dissolved organic matter in aquatic environments [J]. Journal of Environmental Management, 2021, 294: 113041. doi: 10.1016/j.jenvman.2021.113041 [19] AIKEN G R, HSU-KIM H, RYAN J N. Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids [J]. Environmental Science & Technology, 2011, 45(8): 3196-3201. [20] LOUIE S M, TILTON R D, LOWRY G V. Critical review: Impacts of macromolecular coatings on critical physicochemical processes controlling environmental fate of nanomaterials [J]. Environmental Science:Nano, 2016, 3(2): 283-310. doi: 10.1039/C5EN00104H [21] YU S J, LIU J F, YIN Y G, et al. Interactions between engineered nanoparticles and dissolved organic matter: A review on mechanisms and environmental effects [J]. Journal of Environmental Sciences, 2018, 63: 198-217. doi: 10.1016/j.jes.2017.06.021 [22] WANG Z Y, ZHANG L, ZHAO J, et al. Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter [J]. Environmental Science:Nano, 2016, 3(2): 240-255. doi: 10.1039/C5EN00230C [23] POKHREL L R, DUBEY B, SCHEUERMAN P R. Impacts of select organic ligands on the colloidal stability, dissolution dynamics, and toxicity of silver nanoparticles [J]. Environmental Science & Technology, 2013, 47(22): 12877-12885. [24] BADIREDDY A R, FARNER BUDARZ J, MARINAKOS S M, et al. Formation of silver nanoparticles in visible light-illuminated waters: Mechanism and possible impacts on the persistence of AgNPs and bacterial Lysis [J]. Environmental Engineering Science, 2014, 31(7): 338-349. doi: 10.1089/ees.2013.0366 [25] YANG C W, YUAN L, ZHOU H Z, et al. Coating ligand-mediated dynamic formation of natural organic matter (NOM) corona on engineered nanoparticles in natural environments [J]. Environmental Science:Nano, 2021, 8(4): 1029-1041. doi: 10.1039/D0EN01223H [26] 何莹, 刘洋, 陈治廷, 等. 溶解性有机质的表面吸附行为及其对金属基纳米颗粒环境行为的影响 [J]. 环境化学, 2019, 38(8): 1757-1767. doi: 10.7524/j.issn.0254-6108.2018102902 HE Y, LIU Y, CHEN Z T, et al. Surface adsorption of dissolved organic matters and their effects on environmental behaviors of metal-based nanoparticles [J]. Environmental Chemistry, 2019, 38(8): 1757-1767(in Chinese). doi: 10.7524/j.issn.0254-6108.2018102902
[27] SHANG E X, LI Y, NIU J F, et al. Relative importance of humic and fulvic acid on ROS generation, dissolution, and toxicity of sulfide nanoparticles [J]. Water Research, 2017, 124: 595-604. doi: 10.1016/j.watres.2017.08.001 [28] SHI J P, XU B, SUN X, et al. Light induced toxicity reduction of silver nanoparticles to Tetrahymena Pyriformis: Effect of particle size [J]. Aquatic Toxicology, 2013, 132/133: 53-60. doi: 10.1016/j.aquatox.2013.02.001 [29] ZHANG W C, SONG K, DING R R, et al. Role of polystyrene microplastics in sunlight-mediated transformation of silver in aquatic environments: Mechanisms, kinetics and toxicity [J]. Journal of Hazardous Materials, 2021, 419: 126429. doi: 10.1016/j.jhazmat.2021.126429 [30] LIU J Y, HURT R H. Ion release kinetics and particle persistence in aqueous nano-silver colloids [J]. Environmental Science & Technology, 2010, 44(6): 2169-2175. [31] LI Y, NIU J F, SHANG E X, et al. Photochemical transformation and photoinduced toxicity reduction of silver nanoparticles in the presence of perfluorocarboxylic acids under UV irradiation [J]. Environmental Science & Technology, 2014, 48(9): 4946-4953. [32] YU S J, YIN Y G, ZHOU X X, et al. Transformation kinetics of silver nanoparticles and silver ions in aquatic environments revealed by double stable isotope labeling [J]. Environmental Science:Nano, 2016, 3(4): 883-893. doi: 10.1039/C6EN00104A [33] ZOU X Y, SHI J P, ZHANG H W. Morphological evolution and reconstruction of silver nanoparticles in aquatic environments: The roles of natural organic matter and light irradiation [J]. Journal of Hazardous Materials, 2015, 292: 61-69. doi: 10.1016/j.jhazmat.2015.03.005 [34] FERNANDO I, ZHOU Y. Concentration dependent effect of humic acid on the transformations of silver nanoparticles [J]. Journal of Molecular Liquids, 2019, 284: 291-299. doi: 10.1016/j.molliq.2019.04.027 [35] OSTERMEYER A K, KOSTIGEN MUMUPER C, SEMPRINI L, et al. Influence of bovine serum albumin and alginate on silver nanoparticle dissolution and toxicity to Nitrosomonas europaea [J]. Environmental Science & Technology, 2013, 47(24): 14403-14410. [36] ZHANG W C, HUANG J L, LIANG L, et al. Dual impact of dissolved organic matter on cytotoxicity of PVP-Ag NPs to Escherichia coli: Mitigation and intensification [J]. Chemosphere, 2019, 214: 754-763. doi: 10.1016/j.chemosphere.2018.09.179 [37] YANG Y, ZHENG S M, LI R X, et al. New insights into the facilitated dissolution and sulfidation of silver nanoparticles under simulated sunlight irradiation in aquatic environments by extracellular polymeric substances [J]. Environmental Science:Nano, 2021, 8(3): 748-757. doi: 10.1039/D0EN01142H [38] FREITAS D N, MARTINOLICH A J, AMARIS Z N, et al. Beyond the passive interactions at the nano-bio interface: Evidence of Cu metalloprotein-driven oxidative dissolution of silver nanoparticles [J]. Journal of Nanobiotechnology, 2016, 14: 7. doi: 10.1186/s12951-016-0160-6 [39] LIU W, WORMS I A M, HERLIN-BOIME N, et al. Interaction of silver nanoparticles with metallothionein and ceruloplasmin: Impact on metal substitution by Ag(i), corona formation and enzymatic activity [J]. Nanoscale, 2017, 9(19): 6581-6594. doi: 10.1039/C7NR01075C [40] GONDIKAS A P, MORRIS A, REINSCH B C, et al. Cysteine-induced modifications of zero-valent silver nanomaterials: Implications for particle surface chemistry, aggregation, dissolution, and silver speciation [J]. Environmental Science & Technology, 2012, 46(13): 7037-7045. [41] 黄娇龙, 刘夏薇, 黄曼绮, 等. 腐殖酸对沉积物中纳米银的释放及其毒性的影响研究 [J]. 水生生物学报, 2020, 44(5): 1119-1129. doi: 10.7541/2020.130 HUANG J L, LIU X W, HUANG M Q, et al. The release of sediment-associated silver nanoparticles by humic acid and its toxicity on zebrafish [J]. Acta Hydrobiologica Sinica, 2020, 44(5): 1119-1129(in Chinese). doi: 10.7541/2020.130
[42] TENORIO R, FEDDERS A C, STRATHMANN T J, et al. Impact of growth phases on photochemically produced reactive species in the extracellular matrix of algal cultivation systems [J]. Environmental Science:Water Research & Technology, 2017, 3(6): 1095-1108. [43] TONG L, DUAN P, TIAN X, et al. Polystyrene microplastics sunlight-induce oxidative dissolution, chemical transformation and toxicity enhancement of silver nanoparticles [J]. Science of the Total Environment, 2022, 827: 154180. doi: 10.1016/j.scitotenv.2022.154180 [44] DING Y Y, BAI X, YE Z F, et al. Humic acid regulation of the environmental behavior and phytotoxicity of silver nanoparticles to Lemna minor [J]. Environmental Science:Nano, 2019, 6(12): 3712-3722. doi: 10.1039/C9EN00980A [45] ZHOU W, LIU Y L, STALLWORTH A M, et al. Effects of pH, electrolyte, humic acid, and light exposure on the long-term fate of silver nanoparticles [J]. Environmental Science & Technology, 2016, 50(22): 12214-12224. [46] AZODI M, SULTAN Y, GHOSHAL S. Dissolution behavior of silver nanoparticles and formation of secondary silver nanoparticles in municipal wastewater by single-particle ICP-MS [J]. Environmental Science & Technology, 2016, 50(24): 13318-13327. [47] LEVAK M, BURIĆ P, DUTOUR SIKIRIĆ M, et al. Effect of protein Corona on silver nanoparticle stabilization and ion release kinetics in artificial seawater [J]. Environmental Science & Technology, 2017, 51(3): 1259-1266. [48] FU Q L, ZHONG C J, QING T, et al. Effects of extracellular polymeric substances on silver nanoparticle bioaccumulation and toxicity to Triticum aestivum L [J]. Chemosphere, 2021, 280: 130863. doi: 10.1016/j.chemosphere.2021.130863 [49] CÁCERES-VÉLEZ P R, FASCINELI M L, SOUSA M H, et al. Humic acid attenuation of silver nanoparticle toxicity by ion complexation and the formation of a Ag3+ coating [J]. Journal of Hazardous Materials, 2018, 353: 173-181. doi: 10.1016/j.jhazmat.2018.04.019 [50] BOEHMLER D J, O’DELL Z J, CHUNG C, et al. Bovine serum albumin enhances silver nanoparticle dissolution kinetics in a size- and concentration-dependent manner [J]. Langmuir, 2020, 36(4): 1053-1061. doi: 10.1021/acs.langmuir.9b03251 [51] GUNSOLUS I L, MOUSAVI M P S, HUSSEIN K, et al. Effects of humic and fulvic acids on silver nanoparticle stability, dissolution, and toxicity [J]. Environmental Science & Technology, 2015, 49(13): 8078-8086. [52] AFSHINNIA K, MARRONE B, BAALOUSHA M. Potential impact of natural organic ligands on the colloidal stability of silver nanoparticles [J]. Science of the Total Environment, 2018, 625: 1518-1526. doi: 10.1016/j.scitotenv.2017.12.299 [53] POKHREL L R, DUBEY B, SCHEUERMAN P R. Natural water chemistry (dissolved organic carbon, pH, and hardness) modulates colloidal stability, dissolution, and antimicrobial activity of citrate functionalized silver nanoparticles [J]. Environmental Science:Nano, 2014, 1(1): 45-54. doi: 10.1039/C3EN00017F [54] BAALOUSHA M, MOTELICA-HEINO M, COUSTUMER P L. Conformation and size of humic substances: Effects of major cation concentration and type, pH, salinity, and residence time [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2006, 272(1/2): 48-55. [55] GUO Z, CHEN G Q, ZENG G M, et al. Are silver nanoparticles always toxic in the presence of environmental anions? [J]. Chemosphere, 2017, 171: 318-323. doi: 10.1016/j.chemosphere.2016.12.077 [56] ZHAO J, LI Y, WANG X J, et al. Ionic-strength-dependent effect of suspended sediment on the aggregation, dissolution and settling of silver nanoparticles [J]. Environmental Pollution, 2021, 279: 116926. doi: 10.1016/j.envpol.2021.116926 [57] LI P H, SU M, WANG X D, et al. Environmental fate and behavior of silver nanoparticles in natural estuarine systems [J]. Journal of Environmental Sciences, 2020, 88: 248-259. doi: 10.1016/j.jes.2019.09.013 [58] CHAMBERS B A, AFROOZ A R M N, BAE S, et al. Effects of chloride and ionic strength on physical morphology, dissolution, and bacterial toxicity of silver nanoparticles [J]. Environmental Science & Technology, 2014, 48(1): 761-769. [59] LEVARD C, MITRA S, YANG T, et al. Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli [J]. Environmental Science & Technology, 2013, 47(11): 5738-5745. [60] AGNIHOTRI S, MUKHERJI S, MUKHERJI S. Impact of background water quality on disinfection performance and silver release of immobilized silver nanoparticles: Modeling disinfection kinetics, bactericidal mechanism and aggregation behavior [J]. Chemical Engineering Journal, 2019, 372: 684-696. doi: 10.1016/j.cej.2019.04.186 [61] REN M J, HORN H, FRIMMEL F H. Aggregation behavior of TiO2 nanoparticles in municipal effluent: Influence of ionic strengthen and organic compounds [J]. Water Research, 2017, 123: 678-686. doi: 10.1016/j.watres.2017.07.021 [62] HUANG T D, SUI M H, YAN X, et al. Anti-algae efficacy of silver nanoparticles to Microcystis aeruginosa: Influence of NOM, divalent cations, and pH [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2016, 509: 492-503. doi: 10.1016/j.colsurfa.2016.09.009 [63] MOLLEMAN B, HIEMSTRA T. Time, pH, and size dependency of silver nanoparticle dissolution: The road to equilibrium [J]. Environmental Science:Nano, 2017, 4(6): 1314-1327. doi: 10.1039/C6EN00564K [64] LAN T, WU P, LIU Z Y, et al. Understanding the effect of pH on the solubility and aggregation extent of humic acid in solution by combining simulation and the experiment [J]. Environmental Science & Technology, 2022, 56(2): 917-927. [65] LIU S C, TAN M X, GE L Q, et al. Photooxidation mechanism of As(III) by straw-derived dissolved organic matter [J]. Science of the Total Environment, 2021, 757: 144049. doi: 10.1016/j.scitotenv.2020.144049 [66] RONG H Y, GARG S, WAITE T D. Impact of light and suwanee river fulvic acid on O2 and H2O2 mediated oxidation of silver nanoparticles in simulated natural waters [J]. Environmental Science & Technology, 2019, 53(12): 6688-6698. [67] YU S J, YIN Y G, CHAO J B, et al. Highly dynamic PVP-coated silver nanoparticles in aquatic environments: Chemical and morphology change induced by oxidation of Ag0 and reduction of Ag+ [J]. Environmental Science & Technology, 2014, 48(1): 403-411. [68] ADEGBOYEGA N F, SHARMA V K, SISKOVA K, et al. Interactions of aqueous Ag+ with fulvic acids: Mechanisms of silver nanoparticle formation and investigation of stability [J]. Environmental Science & Technology, 2013, 47(2): 757-764. [69] LIU M, GAO X Y, PAN F, et al. Effect of pyrene on formation of natural silver nanoparticles via reduction of silver ions by humic acid under UV irradiation [J]. Chemosphere, 2020, 247: 125937. doi: 10.1016/j.chemosphere.2020.125937 [70] CHEN N, CAO S Y, ZHANG L, et al. Structural dependent Cr(VI) adsorption and reduction of biochar: Hydrochar versus pyrochar [J]. Science of the Total Environment, 2021, 783: 147084. doi: 10.1016/j.scitotenv.2021.147084 [71] SHENG G P, XU J, LI W H, et al. Quantification of the interactions between Ca2+, Hg2+ and extracellular polymeric substances (EPS) of sludge [J]. Chemosphere, 2013, 93(7): 1436-1441. doi: 10.1016/j.chemosphere.2013.07.076 [72] PENG H B, GUO H Y, GAO P, et al. Reduction of silver ions to silver nanoparticles by biomass and biochar: Mechanisms and critical factors [J]. Science of the Total Environment, 2021, 779: 146326. doi: 10.1016/j.scitotenv.2021.146326 [73] HUANG Y N, QIAN T T, DANG F, et al. Significant contribution of metastable particulate organic matter to natural formation of silver nanoparticles in soils [J]. Nature Communications, 2019, 10: 3775. doi: 10.1038/s41467-019-11643-6 [74] YIN Y G, LIU J F, JIANG G B. Sunlight-induced reduction of ionic Ag and Au to metallic nanoparticles by dissolved organic matter [J]. ACS Nano, 2012, 6(9): 7910-7919. doi: 10.1021/nn302293r [75] LIU H T, GE Q, XU F C, et al. Dissolved black carbon induces fast photo-reduction of silver ions under simulated sunlight [J]. Science of the Total Environment, 2021, 775: 145897. doi: 10.1016/j.scitotenv.2021.145897 [76] HOU W C, STUART B, HOWES R, et al. Sunlight-driven reduction of silver ions by natural organic matter: Formation and transformation of silver nanoparticles [J]. Environmental Science & Technology, 2013, 47(14): 7713-7721. [77] LIU H T, GU X Y, WEI C H, et al. Threshold concentrations of silver ions exist for the sunlight-induced formation of silver nanoparticles in the presence of natural organic matter [J]. Environmental Science & Technology, 2018, 52(7): 4040-4050. [78] XIONG S C, CAO X S, FANG H, et al. Formation of silver nanoparticles in aquatic environments facilitated by algal extracellular polymeric substances: Importance of chloride ions and light [J]. Science of the Total Environment, 2021, 775: 145867. doi: 10.1016/j.scitotenv.2021.145867 [79] YIN Y G, SHEN M H, ZHOU X X, et al. Photoreduction and stabilization capability of molecular weight fractionated natural organic matter in transformation of silver ion to metallic nanoparticle [J]. Environmental Science & Technology, 2014, 48(16): 9366-9373. [80] NIE X F, ZHU K C, ZHAO S, et al. Interaction of Ag+ with soil organic matter: Elucidating the formation of silver nanoparticles [J]. Chemosphere, 2020, 243: 125413. doi: 10.1016/j.chemosphere.2019.125413 [81] GUO H Y, HAN F, SHANG H P, et al. New insight into naturally formed nanosilver particles: Role of plant root exudates [J]. Environmental Science:Nano, 2021, 8(6): 1580-1592. doi: 10.1039/D0EN01188F [82] DONG B, LIU G F, ZHOU J T, et al. Transformation of silver ions to silver nanoparticles mediated by humic acid under dark conditions at ambient temperature [J]. Journal of Hazardous Materials, 2020, 383: 121190. doi: 10.1016/j.jhazmat.2019.121190 [83] GUO H Y, MA C X, THISTLE L, et al. Transformation of Ag ions into Ag nanoparticle-loaded AgCl microcubes in the plant root zone [J]. Environmental Science:Nano, 2019, 6(4): 1099-1110. doi: 10.1039/C9EN00088G [84] SINGH A, HOU W C, LIN T F, et al. Roles of silver-chloride complexations in sunlight-driven formation of silver nanoparticles [J]. Environmental Science & Technology, 2019, 53(19): 11162-11169. [85] ADEGBOYEGA N F, SHARMA V K, SISKOVA K M, et al. Enhanced formation of silver nanoparticles in Ag+-NOM-iron(II, III) systems and antibacterial activity studies [J]. Environmental Science & Technology, 2014, 48(6): 3228-3235. [86] YIN Y G, HAN D, TAI C, et al. Catalytic role of iron in the formation of silver nanoparticles in photo-irradiated Ag+-dissolved organic matter solution [J]. Environmental Pollution, 2017, 225: 66-73. doi: 10.1016/j.envpol.2017.03.048 [87] YIN Y G, SHEN M H, TAN Z Q, et al. Particle coating-dependent interaction of molecular weight fractionated natural organic matter: Impacts on the aggregation of silver nanoparticles [J]. Environmental Science & Technology, 2015, 49(11): 6581-6589. [88] GUO B L, ALIVIO T E G, FLEER N A, et al. Elucidating the role of dissolved organic matter and sunlight in mediating the formation of Ag-Au bimetallic alloy nanoparticles in the aquatic environment [J]. Environmental Science & Technology, 2021, 55(3): 1710-1720. [89] CHUTRAKULWONG F, THAMAPHAT K, LIMSUWAN P. Photo-irradiation induced green synthesis of highly stable silver nanoparticles using durian rind biomass: Effects of light intensity, exposure time and pH on silver nanoparticles formation [J]. Journal of Physics Communications, 2020, 4(9): 095015. doi: 10.1088/2399-6528/abb4b5 [90] ZHANG X, YANG C W, YU H Q, et al. Light-induced reduction of silver ions to silver nanoparticles in aquatic environments by microbial extracellular polymeric substances (EPS) [J]. Water Research, 2016, 106: 242-248. doi: 10.1016/j.watres.2016.10.004 [91] TAN Z Q, GUO X R, YIN Y G, et al. Freezing facilitates formation of silver nanoparticles under natural and simulated sunlight conditions [J]. Environmental Science & Technology, 2019, 53(23): 13802-13811. [92] LIU Y J, LI C, LUO S, et al. Inter-transformation between silver nanoparticles and Ag+ induced by humic acid under light or dark conditions [J]. Ecotoxicology, 2021, 30(7): 1376-1385. doi: 10.1007/s10646-020-02284-3 [93] ZHAO J, WANG X J, HOANG S A, et al. Silver nanoparticles in aquatic sediments: Occurrence, chemical transformations, toxicity, and analytical methods [J]. Journal of Hazardous Materials, 2021, 418: 126368. doi: 10.1016/j.jhazmat.2021.126368 [94] YANG F, WANG C P, SUN H W. A comprehensive review of biochar-derived dissolved matters in biochar application: Production, characteristics, and potential environmental effects and mechanisms [J]. Journal of Environmental Chemical Engineering, 2021, 9(3): 105258. doi: 10.1016/j.jece.2021.105258 -



 下载:
下载:
























