-
频繁的工业生产活动排放了越来越多成分复杂的废水,导致了严重的环境问题,造成了极大的环境压力。工业过程产生的废水中大多都含有毒有害的污染物,不适合直接利用传统的污水处理技术进行处置。当前,膜分离、吸附、混凝等技术只能将污染物富集或从水中物理分离,而不能将其降解或矿化。化学氧化技术尽管能将有机污染物转化或矿化,但有可能产生毒性更强的中间产物而带来二次污染。
以半导体催化剂(TiO2、ZnO、Fe2O3)为基础的光催化技术能对持久性有机污染物部分或完全分解,甚至可以彻底矿化成CO2、H2O、
${\rm{NO}}_3^- $ ${\rm{PO}}_4^{3-} $ 2-甲基咪唑锌(又称ZIF-8或MAF-4)是一种具类沸石结构的MOF材料,由中山大学陈小明院士课题组首先合成并报道[8]。研究表明,ZIF-8能通过多种方法制备,例如溶剂热(N,N-二甲基甲酰胺、甲醇为溶剂)、微波辅助、声化学、机械化学、干凝胶等[9]。ZIF-8具有比表面积大、孔径尺寸较小(用于分子筛的ZIF-8的有效孔径在4.0 nm至4.2 nm的范围内[10])等特点,因此可以对H2和CH4等气体进行分离。然而,研究显示,动力学直径大于4.2 nm的大分子也能被ZIF-8缓慢吸附并进入孔笼,表明其结构具有柔性[11]。由于ZIF-8独特的结构和性能,使其在气体存储[12]、吸附与分离[13-17]、(光)催化[18-19]、药物缓释[20]等领域广泛应用。目前,越来越多的研究者对ZIF-8光催化性能产生兴趣,王崇臣课题组首先发现ZIF-8在紫外线照射下可实现光催化降解有机染料亚甲基蓝,基于质谱分析数据分析亚甲基蓝的光催化降解路径,并根据活性物质的种类研究了其光催化反应机理[21]。但是,ZIF-8因具有宽带隙(Eg = 5.1 eV)而仅能被紫外光激发。为进一步增强ZIF-8的光催化性能,相关研究者将其与半导体光催化剂进行复合,使所得到的复合物在可见光照射下能表现出令人满意的光催化性能。
本文将选择一些典型的ZIF-8复合物,介绍其制备的方法,并详细探讨其光催化还原Cr(Ⅵ)和光催化降解染料、农药、药物及个人护理品(PPCPs)的性能与机理,总结其优势与不足,以期为相关研究者提供系统的文献梳理与总结。
-
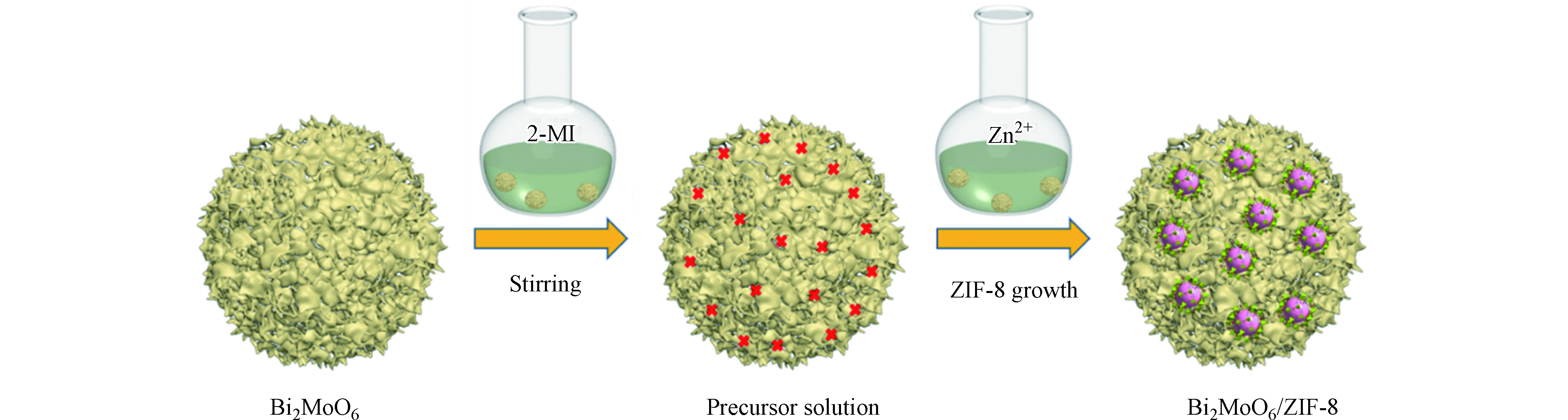
目前,ZIF-8复合物的合成大多是采用原位法。将功能材料均匀溶解/分散于Zn2+(或2-甲基咪唑)的溶液中,然后再引入2-甲基咪唑(或Zn2+),持续搅拌或超声处理,使ZIF-8均匀地与功能材料复合。比如,Xia等[22]用原位自组装法将ZIF-8与金属氧化物Bi2MoO6复合制备了Bi2MoO6/ZIF-8复合材料。制备过程如图1所示,将合成的花状Bi2MoO6球加入到含有2-甲基咪唑的甲醇溶液中,搅拌分散。将Zn(NO3)2·6H2O分散在甲醇中,并与上述溶液混合后充分搅拌。通过离心收集样品,用甲醇洗涤,最后干燥即可获得Bi2MoO6/ZIF-8复合材料。
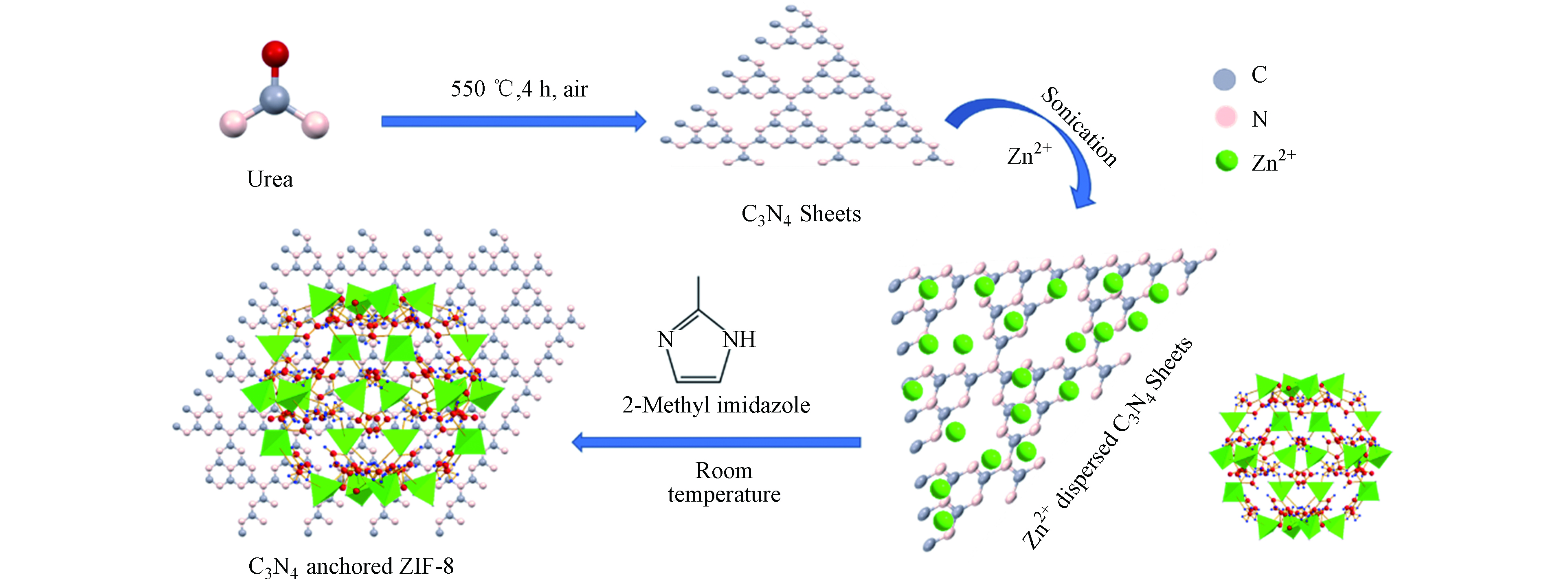
Panneri等[23]通过原位合成C3N4-ZIF-8 (ZC)复合材料的过程如图2所示。在合成中,通过超声处理将C3N4分散在蒸馏水中。将硝酸锌水溶液添加到C3N4分散液中,并将混合物超声处理。然后,将上述溶液加入2-甲基咪唑水溶液中,在室温下保持搅拌一段时间后制得C3N4-ZIF-8 (ZC)复合材料。
-
ZIF-8复合材料在光催化还原Cr(Ⅵ)、降解PPCPs、降解染料废水和有机农药等方面取得了一些进展。本文将结合近年来利用ZIF-8复合物光催化去除水体污染物的案例(表1),来详细阐述一些有代表性的研究,并着重论述一些典型的研究工作。
-
中国是全球最大的金属/类金属生产国和消费国之一。矿产资源的开采对环境造成严重影响,不仅会影响大气[49]、水体和土壤质量,还会通过水-土-气-食物链威胁动物和人类的健康[50]。在所有有毒重金属离子中,铬被广泛用于皮革、电镀、印刷、颜料、抛光和其他行业[51-52]。因此,其是地表水和地下水中的最常见污染物之一,在水生系统和饮用水中的少量存在即可大概率增加腹泻、肝癌、肾癌和皮肤癌的威胁。水体中的铬主要以低毒形式的Cr(Ⅲ)和高毒的Cr(Ⅵ)形式存在。含Cr(Ⅵ)废水的有效处理方法之一是将其转化为Cr(Ⅲ),因为Cr(Ⅲ)不但毒性很低,并且容易以固体形式沉淀和去除。使用光催化剂可将Cr(Ⅵ)还原为Cr(Ⅲ),具有效率较高、成本更低、不产生任何有害物质等优点[53-54]。MOFs及其复合物作为一种新兴的功能性无机-有机杂化材料,是光催化去除水体污染物的备选光催化剂之一。
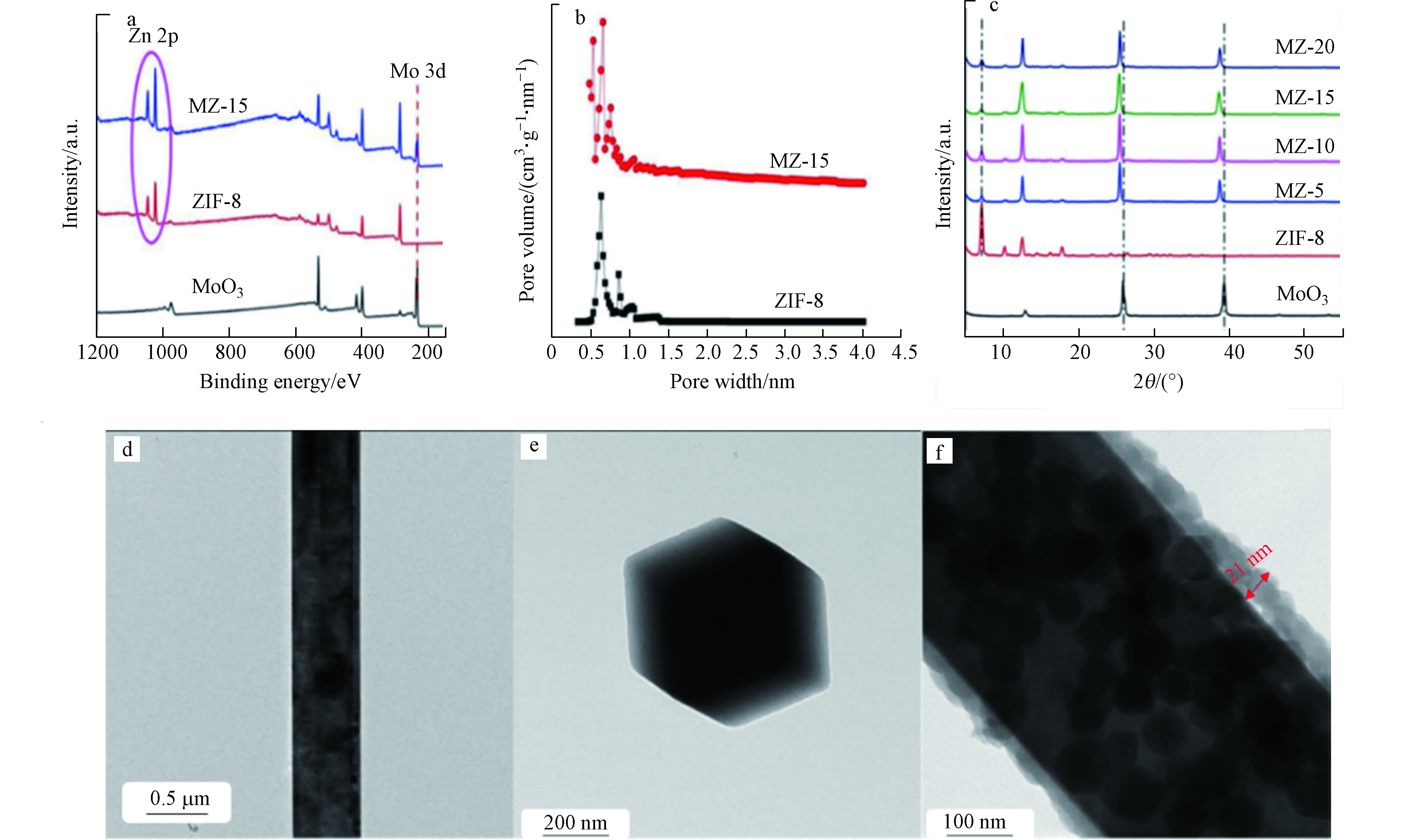
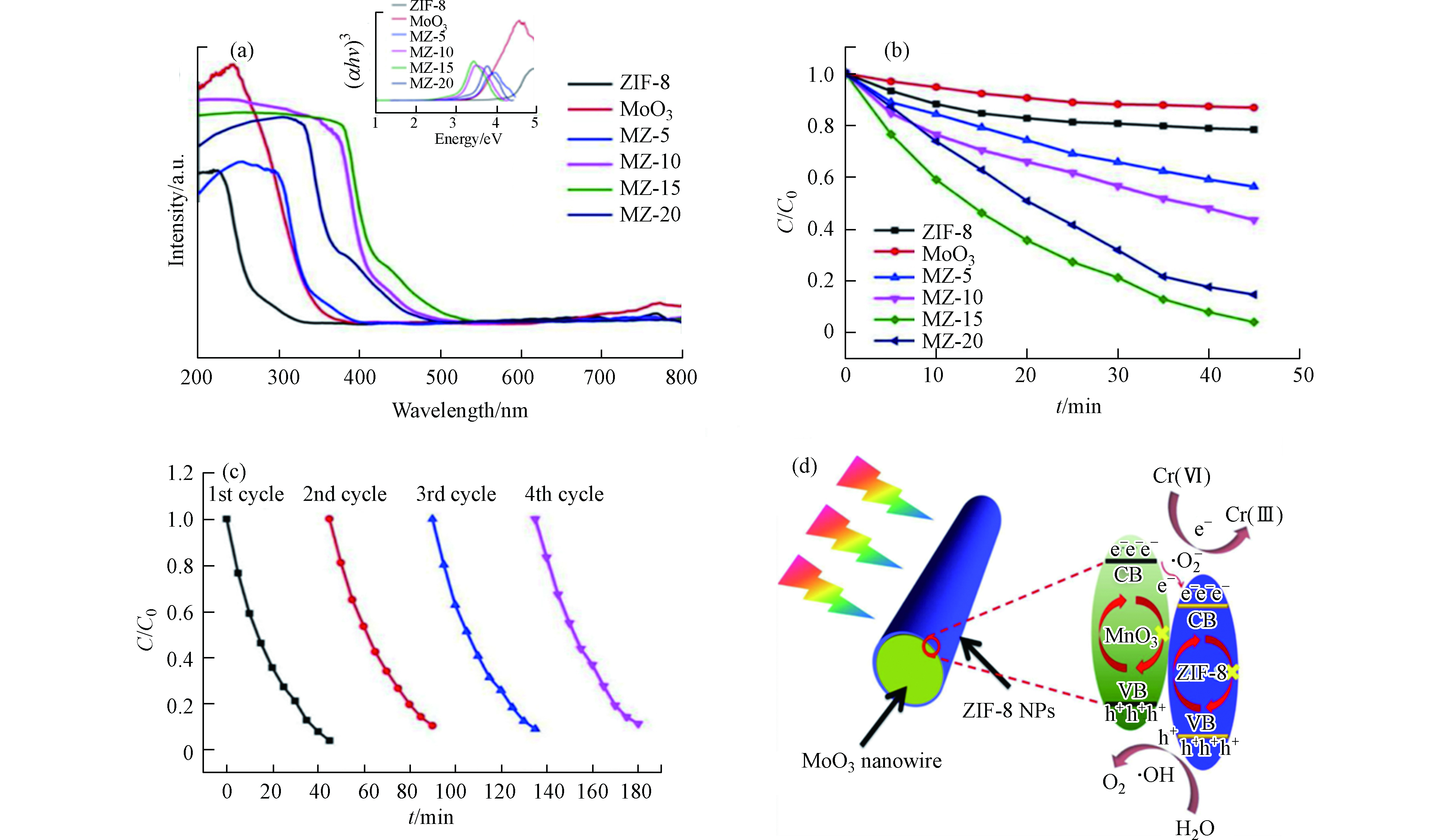
Zhang等制备了三维(3D) MoO3@ZIF-8(MZ-x)核-壳纳米棒复合光催化剂,并将其用于光催化还原Cr(Ⅵ) [41]。通过X-射线光电子能谱(XPS)测定所制备样品的化学状态(如图3a所示)可知,在ZIF-8和MZ-15中均观察到Zn 2p峰,同时在MoO3和MZ-15中观察到Mo 3d峰,这表明MoO3@ZIF-8(MZ-15)核壳纳米棒的成功制备。图3b显示ZIF-8的比表面积高达1531.1 m2·g−1,意味着在后续光催化反应过程中能提供更多活性位点。复合材料MZ-15的XRD衍射峰与原材料基本一致(如图3c所示),但也观察到部分衍射峰显示向较低衍射角度明显偏移,这可归因于逐渐增大的层间间距。在沉积ZIF-8纳米颗粒后,复合物中MoO3的平行原子平面之间的距离明显大于原始的MoO3纳米线中相应的平面距离,从侧面证明了ZIF-8已成功沉积在MoO3纳米线上。图3d和3e显示了MoO3纳米线和ZIF-8 NP的TEM图像。此外,HRTEM还展示了MoO3@ZIF-8的核壳型纳米棒(MZ-15) (图3f),有利于电荷/质量转移和光吸收。
紫外-可见漫反射光谱(图4a)表明所制备MZ-x样品的光吸收特性随着ZIF-8含量的逐渐增强,这表明ZIF-8的引入可提高MoO3的吸光能力,从而有望提高其光催化活性。
图4b表明所有制备的MZ-x均具有光催化还原Cr(Ⅵ)的能力。MZ-15在40 min内对初始浓度为20 mg·L−1的Cr(Ⅵ)还原效率达96%,远高于其他催化剂。经过4个循环后,MZ-15的光催化活性几乎保持不变(图4c),表明在反应过程中催化剂组分和晶体结构稳定。基于上述实验结果,作者提出了增强MZ-15光催化活性的机理机制(如图4d所示)。制备的MZ-15中ZIF-8与MoO3形成的异质结,可有效提高电荷分离效率。更多来自MoO3导带(CB)的光生电子可转移到ZIF-8上,加速了Cr(Ⅵ)向Cr(Ⅲ)的还原过程。换句话说,单纯的MoO3也可被光照射激发产生光生电子(e−)和光生空穴(h+)。
但是,在没有ZIF-8 NP的情况下,e−和h+容易复合。ZIF-8引入后,MoO3与ZIF-8之间的协同效应将增强复合材料的电子利用能力和光催化还原活性。
-
从纺织、造纸和染料工业中排放出来的有色废水含有许多有机染料类化学污染物,容易导致环境和健康问题[55]。有机染料的存在会干扰细胞的正常功能,进而极有可能导致动物的生理和生化机制的变化,引起重要功能(如呼吸、渗透调节、繁殖甚至死亡)的损害。现有研究已经表明,ZIF-8复合物可通过光催化降解过程有效处理含有机染料的有色废水[25, 56]。
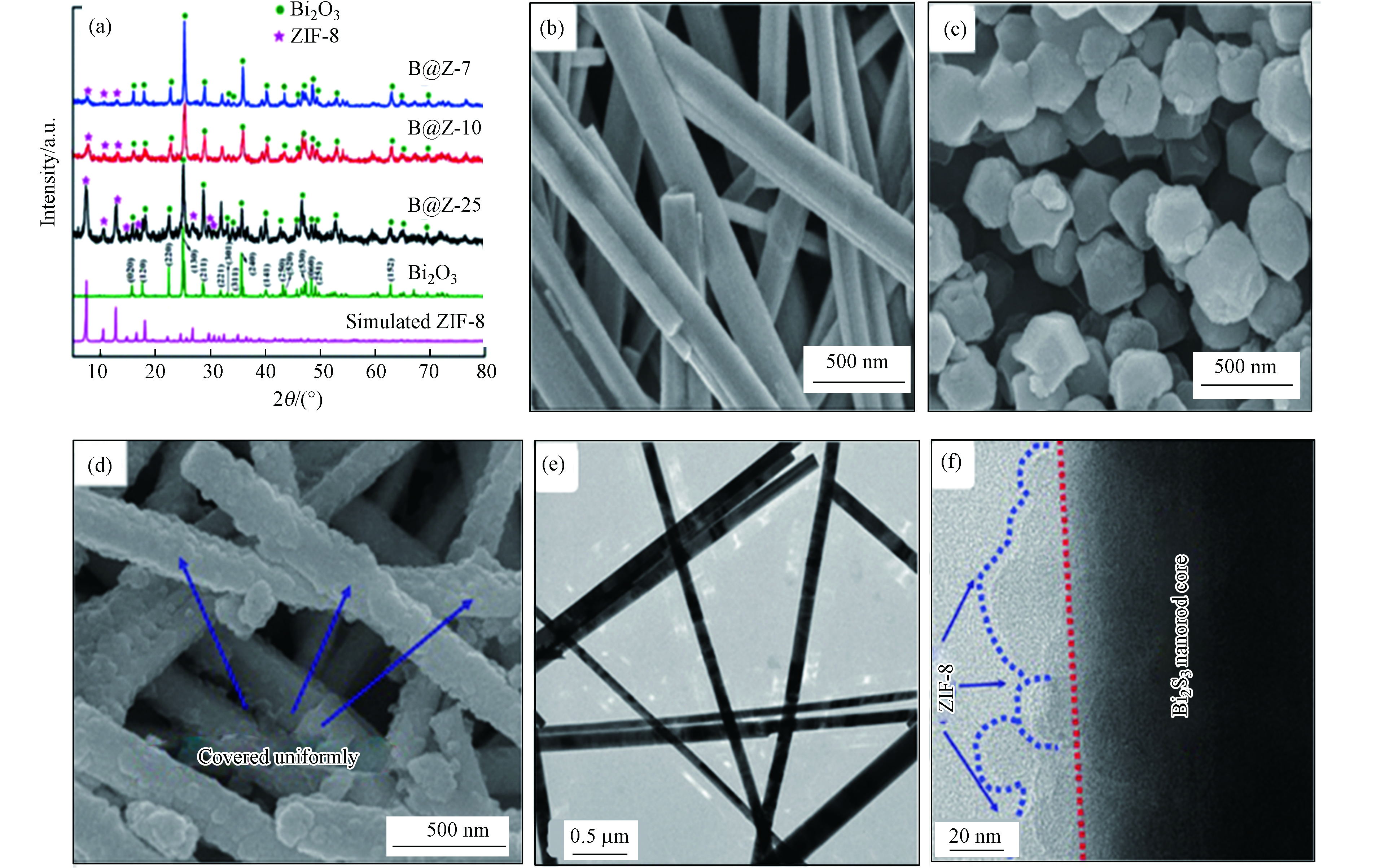
Ding等[37]通过简单有效的自组装策略原位制备了具核-壳异质结构Bi2S3@ZIF-8(B@Z-x)。与原始的Bi2S3纳米棒相比,核-壳Bi2S3@ZIF-8异质结在室温下可实现高效光催化罗丹明B (Rhb)。如图5a所示,B@Z-x复合物的XRD谱图和Bi2S3标准谱图及ZIF-8拟合谱图吻合较好,表明复合物成功制备,且具有较高结晶度。从SEM表征结果来看,合成后的Bi2S3样品呈现为规则的纳米棒(如图5b所示),具有光滑的表面和良好的结晶度。ZIF-8纳米晶体显示出六边形的形态(如图5c所示)。比如,在B@Z-10复合材料(图5d)中Bi2S3仍保持纳米棒特征,但因其表面被ZIF-8纳米晶体牢固包裹而显得表面粗糙(如图5e-f所示)。研究表明B@Z-10复合物中Bi2S3和ZIF-8之间形成的Bi-S-Zn化学键有效提高光生电子的转移速率。
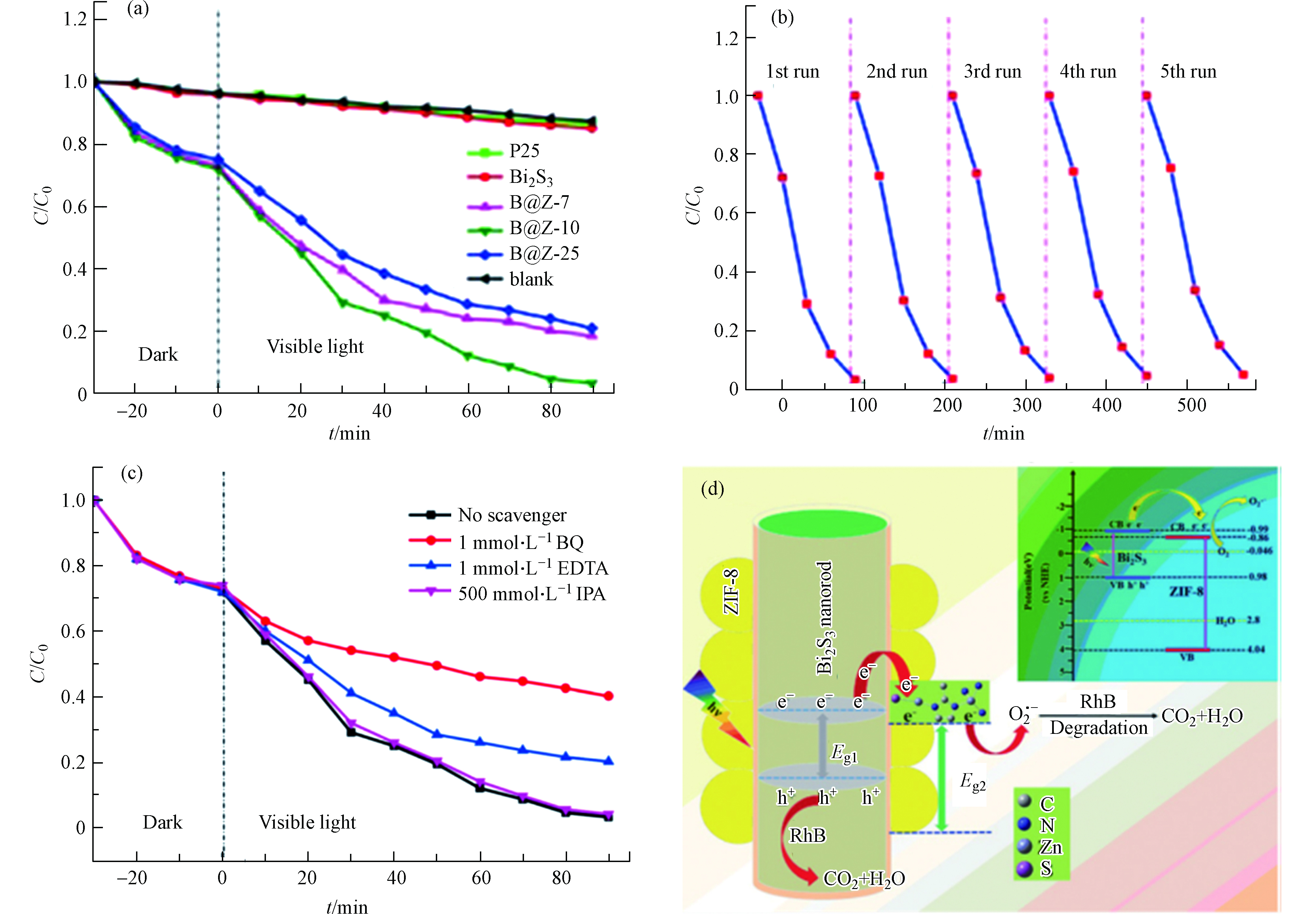
B@Z在可见光照射下降解有机染料RhB的光催化活性如图6a所示。其中B@Z-10复合材料的光催化降解效率最好,在90 min内能将初始浓度为10 mg·L−1的RhB降解97%(同样时间内,无催化剂的空白实验自降解效率为11%)。此外,与单纯Bi2S3和市售Degussa P25样品相比,所有B@Z复合材料均显示出良好的光催化活性,表明Bi2S3和ZIF-8复合具有很大的潜在优势。作者以B@Z-10为例研究了催化剂的稳定性和可重复使用性(如图6b所示)。经过5轮循环实验,B@Z-10的光催化降解RhB的效率仅降低了5%,表明复合物B@Z-10具有良好的水稳定性和可循环利用性。众所周知,超氧自由基(O2·−)、空穴(h+)和羟基自由基(·OH)是光催化反应过程的三种主要活性物质。为了解B@Z-10的光催化机理,作者进行了活性物质捕捉实验(如图6c所示)。添加IPA(羟基自由基捕捉剂)后,RhB降解速率几乎未发生变化,表明·OH不是该体系光催化反应中的关键活性物质。添加BQ(超氧自由基捕捉剂)和EDTA(空穴捕捉剂)后,RhB的降解效率明显降低,表明O2·−和h+是该光催化系统中的主要活性物质。因此,作者提出了B@Z-10光催化剂可能的光催化机理(图6d)。Bi2S3易于被可见光激发产生e−和h+。Bi2S3的CB明显比ZIF-8的CB负,因此Bi2S3纳米棒上的光生电子倾向于通过Bi-S-Zn共价界面转移到ZIF-8的CB。结果,ZIF-8的ECB(−0.86)明显比E0(O2/O2·−)(0.046 eV vs NHE)更负,因此光生电子可以结合溶解氧在ZIF-8上生成O2·−。通常,O2·−几乎不会扩散到本体溶液中攻击污染物,只有那些吸附在催化剂表面的RhB才能被有效降解。在B@Z-10异质结构中,ZIF-8的多孔性有利于RhB分子的快速运输,从而促进O2·−高效利用。这种方式可有效地实现e−和h+的分离,因此B@Z-10复合材料具有更高的光催化性能。
-
作为农业大国,中国是当今世界最大的农药生产和消费国之一[57]。与其他许多发展中国家一样,过去大力鼓励使用农药来提高农业产量,农业活动中大量使用的农药造成地表水和地下水污染,而且多数残留农药在环境中能持久存在,可通过食物链/网进行传递并进行生物富集,实现长距离转移[58],对人类健康构成重大威胁[59]。寻找经济有效的方法去除环境中的有毒、有害农药残留成为亟待解决的环境问题。
为有效去除农药,且快速将催化剂从环境中分离,Joubani等[46]通过将戊二酸酐作为官能团修饰在Fe3O4表面成功地制备了磁性纳米颗粒Fe3O4-COOH。以MNP(磁性纳米颗粒)为模板,诱导ZIF-8在其表面生长。将核壳结构的Fe3O4-COOH@ZIF-8与磷酸银(Ag3PO4)、Ag纳米颗粒(Ag NPs)结合,制备了可受可见光激发的Fe3O4-COOH@ZIF-8/Ag/Ag3PO4光催化剂,并在可见光照射下对有机磷农药二嗪农进行了高效光催化降解。SEM和TEM结果表明,所制备的Fe3O4-COOH@ZIF-8为球形,平均直径约为300 nm;其中,Fe3O4-COOH@ZIF-8主要的形貌是以Fe3O4为核,ZIF-8为壳,厚度约15 nm。但是,形成Fe3O4-COOH@ZIF-8/Ag/Ag3PO4后,Fe3O4-COOH@ZIF-8核-壳和位于ZIF-8壳上的Ag/Ag3PO4 NPs的形态没有明显变化。
如表2所示,在没有光催化剂的条件下,二嗪农杀虫剂并未发生明显降解行为,表明该农药具有较强的稳定性。Fe3O4-COOH@ZIF-8/Ag/Ag3PO4 光催化剂对二嗪农杀虫剂的降解效率最佳(去除率99.7%),物理混合粉末样品的杀虫剂去除率(48.9%)低于Ag/Ag3PO4 NPs (86.3%)和Fe3O4-COOH@ZIF-8/Ag/Ag3PO4 NC (99.7%)的去除率。对活性物质进行了捕捉实验测试,结果表明光催化活性物质主要是h+和·
${\rm{O}}_2^- $ ${\rm{O}}_2^- $ -
近几十年来,由于药物和个人护理产品(PPCPs)的广泛使用而导致其在水生环境中持续存在,已成为影响生态环境和人类健康不容忽视的新兴污染物[60]。PPCPs可以通过多种途径释放到水环境中,包括生活污水、医院排放物、制药废水等。与生活污水相比,医院和制药企业污水一般具有更高的检测频率和药物浓度。排放的PPCPs可能会在水生基质中保留其原始浓度和结构,或者被动转化为其他活性(或非活性)化合物。由于近年来经济发展和人口增长的原因,中国已然成为PPCPs生产和消费量很高的国家,这可能对生态系统和人类健康带来极大的潜在威胁[61]。抗生素作为PPCPs的重要一类在我国长期滥用,被不断排入水体并以残留形式在水中持续存在[62]。
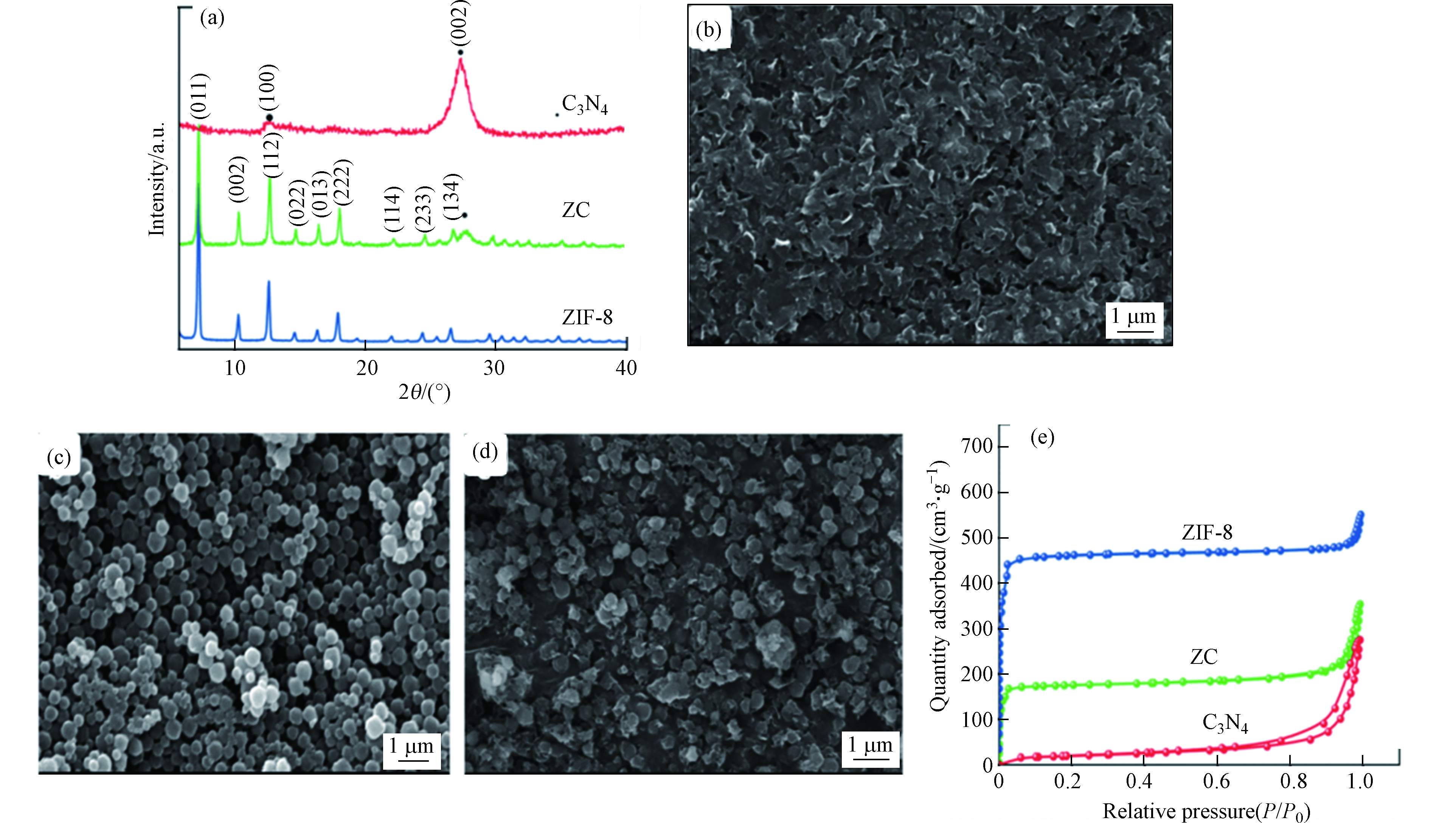
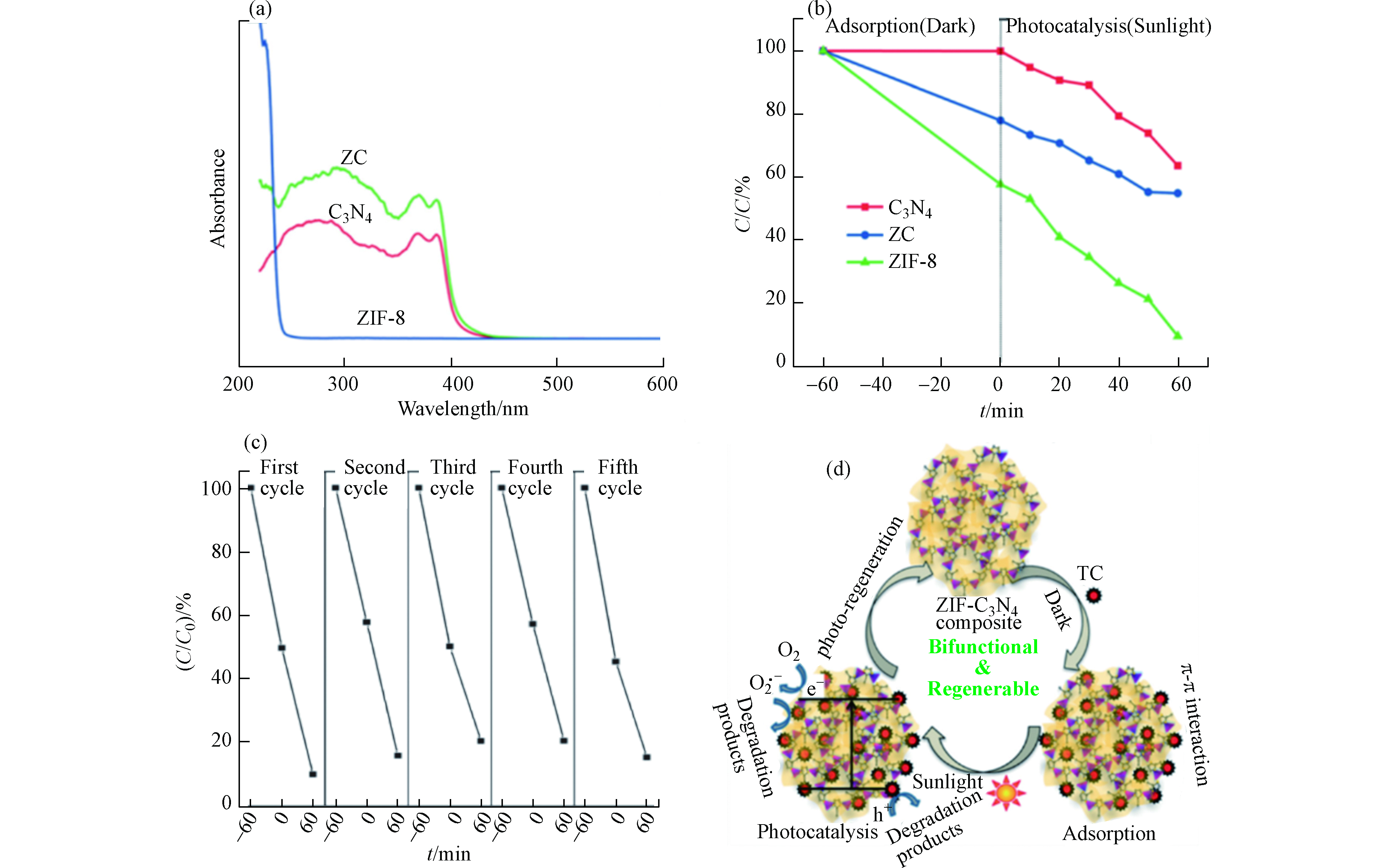
Panneri等[23]通过将C3N4与ZIF-8复合开发了双功能复合材料(ZC),用于从溶液中高效吸附和光催化降解四环素(TC)。从样品的粉末X-射线衍射(PXRD)图可以看出,样品ZC呈现出代表g-C3N4和ZIF-8成分的峰,表明纳米复合材料的形成(如图7a所示)。利用扫描电镜对制备样品的显微结构特征进行了分析,观察到典型的片层状形貌(图7b),ZIF-8为高度结晶的菱形十二面体晶体,尺寸为400 nm左右(图7c)。图7d的SEM显微相片揭示了在C3N4基体中ZIF-8晶体的原位形成。其中ZIF-8晶体被C3N4纳米片所包裹。对样品进行N2吸附-解吸分析,探究了其孔隙特征(图7e),结果表明ZIF-8和C3N4两种多孔结构在复合材料中共存。
图8a所示的紫外-可见吸收光谱显示了各种样品的光学性能。g-C3N4是可见光激发的光催化剂,而ZIF-8是一种紫外光激发的光催化剂。该复合材料ZC表现出一种混合光吸收性质,并显示轻微红移。纯C3N4、ZIF-8和ZC复合材料对TC的降解效率如图8b所示。单独的C3N4对TC的吸附微不足道,降解效率也不高, 在120 min内仅有30%的TC被降解。这主要归因于C3N4纳米片的比表面积较小且光生电子-空穴复合率较高。ZIF-8对TC的吸附效率为25%,在120 min内降解效率为60%。而复合物ZC对TC的吸附效率高达45%,且在120 min内对TC的降解率为90%。可见光响应的光催化剂C3N4和高比表面积、紫外光响应的催化剂ZIF-8的有效复合可实现协同作用而有助于同时增强吸附和光催化性能。复合物ZC能被循环使用5次,其间,对TC的吸附量保持在45%—55%之间,其光催化降解率从91%下降到86%。这表明其具有良好的水稳定性和可循环利用性(图8c)。作者提出一种可能的吸附和光催化机理如图8d所述。复合材料ZC对TC具有良好的吸附效果,能将TC吸附在催化剂的活性位点上。在可见光照射下,溶液中未被吸附的TC与吸附在催化剂表面的TC将进一步被光催化降解。复合材料ZC中稳定的微介孔结构为增强吸附和随后的光催化活性提供了大量的活性位点,从而进一步促进了吸附过程。此外,ZC复合材料对可见光吸收能力的增加也提高了其光催化性能和效率。在该反应体系中,主导光催化过程的活性物质是h+、O2·−和·OH。在可见光照射下,复合物ZC表面产生e−和h+,并分别于溶解氧分子和水分子反应,产生O2·−和·OH。在该反应体系中,光催化活性增强的可能机制为:在光线照射下,ZIF-8的CB中的e−被转移到C3N4的相对较低的CB中,并与O2反应并形成O2·−。这些O2·−自由基使TC分子降解。另一方面,与I型异质结构不同,由于ZIF-8和C3N4的VB电位非常接近,所以ZIF-8的VB中的h+直接降解TC分子,而不会移动到C3N4的VB上。因此,由于这些成分之间的协同作用,致使e−和h+复合机会减少,光催化活性增强。
-
从当前的研究进展来看,ZIF-8及其复合物的高效合成可不借助有毒有害的有机溶剂而仅使用甲醇、乙醇甚至水做溶剂即可实现。由于其具备制备成本较低、合成方法简便、耗时短且其生物兼容性较好等优点,ZIF-8及其复合物作为多功能新材料已经在吸附、分离、光催化、传感和药物缓释等多个领域取得不凡的表现。但是,ZIF-8带隙大、只能被紫外光激发,因此与功能材料复合拓宽ZIF-8对光的吸收效率及电子转移效率是制备ZIF-8系列复合材料的研究热点。虽然越来越多的研究证实ZIF-8复合材料在水处理尤其是光催化去除污染物领域具有巨大的应用前景,但是当下的研究还处于实验室阶段,如何更加高效地使用ZIF-8复合材料光催化去除水体污染物,并大规模推广到工业水处理中,仍然是当前科研工作者面临的难题。同时,研发新型多功能ZIF-8复合物材料来光催化降解多种水体污染物也将是具有挑战性和现实意义的科研方向。为进一步增强ZIF-8使其在水环境中能持久发挥作用,引入疏水基团/材料提高其水稳定性也是未来值得研究的方向。另外,研究者还应关注ZIF-8及其复合物材料在内的MOFs材料在环境中的迁移、转化、对应的环境效应和生物毒性,同时使用过的MOFs材料的再利用和处置问题也应该加以关注,避免MOFs材料带来二次污染问题。
Photocatalytic removal of water pollutants in ZIF-8 composites
- Received Date: 14/03/2021
- Available Online: 27/07/2022
-
Key words:
- metal-organic framework /
- ZIF-8 composite /
- photocatalysis /
- Cr(Ⅵ) reduction /
- organic pollutant degradation
Abstract: Metal-organic frameworks and its composite materials have been paid more and more attention in the field of water treatment due to their unique structure and excellent properties. In this review, the general synthesis methods of ZIF-8 composite materials and the research progress of photocatalytic removal of various pollutants from water are discussed in detail, aiming to provide a convenient overview for the research direction of photocatalytic removal of pollutants from water by ZIF-8 composite materials. According to the research status and progress of ZIF-8 composites, the development trend of its photocatalytic removal of environmental pollutants is prospected in this paper.




 DownLoad:
DownLoad:
