-
随着经济的快速发展,工业生产如炼焦炼油、冶金制药等过程中都会产生高浓度的含酚废水[1 − 6]. 酚类污染物由于其广泛的用途、高稳定性及环境毒性,成为水环境中最有害的一类污染物,对人类健康和生态环境产生严重的影响[7 − 16]. 因此如何绿色环保地去降解酚类污染物是研究的热点之一,对保护环境具有极其重要的现实意义[17]. 废水处理方法包括自然衰减法[18]、物理法[19 − 20]、化学法[21 − 24]、生物法[21, 25 − 26]、物理化学法[27- 28]、物理生物法[29]、生物化学法[26, 30]等,但这些废水处理方法可能存在二次污染,处理不彻底、处理时间长、经济成本较高等缺点[31]. 二十大提出“要加快发展方式绿色转型,推动形成绿色低碳的生产方式和生活方式”,凸显出开发一种绿色高效降解含酚废水技术的必要性和紧迫性. 电化学降解可以使用来自风能和太阳能的可再生电力,是一种绿色环保去除酚类污染物的替代方法,具有处理效率高、无二次污染、环境友好、操作条件温和、适用范围广等优点[32 − 34].
传统电化学降解污染物多采用的恒压或恒流供电模式,但是在污染物浓度不高,共存物质较多的情况下,竞争副反应严重导致电流效率低、能耗高,而方波脉冲供电方式可以减少周期内的供电时间[35 − 36];增大电流效率,提高电能利用率[37 − 39];通过调节脉冲参数减少副反应、提高反应选择性[40 − 42],因此方波脉冲电化学处理技术是一种具有更高能源效率且有前途的替代方法[37, 43]. 本研究以绿色低耗去除酚类污染物为目标,开展方波脉冲电化学处理废水中酚类污染物的降解研究,考察方波脉冲体系(电压波形、脉冲占空比、脉冲频率等)对酚类污染物的去除率及能耗的影响规律,综合评估方波脉冲电化学处理技术对酚类污染物的去除效能,为建立低耗高效去除废水中酚类污染物的方波脉冲电化学处理技术提供基础数据和理论指导.
-
实验试剂:苯酚、无水硫酸钠、5,5-二甲基-1-氧化吡咯啉和叔丁醇等均为分析纯,购自上海阿拉丁生化科技股份有限公司,甲醇为色谱纯,购自北京百灵威科技有限公司.
实验仪器:Wavedriver 100 电化学工作站,美国 Pine 公司;脉冲电源,深圳市荣达信电源科技有限公司;Shimadzu2010-AT高效液相色谱仪,日本岛津株式会社.
-
利用方波脉冲电化学处理苯酚污染废水,实验在100 mL的电化学反应器中进行,以不锈钢材料和铂片分别作为阴极和阳极(2 cm×2 cm),电极间距为2.5 cm,电解液为0.05 mol·L−1 Na2SO4,反应溶液体积为80 mL,电解池内苯酚的初始浓度为20 mg·L−1,pH为6.5. 反应器外加恒定直流电源或者脉冲电源. 反应持续时间为240 min. 采样时间:0、15、30、60、90、120、180、240 min,采样量1 mL. 采用高效液相色谱法分析苯酚的浓度,使用的仪器为 Shimadzu2010-AT,色谱柱:Shim-pack GIST C18 (4.6 mm×150 mm×5 μm),甲醇和水的混合物(体积比: 60/40)作为流动相,流速为1 mL·min−1; 柱温30 ℃;进样量5 μL;检测波长270 nm.
-
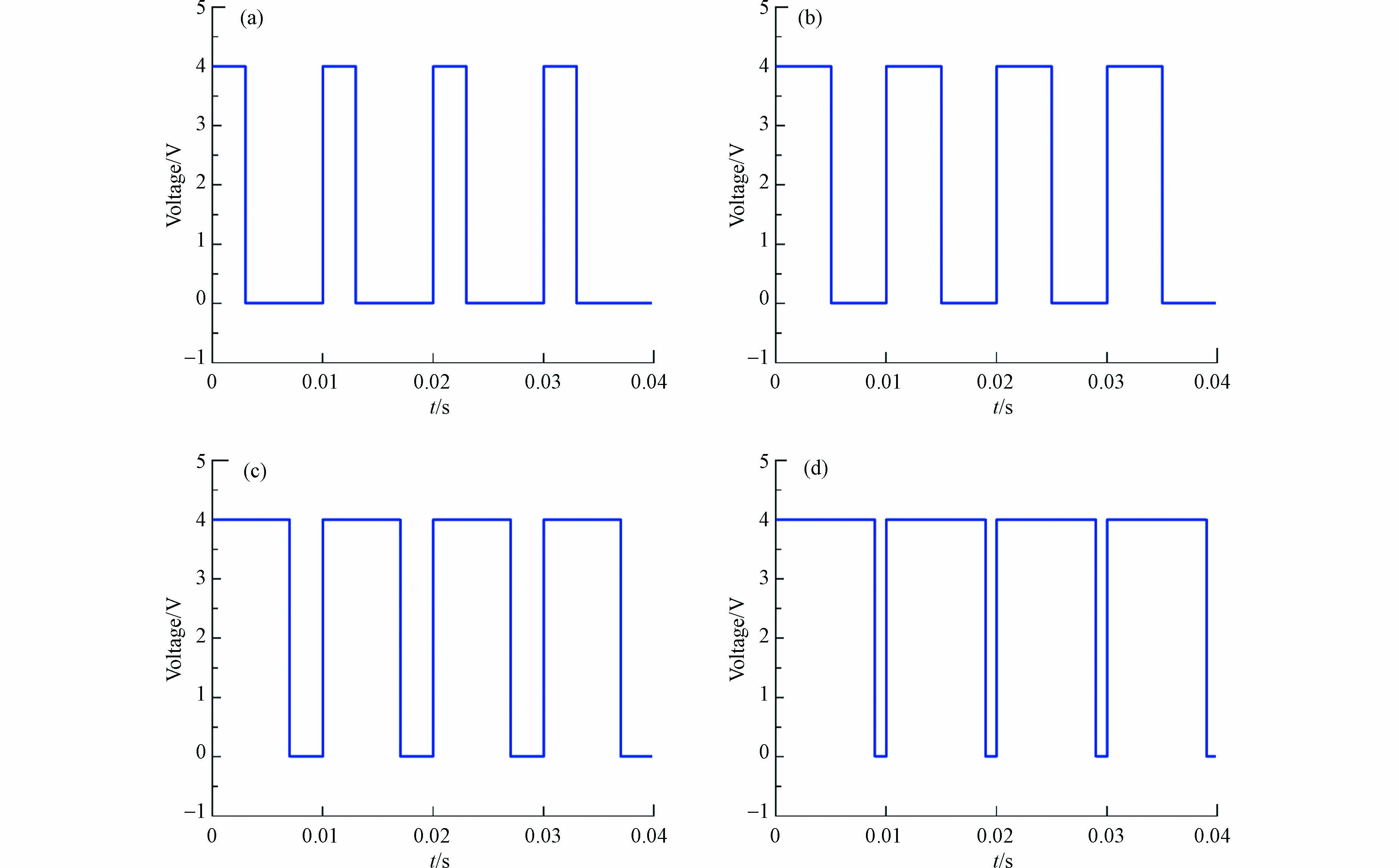
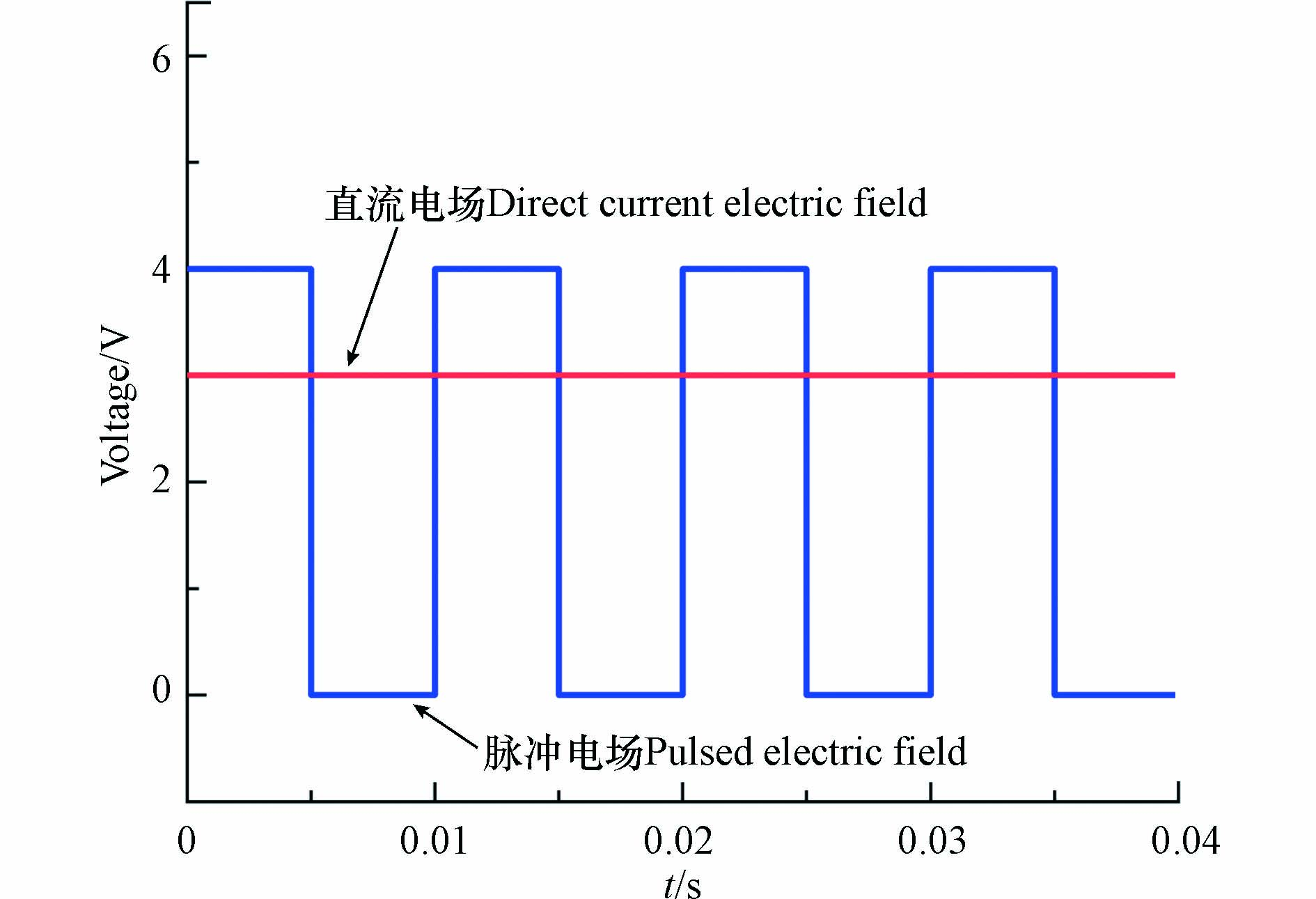
脉冲通电电压分别控制在3.0、3.5、4.0、4.5 V进行电化学降解实验(图1),脉冲通电时间为ton,断电时间为toff,脉冲周期为t(t=ton + toff),脉冲占空比
$ \phi $ ($ \phi $ =ton / t),频率f(f=1/t). 实验条件如下:CP=20 mg·L−1,${C_{{\rm{N}}{{\rm{a}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}}} $ =0.05 mol·L−1,初始pH=6.5±0.2,脉冲频率为100 Hz,脉冲占空比为50%,反应持续240 min. -
脉冲占空比分别控制在30%、50%、70%和90%进行电化学降解实验,不同脉冲占空比下电压变化示意图如图2所示. 实验条件如下:脉冲电压4.0 V,CP=20 mg·L−1,
${C_{{\rm{N}}{{\rm{a}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}}} $ =0.05 mol·L−1,初始pH=(6.5±0.2),脉冲频率为100 Hz,反应持续240 min. -
脉冲频率控制在10、50、100和200 Hz进行电化学降解实验,不同脉冲频率下电流变化示意图如图3所示. 实验条件如下:脉冲电压4.0 V,CP=20 mg·L−1,
$C_{{\mathrm{Na}}_2{\mathrm{SO}}_4} $ =0.05 mol·L−1,初始pH=6.5±0.2,脉冲占空比为50%,反应持续240 min. -
直流电场和脉冲电场的电压分别控制在3.0、3.5、4.0、4.5V进行电化学降解实验(图1). 实验条件如下:CP=20 mg·L−1,
$C_{{\mathrm{Na}}_2{\mathrm{SO}}_4} $ =0.05 mol·L−1,初始pH=6.5±0.2,脉冲频率为100 Hz,脉冲占空比为50%,反应持续240 min. -
(1)苯酚的去除率
式中,η为去除率,%;C0为苯酚初始时刻浓度,mg·L−1;Ct为反应t时刻后的苯酚浓度,mg·L−1.
假定电化学处理苯酚废水遵循伪一级动力学,由−ln(Ct/C0)-t斜率得到表观动力学速率(k,min−1).
(2)处理苯酚废水的平均电流效率和比能耗
式中,CE为平均电流效率,%; ECP 为比能耗,去除1 kg 苯酚所需要消耗的能量, kWh·kg−1;F为法拉第常数,取
96487 C·mol−1;U为电压,V;I为电流,A;V为电解液体积,L;t为处理时间,min;φ表示占空比. -
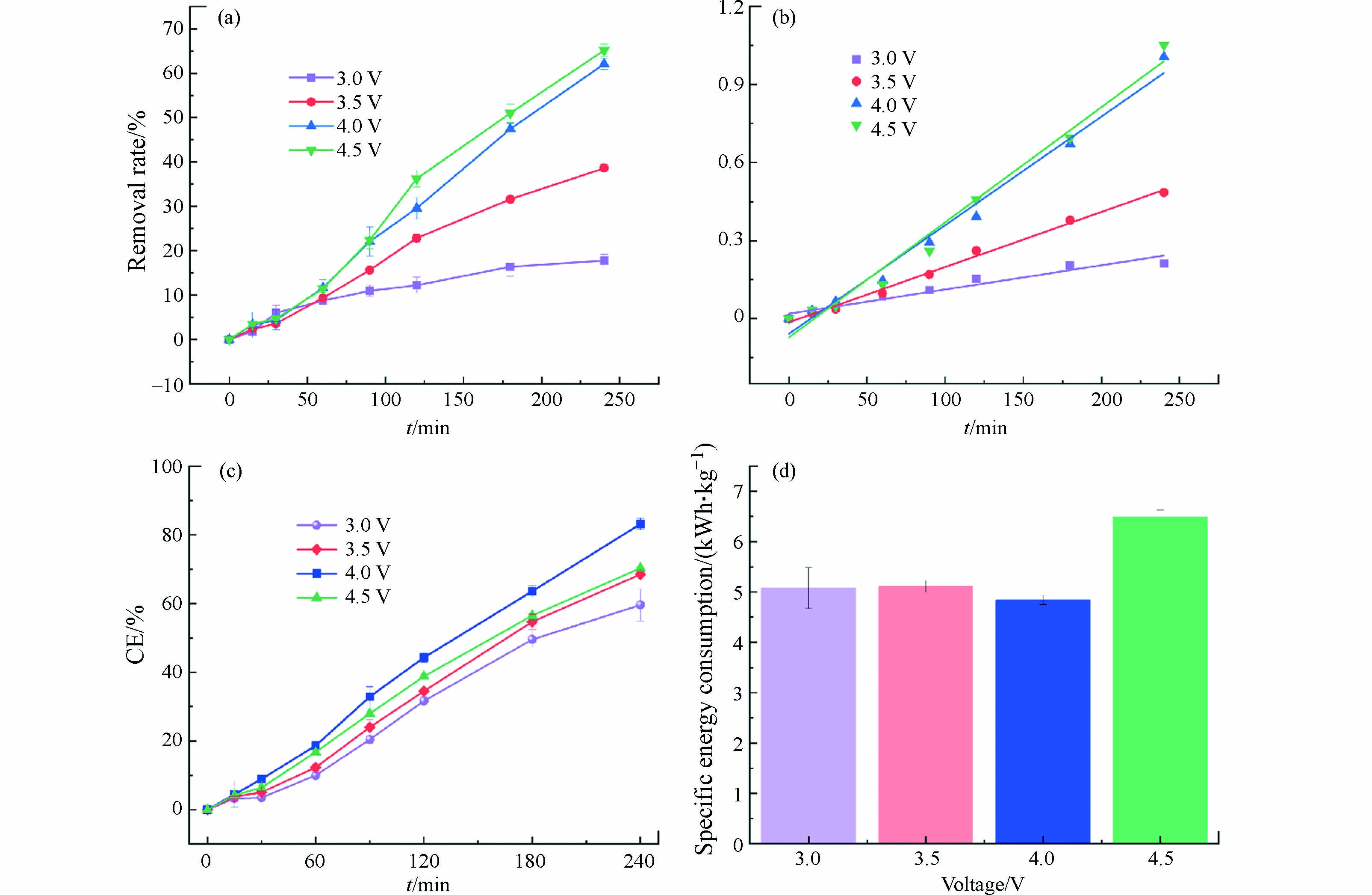
电化学反应的动力学与热力学数据都与电压、电流密切相关,可以通过控制外部电压和电流控制电化学反应,进行其相关催化机理的研究. 电压是控制反应速率的关键参数,决定苯酚的去除效果和比能耗. 不同电压下苯酚的去除率见图4(a),在脉冲供电模式下,苯酚的去除率随着电压的升高而增大. 当电压为3.0 V时,苯酚的去除率仅为17.79%,电压4.5 V下苯酚去除率为65.11%,苯酚去除率大幅度提升. 这是因为电压越高,反应体系中电流越大,使得供给反应发生的电子数越多,反应速率越快,故苯酚的去除率随着电压的升高而升高.
从图4(b)和表1可以得出,不同电压下处理苯酚废水拟合曲线具有较好的相关性,证实苯酚的去除反应符合伪一级反应动力学规律. 随着电压的升高,反应常数呈现升高的趋势,电压为4.5 V时, 反应常数值最大,为
0.00442 min−1,是电压3.0 V时的4.75倍,此时苯酚的降解速率最快.不同电压下的平均电流效率如图4(c)所示,随着电压的升高,平均电流效率呈现先增大再减小的趋势,当电压为4.0 V,平均电流效率最高,为83.14%,此时反应体系中能源利用效率最高,而当电压为4.5 V时,尽管此时反应体系中电流最大,平均电流效率却随电压升高而降低,这是因为当电压进一步升高时,超出阳极的析氧电位,加剧副反应的发生,此外,由于副反应产生气泡附着于电极表面,减小了电极的活性面积,对苯酚的去除产生不良影响,使得平均电流效率降低,能源利用率也随之降低.
不同电压下的比能耗如图4(d)所示,当电压为4.0 V,比能耗最小,即去除1 kg的苯酚需要4.84 kWh的能耗,电压3.0 V所需能耗次之,为5.08 kWh,而电压4.5 V时,所需能耗最大,为6.48 kWh,是电压4.0 V所需能耗的1.33倍,这是因为副反应发生,使得能源利用率降低. 综合考虑. 苯酚的去除率、平均电流效率及比能耗,确定电压4.0 V为最合适的脉冲电压进行后续的实验,此时苯酚的去除率(62.03%)与电压为4.5 V时苯酚的去除率(65.11%)相差较小,且平均电流效率最高,能耗最低.
-
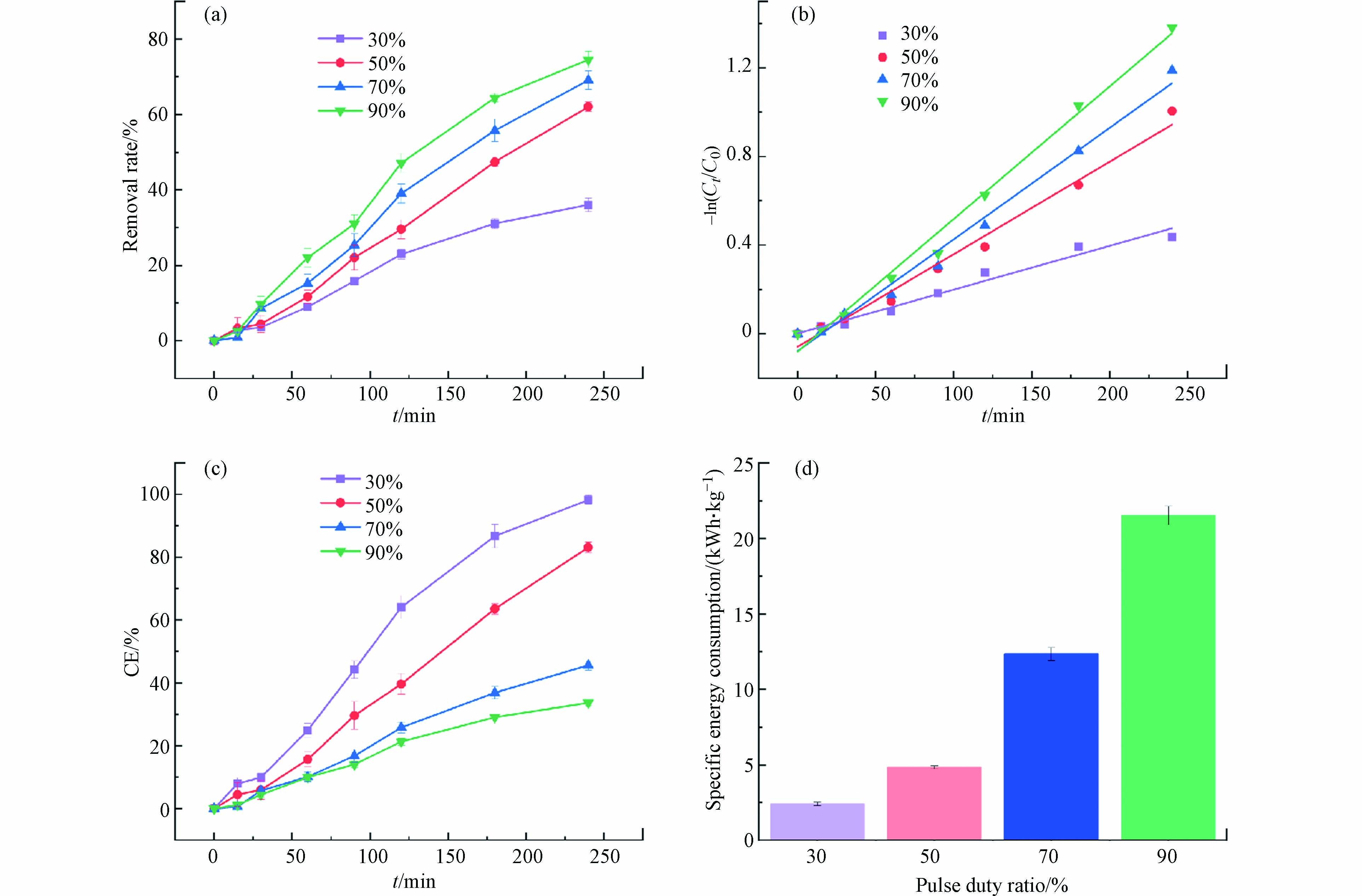
在方波脉冲供电模式下,脉冲占空比是一个脉冲循环中通电时间与单周期总时间之比. 在单位时间内,占空比越大,在反应器中注入的能量越多. 脉冲占空比是控制反应速率的重要参数,依据通电-断电的反应特性,对苯酚的去除效率产生显著影响. 不同脉冲占空比条件下苯酚的去除率如图5(a)所示,在电压为4 V时,随着脉冲占空比从30%增加至90%,去除率随之增大. 占空比90%时,去除率最大,为74.54%,占空比为30%时,去除率最小,为36.03%. 脉冲占空比50%时苯酚的去除率约占脉冲占空比70%的89.74%,约占脉冲占空比90%的83.22%. 结果表明,在方波脉冲供电模式下,脉冲占空比成为反应体系中影响去除率和比能耗的进一步限制因素. 在单位时间内,占空比越大,在反应器中注入的能量越多,苯酚的去除率越高. 但由于苯酚的电化学氧化受传质推动力和电荷转移速率控制,因此,在适当的占空比下,苯酚可通过传质作用到达电极表面,并在下一个通电阶段发生电化学氧化,从而促进苯酚的去除. 然而,过高的占空比使得阳极附近的苯酚没有充足时间得到补偿,使得苯酚的去除率并没有显著增加[44].
从图5(b)和表2可以得出,不同脉冲占空比下处理苯酚废水拟合曲线具有较好的相关性,证实苯酚的去除反应符合伪一级反应动力学规律. 随着脉冲占空比从30%增大到90%,反应速率常数也随之增大,占空比为90%时,k值最大,为
0.00598 min−1,占空比为30%时,k值最小,为0.00198 min−1,仅占占空比50%时反应速率的47.48%.不同脉冲占空比条件下的平均电流效率如图5(c)所示,在方波脉冲供电模式下,占空比不同,反应体系中平均电流值发生显著变化,导致单位时间内输入的电量不同,进而影响苯酚的去除效果. 在相同电压条件下,占空比越大,平均电流效率越小,当占空比为30%时,此时的平均电流效率最大,能源利用率最高,占空比为50%时平均电流效率次之,为83.13%,而占空比为90%时,仅为33.63%,此时能源利用率最低.
如图5(d)所示,在电压为4 V时,随着脉冲占空比从30%增加至90%,比能耗呈现不断升高的趋势,在反应体系中,去除1 kg的苯酚,占空比90%时所需要的能耗最大,为21.53 kWh,而占空比30%时,所需能耗最小,需2.40 kWh(仅占脉冲占空比90%时所需能耗的11.15%)因此,在电压为4 V时,当电化学处理苯酚废水的去除率相同时,占空比越小,能耗越低,能源利用率越高,所消耗的能源就越少,更加节能. 在脉冲电场条件下,适当的脉冲占空比可以同时获得较高的苯酚去除率和较低的能耗. 综合考虑,选择50%作为最佳脉冲占空比,此时废水中苯酚的去除率较高,能源利用率较高,比能耗较低,能够充分发挥脉冲电场通电-断电-通电的优势.
-
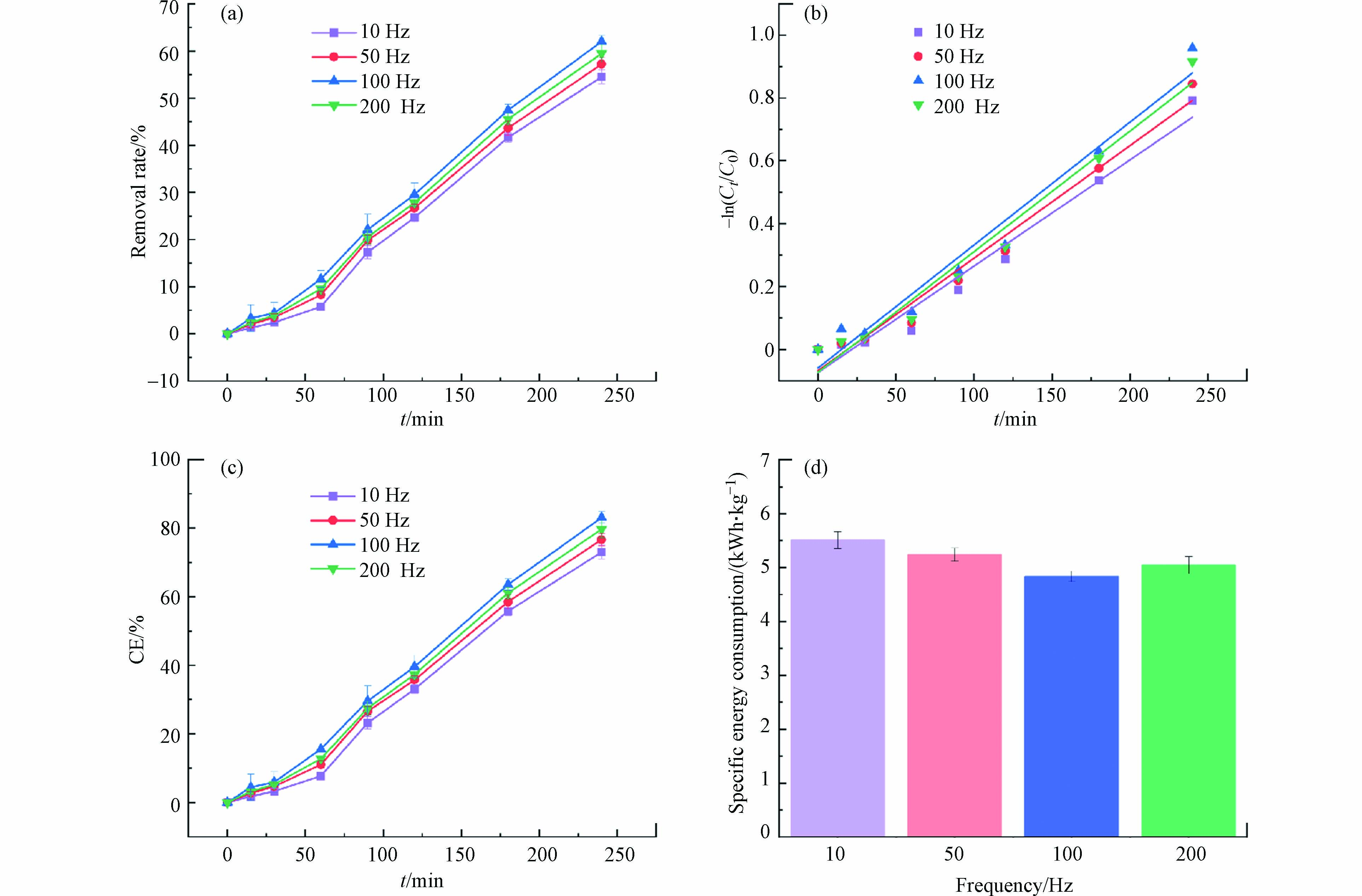
在方波脉冲供电模式下,脉冲频率是在单位时间内完成周期性变化的次数,即f=1/T,在反应器中,单位时间内有效放电的频率越小,放电次数就越多. 通过控制脉冲频率来决定能量的注入效率和电化学反应速率,进而影响苯酚的去除效率和比能耗. 不同脉冲频率下苯酚的去除率如图6(a)所示,在电压为4 V,脉冲占空比50%,脉冲频率从10 Hz增加至200 Hz时,苯酚的去除率分别为54.50%、57.23%、62.03%和59.50%,呈现出先增大后减小的变化规律,当脉冲频率为100 Hz时,苯酚的去除率最高. 但脉冲频率变化时,苯酚去除率并没有显著变化,可知脉冲频率对苯酚去除率的影响较小. 结果表明,在相对较低的脉冲频率范围内,脉冲频率的增加,增强了传质推动力,从而促进了苯酚的去除率,然而,在过高的脉冲频率下,粒子由于惯性在同一地方振荡,净迁移显著减少[44],使得去除率减小.
从图6(b)和表3可以得出,不同脉冲频率下处理苯酚废水拟合曲线具有较好的相关性,证实苯酚的去除反应符合伪一级反应动力学规律. 随着脉冲频率增大,反应速率常数也是先增大后减小,频率为100 Hz时,k值最大,为
0.00391 min−1,观察k值,也并没有随频率变化产生显著的变化,证实了脉冲频率对处理苯酚废水的反应速率影响较小.不同脉冲频率下的平均电流效率如图6(c)所示,在方波脉冲供电模式下,频率发生变化时,反应体系中平均电流值相差较小,平均电流效率也未发生明显变化,因此,调整脉冲频率只是改变单位时间内的放电数量,并不会显著影响反应体系中的平均电流效率.
如图6(d)所示,随着脉冲频率增大,比能耗呈现出先降低后升高的变化规律,与去除率的变化规律相反. 当频率为100 Hz时,比能耗最小. 但总体上,脉冲频率对降解苯酚的比能耗并没有产生显著影响,说明在较大的频率下,苯酚化合物的扩散并不能随电位发生显著变化[43]. 综上所述,当电压为4 V,脉冲占空比50%时,选择100 Hz作为最佳脉冲频率,此时去除效率最高,比能耗最低.
-
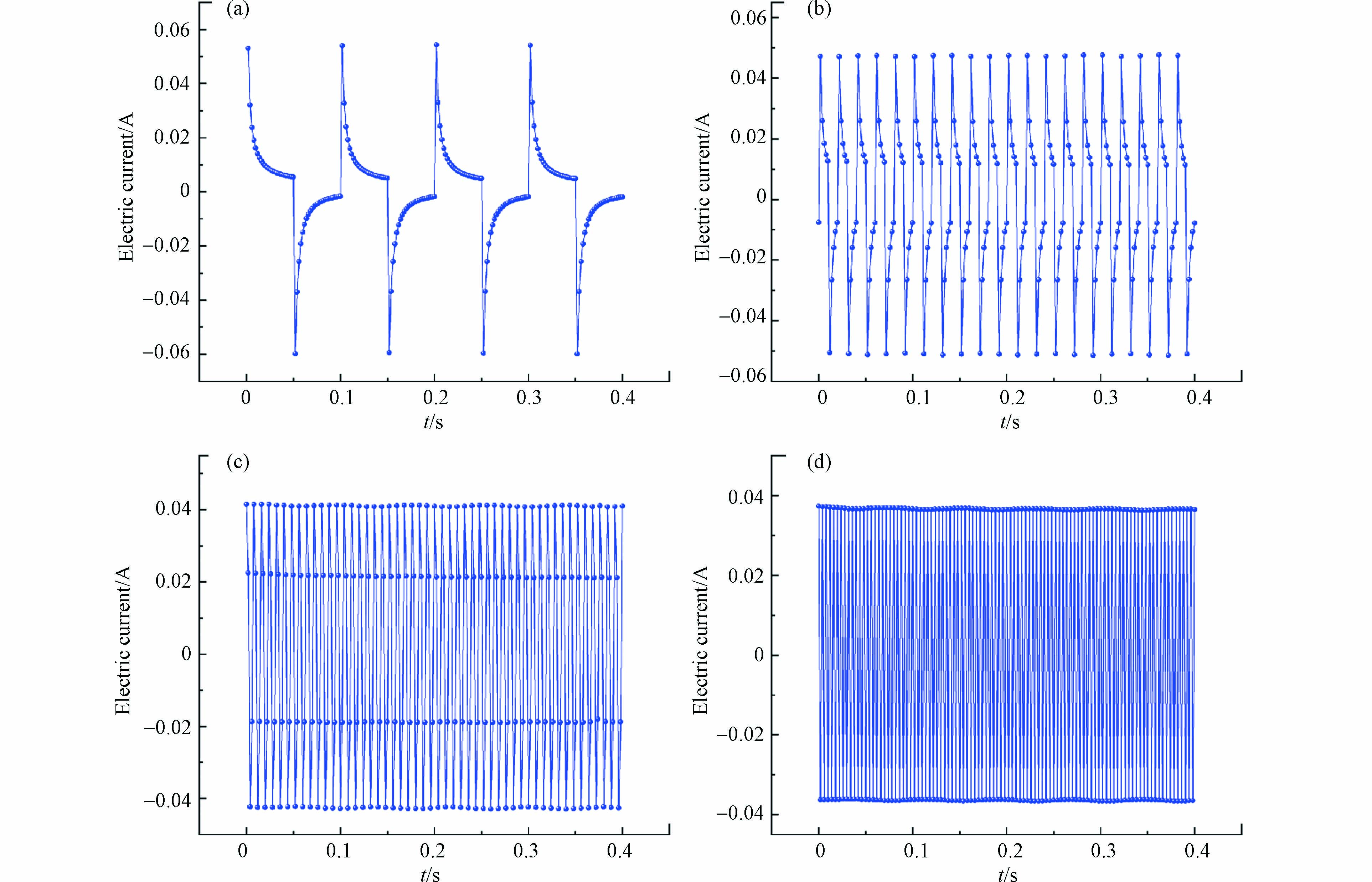
当直流电源持续向反应体系供电时,电流呈现相对稳定的范围内(约0.037 A),如图7a所示. 此时,阳极表面会形成扩散双电层(EDL),持续通电使得电解质溶液中非活性阴离子(SO42−)受电迁移作用,定向移动至阳极表面,阴离子的持续积累与污染物局部浓度降低会限制反应速率,而脉冲电场可以通过通电-断电-通电的特性使得污染物浓度得到补偿来缓解这一缺陷. 当外加脉冲电源,电压为4 V,占空比50%时,脉冲电流对电压的响应在−0.04—0.04 A范围内周期性振荡(图7b),在通电阶段电流约0—0.04 A,断电阶段时,电流方向发生变化. 以方波脉冲供电模式为例,通电阶段利用阳极表面得电子形成的活性自由基进行间接氧化,阳极由于外加电场作用带正电荷,电解质中的阴离子定向迁移到阳极,对EDL组成产生影响(图7c),而EDL在断电阶段易消失,扩散层厚度减少,由于传质作用污染物浓度得到补偿,与此同时,阴离子解吸回到电解质溶液中(图7d). 在脉冲供电模式下使得污染物浓度得到间歇的更新与补偿[45].
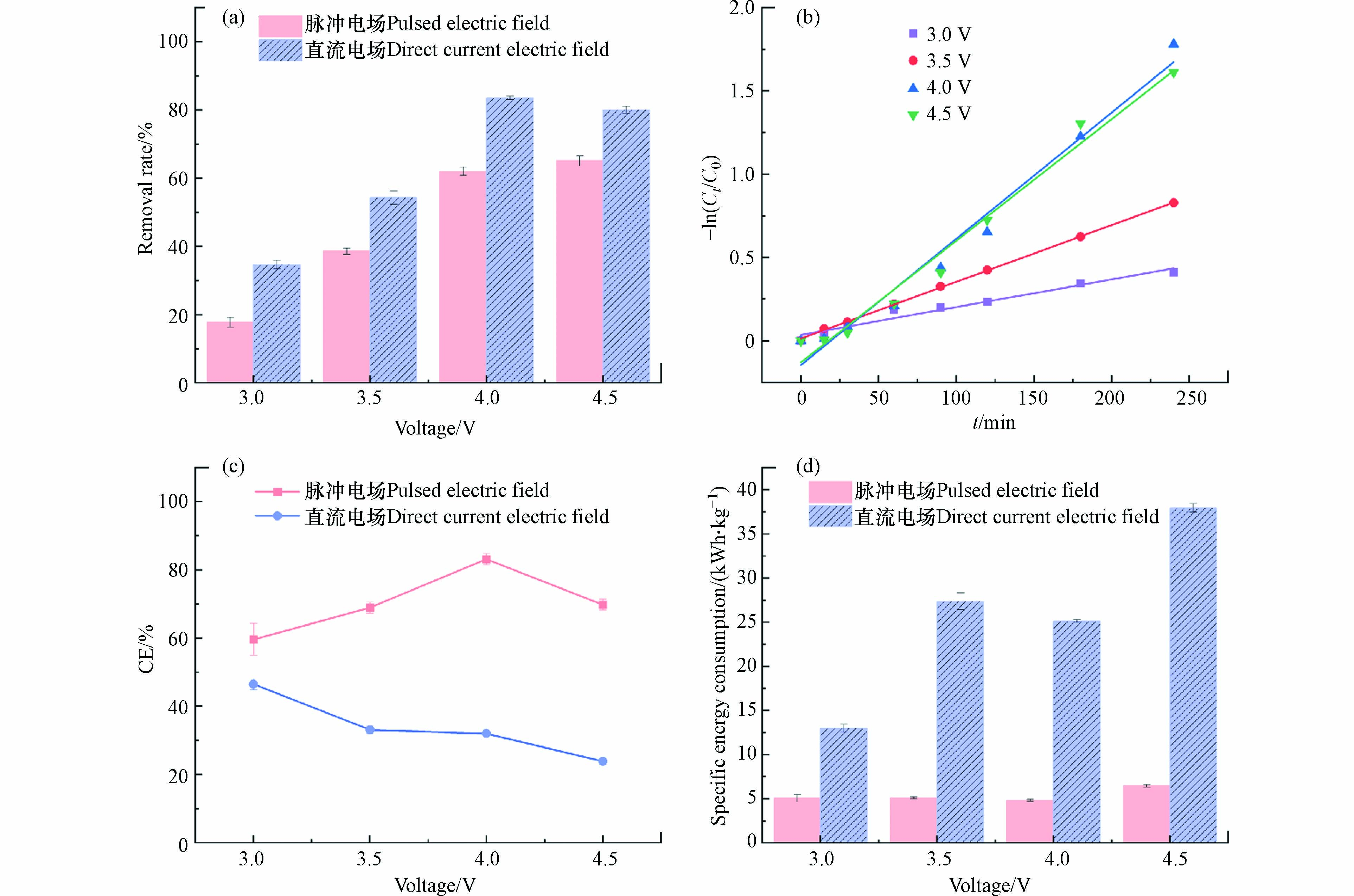
不同电压波形下苯酚的去除率如图8(a)所示,在直流供电模式下,随着电压的升高,去除率呈现先升高再降低的趋势,当电压为4.0 V下83.55%的去除率最高,而当电压进一步升高时,苯酚的去除率呈现降低的趋势,超出阳极的析氧电位,加剧副反应的发生,进而导致苯酚的去除率降低. 此外,由于副反应产生气泡附着于电极表面,降低了电极的活性面积,对苯酚的去除产生不良影响. 综合对比脉冲电场和直流电场对苯酚去除率的影响,当电压分别为3.0、3.5、4.0、4.5 V时,脉冲供电模式下苯酚的去除率是直流供电模式下去除率的51.97%、70.86%、74.34%、74.94%. 当电压4.0 V时,在脉冲供电模式下,经过4 h反应后苯酚的去除率达到62.05%;在直流供电模式下,4 h后苯酚的去除率达到83.55%. 尽管4 h后方波脉冲供电模式的苯酚去除率比直流供电的苯酚去除率低了21.52%,而脉冲供电模式下实际通电时间为2 h,而直流供电模式下苯酚的去除率仅有50.17%. 由此可知,就实际通电时间而言,脉冲供电模式下苯酚的去除率是高于直流供电模式的. 结果表明,在低电压阶段,直流电场和脉冲电场输入能量之比与去除率之比呈线性关系,这是因为,低电压时,体系中产生的自由基的数量较少,使得电化学处理苯酚废水的处理效率和能量利用效率偏低,此时影响去除速率和比能耗的主要因素是电压值. 而在较高反应电压阶段,直流电场和脉冲电场输入能量之比与去除率之比呈非线性关系,是因为随着电压的升高,反应速率加快,体系中能量利用效率也随之升高,此时反应体系中影响去除率和比能耗的主要因素是电压波形,即直流电场和脉冲电场.
从图8(b)和表4可以得出,直流供电模式下处理苯酚废水拟合曲线具有较好的相关性,证实苯酚的去除反应符合伪一级反应动力学规律. 随着电压的升高,反应常数先升高再降低,电压为4.0 V时,苯酚的降解速率最快. 对比表1,可知相同电压下,直流供电模式下的反应速率均高于脉冲供电模式.
不同电压波形下的平均电流效率如图8(c)所示,当电压相同时,脉冲供电模式下的平均电流效率始终高于直流供电模式下的平均电流效率,平均电流效率的差值随着电压的升高呈现先增大后减小的趋势. 低电压阶段,脉冲供电模式下的平均电流效率略高于直流供电模式下的平均电流效率,而在较高电压阶段下,平均电流效率相差较大. 因此,电压波形发生变化,平均电流效率也随之变化,进而影响反应体系中的传质推动力,影响苯酚的降解速率,导致苯酚的去除效果不同.
比能耗如图8(d)所示,方波脉冲供电模式下所需的比能耗远低于直流供电模式,当电压分别为3.0、3.5、4.0、4.5 V时,去除1 kg的苯酚,脉冲供电模式下所需能耗仅占直流供电模式的39.11%、18.65%、19.24%和17.07%. 由此可知,证明方波脉冲供电模式处理苯酚废水大大节约了能源,相比直流供电模式降解苯酚的效率更高,这是因为脉冲电流使副反应和浓度极化明显减少,提高了能源利用率,降低了化学成本. 在脉冲供电模式下,苯酚不仅可以在通电阶段发生降解,在断电阶段也会发生降解. 通电阶段产生的·OH氧化降解苯酚,使阳极附近污染物浓度逐渐降低,随后在断电阶段,阳极附近的污染物浓度因传质作用而增加,使得在通电阶段,产生的·OH与苯酚充分反应,·OH氧化成O2的副反应较弱. 而且在断电阶段,由于电容的存在,体系仍能放电,激发自由基进行氧化降解苯酚. 此外,脉冲供电模式下,在断电—通电的瞬间会产生强电流,因而能产生更多·OH氧化有机物,从而提高了苯酚的去除率. 因此,在脉冲供电模式下比能耗较低,另一方面,在直流供电模式下,阳极附近因为没有足够的污染物,使得·OH的利用率较低,从而产生较高的能耗,而在脉冲供电模式下,由于支持电解液的脉动补偿,使浓差极化减小,因此,与适当的脉冲间歇电流供电方式相比,连续电流供电方式很难获得更低能耗. 在脉冲供电模式下,电化学反应将实现较低的反应电位和能耗. 同时还可能由于在直流供电模式下通电时间过长易使电极损坏导致电极性能变差,且在电极上容易发生腐蚀,产生氧化膜,阻碍苯酚的降解,使得苯酚的去除效率降低,而脉冲供电模式通过“通电-断电-通电”方式避免电极受损,且减少氧化膜的产生,从而提高苯酚的降解效率.
-
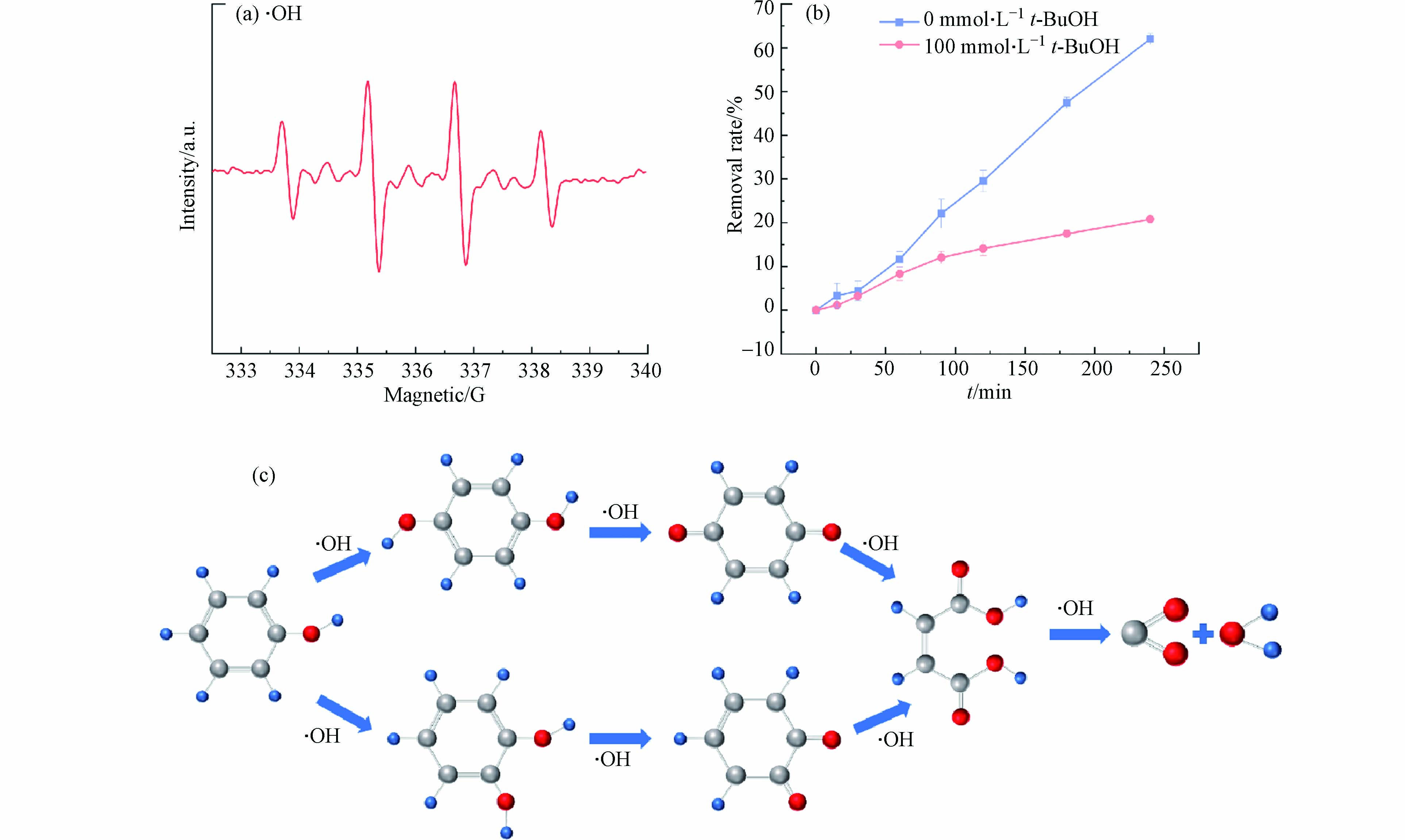
电化学降解苯酚的主要氧化反应是依靠电解过程中电极表面的水分子氧化而产生羟基自由基,进行氧化降解苯酚(式4、5). 通过放电产生瞬间寿命(小于10−9 s)的羟基自由基,在阳极表面附近与苯酚发生反应[46]. 利用电子自旋共振方法来测定电催化反应体系中活性物种的检测. 以5,5-二甲基-1-氧化吡咯啉(DMPO)作为自由基的捕获剂,证实反应体系中存在·OH(图9a). 以叔丁醇(t-BuOH)作为·OH淬灭剂,通过淬灭实验进一步验证·OH在电化学降解苯酚所起的作用. 如图9b所示,当反应体系中100 mmol·L−1 t-BuOH存在下,苯酚的去除率由62.50%下降到20.78%,进一步说明电化学降解苯酚主要依靠反应体系中的·OH. 与直流供电模式相比,脉冲供电并没有改变苯酚的氧化途径,但选择性更强,这是由于通电阶段产生的·OH氧化降解苯酚,而在断电阶段,阳极附近的苯酚受传质作用得到补偿,使得·OH可与苯酚充分反应,避免了副反应的发生.
通过高效液相色谱-质谱联用,推测苯酚降解的中间产物中可能存在邻苯二酚或对苯二酚,邻苯醌和对苯醌,顺丁烯二酸酐,顺丁烯二酸,反丁烯二酸和草酸等中间体. 推测电化学降解苯酚反应路径示意图如图9c所示,苯酚通过·OH进行电化学氧化降解,少部分羟基自由基将苯酚氧化后使苯酚首先羟基化,生成邻苯二酚,间苯二酚或对苯二酚,·OH可能将产物氧化成苯醌,随后苯环打开,最终降解为CO2和H2O.
-
通过开展方波脉冲电化学处理苯酚废水的研究,考察了电压波形、脉冲占空比、脉冲频率对苯酚去除率及能耗的影响规律,通过机理探究,厘清电化学降解苯酚的反应路径. 主要结论如下:
(1)通过考察脉冲电压、脉冲占空比、脉冲频率对苯酚去除率、平均电流效率和比能耗的影响规律,脉冲电压、脉冲占空比对去除率和比能耗影响较为显著,而脉冲频率的影响较小. 在脉冲电场条件下,适当的脉冲占空比条件下,可实现高效低耗的目的. 方波脉冲电化学处理苯酚废水的最佳工艺条件为:电压4.0 V,脉冲占空比50%,频率100 Hz,此时苯酚的去除率为62.03%,比能耗为4.84 kWh·kg−1.
(2)脉冲供电模式下实际通电时间为2 h,去除率达62.03%,而直流供电模式下苯酚的去除率仅有50.17%. 直流供电模式去除1 kg的苯酚需要25.14 kWh的能耗,而脉冲供电模式仅需要4.84 kWh(仅占直流供电模式的19.24%),由此可知,就实际通电时间而言,脉冲供电模式下苯酚的去除率是高于直流供电模式的,而比能耗相比直流供电模式明显降低,这是由于脉冲电流使副反应和浓度极化明显减少. 与直流供电模式相比,脉冲供电并没有改变苯酚的氧化途径,但选择性更强,减少副反应发生. 研究表明,脉冲供电模式的电能利用率更高,能耗更低,是一种经济可行的电化学氧化技术.
方波脉冲电化学处理含酚废水
Research on the process of square wave pulse electrochemical treatment of phenol-containing wastewater
-
摘要: 以绿色低耗去除酚类污染物为目标,开展方波脉冲电化学处理废水中酚类污染物的降解研究,考察方波脉冲体系(电压波形、脉冲占空比、脉冲频率等参数)对酚类污染物的去除率及比能耗的影响规律,提出最佳工艺及实验参数. 结果表明,在电化学反应体系中,脉冲电压、电压波形和脉冲占空比对去除率、平均电流效率和比能耗的影响较为显著,而脉冲频率的影响较小. 在脉冲电压4 V,占空比为50%,脉冲频率为100 Hz的最佳工艺条件下,对苯酚的去除率为62.03%,比能耗为4.84 kWh·kg−1. 因此,相比于直流供电模式,方波脉冲供电模式降解苯酚废水的电能利用率更高,能耗更低,选择性更强,为电化学处理废水提供了一种绿色环保的方法.Abstract: The degradation of phenolic pollutants in wastewater by square wave pulse electrochemical treatment was studied with the goal of green and low consumption removal of phenolic pollutants. The effects of square wave pulse system ( voltage waveform, pulse duty cycle, pulse frequency and other parameters ) on the removal rate and specific energy consumption of phenolic pollutants were investigated, and the optimum process and experimental parameters were proposed. The results show that the pulse voltage, voltage waveform and pulse duty cycle have significant effects on the removal rate, average current efficiency and specific energy consumption in the electrochemical reaction system, while the pulse frequency has little effect. Under the optimum conditions of pulse voltage 4 V, duty cycle 50% and pulse frequency 100 Hz, the removal rate of phenol was 62.03%, and the specific energy consumption was 4.84 kWh·kg−1. Therefore, compared with the DC power supply mode, the square wave pulse power supply mode has higher power utilization rate, lower energy consumption and stronger selectivity for the degradation of phenol wastewater, which provides a green and environmentally friendly method for electrochemical treatment of wastewater.
-
Key words:
- green low consumption /
- square wave pulse /
- electrochemical oxidation /
- duty cycle /
- frequency.
-

-
表 1 不同电压下苯酚去除的伪一级动力学拟合分析表
Table 1. Pseudo-first-order kinetic fitting analysis of phenol removal at different voltage
电压/V
Voltage拟合方程
Fitting equation反应常数k/min−1
Reaction constant拟合系数
R23.0 y= 0.00093 x+0.01899 0.00093 0.93826 3.5 y= 0.00212 x−0.01377 0.00212 0.99332 4.0 y= 0.00417 x−0.05806 0.00417 0.98183 4.5 y= 0.00442 x−0.07184 0.00442 0.97660 表 2 不同脉冲占空比下苯酚去除的伪一级动力学拟合分析表
Table 2. Pseudo-first-order kinetic fitting analysis of phenol removal at different pulse duty ratios
脉冲占空比/%
Pulse duty ratios拟合方程
Fitting equation反应常数k/min−1
Reaction constant拟合系数
R230 y= 0.00198 x+0.00173 0.00198 0.97025 50 y= 0.00417 x−0.05806 0.00417 0.98183 70 y= 0.00503 x−0.07707 0.00503 0.98306 90 y= 0.00598 x−0.08038 0.00598 0.98797 表 3 不同脉冲频率下苯酚去除的伪一级动力学拟合分析表
Table 3. Pseudo-first-order kinetic fitting analysis of phenol removal at different pulse frequencies
脉冲频率/Hz
Pulse frequencies拟合方程
Fitting equation反应常数k/min−1
Reaction constant拟合系数
R210 y= 0.00338 x−0.0735 0.00338 0.96333 50 y= 0.00359 x−0.06838 0.00359 0.97113 100 y= 0.00391 x−0.05932 0.00391 0.96189 200 y= 0.00384 x−0.07331 0.00384 0.96776 表 4 直流供电模式下不同电压苯酚去除的伪一级动力学拟合分析表
Table 4. Pseudo-level kinetic fitting analysis table for different voltage phenol removal in DC supply mode
电压/V
Voltage拟合方程
Fitting equation反应常数k/min−1
Reaction constant拟合系数
R23.0 y= 0.00166 x+0.03554 0.00166 0.96027 3.5 y= 0.00341 x+0.01281 0.00341 0.99934 4.0 y= 0.00758 x−0.14563 0.00758 0.97333 4.5 y= 0.00730 x−0.12982 0.0073 0.97619 -
[1] BELTRAN DE HEREDIA J, TORREGROSA J, DOMINGUEZ J R, et al. Kinetic model for phenolic compound oxidation by Fenton’s reagent[J]. Chemosphere, 2001, 45(1): 85-90. doi: 10.1016/S0045-6535(01)00056-X [2] CHENG S N, YUAN Z S, LEITCH M, et al. Highly efficient de-polymerization of organosolv lignin using a catalytic hydrothermal process and production of phenolic resins/adhesives with the depolymerized lignin as a substitute for phenol at a high substitution ratio[J]. Industrial Crops and Products, 2013, 44: 315-322. doi: 10.1016/j.indcrop.2012.10.033 [3] MICHALOWICZ J, DUDA W. Phenols- sources and toxicity[J]. Polish Journal of Environmental Studies, 2008, 16(3): 347-362. [4] MA H P, WANG H L, TIAN C C, et al. An integrated membrane- and thermal-based system for coal chemical wastewater treatment with near-zero liquid discharge[J]. Journal of Cleaner Production, 2021, 291: 125842. doi: 10.1016/j.jclepro.2021.125842 [5] LI B, HU X Q, LIU R X, et al. Occurrence and distribution of phthalic acid esters and phenols in Hun River Watersheds[J]. Environmental Earth Sciences, 2015, 73(9): 5095-5106. doi: 10.1007/s12665-015-4299-5 [6] CHEN K C, LIN Y H, CHEN W H, et al. Degradation of phenol by PAA-immobilized Candida tropicalis[J]. Enzyme and Microbial Technology, 2002, 31(4): 490-497. doi: 10.1016/S0141-0229(02)00148-5 [7] BIGLARI H, AFSHARNIA M, ALIPOUR V, et al. A review and investigation of the effect of nanophotocatalytic ozonation process for phenolic compound removal from real effluent of pulp and paper industry[J]. Environmental Science and Pollution Research, 2017, 24(4): 4105-4116. doi: 10.1007/s11356-016-8079-x [8] BAO K, YAN C C, NIU D L, et al. Persulfate oxidation enhanced extraction to improve the removal of high concentration phenol wastewater[J]. Environmental Science:Water Research & Technology, 2022, 8(5): 981-997. [9] XU R, ZHAO Y H, HAN Q Z, et al. Computer-aided blended extractant design and screening for co-extracting phenolic, polycyclic aromatic hydrocarbons and nitrogen heterocyclic compounds pollutants from coal chemical wastewater[J]. Journal of Cleaner Production, 2020, 277: 122334. doi: 10.1016/j.jclepro.2020.122334 [10] REN S, DENG J, MENG Z F, et al. Enhanced removal of phenol by novel magnetic bentonite composites modified with amphoteric-cationic surfactants[J]. Powder Technology, 2019, 356: 284-294. doi: 10.1016/j.powtec.2019.08.024 [11] STRIKWOLD M, SPENKELINK B, DE HAAN L H J, et al. Integrating in vitro data and physiologically based kinetic (PBK) modelling to assess the in vivo potential developmental toxicity of a series of phenols[J]. Archives of Toxicology, 2017, 91(5): 2119-2133. doi: 10.1007/s00204-016-1881-x [12] SUN J L, ZENG H, NI H G. Halogenated polycyclic aromatic hydrocarbons in the environment[J]. Chemosphere, 2013, 90(6): 1751-1759. doi: 10.1016/j.chemosphere.2012.10.094 [13] FERNÁNDEZ L, BORRÁS C, CARRERO H. Electrochemical behavior of phenol in alkaline media at hydrotalcite-like clay/anionic surfactants/glassy carbon modified electrode[J]. Electrochimica Acta, 2006, 52(3): 872-884. doi: 10.1016/j.electacta.2006.06.021 [14] NURDIN M, MAULIDIYAH M, WATONI A H, et al. Nanocomposite design of graphene modified TiO2 for electrochemical sensing in phenol detection[J]. Korean Journal of Chemical Engineering, 2022, 39(1): 209-215. doi: 10.1007/s11814-021-0938-6 [15] DUAN W Y, MENG F P, CUI H W, et al. Ecotoxicity of phenol and cresols to aquatic organisms: A review[J]. Ecotoxicology and Environmental Safety, 2018, 157: 441-456. doi: 10.1016/j.ecoenv.2018.03.089 [16] GONG Y, DING P, XU M J, et al. Biodegradation of phenol by a halotolerant versatile yeast Candida tropicalis SDP-1 in wastewater and soil under high salinity conditions[J]. Journal of Environmental Management, 2021, 289: 112525. doi: 10.1016/j.jenvman.2021.112525 [17] WANG Y X, SUN H Q, DUAN X G, et al. A new magnetic nano zero-valent iron encapsulated in carbon spheres for oxidative degradation of phenol[J]. Applied Catalysis B:Environmental, 2015, 172/173: 73-81. doi: 10.1016/j.apcatb.2015.02.016 [18] van BREUKELEN B M. Quantifying the degradation and dilution contribution to natural attenuation of contaminants by means of an open system Rayleigh equation[J]. Environmental Science & Technology, 2007, 41(14): 4980-4985. [19] XU H J, LI Y Z, GAO L J, et al. Planned heating control strategy and thermodynamic modeling of a natural gas thermal desorption system for contaminated soil[J]. Energies, 2020, 13(3): 642. doi: 10.3390/en13030642 [20] ERTO A, BORTONE I, DI NARDO A, et al. Permeable Adsorptive Barrier (PAB) for the remediation of groundwater simultaneously contaminated by some chlorinated organic compounds[J]. Journal of Environmental Management, 2014, 140: 111-119. [21] SUTTON N B, ATASHGAHI S, VAN DER WAL J, et al. Microbial dynamics during and after in situ chemical oxidation of chlorinated solvents[J]. Ground Water, 2015, 53(2): 261-270. doi: 10.1111/gwat.12209 [22] DOMINGUEZ C M, ROMERO A, LORENZO D, et al. Thermally activated persulfate for the chemical oxidation of chlorinated organic compounds in groundwater[J]. Journal of Environmental Management, 2020, 261: 110240. doi: 10.1016/j.jenvman.2020.110240 [23] CHENG M, ZENG G M, HUANG D L, et al. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review[J]. Chemical Engineering Journal, 2016, 284: 582-598. doi: 10.1016/j.cej.2015.09.001 [24] DOMINGUEZ C M, OTURAN N, ROMERO A, et al. Removal of organochlorine pesticides from lindane production wastes by electrochemical oxidation[J]. Environmental Science and Pollution Research, 2018, 25(35): 34985-34994. doi: 10.1007/s11356-018-1425-4 [25] ZHANG H Y, YUAN X Z, XIONG T, et al. Bioremediation of co-contaminated soil with heavy metals and pesticides: Influence factors, mechanisms and evaluation methods[J]. Chemical Engineering Journal, 2020, 398: 125657. doi: 10.1016/j.cej.2020.125657 [26] OSSAI I C, AHMED A, HASSAN A, et al. Remediation of soil and water contaminated with petroleum hydrocarbon: A review[J]. Environmental Technology & Innovation, 2020, 17: 100526. [27] ZHANG J, ZHAO Y, LIU Y Q, et al. Pd/PANI/Ti composite electrocatalyst with efficient electrocatalytic performance: Synthesis, characterization, stability, kinetic studies, and degradation mechanism[J]. Journal of Alloys and Compounds, 2022, 902: 163723. doi: 10.1016/j.jallcom.2022.163723 [28] HASSAN M F, SABRI M A, FAZAL H, et al. Recent trends in activated carbon fibers production from various precursors and applications—a comparative review[J]. Journal of Analytical and Applied Pyrolysis, 2020, 145: 104715. doi: 10.1016/j.jaap.2019.104715 [29] MUHAMAD M H, SHEIKH ABDULLAH S R, MOHAMAD A B, et al. Application of response surface methodology (RSM) for optimisation of COD, NH3–N and 2, 4-DCP removal from recycled paper wastewater in a pilot-scale granular activated carbon sequencing batch biofilm reactor (GAC-SBBR)[J]. Journal of Environmental Management, 2013, 121: 179-190. [30] Dell' ARMI E, ZEPPILLI M, Di FRANCA M L, et al. Evaluation of a bioelectrochemical reductive/oxidative sequential process for chlorinated aliphatic hydrocarbons (CAHs) removal from a real contaminated groundwater[J]. Journal of Water Process Engineering, 2022, 49: 103101. doi: 10.1016/j.jwpe.2022.103101 [31] DU C M, SHANG C, WANG T, et al. Study of the process and mechanism of the remediation of phenol contaminated soil by plasma vibrated bed[J]. Plasma Chemistry and Plasma Processing, 2017, 37(6): 1635-1653. doi: 10.1007/s11090-017-9850-6 [32] FALLAHPOUR N, MAO X H, RAJIC L, et al. Electrochemical dechlorination of trichloroethylene in the presence of natural organic matter, metal ions and nitrates in a simulated Karst media[J]. Journal of Environmental Chemical Engineering, 2017, 5(1): 240-245. doi: 10.1016/j.jece.2016.11.046 [33] YANG K X, KONG Y J, HUANG L Z, et al. Catalytic elimination of chlorinated organic pollutants by emerging single-atom catalysts[J]. Chemical Engineering Journal, 2022, 450: 138467. doi: 10.1016/j.cej.2022.138467 [34] XIE J Z, ZHANG C Y, WAITE T D. Hydroxyl radicals in anodic oxidation systems: Generation, identification and quantification[J]. Water Research, 2022, 217: 118425. doi: 10.1016/j.watres.2022.118425 [35] ROJO A, HANSEN H K, MONÁRDEZ O. Electrokinetic remediation of mine tailings by applying a pulsed variable electric field[J]. Minerals Engineering, 2014, 55: 52-56. doi: 10.1016/j.mineng.2013.09.004 [36] XU J W, LIU C, HSU P C, et al. Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry[J]. Nature Communications, 2019, 10: 2440. doi: 10.1038/s41467-019-10472-x [37] HRISTOVA D, BETOVA I, TZVETKOFF T. An electrochemical and analytical characterization of surface films on AISI 316 as electrode material for pulse electrolysis of water[J]. International Journal of Hydrogen Energy, 2013, 38(20): 8232-8243. doi: 10.1016/j.ijhydene.2013.04.127 [38] RYU B G, YANG J S, KIM D H, et al. Pulsed electrokinetic removal of Cd and Zn from fine-grained soil[J]. Journal of Applied Electrochemistry, 2010, 40(6): 1039-1047. doi: 10.1007/s10800-009-0046-5 [39] GENG Z N, LIU B, LI G H, et al. Enhancing DNAPL removal from low permeability zone using electrical resistance heating with pulsed direct current[J]. Journal of Hazardous Materials, 2021, 413: 125455. doi: 10.1016/j.jhazmat.2021.125455 [40] JUHL A D. Flipping the switch on anodizing[J]. Products Finishing, 2012, 76(6): 28-31. [41] SHU J C, SUN X L, LIU R L, et al. Enhanced electrokinetic remediation of manganese and ammonia nitrogen from electrolytic manganese residue using pulsed electric field in different enhancement agents[J]. Ecotoxicology and Environmental Safety, 2019, 171: 523-529. doi: 10.1016/j.ecoenv.2019.01.025 [42] RYU B G, PARK S W, BAEK K, et al. Pulsed electrokinetic decontamination of agricultural lands around abandoned mines contaminated with heavy metals[J]. Separation Science and Technology, 2009, 44(10): 2421-2436. doi: 10.1080/01496390902983778 [43] WEI J J, ZHU X P, NI J R. Electrochemical oxidation of phenol at boron-doped diamond electrode in pulse current mode[J]. Electrochimica Acta, 2011, 56(15): 5310-5315. doi: 10.1016/j.electacta.2011.04.006 [44] LU Z H, TANG J L, de LOURDES MENDOZA M, et al. Electrochemical decrease of sulfide in sewage by pulsed power supply[J]. Journal of Electroanalytical Chemistry, 2015, 745: 37-43. doi: 10.1016/j.jelechem.2015.02.014 [45] PEI S Z, YOU S J, ZHANG J N. Application of pulsed electrochemistry to enhanced water decontamination[J]. ACS ES& T Engineering, 2021, 1(11): 1502-1508. [46] YUAN C, CHEN C Y, HUNG C H. Electrochemical remediation of BPA in a soil matrix by Pd/Ti and RuO2/Ti electrodes[J]. Journal of Applied Electrochemistry, 2013, 43(12): 1163-1174. doi: 10.1007/s10800-013-0600-z -



 下载:
下载: